Abstract
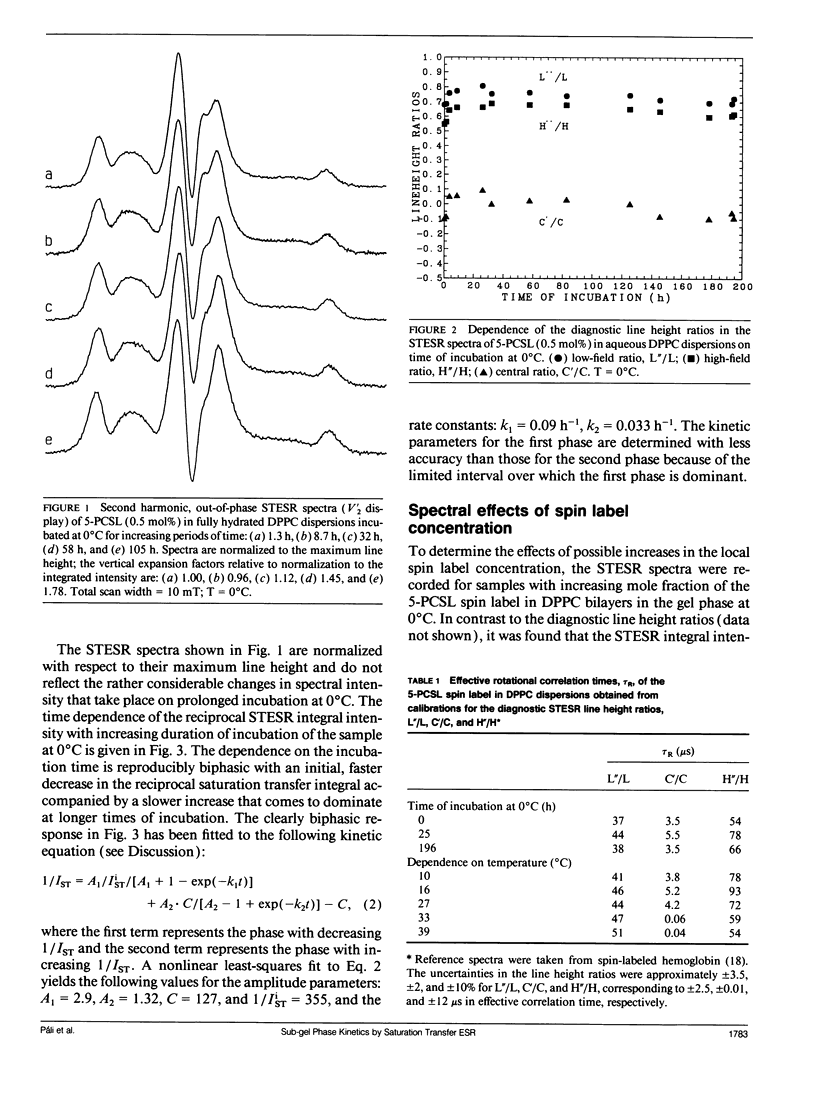
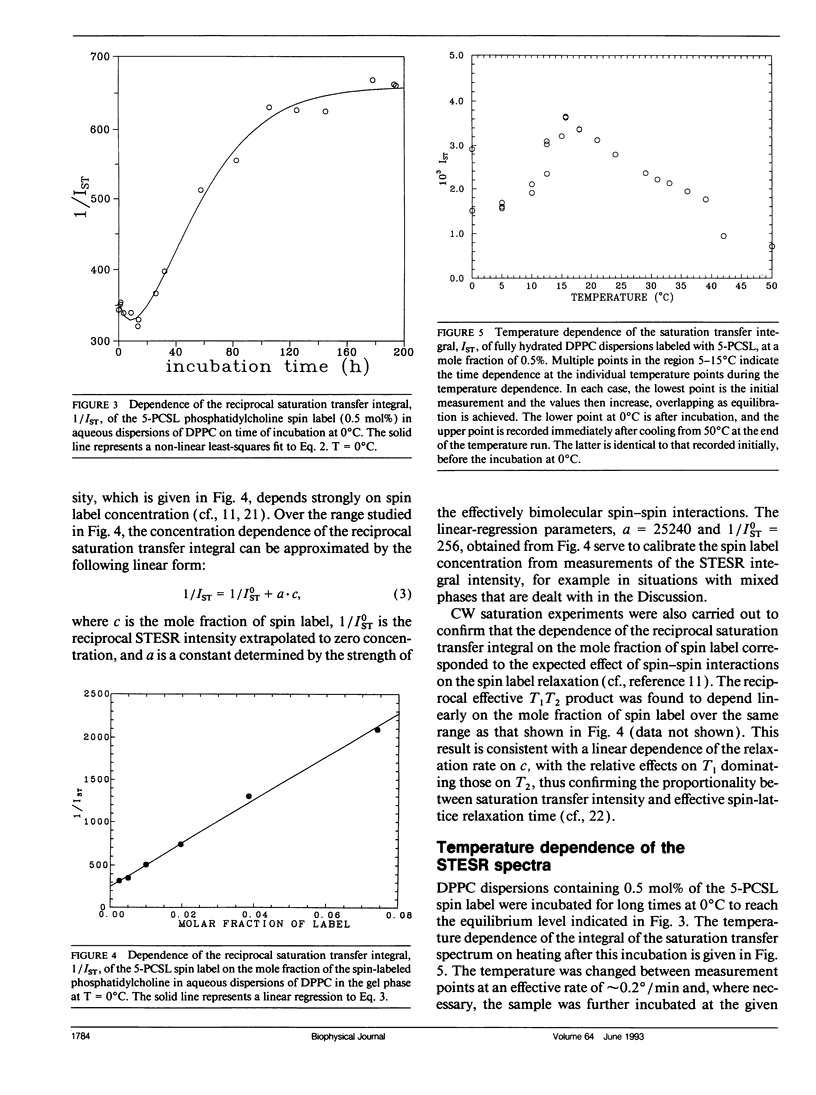
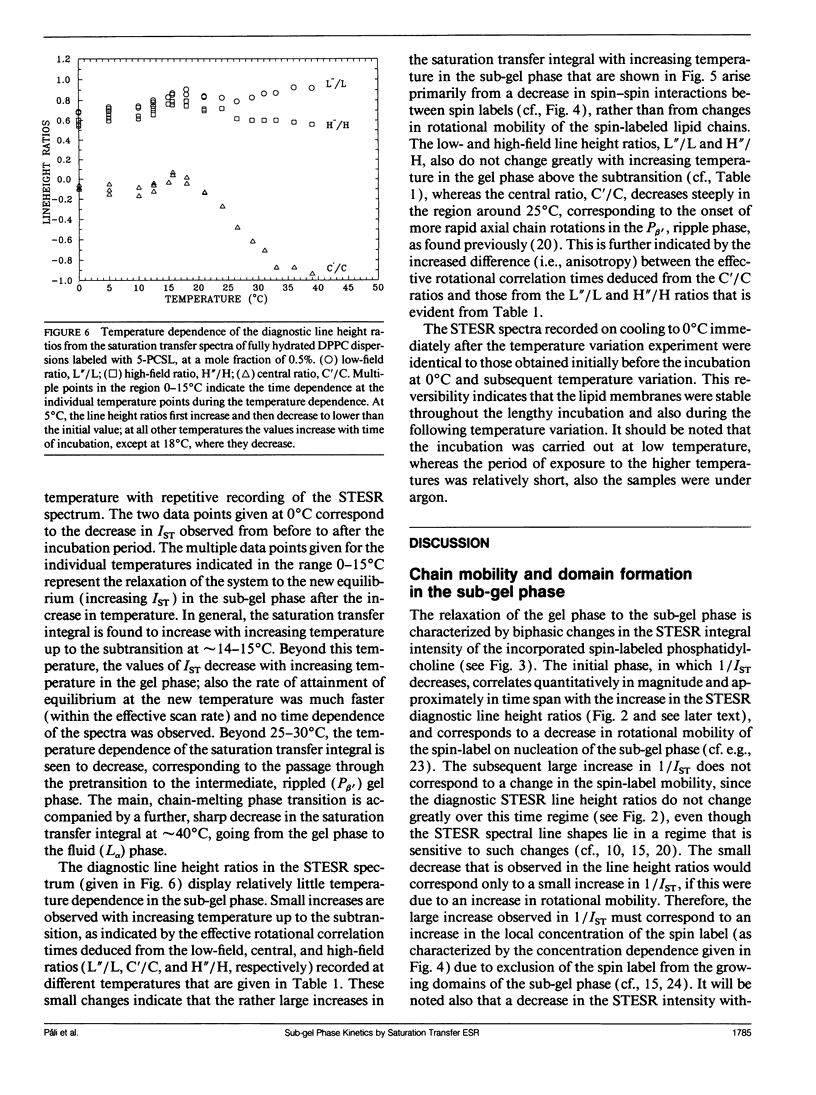
The saturation transfer electron spin resonance (STESR) spectra of spin-labeled phosphatidylcholine have been used to follow the kinetics of conversion from the gel phase to the sub-gel phase in aqueous bilayers of dipalmitoyl phosphatidylcholine. This is a simple, well-defined model system for lipid domain formation in membranes. The integrated intensity of the STESR spectrum from the chain-labeled lipid first increases and then decreases with time of incubation in the gel phase at 0°C. The first, more rapid phase of the kinetics is attributed to the conversion of germ nuclei to growth nuclei of the sub-gel phase. The increase in STESR intensity corresponds to the reduction in chain mobility of spin labels located in the gel phase at the boundaries of the growth nuclei and correlates with the increase in the diagnostic STESR line height ratios over this time range. The second, slower phase of the kinetics is attributed to growth of the domains of the sub-gel phase. The decrease in STESR intensity over this time regime corresponds to exclusion of the spin-labeled lipids from the tightly packed sub-gel phase and correlates quantitatively with calibrations of the spin label concentration dependence of the STESR intensity in the gel phase. The kinetics of formation of the sub-gel phase are consistent with the classical model for domain formation and growth. At 0°C, the half-time for conversion of germ nuclei to growth nuclei is ∼7.7 h and domain growth of the sub-gel phase is characterized by a rate constant of 0.025 h-1. The temperature dependence of the STESR spectra from samples annealed at 0°C suggests that the subtransition takes place via dissolution of sub-gel phase domains, possibly accompanied by domain fission.