Abstract
Memory CD4+ T cells are considered a stable latent reservoir for human immunodeficiency virus type 1 (HIV-1) and a barrier to eradication of this retroviral infection in patients under therapy. It has been shown that memory CD4+ T cells are preferentially infected with HIV-1, but the exact mechanism(s) responsible for this higher susceptibility remains obscure. Previous findings indicate that incorporation of host-derived intercellular adhesion molecule 1 (ICAM-1) in HIV-1 increases virus infectivity. To measure the putative involvement of virus-anchored ICAM-1 in the preferential infection of memory cells by HIV-1, quiescent and activated naive and memory T-cell subsets were exposed to isogenic virions either lacking or bearing ICAM-1. Memory CD4+ T cells were found to be more susceptible than naive CD4+ T cells to infection with ICAM-1-bearing virions, as exemplified by a more important virus replication, an increase in integrated viral DNA copies, and a more efficient entry process. Interactions between virus-associated host ICAM-1 and cell surface LFA-1 under a cluster formation seem to be responsible for the preferential HIV-1 infection of the memory cell subset. Altogether, these data shed light on a potential mechanism by which HIV-1 preferentially targets long-lived memory CD4+ T cells.
There is an extensive diversity displayed by CD4+ T cells in terms of phenotype, function, and anatomical distribution. This cellular subtype is heterogeneous and can be subdivided into naive (CD45RA+) and memory (CD45RO+) subsets (reviewed in reference 44). Naive T lymphocytes exit the thymus, enter into the bloodstream, and get in lymphoid tissues through high endothelial venules. They circulate in both compartments until they encounter their cognate antigen. Maintained in a G0 state, naive T cells require a stimulation of ∼20 h from dendritic cells exposing the related antigen to be committed to proliferate (26). Depending on the duration of T-cell receptor stimulation mediated by dendritic cells in combination with some cytokines, the activated T cells differentiate and reach distinct effector functions and homing and survival capacity. Cells receiving a weak stimulation die by apoptosis, whereas those receiving a strong stimulation become effector or enter the memory pool. The memory subset remains in a nondividing state (quiescent), expresses lymph node homing receptors, and has a higher sensitivity to antigenic stimulation compared to the naive one. Enhanced expression of adhesion molecules and cellular factors is mainly involved in their ability to rapidly undergo terminal differentiation upon exposure to a recall antigen (32, 46).
Although human immunodeficiency virus type 1 (HIV-1) replicates predominantly in activated CD4+ T lymphocytes (35), quiescent CD4+ T cells likely represent the major target for initial infection among T cells. Indeed, most T cells in the body are in a quiescent G0 state with a low metabolic rate. However, numerous studies have reported that quiescent T cells are mainly nonpermissive to HIV-1 replication. In spite of this, integrated HIV-1 DNA has been largely found in resting CD4+ T lymphocytes from infected individuals (7, 16, 39). Interestingly, the majority of quiescent T cells carrying integrated HIV-1 genome displays a memory phenotype (10). While it is recognized that memory CD4+ T cells constitute the main cellular reservoir for HIV-1, it remains unclear whether these cells are more susceptible to the initial steps of viral life cycle.
Based on comparable surface levels of both primary cellular receptor and coreceptor (i.e., CD4 and CXCR4) on naive and memory T lymphocytes (43), it is usually thought that entry of HIV-1 occurs at similar rates in these two distinct cell subsets. However, besides interactions between the external virus-encoded envelope glycoprotein gp120 and CD4/CXCR4, it has been recognized that other interactions can promote the initial events in HIV-1 replication. Indeed, convincing studies have revealed that HIV-1 incorporates a plethora of host-derived cell surface molecules during the budding process, including the intercellular adhesion molecule 1 (ICAM-1) (9, 21, 57). Interestingly, the adhesion molecule ICAM-1 is efficiently acquired by all tested laboratory and clinical variants of HIV-1 bearing different tropisms (i.e., R5, X4, and R5X4) once amplified either in established cell lines or in primary human cells (2, 9, 12, 14, 23, 36). Moreover, it has also been demonstrated that virus-associated ICAM-1 influences HIV-1 biology since the natural ability of ICAM-1 to associate with its natural counterligand LFA-1 is preserved and leads to a severalfold increase in virus infectivity (21, 22, 55). Such a significant enhancement of HIV-1 infectivity is due to a more efficient virus adsorption onto target cells and a preferential entry process by fusion rather than through endocytosis (16).
