Abstract
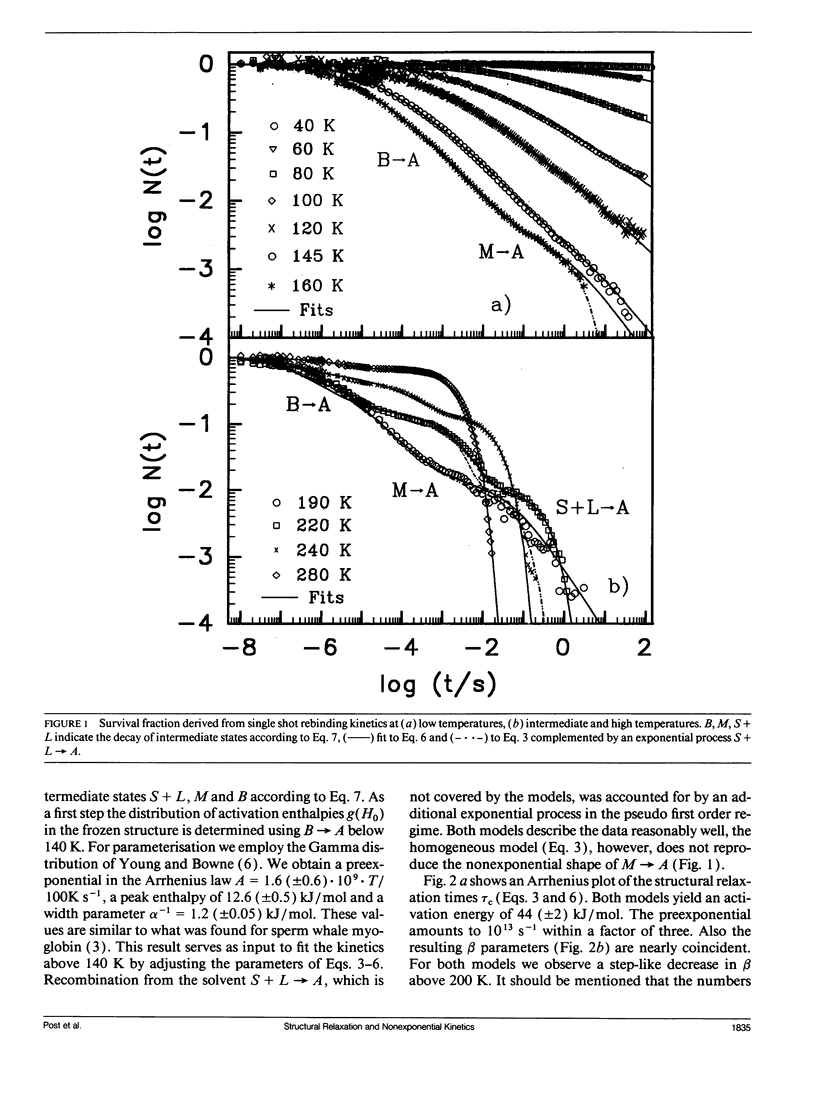
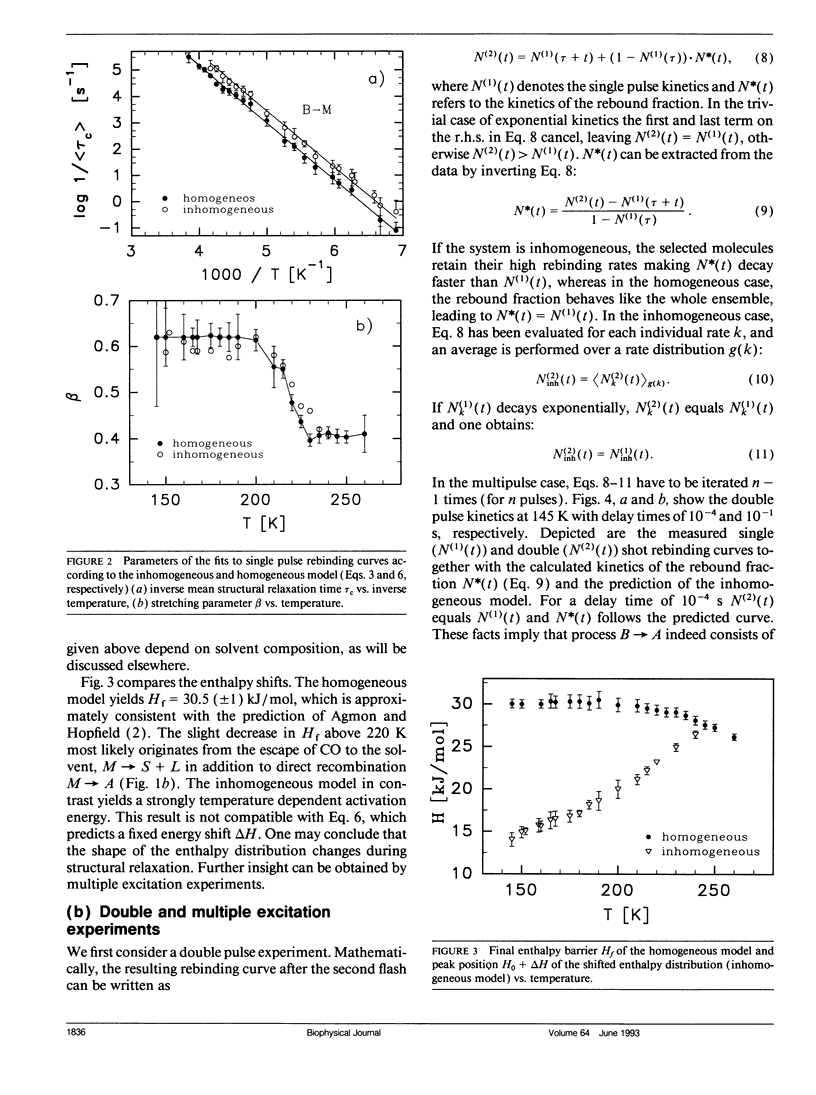
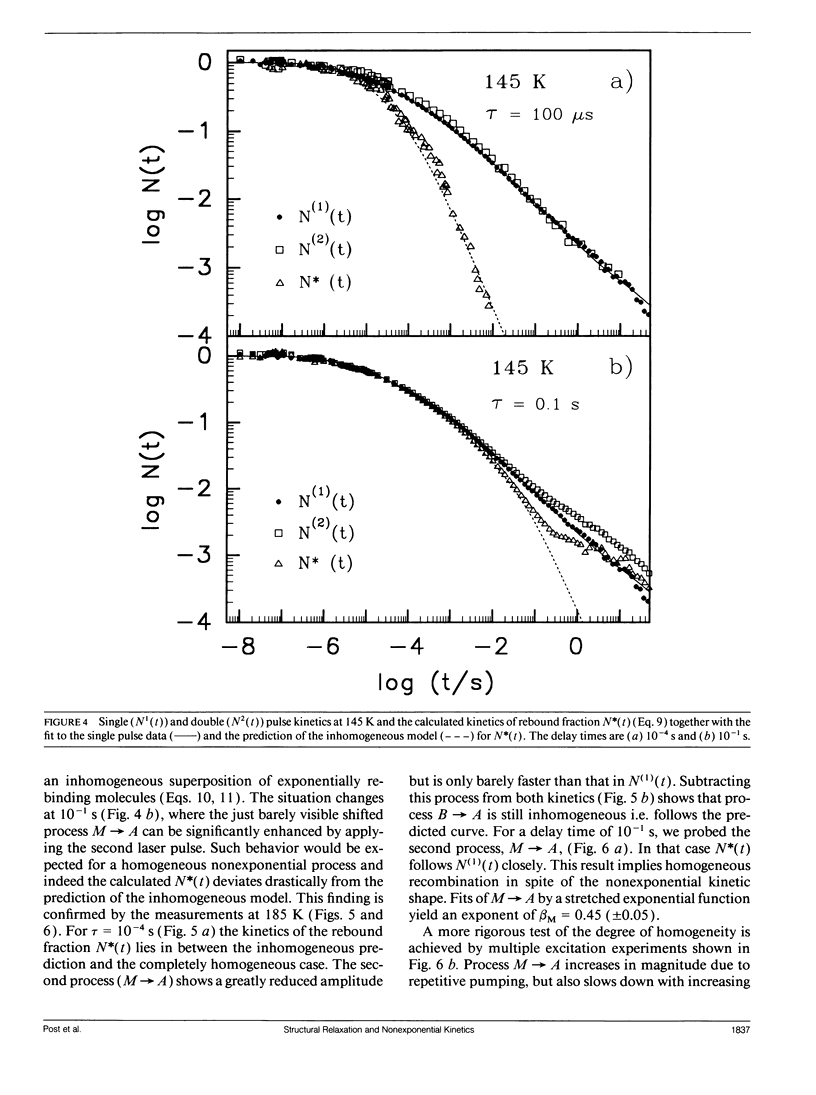
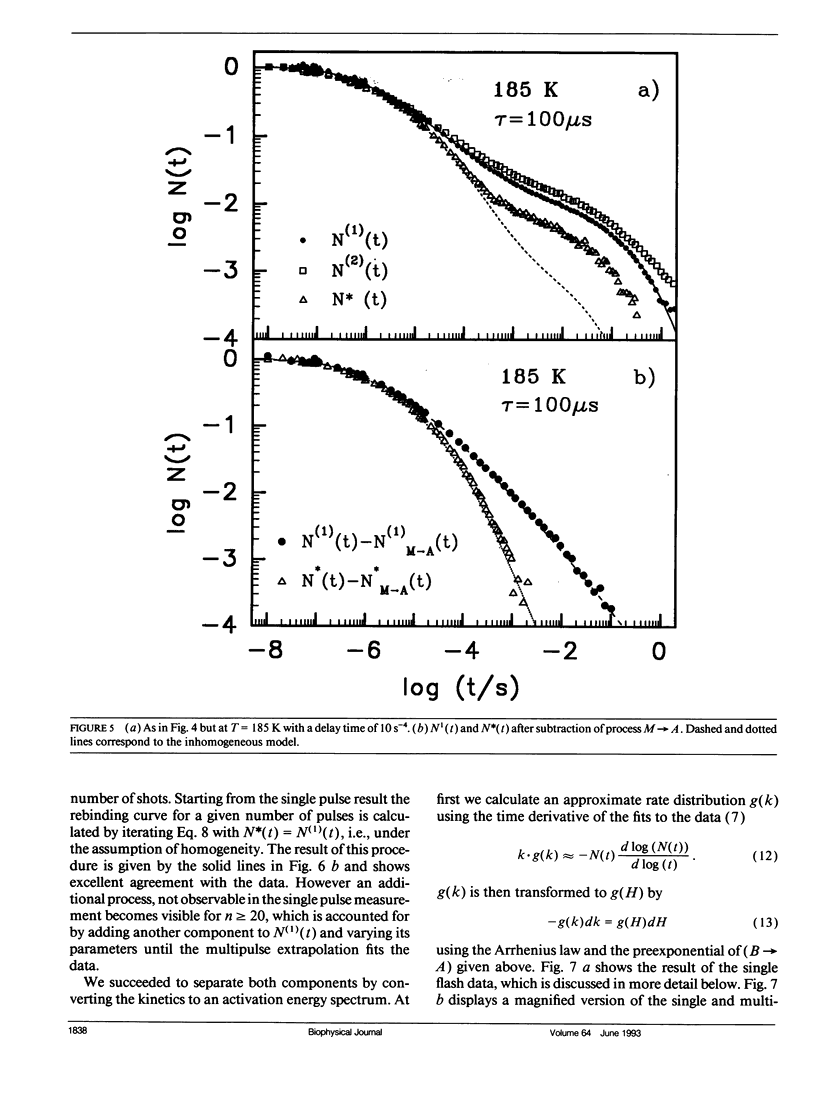
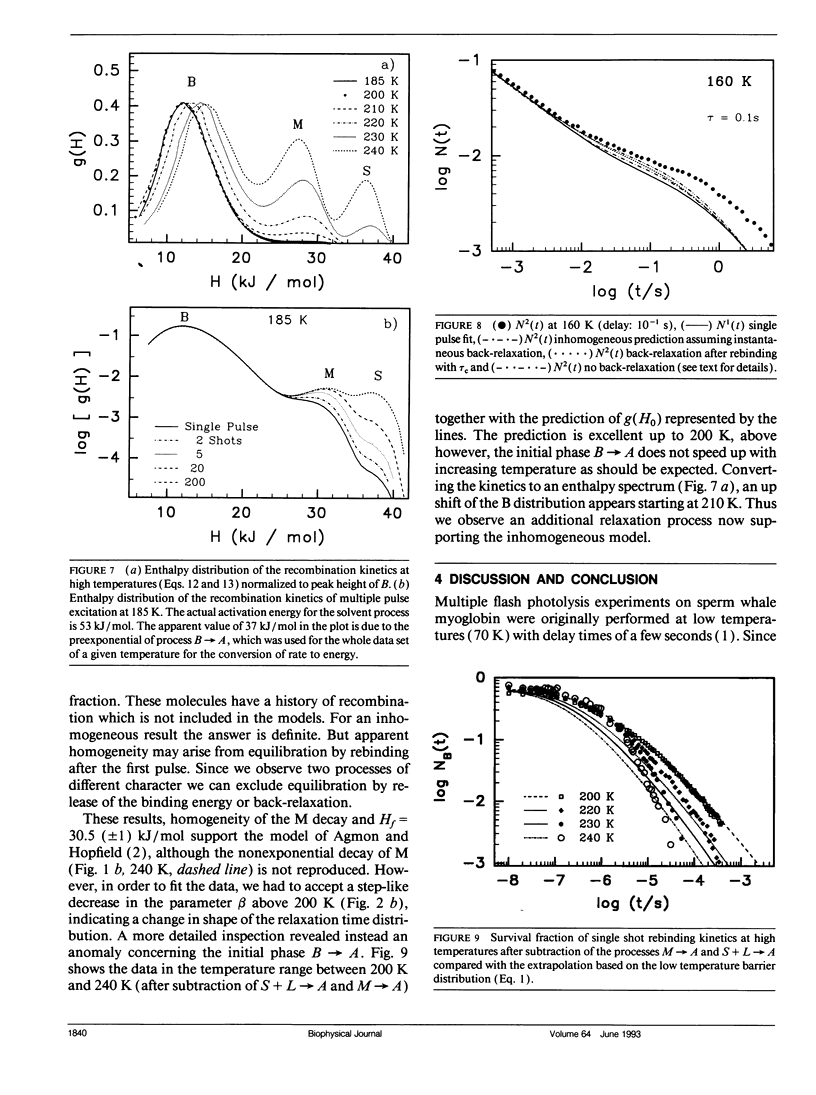
The geminate recombination kinetics of CO-myoglobin strongly deviates from single exponential behavior in contrast to what is expected for unimolecular reactions (1). At low temperatures, this result was attributed to slowly exchanging conformational states which differ substantially in barrier height for ligand binding. Above 160 K the kinetics apparently slow down with temperature increase. Agmon and Hopfield (2) explain this result in terms of structural relaxation perpendicular to the reaction coordinate, which enhances the activation energy. In their model, structural relaxation homogenizes the kinetic response. Recently, Steinbach