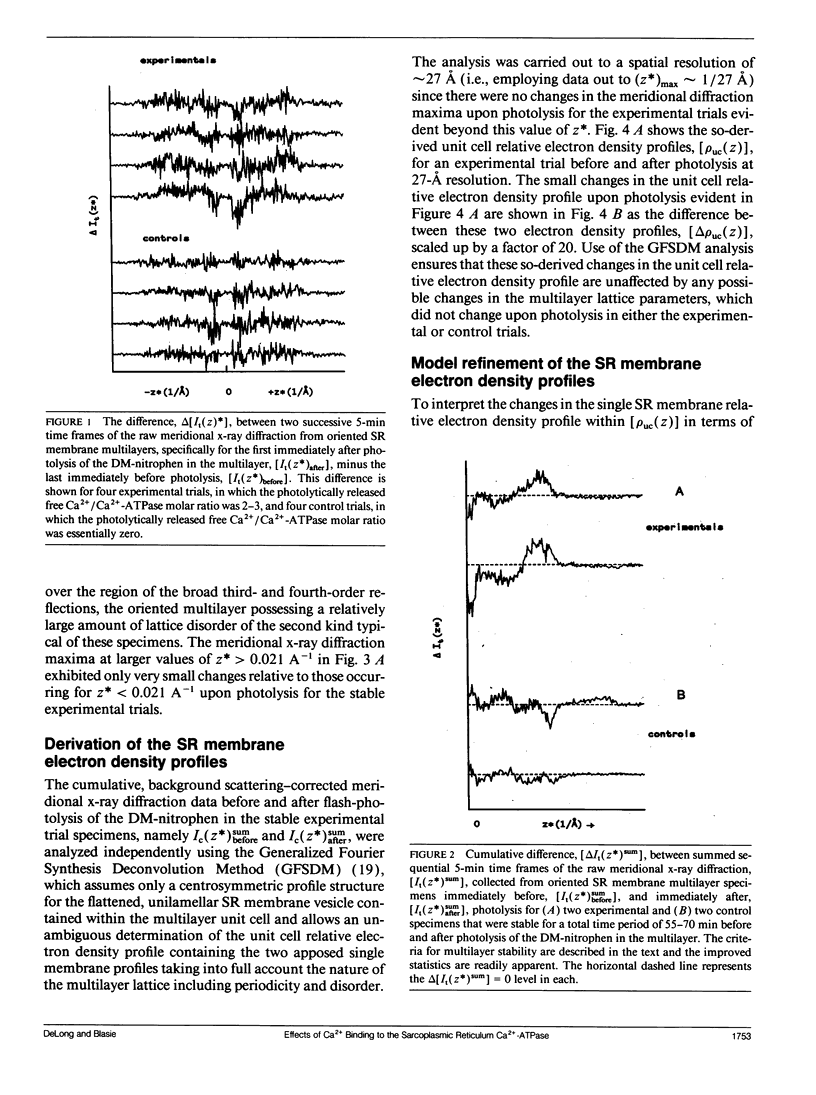
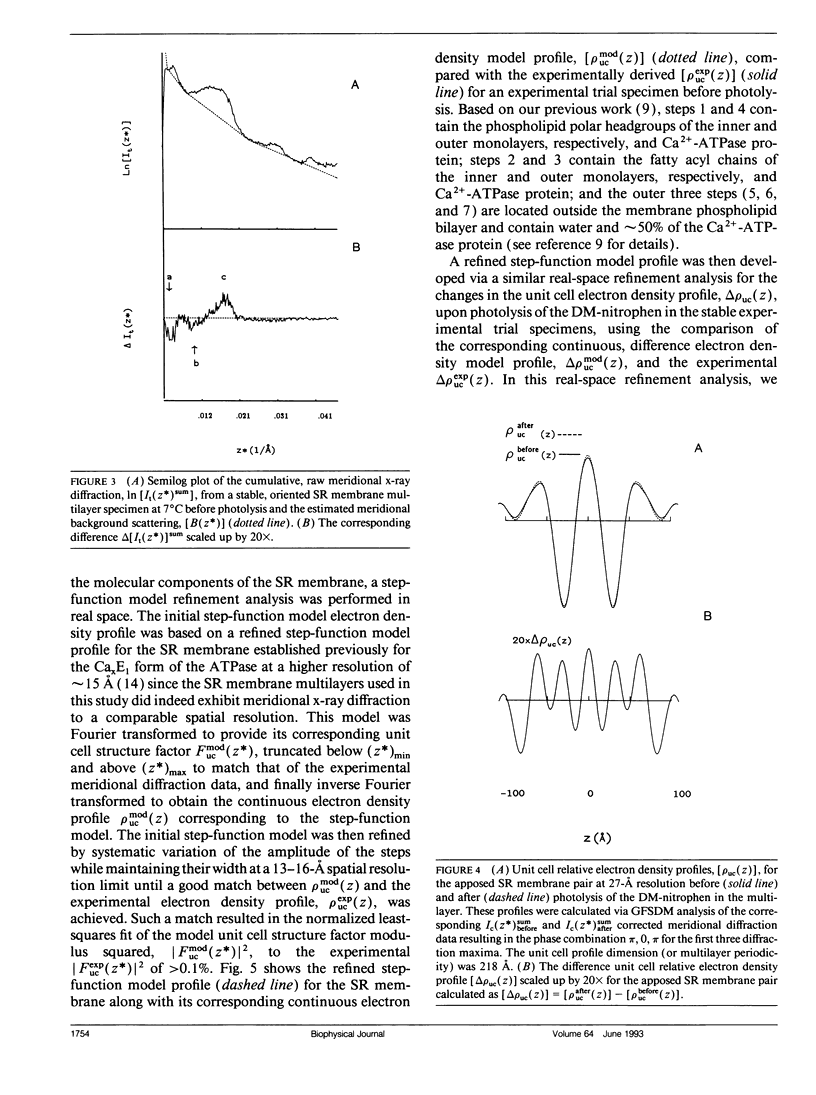
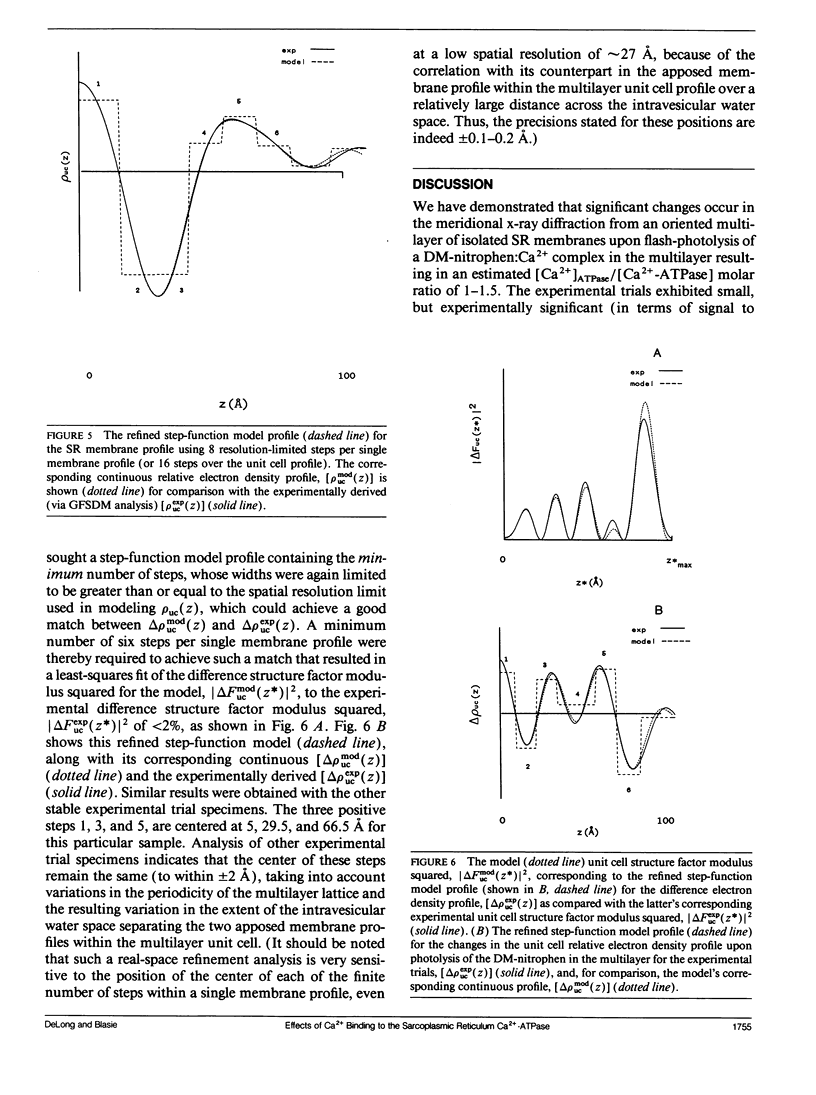
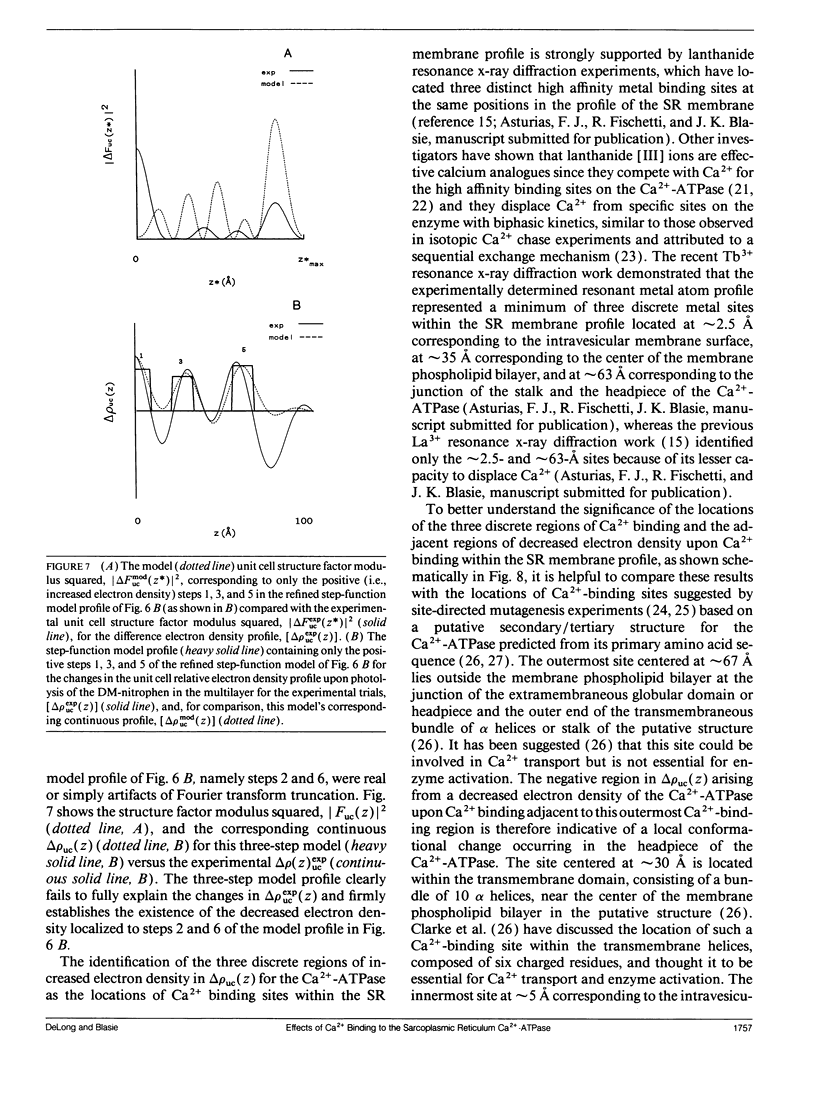
Abstract
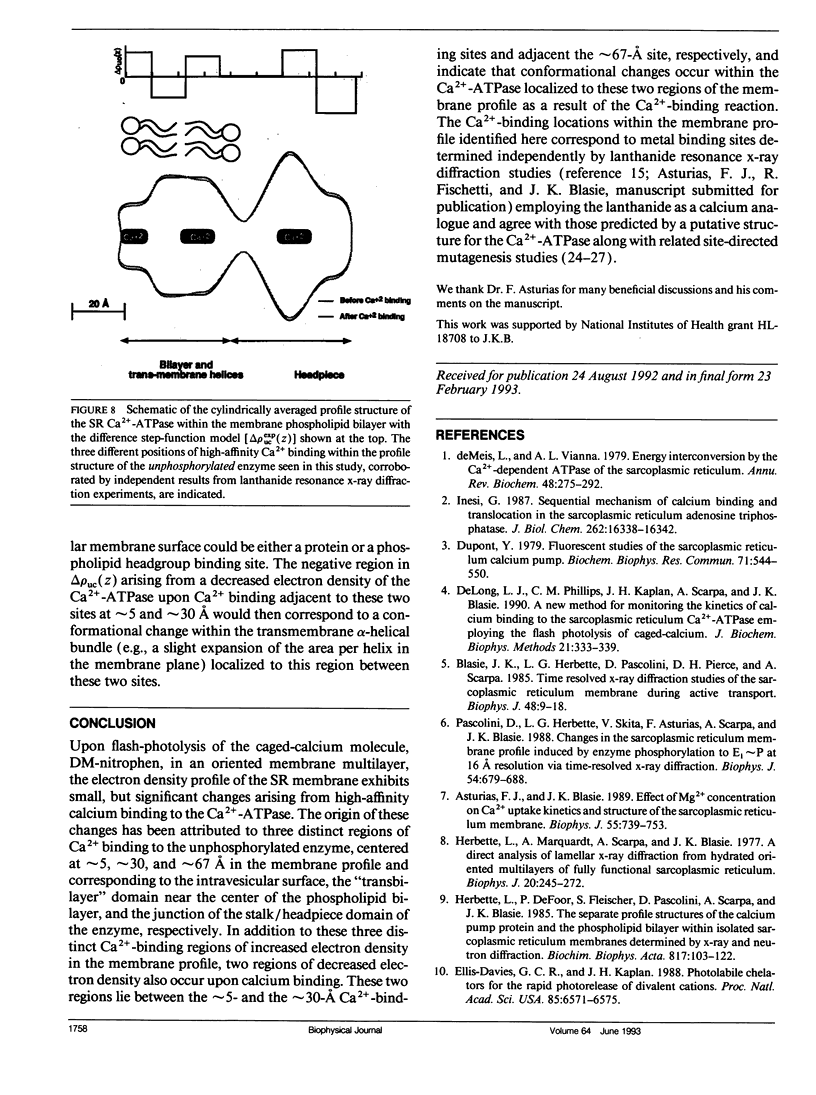
A number of studies have indicated that Ca(2+)-ATPase, the integral membrane protein of the sarcoplasmic reticulum (SR) membrane, undergoes some structural change upon Ca2+ binding to its high affinity binding sites (i.e., upon conversion of the E1 to the CaxE1 form of the enzyme). We have used x-ray diffraction to study the changes in the electron density profile of the SR membrane upon high-affinity Ca2+ binding to the enzyme in the absence of enzyme phosphorylation. The photolabile Ca2+ chelator DM-nitrophen was used to rapidly release Ca2+ into the extravesicular spaces throughout an oriented SR membrane multilayer and thereby synchronously in the vicinity of the high affinity binding sites of each enzyme molecule in the multilayer. A critical control was developed to exclude possible artifacts arising from heating and non-Ca2+ photolysis products in the membrane multilayer specimens upon photolysis of the DM-nitrophen. Upon photolysis, changes in the membrane electron density profile arising from high-affinity Ca2+ binding to the enzyme are found to be localized to three different regions within the profile. These changes can be attributed to the added electron density of the Ca2+ bound at three discrete sites centered at 