Abstract
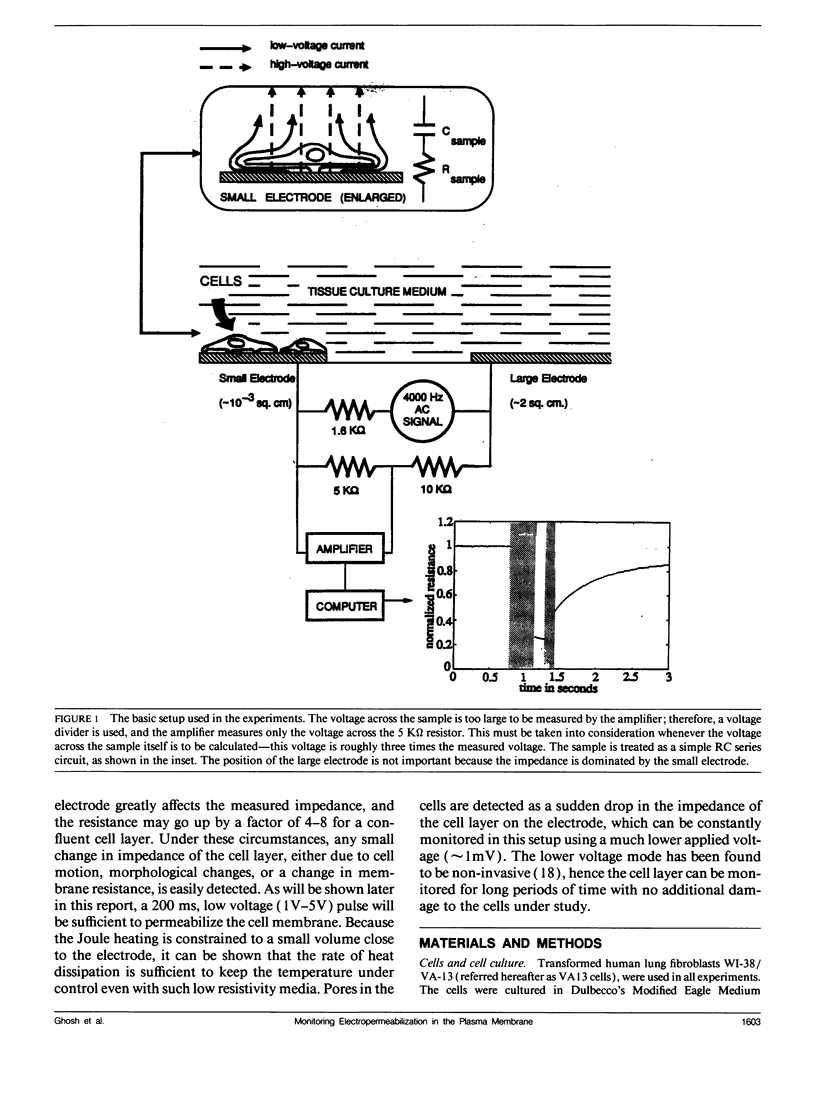
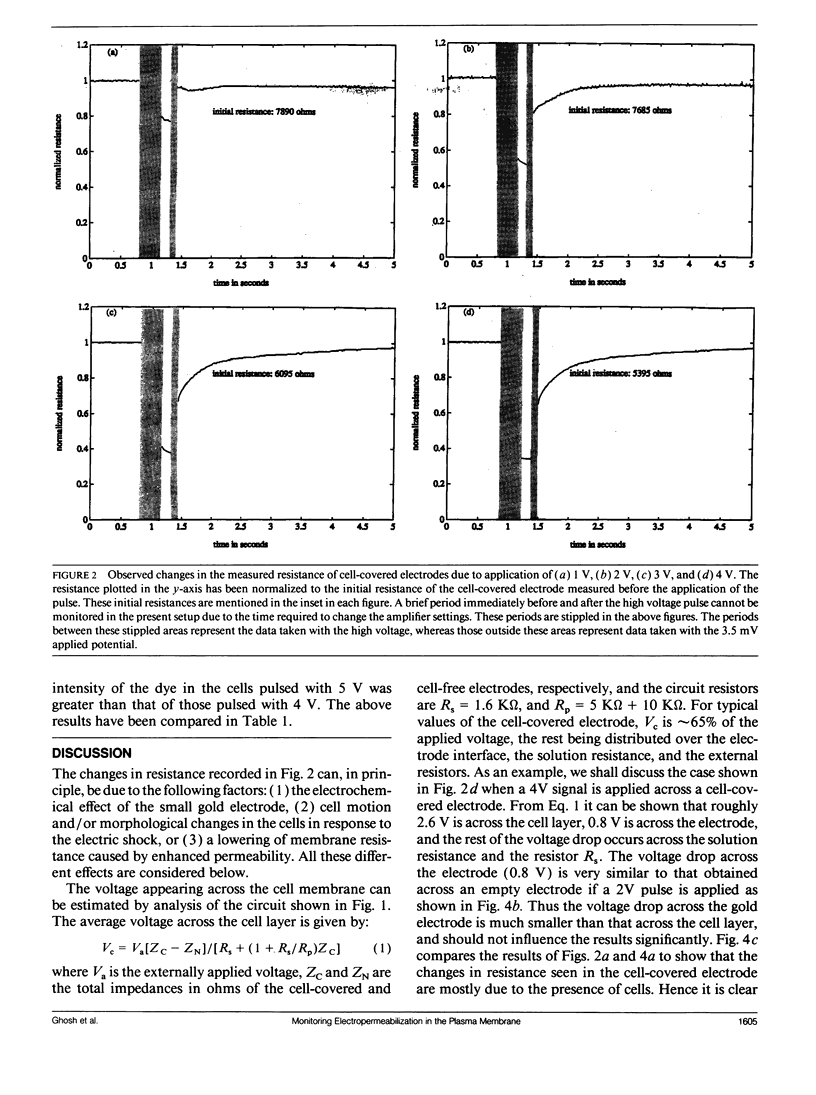
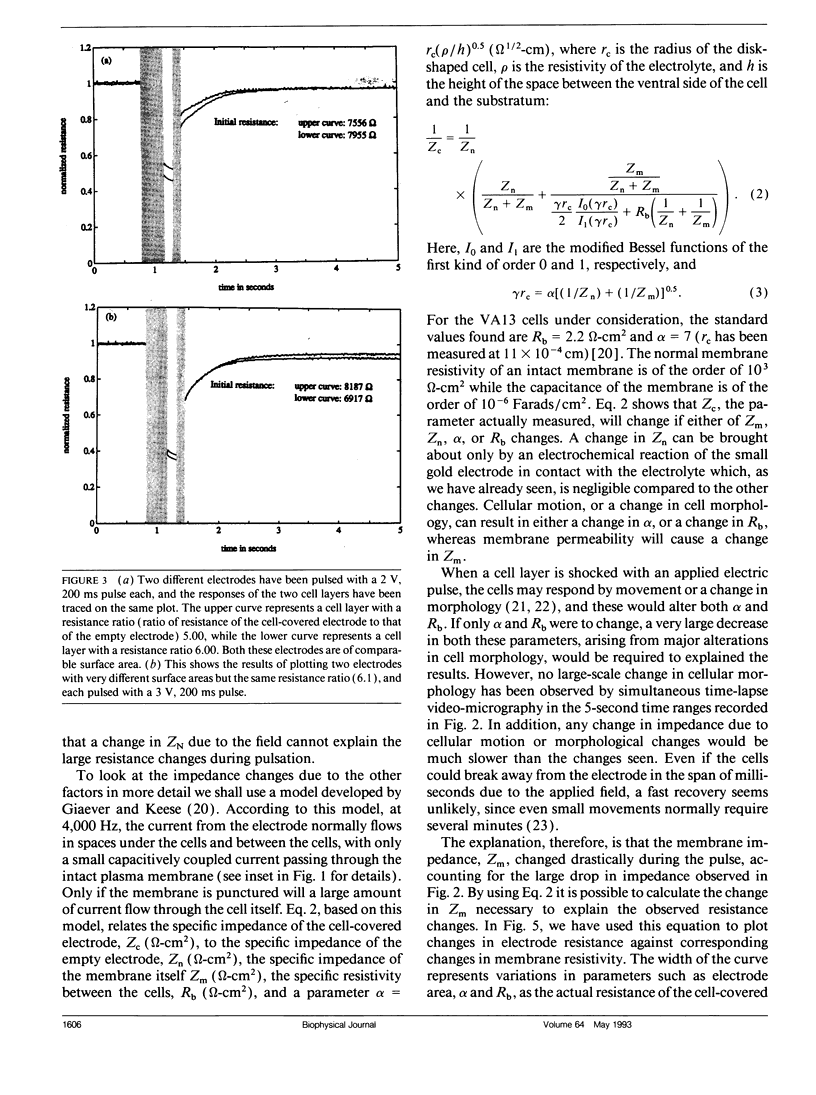
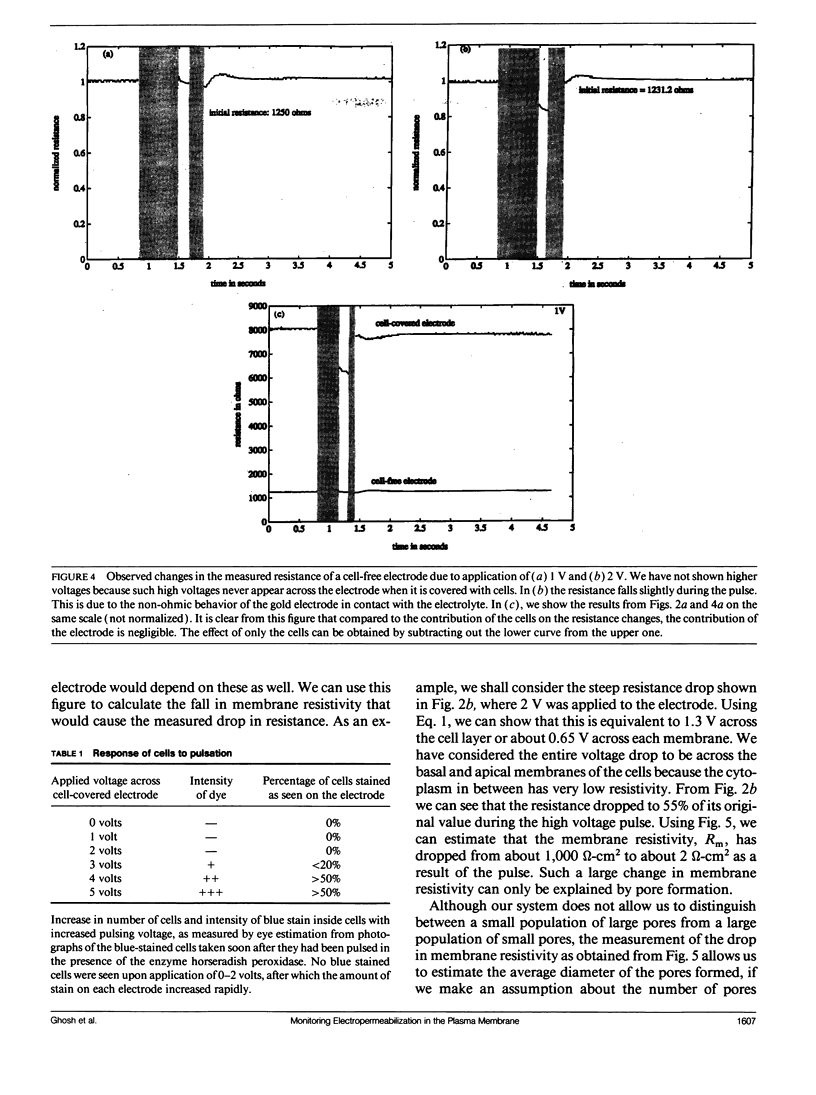
When an electrical potential of order one volt is induced across a cell membrane for a fraction of a second, temporary breakdown of ordinary membrane functions may occur. One result of such a breakdown is that molecules normally excluded by the membrane can now enter the cells. This phenomenon, generally referred to as electropermeabilization, is known as electroporation when actual pores form in the membrane. This paper presents a unique approach to the measurement of pore formation and closure in anchored mammalian cells. The cells are cultured on small gold electrodes, and by constantly monitoring the impedance of the electrode with a low-amplitude AC signal, small changes in cell morphology, cell motion, and membrane resistance can be detected. Because the active electrode is small, the application of a few volts across the cell-covered electrode causes pore formation in the cell membrane. In addition, the heat transfer is very efficient, and the cells can be porated in their regular growth medium. By this method, the formation and resealing of pores due to applied electric fields can be followed in real time for anchorage-dependent cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang D. C., Reese T. S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990 Jul;58(1):1–12. doi: 10.1016/S0006-3495(90)82348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Sokolov A. V., Budker V. G. Electrostimulated uptake of DNA by liposomes. Biochim Biophys Acta. 1990 May 9;1024(1):179–183. doi: 10.1016/0005-2736(90)90222-a. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Sukharev S. I., Popov S. V., Pastushenko V. F., Sokirko A. V., Abidor I. G., Chizmadzhev Y. A. The electrical breakdown of cell and lipid membranes: the similarity of phenomenologies. Biochim Biophys Acta. 1987 Sep 3;902(3):360–373. doi: 10.1016/0005-2736(87)90204-5. [DOI] [PubMed] [Google Scholar]
- DeRosa J. F., Beard R. B. Linear AC electrode polarization impedance at smooth noble metal interfaces. IEEE Trans Biomed Eng. 1977 May;24(3):260–268. doi: 10.1109/TBME.1977.326211. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Sowers A. E. Membrane electroporation--fast molecular exchange by electroosmosis. Biochim Biophys Acta. 1990 Mar;1022(3):381–392. doi: 10.1016/0005-2736(90)90289-z. [DOI] [PubMed] [Google Scholar]
- Escande-Géraud M. L., Rols M. P., Dupont M. A., Gas N., Teissié J. Reversible plasma membrane ultrastructural changes correlated with electropermeabilization in Chinese hamster ovary cells. Biochim Biophys Acta. 1988 Apr 7;939(2):247–259. doi: 10.1016/0005-2736(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Gass G. V., Chernomordik L. V. Reversible large-scale deformations in the membranes of electrically-treated cells: electroinduced bleb formation. Biochim Biophys Acta. 1990 Mar 30;1023(1):1–11. doi: 10.1016/0005-2736(90)90002-6. [DOI] [PubMed] [Google Scholar]
- Giaever I., Keese C. R. Micromotion of mammalian cells measured electrically. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7896–7900. doi: 10.1073/pnas.88.17.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever I., Keese C. R. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3761–3764. doi: 10.1073/pnas.81.12.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever I., Keese C. R. Use of electric fields to monitor the dynamical aspect of cell behavior in tissue culture. IEEE Trans Biomed Eng. 1986 Feb;33(2):242–247. doi: 10.1109/TBME.1986.325896. [DOI] [PubMed] [Google Scholar]
- Hibino M., Shigemori M., Itoh H., Nagayama K., Kinosita K., Jr Membrane conductance of an electroporated cell analyzed by submicrosecond imaging of transmembrane potential. Biophys J. 1991 Jan;59(1):209–220. doi: 10.1016/S0006-3495(91)82212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Voltage-induced conductance in human erythrocyte membranes. Biochim Biophys Acta. 1979 Jul 5;554(2):479–497. doi: 10.1016/0005-2736(79)90386-9. [DOI] [PubMed] [Google Scholar]
- Marszalek P., Liu D. S., Tsong T. Y. Schwan equation and transmembrane potential induced by alternating electric field. Biophys J. 1990 Oct;58(4):1053–1058. doi: 10.1016/S0006-3495(90)82447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P., Keese C. R., Giaever I. Electric measurements can be used to monitor the attachment and spreading of cells in tissue culture. Biotechniques. 1991 Oct;11(4):504–510. [PubMed] [Google Scholar]
- O'Neill R. J., Tung L. Cell-attached patch clamp study of the electropermeabilization of amphibian cardiac cells. Biophys J. 1991 May;59(5):1028–1039. doi: 10.1016/S0006-3495(91)82318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis L., Firth K. L. Electroporation of adherent cells in situ. DNA Cell Biol. 1990 Oct;9(8):615–621. doi: 10.1089/dna.1990.9.615. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Electroporation of cell membranes. Biophys J. 1991 Aug;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q. A., Chang D. C. High-efficiency gene transfection by in situ electroporation of cultured cells. Biochim Biophys Acta. 1991 Jan 17;1088(1):104–110. doi: 10.1016/0167-4781(91)90158-i. [DOI] [PubMed] [Google Scholar]