Considering that memory CD4+ T cells express a higher surface level of LFA-1 compared to the naive subset, we tested the possibility that they might be preferentially targeted by ICAM-1-bearing HIV-1 particles. Our data indicate that memory CD4+ T lymphocytes are indeed more susceptible to a productive HIV-1 infection than the naive population, a process that seems to be due partly to the presence of LFA-1 clusters. This study provides novel insights on the identity of cellular factors responsible for the reported preferential HIV-1 infection of memory CD4+ T cells and highlights the dominant role played by LFA-1 in the first step of the virus life cycle.
(This work was performed by M. R. Tardif in partial fulfillment of her Ph.D. degree at the Faculty of Medicine, Laval University.)
MATERIALS AND METHODS
Antibodies and reagents.
The anti-CD11a (clone MEM25) monoclonal antibody was purchased from EXBIO Praha (Vestec, Czech Republic), whereas the anti-CD18 (CTB104) monoclonal antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The hybridoma cell line that produces the anti-CD4 SIM.2 monoclonal antibody was provided by the AIDS Repository Reagent Program (Germantown, MD). Anti-CD45RA (clone 5H9) and anti-CD45RO (clone UHCL-1) were purchased from BD Pharmingen (Mississauga, Ontario, Canada). R-phycoerythrin (R-PE)-conjugated and fluorescein isothiocyanate-tagged goat anti-mouse immunoglobulin G (IgG) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The following antibodies were obtained from Molecular Probes (Eugene, OR): Cy5 goat anti-mouse IgG, Alexa Fluor 488 goat anti-human IgG, and Alexa Fluor 546 goat anti-human IgG. Azidothymidine (AZT) was purchased from Sigma-Aldrich (Oakville, Ontario, Canada). The fusion inhibitor T-20 was provided by Roche Bioscience (Palo Alto, CA), while lovastatin and pravastatin were purchased from Calbiochem (San Diego, CA).
Cells.
293T cells were provided by W. C. Greene (The J. Gladstone Institutes, San Francisco, CA). These cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque gradient centrifugation, and CD4+ T cells were purified from freshly isolated PBMCs by immunomagnetic negative selection as indicated by the manufacturer (Stem Cell Technologies Inc., Vancouver, British Columbia, Canada). Purified CD4+ cells were further separated into pure CD45RA and CD45RO populations by negative selection as described by the manufacturer (Miltenyi Biotec Inc., Auburn, CA). The purity of the T-cell subsets was determined by cytofluorometry analyses and was always ≥98%. Lymphocytes were cultured for 24 h in RPMI 1640 medium supplemented with 10% FBS in the absence or presence of phytohemagglutinin (PHA; 1 μg/ml) and recombinant human interleukin-2 (rhIL-2; 50 U/ml).
Plasmids and production of viral stocks.
pNL4-3 is a full-length infectious molecular clone of HIV-1 (provided by the AIDS Repository Reagent Program). pNLENG1-EGFP is a full-length infectious molecular clone of HIV-1 containing enhanced green fluorescent protein (EGFP) between the env and nef genes. This vector was kindly given by D. N. Levy (The Howard Hughes Medical Institute, Birmingham, AL). pCD1.8 is a eukaryotic expression vector containing the entire human ICAM-1 (a generous gift from T. Springer, The Center for Blood Research, Boston, MA). Viruses differing by only the absence (called NL4-3) or the presence of host-derived ICAM-1 proteins on their surface (called NL4-3/ICAM-1) were produced by the calcium phosphate coprecipitation method in 293T cells as described previously (21). Virus preparations were normalized for virion content using an in-house enzymatic assay specific for the major viral p24 protein (9).
Fluorescence-activated cell sorter (FACS) analysis.
