Abstract
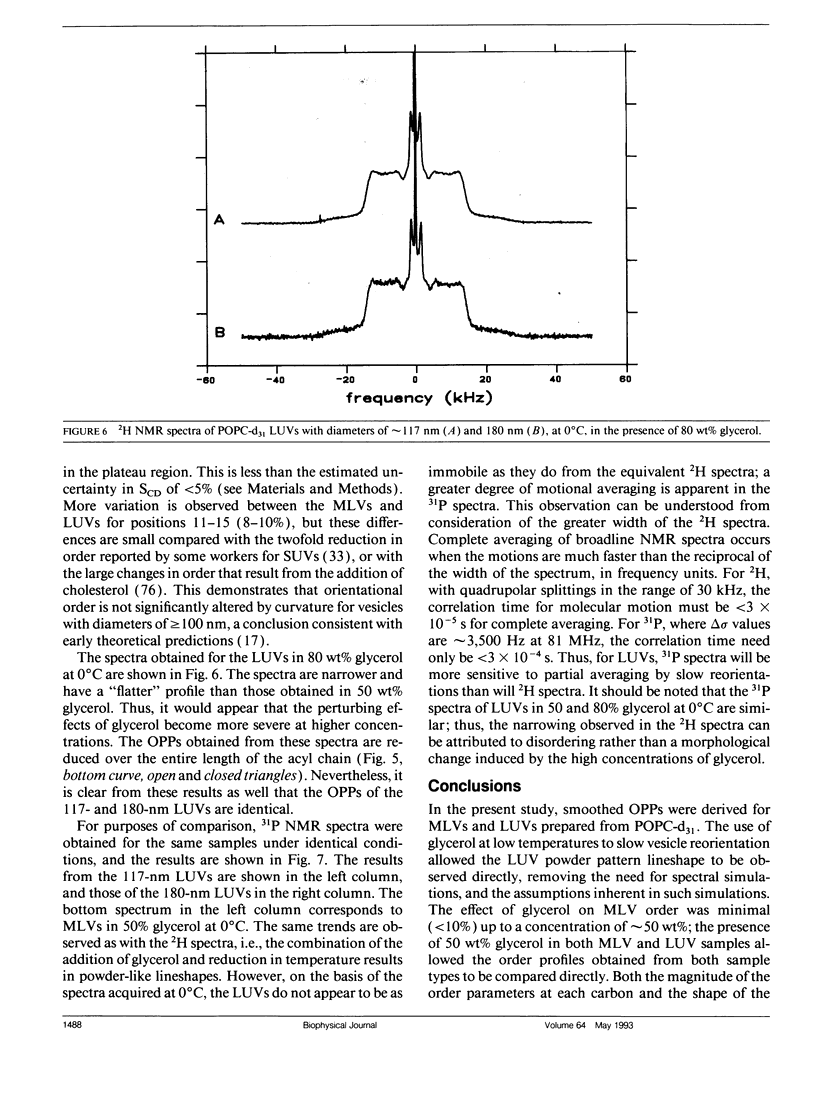
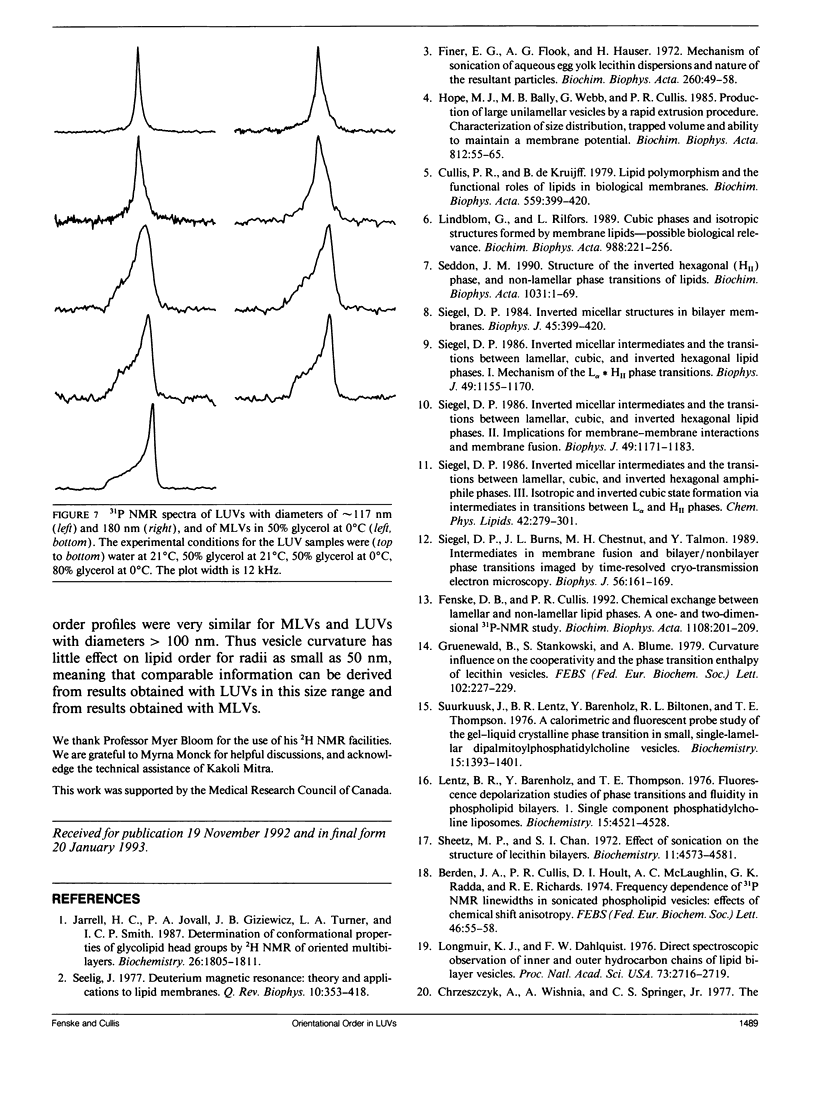
Large unilamellar vesicles (LUVs) composed of 1-[2H31]palmitoyl-2-oleoyl phosphatidylcholine (POPC-d31), with diameters of approximately 117 +/- 31 and 180 +/- 44 nm, were prepared by extrusion through polycarbonate filters with pore sizes of 0.1 and 0.2 microns, respectively. The 2H nuclear magnetic resonance (NMR) spectra obtained at 21 degrees C contain two components: a broad component (approximately 17 kHz linewidth) corresponding to the methylene groups and a narrower component originating from the methyl groups. Spectra with increasing powder pattern characteristics were obtained by reducing the rate of phospholipid reorientations by addition of glycerol (to increase the solvent viscosity) and by lowering the temperature. Full powder spectra, characteristic of liquid-crystalline bilayers, were obtained for both LUV samples at 0 degrees C in the presence of 50 wt% glycerol. Individual quadrupolar splittings were not resolved in these spectra, due to broader linewidths in the LUVs, which have significantly shorter values for spin-spin relaxation time T2 measured from the decay of the quadrupolar echo (90 microseconds) than the multilmellar vesicles (MLVs; 540 microseconds). Smoothed order parameter profiles (OPPs) were obtained for these samples by integration of the dePaked spectra. The OPPs were very similar to the OPP of POPC-d31 MLVs in 50 wt% glycerol at the same temperature, indicating that orientational order in MLVs and LUVs with a diameter of > or = 100 nm is essentially the same. The presence of 80 wt% glycerol was found to have a disordering effect on the vesicles.