5, approximately 30, and approximately 67 A in the membrane profile, but they also require decreased electron density within the cylindrically averaged profile structure of the Ca(2+)-ATPase immediately adjacent (< 15 A) to these sites. The locations of these three Ca2+ binding sites in the SR membrane profile span most of the membrane profile in the absence of enzyme phosphorylation,in agreement with the locations of lanthanide (Tb3+ and La3+) binding sites in the membrane profile determined independently by using resonance x-ray diffraction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asturias F. J., Blasie J. K. Effect of Mg2+ concentration on Ca2+ uptake kinetics and structure of the sarcoplasmic reticulum membrane. Biophys J. 1989 Apr;55(4):739–753. doi: 10.1016/S0006-3495(89)82873-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asturias F. J., Blasie J. K. Location of high-affinity metal binding sites in the profile structure of the Ca+2-ATPase in the sarcoplasmic reticulum by resonance x-ray diffraction. Biophys J. 1991 Feb;59(2):488–502. doi: 10.1016/S0006-3495(91)82242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasie J. K., Herbette L. G., Pascolini D., Skita V., Pierce D. H., Scarpa A. Time-resolved x-ray diffraction studies of the sarcoplasmic reticulum membrane during active transport. Biophys J. 1985 Jul;48(1):9–18. doi: 10.1016/S0006-3495(85)83756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., Inesi G., MacLennan D. H. Location of high affinity Ca2+-binding sites within the predicted transmembrane domain of the sarcoplasmic reticulum Ca2+-ATPase. Nature. 