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs J. M., Mason J. T. Calorimetric and fatty acid spin label study of subgel and interdigitated gel phases formed by asymmetric phosphatidylcholines. Biochim Biophys Acta. 1986 Dec 16;863(2):231–242. doi: 10.1016/0005-2736(86)90263-4. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Mantsch H. H. Metastability and polymorphism in the gel phase of 1,2-dipalmitoyl-3-SN-phosphatidylcholine. A Fourier transform infrared study of the subtransition. Biophys J. 1982 May;38(2):175–184. doi: 10.1016/S0006-3495(82)84544-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. C., Sturtevant J. M., Gaffney B. J. Scanning calorimetric evidence for a third phase transition in phosphatidylcholine bilayers. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5060–5063. doi: 10.1073/pnas.77.9.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer P., Watts A., Marsh D. Saturation transfer, continuous wave saturation, and saturation recovery electron spin resonance studies of chain-spin labeled phosphatidylcholines in the low temperature phases of dipalmitoyl phosphatidylcholine bilayers. Effects of rotational dynamics and spin-spin interactions. Biophys J. 1992 Apr;61(4):879–891. doi: 10.1016/S0006-3495(92)81895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füldner H. H. Characterization of a third phase transition in multilamellar dipalmitoyllecithin liposomes. Biochemistry. 1981 Sep 29;20(20):5707–5710. doi: 10.1021/bi00523a011. [DOI] [PubMed] [Google Scholar]