Naive and memory CD4+ T cells were incubated with anti-CD11a, anti-CD18, or an appropriate isotype-matched irrelevant control antibody for 30 min at 4°C. Cells were next washed with phosphate-buffered saline (PBS) and then incubated with a secondary R-PE-conjugated goat anti-mouse IgG for 30 min at 4°C. After two washes with PBS, cells were fixed in 2% paraformaldehyde and analyzed by cell sorting (Epics ELITE ESP; Coulter Electronics, Burlington, Ontario, Canada).
Virus capture assay.
We used a modified version of our previously described virus precipitation assay which is based on the capture of HIV-1 particles using immunomagnetic beads (13). Briefly, commercially available streptavidin-coated magnetic beads (8.4 × 106 beads; Dynal Biotech Inc., Brown Deer, WI) were mixed with 2 μg of biotinylated monoclonal antibodies in a final volume of 1 ml PBS plus 10% bovine serum albumin (PBSA) for 1 h at room temperature on a rocking plate. Immunomagnetic beads were next washed three times in PBSA with a magnet support (Dynal Biotech Inc.) and resuspended in 50 μl PBSA. HIV-1 (2 ng of p24) was added to the antibodies-beads mixture (50 μl), and the mixture was incubated overnight at 4°C on a rocking plate. Thereafter, immunomagnetic beads were washed four times in 200 μl of PBSA and finally resuspended in 200 μl of PBSA. Viruses captured on magnetic beads were disrupted by incubation for 30 min at room temperature in 50 μl of lysis buffer (PBS containing 2.5% Triton X-100). Magnetic beads were pelleted with a magnetic plate (Dynal Biotech Inc.), and 125 μl of the cleared supernatants was loaded on an enzyme-linked immunosorbent assay plate for p24 measurement as mentioned above.
Virus entry assay.
Quiescent and activated naive and memory CD4+ T lymphocytes (5 × 105) were resuspended in 0.1 ml of culture medium containing isogenic HIV-1 particles, either lacking or bearing host-derived ICAM-1 (2.5 ng of p24 per 105 cells), and were incubated at 37°C for 1 h or 3 h. To monitor the role played by LFA-1 in the process of virus entry, cells were pretreated for 30 min at 37°C with an antibody known to abolish ICAM-1/LFA-1 interactions (i.e., MEM25) before addition of viruses. In some experiments, T-cell subsets were pretreated with an anti-CD4 antibody (i.e., SIM.2 at 20 μg/ml) for 30 min at 37°C to abrogate interactions between gp120 and CD4 while virus fusion was blocked by using the fusion inhibitor T-20 (20 μg/ml). Following virus exposure, cells were washed and trypsinized for 5 min at 37°C to remove noninternalized viruses. Next, cells were first washed once with RPMI-1640 supplemented with 10% FBS and then twice with PBS before lysis in 200 μl of ice-cold lysis buffer (20 mM HEPES [pH 7.4], 150 mM NaCl, 0.5% Triton X-100). The amount of viruses entering cells was estimated by the p24 assay.
Detection of integrated HIV-1 DNA by real-time PCR.
Naive and memory CD4+ T cells were infected with isogenic NL4-3 or NL4-3/ICAM-1 particles for 3 days before extraction of genomic DNA by using the QIAGEN kit (Mississauga, Ontario, Canada). DNA was quantified and subjected to a combined Alu-HIV-1 PCR and real-time PCR as described by Suzuki and coworkers (54). Briefly, genomic DNA (100 ng) was first amplified with an Alu-sequence-specific sense primer (16) and HIV-1-specific antisense primer (i.e., M661). Next, the PCR products were diluted 25-fold and subjected to a real-time PCR assay with the sense primer M667, the antisense primer AA55, and the TaqMan probe HIV-6-carboxyfluorescein (54, 58). The cycling conditions included a hot start (95°C 5 min), followed by 40 cycles of denaturation (95°C for 1 min) and extension (63°C for 1 min). NL4-3 DNA was used for the standard curve (i.e., from 938 to 60,000 copies). HIV-1 standards contain 1 ng of DNA from uninfected cells as carrier. Real-time PCR was performed with Rotor Gene RG-3000 (Corbett Research, distributed by Montreal Biotech Inc., Montreal, Quebec, Canada).
Infectivity analysis.