Full text
PDF

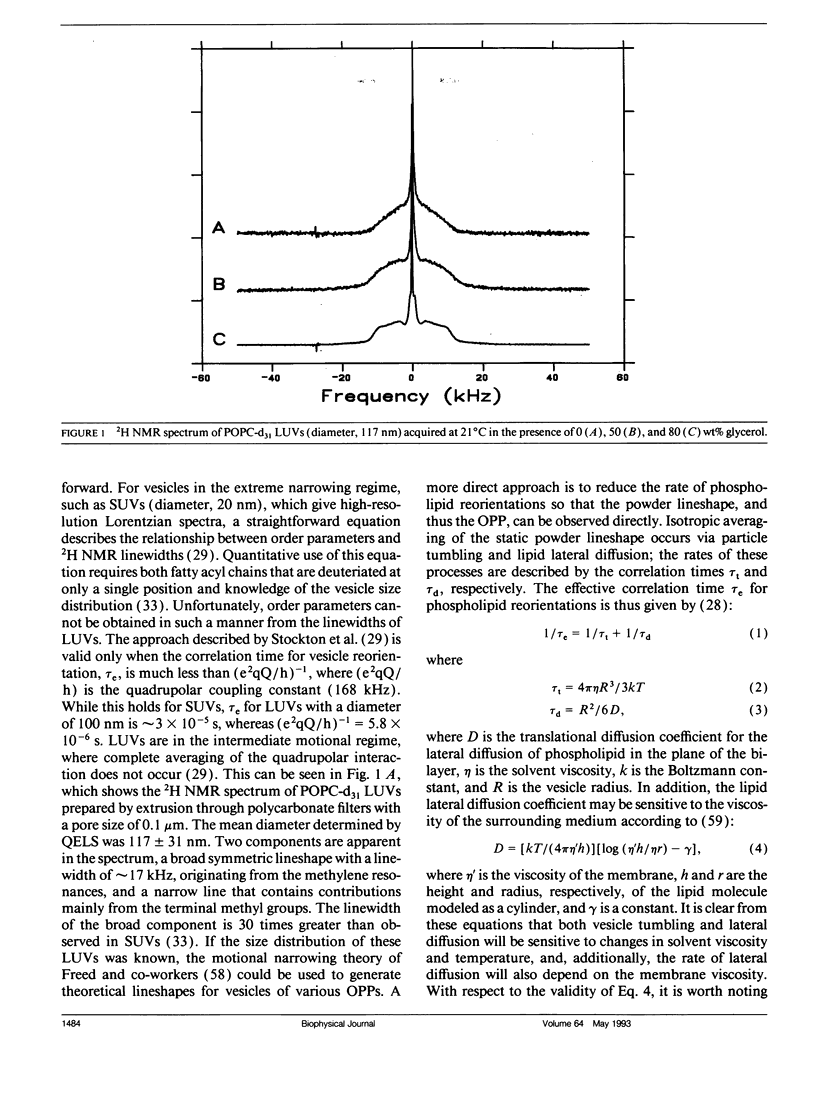
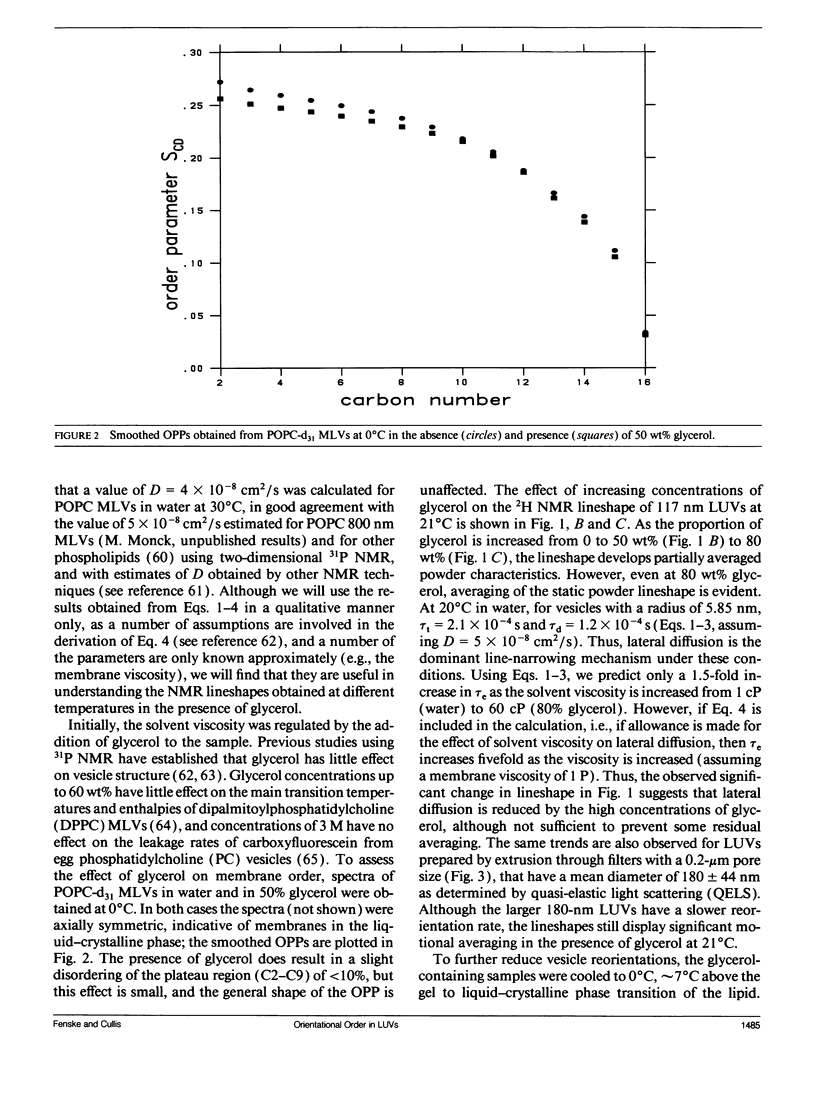
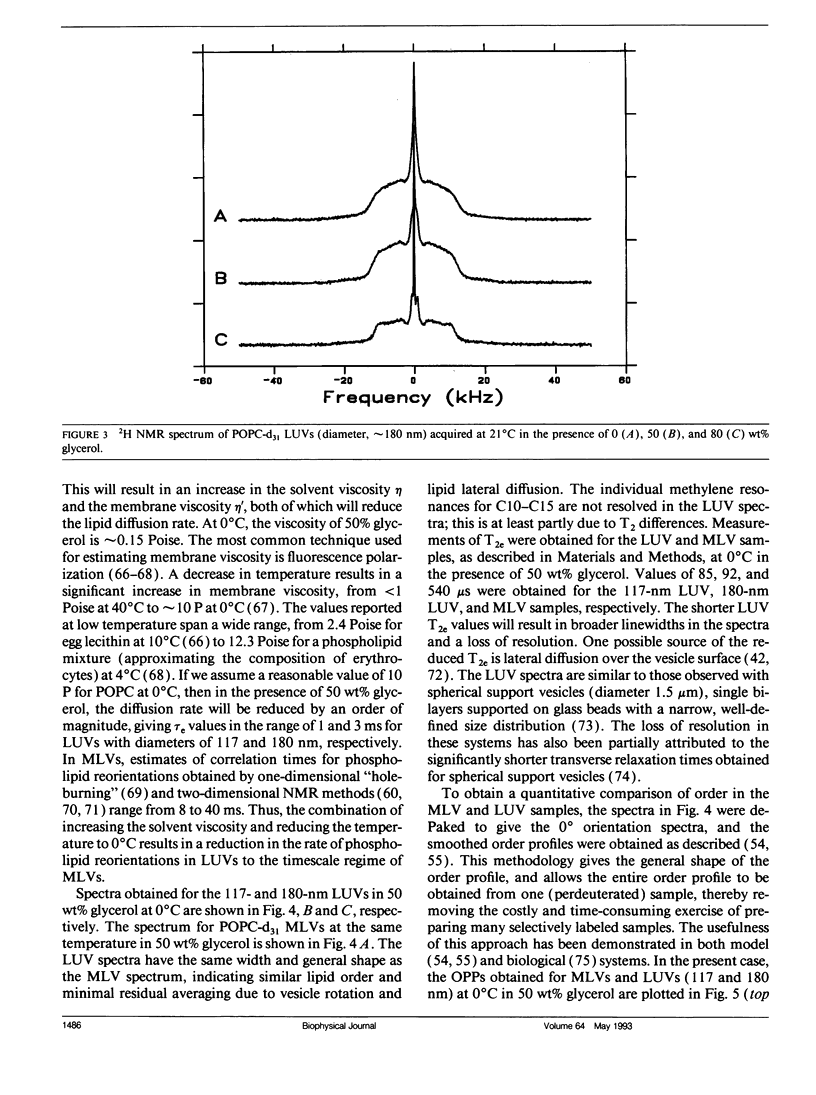
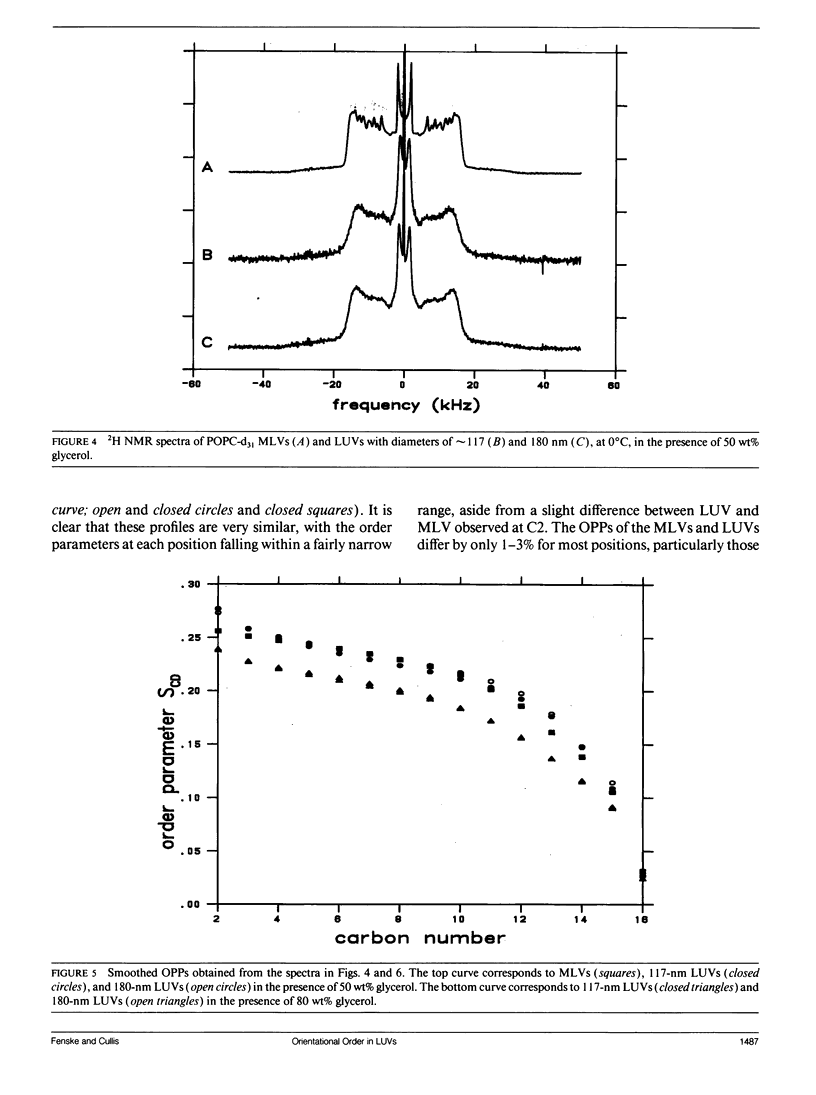


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anchordoguy T. J., Carpenter J. F., Cecchini C. A., Crowe J. H., Crowe L. M. Effects of protein perturbants on phospholipid bilayers. Arch Biochem Biophys. 1990 Dec;283(2):356–361. doi: 10.1016/0003-9861(90)90654-h. [DOI] [PubMed] [Google Scholar]
- Andrasko J., Frosén S. NMR study of rapid water diffusion across lipid bilayers in dipalmitoyl lecithin vesicles. Biochem Biophys Res Commun. 1974 Sep 23;60(2):813–819. doi: 10.1016/0006-291x(74)90313-1. [DOI] [PubMed] [Google Scholar]
- Auger M., Smith I. C., Jarrell H. C. Slow motions in lipid bilayers. Direct detection by two-dimensional solid-state deuterium nuclear magnetic resonance. Biophys J. 1991 Jan;59(1):31–38. doi: 10.1016/S0006-3495(91)82195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayerl T. M., Bloom M. Physical properties of single phospholipid bilayers adsorbed to micro glass beads. A new vesicular model system studied by 2H-nuclear magnetic resonance. Biophys J. 1990 Aug;58(2):357–362. doi: 10.1016/S0006-3495(90)82382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berden J. A., Cullis P. R., Hoult D. I., McLaughlin A. C., Radda G. K., Richards R. E. Frequency dependence of 31P NMR linewidths in sonicated phospholipid vesicles: effects of chemical shift anisotropy. FEBS Lett. 1974 Sep 15;46(1):55–58. doi: 10.1016/0014-5793(74)80333-9. [DOI] [PubMed] [Google Scholar]
- Bloom M., Burnell E. E., MacKay A. L., Nichol C. P., Valic M. I., Weeks G. Fatty acyl chain order in lecithin model membranes determined from proton magnetic resonance. Biochemistry. 1978 Dec 26;17(26):5750–5762. doi: 10.1021/bi00619a024. [DOI] [PubMed] [Google Scholar]