- Horváth L. I., Dux L., Hankovszky H. O., Hideg K., Marsh D. Saturation transfer electron spin resonance of Ca2(+)-ATPase covalently spin-labeled with beta-substituted vinyl ketone- and maleimide-nitroxide derivatives. Effects of segmental motion and labeling levels. Biophys J. 1990 Jul;58(1):231–241. doi: 10.1016/S0006-3495(90)82368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. Molecular motion in phospholipid bilayers in the gel phase: long axis rotation. Biochemistry. 1980 Apr 15;19(8):1632–1637. doi: 10.1021/bi00549a017. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Wilkinson D. A. Dilatometric studies of the subtransition in dipalmitoylphosphatidylcholine. Biochemistry. 1982 Aug 3;21(16):3817–3821. doi: 10.1021/bi00259a015. [DOI] [PubMed] [Google Scholar]
- Páli T., Bartucci R., Horváth L. I., Marsh D. Distance measurements using paramagnetic ion-induced relaxation in the saturation transfer electron spin resonance of spin-labeled biomolecules: Application to phospholipid bilayers and interdigitated gel phases. Biophys J. 1992 Jun;61(6):1595–1602. doi: 10.1016/S0006-3495(92)81963-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenchov B. G., Yao H., Hatta I. Time-resolved x-ray diffraction and calorimetric studies at low scan rates: I. Fully hydrated dipalmitoylphosphatidylcholine (DPPC) and DPPC/water/ethanol phases. Biophys J. 1989 Oct;56(4):757–768. doi: 10.1016/S0006-3495(89)82723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristram-Nagle S., Wiener M. C., Yang C. P., Nagle J. F. Kinetics of the subtransition in dipalmitoylphosphatidylcholine. Biochemistry. 1987 Jul 14;26(14):4288–4294. doi: 10.1021/bi00388a016. [DOI] [PubMed] [Google Scholar]
- Yang CP, Nagle JF. Phase transformations in lipids follow classical kinetics with small fractional dimensionalities. Phys Rev A Gen Phys. 1988 May 15;37(10):3993–4000. doi: 10.1103/physreva.37.3993. [DOI] [PubMed] [Google Scholar]