Quiescent and activated T-cell subsets (105 cells) were incubated for 2 h at 37°C with isogenic NL4-3 or NL4-3/ICAM-1 virions (2.5 ng of p24). Cells were then extensively washed with PBS and resuspended in 200 μl of culture medium supplemented with rhIL-2. Cells were then transferred into 96-well flat-bottom tissue culture plates and incubated at 37°C. In some experiments, cells were treated with 10 μM AZT after an incubation of 4 h with viruses. Following an additional incubation time period of 16 h, cells were washed with culture medium and resuspended in 200 μl of culture medium supplemented with rhIL-2. Supernatants (100 μl) were harvested at different time points and frozen at −20°C until assayed for p24 contents.
Separation of naive and memory CD4+ T cells following exposure to HIV-1.
Quiescent CD4+ T lymphocytes (2 × 106 cells) were incubated for 6 h at 37°C with HIV-1 particles either lacking or bearing host-derived ICAM-1 (5 ng of p24 per 105 cells in a final volume of 0.5 ml of culture medium). In some experiments, cells were pretreated with lovastatin (50 μM) for 30 min at 37°C to block the interaction between LFA-1 and ICAM-1 or with the control compound pravastatin (50 μM) before exposure to viruses. Cells were washed twice with ice-cold PBS-2% FBS (binding buffer) and next incubated with magnetic beads to achieve isolation of cell subpopulations (Dynabeads M-450; Dynal Biotech Inc., Brown Deer, WI). Briefly, magnetic beads (1.5 μl per assay) were first coated with an anti-CD45RA or anti-CD45RO (1 ng per assay) antibody for 1 h at room temperature on a rotating plate. Beads were next washed twice with binding buffer through the use of a magnetic separation unit and resuspended in 100 μl of binding buffer. Beads were then mixed with unseparated CD4+ T cells that were exposed to the tested virus preparations. The bead-cell mixture was incubated for 1 h at room temperature on a rotating plate in a final volume of 1 ml of binding buffer. Beads were finally washed twice with binding buffer with a magnetic separation unit. Naive and memory quiescent CD4+ T lymphocytes, which were positively selected, were resuspended in 400 μl of culture medium containing PHA and IL-2 and incubated at 37°C. Supernatants were harvested after 3 days, and the amount of released viruses was quantified by measuring p24 contents.
Confocal microscopy.
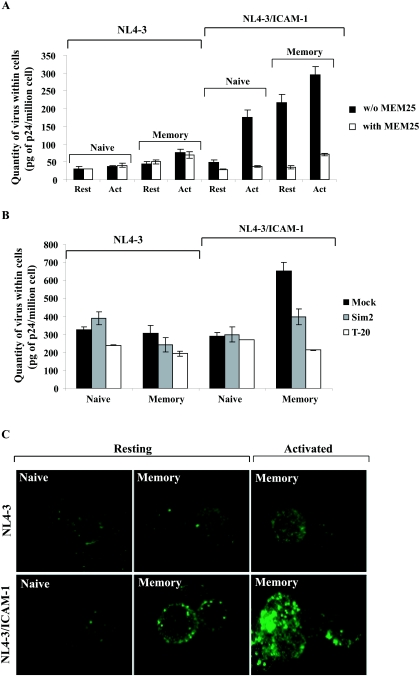
To analyze the distribution of LFA-1 on CD4+ T cells, quiescent and activated naive and memory CD4+ T cells were incubated with MEM25 for 30 min on ice followed by incubation with a Cye5-conjugated secondary antibody for 30 min on ice. After several washes, slides were mounted in 90% glycerol in PBS before confocal microscopy analysis. HIV-1 was labeled as described previously (55). Bound and internalized viruses were visualized by confocal laser scanning microscopy (Fluoview FV300; Olympus, Melville, NY). Digital images were processed with Adobe Photoshop (version 8). All the images were taken under similar experimental conditions (i.e., exposure time, magnification, and intensification), and processing was also the same for all the images shown in this study.
RESULTS
To define the possible contribution of virus-anchored host ICAM-1 in the reported ability of HIV-1 to replicate preferentially within memory CD4+ T lymphocytes, we produced isogenic HIV-1 particles (NL4-3 strain/X4 tropic) differing only by the absence or presence of host-derived ICAM-1 using a well-established transient transfection-and-expression system (21). The efficient incorporation of ICAM-1 onto progeny virus was monitored by a previously described virus capture assay (data not shown) (13).