et al. (3) proposed a relaxation model which conserves the kinetic inhomogeneity. Below we test these conjectures by single and multiple excitation experiments. This method allows for discrimination between parallel (inhomogeneous) and sequential (homogeneous) kinetic schemes. The kinetic anomaly above 160 K is shown to result from a homogeneous, structurally relaxed intermediate. However a second anomaly is found above 210 K concerning the inhomogeneous phase which may indicate either a shift in activation energy or entropy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Di Iorio E. E., Hiltpold U. R., Filipovic D., Winterhalter K. H., Gratton E., Vitrano E., Cupane A., Leone M., Cordone L. Protein dynamics. Comparative investigation on heme-proteins with different physiological roles. Biophys J. 1991 Mar;59(3):742–754. doi: 10.1016/S0006-3495(91)82287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- Doster W., Bowne S. F., Frauenfelder H., Reinisch L., Shyamsunder E. Recombination of carbon monoxide to ferrous horseradish peroxidase types A and C. J Mol Biol. 1987 Mar 20;194(2):299–312. doi: 10.1016/0022-2836(87)90377-9. [DOI] [PubMed] [Google Scholar]
- Ehrenstein D., Nienhaus G. U. Conformational substates in azurin. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9681–9685. doi: 10.1073/pnas.89.20.9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Hong MK, Shyamsunder E, Austin RH, Gerstman BS, Chan SS. Time-resolved infrared studies of molecular diffusion in myoglobin. Phys Rev Lett. 1991 May 20;66(20):2673–2676. doi: 10.1103/PhysRevLett.66.2673. [DOI] [PubMed] [Google Scholar]
- Powers L., Sessler J. L., Woolery G. L., Chance B. CO bond angle changes in photolysis of carboxymyoglobin. Biochemistry. 1984 Nov 6;23(23):5519–5523. doi: 10.1021/bi00318a021. [DOI] [PubMed] [Google Scholar]
- Srajer V., Reinisch L., Champion P. M. Investigation of laser-induced long-lived states of photolyzed MbCO. Biochemistry. 1991 May 21;30(20):4886–4895. doi: 10.1021/bi00234a008. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Chu K., Frauenfelder H., Johnson J. B., Lamb D. C., Nienhaus G. U., Sauke T. B., Young R. D. Determination of rate distributions from kinetic experiments. Biophys J. 1992 Jan;61(1):235–245. doi: 10.1016/S0006-3495(92)81830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian WD, Sage JT, Srajer V, V, Champion PM. Relaxation dynamics of myoglobin in solution. Phys Rev Lett. 1992 Jan 20;68(3):408–411. doi: 10.1103/PhysRevLett.68.408. [DOI] [PubMed] [Google Scholar]