1989 Jun 8;339(6224):476–478. doi: 10.1038/339476a0. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Maruyama K., Loo T. W., Leberer E., Inesi G., MacLennan D. H. Functional consequences of glutamate, aspartate, glutamine, and asparagine mutations in the stalk sector of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Jul 5;264(19):11246–11251. [PubMed] [Google Scholar]
- DeLong L. J., Phillips C. M., Kaplan J. H., Scarpa A., Blasie J. K. A new method for monitoring the kinetics of calcium binding to the sarcoplasmic reticulum Ca(2+)-ATPase employing the flash-photolysis of caged-calcium. J Biochem Biophys Methods. 1990 Nov-Dec;21(4):333–339. doi: 10.1016/0165-022x(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Dipolo R., Requena J., Brinley F. J., Jr, Mullins L. J., Scarpa A., Tiffert T. Ionized calcium concentrations in squid axons. J Gen Physiol. 1976 Apr;67(4):433–467. doi: 10.1085/jgp.67.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont Y. Fluorescence studies of the sarcoplasmic reticulum calcium pump. Biochem Biophys Res Commun. 1976 Jul 26;71(2):544–550. doi: 10.1016/0006-291x(76)90821-4. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbette L., DeFoor P., Fleischer S., Pascolini D., Scarpa A., Blasie J. K. The separate profile structures of the functional calcium pump protein and the phospholipid bilayer within isolated sarcoplasmic reticulum membranes determined by X-ray and neutron diffraction. Biochim Biophys Acta. 1985 Jul 11;817(1):103–122. doi: 10.1016/0005-2736(85)90073-2. [DOI] [PubMed] [Google Scholar]
- Herbette L., Marquardt J., Scarpa A., Blasie J. K. A direct analysis of lamellar x-ray diffraction from hydrated oriented multilayers of fully functional sarcoplasmic reticulum. Biophys J. 1977 Nov;20(2):245–272. doi: 10.1016/S0006-3495(77)85547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S. R., Head M. R. Tb3+ binding to Ca2+ and Mg2+ binding sites on sarcoplasmic reticulum ATPase. J Biol Chem. 1983 Jun 10;258(11):6858–6862. [PubMed] [Google Scholar]
- Inesi G. Sequential mechanism of calcium binding and translocation in sarcoplasmic reticulum adenosine triphosphatase. J Biol Chem. 1987 Dec 5;262(34):16338–16342. [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H. Photochemical manipulation of divalent cation levels. Annu Rev Physiol. 1990;52:897–914. doi: 10.1146/annurev.ph.52.030190.004225. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Pascolini D., Blasie J. K. Moderate resolution profile structure of the sarcoplasmic reticulum membrane under low temperature conditions for the transient trapping of E1 approximately P. Biophys J. 1988 Oct;54(4):669–678. doi: 10.1016/S0006-3495(88)83002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolini D., Herbette L. G., Skita V., Asturias F., Scarpa A., Blasie J. K. Changes in the sarcoplasmic reticulum membrane profile induced by enzyme phosphorylation to E1 approximately P at 16 A resolution via time-resolved x-ray diffraction. Biophys J. 1988 Oct;54(4):679–687. doi: 10.1016/S0006-3495(88)83003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce D. H., Scarpa A., Topp M. R., Blasie J. K. Kinetics of calcium uptake by isolated sarcoplasmic reticulum vesicles using flash photolysis of caged adenosine 5'-triphosphate. Biochemistry. 1983 Nov 8;22(23):5254–5261. doi: 10.1021/bi00292a003. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Cain J. E., Dratz E. A., Blasie J. K. An analysis of lamellar x-ray diffraction from disordered membrane multilayers with application to data from retinal rod outer segments. Biophys J. 1975 Dec;15(12):1201–1233. doi: 10.1016/S0006-3495(75)85895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T. L. Luminescence studies of Tb3+ bound to the high affinity sites of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1984 Apr 10;259(7):4035–4037. [PubMed] [Google Scholar]
- Squier T. C., Bigelow D. J., Fernandez-Belda F. J., deMeis L., Inesi G. Calcium and lanthanide binding in the sarcoplasmic reticulum ATPase. J Biol Chem. 1990 Aug 15;265(23):13713–13720. [PubMed] [Google Scholar]
- de Meis L., Vianna A. L. Energy interconversion by the Ca2+-dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]