Susceptibility of quiescent and activated CD4+-T-cell subsets to HIV-1 infection.
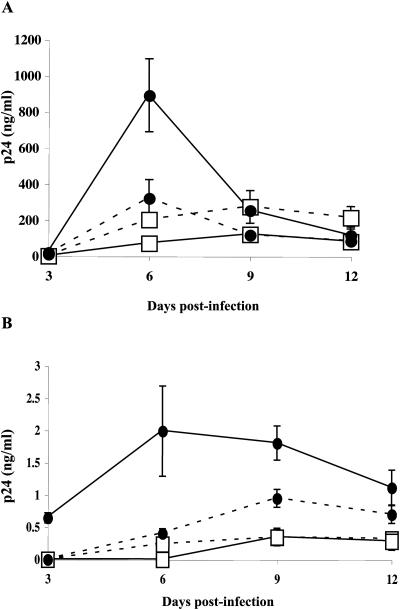
Previous studies have clearly demonstrated that interactions between virus-associated ICAM-1 and cell surface LFA-1 play a dominant role in the initial steps of HIV-1 life cycle once infection is allowed to proceed in PHA/IL-2-activated CD4+ T lymphocytes (9, 21, 41). However, given that CD4+ T cells are heterogeneous both functionally and phenotypically, we were interested in monitoring whether the insertion of host ICAM-1 within HIV-1 can direct infection toward a specific cellular subset. Data from studies on HIV-1 replication kinetics demonstrated that levels of virus replication in activated naive and memory CD4+ T cells were comparable when using virions lacking ICAM-1 (Fig. 1A). Interestingly, a more massive virus production in the memory subset compared to the naive subpopulation was seen following infection with isogenic ICAM-1-bearing HIV-1 particles. Although Fig. 1A shows that the infection of activated naive T cells with ICAM-1-bearing virions is lower than the ones infected with HIV-1 lacking the adhesion molecule, this result is not representative since we have not observed it with other donors. Next, we analyzed the impact of virus-associated ICAM-1 on the infection of quiescent naïve and memory T-cell subsets. Even though virus production was significantly reduced when infections were carried out in quiescent CD4+ T cells, observations similar to the ones obtained with activated cells were made. Indeed, resting memory CD4+ T lymphocytes produced an average of 3.4-fold more viruses than did the naive subpopulation at 6 days postinfection (Fig. 1B).
FIG. 1.
Virus production following infection of activated and quiescent CD4+-T-cell subsets. Naive (□) and memory (•) CD4+ T cells were either treated with PHA and IL-2 for 24 h (activated) (A) or left untreated (quiescent) (B) before incubation at 37°C for 2 h with similar amounts of isogenic NL4-3 particles, either lacking (dotted lines) or bearing (continuous lines) host-derived ICAM-1 (5 ng of p24 per 105 cells). Cells were extensively washed and resuspended in complete culture medium containing IL-2, and cell-free supernatants were collected at days 3, 6, 9, and 12 postinfection. Virus production was estimated by assessing p24 production. Experiments were performed in triplicate, and standard deviations are indicated. Data shown are representative of three separate experiments with different donors.
Upon infection with ICAM-1-bearing virions, memory CD4+ T cells in a resting state carry more integrated HIV-1 DNA than the naive subset.