- Bloom M., Evans E., Mouritsen O. G. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991 Aug;24(3):293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- Brumm T., Möps A., Dolainsky C., Brückner S., Bayerl T. M. Macroscopic orientation effects in broadline NMR-spectra of model membranes at high magnetic field strength: A method preventing such effects. Biophys J. 1992 Apr;61(4):1018–1024. doi: 10.1016/S0006-3495(92)81909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell E. E., Cullis P. R., de Kruijff B. Effects of tumbling and lateral diffusion on phosphatidylcholine model membrane 31P-NMR lineshapes. Biochim Biophys Acta. 1980 Dec 2;603(1):63–69. doi: 10.1016/0005-2736(80)90391-0. [DOI] [PubMed] [Google Scholar]
- Chrzeszczyk A., Wishnia A., Springer C. S., Jr The intrinsic structural asymmetry of highly curved phospholipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 17;470(2):161–169. doi: 10.1016/0005-2736(77)90097-9. [DOI] [PubMed] [Google Scholar]
- Cogan U., Shinitzky M., Weber G., Nishida T. Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes. Biochemistry. 1973 Jan 30;12(3):521–528. doi: 10.1021/bi00727a026. [DOI] [PubMed] [Google Scholar]
- Cullis P. R. Lateral diffusion rates of phosphatidylcholine in vesicle membranes: effects of cholesterol and hydrocarbon phase transitions. FEBS Lett. 1976 Nov;70(1):223–228. doi: 10.1016/0014-5793(76)80762-4. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Mayer C., Stohrer J., Althoff G., Kothe G. Dynamics of phosphate head groups in biomembranes. Comprehensive analysis using phosphorus-31 nuclear magnetic resonance lineshape and relaxation time measurements. Biophys J. 1992 Jan;61(1):42–57. doi: 10.1016/S0006-3495(92)81814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman S. J., Hope M. J., Cullis P. R. Transbilayer transport of phosphatidic acid in response to transmembrane pH gradients. Biochemistry. 1991 Feb 19;30(7):1740–1745. doi: 10.1021/bi00221a002. [DOI] [PubMed] [Google Scholar]
- Eastman S. J., Hope M. J., Wong K. F., Cullis P. R. Influence of phospholipid asymmetry on fusion between large unilamellar vesicles. Biochemistry. 1992 May 5;31(17):4262–4268. doi: 10.1021/bi00132a016. [DOI] [PubMed] [Google Scholar]
- Eigenberg K. E., Chan S. I. The effect of surface curvature on the head-group structure and phase transition properties of phospholipid bilayer vesicles. Biochim Biophys Acta. 1980 Jun 20;599(1):330–335. doi: 10.1016/0005-2736(80)90079-6. [DOI] [PubMed] [Google Scholar]