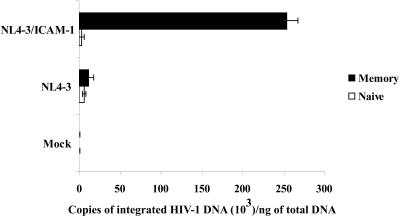
Integration represents an essential step in the replicative cycle of retroviruses (19, 34, 62). It is generally considered that reverse transcription is impaired in naive CD4+ T cells and that no integration can be detected in vitro. Nevertheless, it has been shown that, in HIV-1-seropositive patients, both quiescent naive and memory CD4+ T lymphocytes carry integrated proviral DNA that can support virus replication (39). Thus, we next estimated the levels of integrated HIV-1 DNA in quiescent naive and memory CD4+ T cells. This goal was achieved by performing a first round of PCR amplification using primers specific for Alu and HIV-1 sequences. The first PCR product was subsequently subjected to real-time PCR using primers specific for HIV-1 R/U5 regions. The Alu sequence is a ubiquitous repeat element found in the human genome (about 106 copies). By amplifying the junction between the nearest Alu sequence and the viral long terminal repeat (LTR), followed by a second round of LTR amplification, the presence of integrated HIV-1 genome can be specifically demonstrated. As shown in Fig. 2, the numbers of integrated viral DNA copies per ng of total DNA were comparable in resting naive (6,343 ± 1,987) and memory (11,218 ± 7,027) CD4+ T cells following infection with NL4-3 lacking host ICAM-1 as well as in quiescent naive CD4+ T cells inoculated with virions bearing the adhesion molecule (2,781 ± 3,932). However, a significant increase in the levels of integrated viral DNA was detected in memory CD4+ T cells that were infected with NL4-3/ICAM-1 particles (i.e., a mean increase of 22-fold; 254,000 ± 138,592). These data were confirmed when samples from a second healthy donor were used, since memory CD4+ T cells infected with ICAM-1-bearing viruses carried about 14-fold more integrated HIV-1 DNA copies than did the three other samples tested (data not shown).
FIG. 2.
Measurements of integrated HIV-1 DNA in resting naive and memory CD4+ T cells. The freshly separated naive and memory subsets of CD4+ T cells were incubated with virus preparations for 6 h at 37°C, washed, and maintained in culture for an additional 72 h. Genomic DNA was extracted and subjected in duplicate to a first PCR with Alu and M661 primers to amplify integrated proviruses. Next, the first PCR products were diluted 25-fold and subjected to real-time PCR using AA55 and M667 primers to further amplify the integrated HIV-1 LTR. The number of NL4-3 copies was determined by a standard curve prepared with the NL4-3 vector. Results shown are representative of two experiments made with different donors.
Reverse transcription of ICAM-1-bearing virions is more efficient in memory CD4+ T cells.
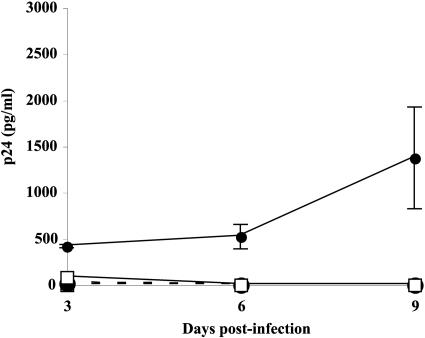
Following virus-cell fusion and uncoating, HIV-1 RNA undergoes reverse transcription to a DNA intermediate that can remain under either a nonintegrated or an integrated form. It has been recently suggested that the nonintegrated HIV-1 DNA can serve as a template for viral gene expression and has the full capacity to produce all classes of viral transcripts (50, 51, 63). However, it was shown that reverse transcription of the HIV-1 genome in quiescent T cells remains incomplete. Thus, if we detect the integrated HIV-1 genome in memory CD4+ T cells infected with NL4-3/ICAM-1, it would imply a more efficient reverse transcription process in this particular cell subset. To address this question, naive and memory CD4+ T cells were incubated with the studied virus preparations (i.e., NL4-3 and NL4-3/ICAM-1) for a time period sufficient to allow the binding, entry, and formation of partially or completely reverse-transcribed DNA before the addition of AZT (i.e., 4 h). Treatment with AZT will impair DNA synthesis from partially reverse-transcribed forms of viral genomic RNA, but not from completely synthesized viral DNA. Studies performed with this experimental strategy indicated that the release of progeny virus was observed only in AZT-treated memory CD4+ T cells that were infected with ICAM-1-bearing virions but not in the other samples subjected to AZT treatment (Fig. 3).
FIG. 3.
Efficiency of reverse transcription following inoculation of resting CD4+ T-cell subsets. Naive (□) and memory (•) CD4+ T cells were incubated at 37°C for 4 h with NL4-3 (dotted lines) or NL4-3/ICAM-1 (continuous lines), washed, and treated with 10 μM AZT for 16 h. Cells were washed twice to remove excess AZT, and cultures were maintained for 8 additional days. Cell-free supernatants were harvested at days 3, 6, and 9, and released viruses were quantified by p24 testing. Experiments were performed in triplicate, and standard deviations are indicated. Data shown are representative of six separate experiments made with different donors.
Higher levels of LFA-1 clusters are found on memory compared to naive CD4+ T cells.
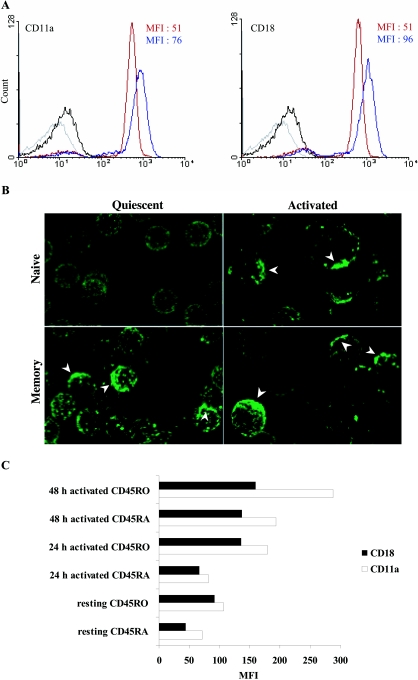
Reorganization of LFA-1 molecules into clusters represents one of the major mechanisms that regulate ICAM-1 binding (18, 31, 59-61). In naive CD4+ T cells, LFA-1 is maintained in a low avidity state (45, 48). Cellular activation favors lateral diffusion of the integrin and formation of LFA-1 clusters (18, 28, 52, 64). Since memory CD4+ T cells originate from activated naive CD4+ T cells, on which LFA-1 molecules are found in clusters, we wanted to investigate whether this patching is preserved and can play a role in the observed higher susceptibility of memory CD4+ T cells to a productive infection with ICAM-1-bearing virions. FACS analyses demonstrate that all naive and memory CD4+ T cells express LFA-1 subunits (CD11a and CD18) but at different levels (Fig. 4A). The mean fluorescence intensities (MFI) of CD11a and CD18 are higher on the memory subset (blue line) than on naive ones (red line), as was previously demonstrated by others groups (reviewed in references 4 and 11). Distinct spatial distribution of LFA-1 on naive and memory T cells is observed when performing confocal microscopy analyses. Indeed, a weak but homogenous distribution of LFA-1 was found on the surface of naive CD4+ T cells (Fig. 4B). Interestingly, LFA-1 was localized in patches on memory CD4+ T lymphocytes in a quiescent state as well as on the two studied activated T-cell subpopulations. These results suggest that LFA-1 is distributed in clusters onto resting memory CD4+ T cells. To compare densities of LFA-1 clusters upon stimulation of both CD4+ T-cell subsets, cells were stimulated with PHA for 24 and 48 h prior to analysis by flow cytometry. Results depicted in Fig. 4C demonstrate that levels of LFA-1 clusters are only marginally augmented following activation of naive CD4+ T lymphocytes, which is in sharp contrast to the situation seen with the activated memory cell subset.
FIG.4.
Distribution of LFA-1 on naive and memory CD4+ T cells. (A) Freshly isolated naive and memory CD4+ T cells were labeled with an anti-CD11a or an anti-CD18 antibody followed by an R-PE-conjugated goat anti-mouse antibody as described in Materials and Methods. The expression of both subunits was measured by flow cytometry analyses (MFI). Blue line, memory subset; red line, naive subset. (B) Resting naive and memory CD4+ T lymphocytes as well as naive CD4+ T cells stimulated for 24 h with PHA/IL-2 were labeled with a mouse anti-LFA-1 antibody (MEM25) followed by a Cy5-conjugated goat anti-mouse antibody. Cells were visualized by confocal microscopy. The images shown are a three-dimensional reconstructed Z series, and the individual sections are taken along the x and y axes. Arrows indicate LFA-1 clusters. Data shown are representative of three separate experiments made with different donors. (C) Resting and activated (24 or 48 h) CD4+-T-cell subsets were labeled with an anti-CD11a or an anti CD18 antibody followed by an R-PE-conjugated goat anti-mouse antibody. The MFI of both subunits was evaluated by FACS analysis.