- Fenske D. B., Cullis P. R. Chemical exchange between lamellar and non-lamellar lipid phases. A one- and two-dimensional 31P-NMR study. Biochim Biophys Acta. 1992 Jul 27;1108(2):201–209. doi: 10.1016/0005-2736(92)90026-i. [DOI] [PubMed] [Google Scholar]
- Fenske D. B., Jarrell H. C. Phosphorus-31 two-dimensional solid-state exchange NMR. Application to model membrane and biological systems. Biophys J. 1991 Jan;59(1):55–69. doi: 10.1016/S0006-3495(91)82198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske D. B., Letellier M., Roy R., Smith I. C., Jarrell H. C. Effect of calcium on the dynamic behavior of sialylglycerolipids and phospholipids in mixed model membranes. A 2H and 31P NMR study. Biochemistry. 1991 Oct 29;30(43):10542–10550. doi: 10.1021/bi00107a025. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Flook A. G., Hauser H. Mechanism of sonication of aqueous egg yolk lecithin dispersions and nature of the resultant particles. Biochim Biophys Acta. 1972 Jan 27;260(1):49–58. doi: 10.1016/0005-2760(72)90073-2. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Flook A. G., Hauser H. The nature and origin of the NMR spectrum of unsonicated and sonicated aqueous egg yolk lecithin dispersions. Biochim Biophys Acta. 1972 Jan 27;260(1):59–69. doi: 10.1016/0005-2760(72)90074-4. [DOI] [PubMed] [Google Scholar]
- Griffin R. G. Solid state nuclear magnetic resonance of lipid bilayers. Methods Enzymol. 1981;72:108–174. doi: 10.1016/s0076-6879(81)72010-x. [DOI] [PubMed] [Google Scholar]
- Gruenewald B., Stankowski S., Blume A. Curvature influence on the cooperativity and the phase transition enthalpy of lecithin vesicles. FEBS Lett. 1979 Jun 15;102(2):227–229. doi: 10.1016/0014-5793(79)80006-x. [DOI] [PubMed] [Google Scholar]
- Huang C., Mason J. T. Geometric packing constraints in egg phosphatidylcholine vesicles. Proc Natl Acad Sci U S A. 1978 Jan;75(1):308–310. doi: 10.1073/pnas.75.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell H. C., Jovall P. A., Giziewicz J. B., Turner L. A., Smith I. C. Determination of conformational properties of glycolipid head groups by 2H NMR of oriented multibilayers. Biochemistry. 1987 Apr 7;26(7):1805–1811. doi: 10.1021/bi00381a003. [DOI] [PubMed] [Google Scholar]
- Kintanar A., Kunwar A. C., Oldfield E. Deuterium nuclear magnetic resonance spectroscopic study of the fluorescent probe diphenylhexatriene in model membrane systems. Biochemistry. 1986 Oct 21;25(21):6517–6524. doi: 10.1021/bi00369a027. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Ahkong Q. F., Brown R. D., 3rd, Lafleur M., Spiller M., Unger E., Tilcock C. Permeability of liposomal membranes to water: results from the magnetic field dependence of T1 of solvent protons in suspensions of vesicles with entrapped paramagnetic ions. Magn Reson Med. 1992 Feb;23(2):275–286. doi: 10.1002/mrm.1910230208. [DOI] [PubMed] [Google Scholar]
- Korstanje L. J., van Faassen E. E., Levine Y. K. Reorientational dynamics in lipid vesicles and liposomes studied with ESR: effects of hydration, curvature and unsaturation. Biochim Biophys Acta. 1989 Jul 10;982(2):196–204. doi: 10.1016/0005-2736(89)90055-2. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Cullis P. R., Bloom M. Modulation of the orientational order profile of the lipid acyl chain in the L alpha phase. Eur Biophys J. 1990;19(2):55–62. doi: 10.1007/BF00185086. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Cullis P. R., Fine B., Bloom M. Comparison of the orientational order of lipid chains in the L alpha and HII phases. Biochemistry. 1990 Sep 11;29(36):8325–8333. doi: 10.1021/bi00488a018. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Fine B., Sternin E., Cullis P. R., Bloom M. Smoothed orientational order profile of lipid bilayers by 2H-nuclear magnetic resonance. Biophys J. 1989 Nov;56(5):1037–1041. doi: 10.1016/S0006-3495(89)82749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4521–4528. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- Lepore L. S., Ellena J. F., Cafiso D. S. Comparison of the lipid acyl chain dynamics between small and large unilamellar vesicles. Biophys J. 1992 Mar;61(3):767–775. doi: 10.1016/S0006-3495(92)81881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmuir K. J., Dahlquist F. W. Direct spectroscopic observation of inner and outer hydrocarbon chains of lipid bilayer vesicles. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2716–2719. doi: 10.1073/pnas.73.8.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay A. L., Burnell E. E., Nichol C. P., Weeks G., Bloom M., Valic M. I. Effect of viscosity on the width of the methylene proton magnetic resonance line in sonicated phospholipid bilayer vesicles. FEBS Lett. 1978 Apr 1;88(1):97–100. doi: 10.1016/0014-5793(78)80616-4. [DOI] [PubMed] [Google Scholar]
- Monck M. A., Bloom M., Lafleur M., Lewis R. N., McElhaney R. N., Cullis P. R. Influence of lipid composition on the orientational order in Acholeplasma laidlawii strain B membranes: a deuterium NMR study. Biochemistry. 1992 Oct 20;31(41):10037–10043. doi: 10.1021/bi00156a025. [DOI] [PubMed] [Google Scholar]
- Ostro M. J., Cullis P. R. Use of liposomes as injectable-drug delivery systems. Am J Hosp Pharm. 1989 Aug;46(8):1576–1587. [PubMed] [Google Scholar]
- Redelmeier T. E., Hope M. J., Cullis P. R. On the mechanism of transbilayer transport of phosphatidylglycerol in response to transmembrane pH gradients. Biochemistry. 1990 Mar 27;29(12):3046–3053. doi: 10.1021/bi00464a022. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh J. R., Banerjee U., Müller L., Chan S. I. The phospholipid packing arrangement in small bilayer vesicles as revealed by proton magnetic resonance studies at 500 MHz. Biochim Biophys Acta. 1982 May 7;687(2):219–225. doi: 10.1016/0005-2736(82)90549-1. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Burns J. L., Chestnut M. H., Talmon Y. Intermediates in membrane fusion and bilayer/nonbilayer phase transitions imaged by time-resolved cryo-transmission electron microscopy. Biophys J. 1989 Jul;56(1):161–169. doi: 10.1016/S0006-3495(89)82661-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal amphiphile phases. III. Isotropic and inverted cubic state formation via intermediates in transitions between L alpha and HII phases. Chem Phys Lipids. 1986 Dec 31;42(4):279–301. doi: 10.1016/0009-3084(86)90087-3. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. I. Mechanism of the L alpha----HII phase transitions. Biophys J. 1986 Jun;49(6):1155–1170. doi: 10.1016/S0006-3495(86)83744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. II. Implications for membrane-membrane interactions and membrane fusion. Biophys J. 1986 Jun;49(6):1171–1183. doi: 10.1016/S0006-3495(86)83745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar structures in bilayer membranes. Formation rates and half-lives. Biophys J. 1984 Feb;45(2):399–420. doi: 10.1016/S0006-3495(84)84164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternin E., Fine B., Bloom M., Tilcock C. P., Wong K. F., Cullis P. R. Acyl chain orientational order in the hexagonal HII phase of phospholipid-water dispersions. Biophys J. 1988 Oct;54(4):689–694. doi: 10.1016/S0006-3495(88)83004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton G. W., Polnaszek C. F., Tulloch A. P., Hasan F., Smith I. C. Molecular motion and order in single-bilayer vesicles and multilamellar dispersions of egg lecithin and lecithin-cholesterol mixtures. A deuterium nuclear magnetic resonance study of specifically labeled lipids. Biochemistry. 1976 Mar 9;15(5):954–966. doi: 10.1021/bi00650a003. [DOI] [PubMed] [Google Scholar]
- Suurkuusk J., Lentz B. R., Barenholz Y., Biltonen R. L., Thompson T. E. A calorimetric and fluorescent probe study of the gel-liquid crystalline phase transition in small, single-lamellar dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1976 Apr 6;15(7):1393–1401. doi: 10.1021/bi00652a007. [DOI] [PubMed] [Google Scholar]
- Tauskela J. S., Thompson M. A 31P-NMR spin-lattice relaxation and 31P[1H] nuclear Overhauser effect study of sonicated small unilamellar phosphatidylcholine vesicles. Biochim Biophys Acta. 1992 Feb 17;1104(1):137–146. doi: 10.1016/0005-2736(92)90142-9. [DOI] [PubMed] [Google Scholar]
- Wu W. G., Dowd S. R., Simplaceanu V., Peng Z. Y., Ho C. 19F NMR investigation of molecular motion and packing in sonicated phospholipid vesicles. Biochemistry. 1985 Dec 3;24(25):7153–7161. doi: 10.1021/bi00346a020. [DOI] [PubMed] [Google Scholar]