ICAM-1-bearing viruses attach to and enter more efficiently in memory compared to naive CD4+ T cells.
Given that higher amounts of integrated HIV-1 DNA are found in memory CD4+ T lymphocytes, we addressed the possibility that the entry of ICAM-1-bearing virions is more efficient in this cellular subtype. To this end, entry of the studied virus preparations in purified CD4+-T-cell subsets was estimated in the absence or presence of an anti-LFA-1 antibody that can abrogate ICAM-1/LFA-1 interactions (MEM25). The process of entry of viruses lacking ICAM-1 was not affected by the anti-LFA-1 antibody, and slight differences were detected between naive and memory CD4+ T cells (Fig. 5A). On the contrary, larger amounts of isogenic ICAM-1-bearing virions were found inside memory CD4+ T cells than in cells bearing the naive phenotype (4.7-fold increase). The contribution of ICAM-1/LFA-1 interactions in the observed enhancement of virus entry is clearly illustrated through the use of the LFA-1 neutralizing antibody. Cellular activation of both naive and memory CD4+ T cells led to an increase in the rate of entry of NL4-3/ICAM-1, suggesting that enhanced activation of LFA-1 as well as LFA-1 expression might be responsible for this phenomenon. Differences observed in the entry of ICAM-1-bearing virions into activated naive and memory T cells are linked to the levels of LFA-1 clusters. Indeed, resting memory and activated naive CD4+ T cells (24 h poststimulation) contain similar amounts of LFA-1 clusters (Fig. 4C). Interestingly, similar amounts of ICAM-1-bearing viruses are detected inside these cell subpopulations. In contrast, the amount of virions detected within activated memory CD4+ T lymphocytes is the largest among samples tested, a phenomenon associated with the more important level of LFA-1 clusters observed on these cells. Next, we assessed the contribution of CD4 and CXCR4 in entry of the studied virus preparations in resting naive and memory CD4+ T cells. We found that the entry of virions lacking ICAM-1 in naive and memory CD4+ T cells is weakly affected by the anti-CD4 antibody SIM.2 and the fusion inhibitor T-20 (Fig. 5B). These data suggest that, at least under the tested experimental conditions (i.e., virus entry monitored after 3 h following infection), virions lacking host ICAM-1 are mainly internalized by endocytosis and not by fusion in the studied target cells. Similar results were obtained when assessing the entry of ICAM-1-bearing virions inside resting naive CD4+ T cells. In contrast, entry of ICAM-1-bearing viruses within resting memory CD4+ T cells was found to be sensitive to the two blocking agents studied. These results are in agreement with our previous work demonstrating that ICAM-1 incorporation favors the entry of HIV-1 particles through fusion, a process known to result in productive infection (55, 56).
FIG.5.
Attachment and entry of viruses either lacking or bearing host-derived ICAM-1 in quiescent and activated CD4+-T-cell subsets. (A) Naive and memory quiescent or activated CD4+ T cells (i.e., treated with PHA and IL-2 for 24 h) were either untreated or treated for 30 min at 37°C with the blocking anti-LFA-1 antibody MEM25 before incubation for 1 h with the studied virus preparations. (B) Resting naive and memory CD4+ T cells were either left untreated or were treated with SIM.2 or T-20, followed by incubation with the listed virus preparations for 3 h at 37°C. Virus entry assays were performed as described in Materials and Methods. Data shown are representative of three separate experiments. (C) Viruses were stained on both cellular subsets as described in Materials and Methods and visualized by confocal microscopy. The images are three-dimensional reconstructed Z series, and the individual sections are taken along the x and y axes. Data shown are representative of three separate experiments.
Confocal microscopy studies were next carried out to provide additional evidence of the capacity of ICAM-1-bearing HIV-1 particles to interact more efficiently with CD4+ T cells compared to the naive subset. As depicted in Fig. 5C, similar low levels of virions lacking host ICAM-1 were found to associate with both CD4+ T-cell subsets. A larger amount of NL4-3/ICAM-1 viruses was found to associate with resting memory CD4+ T lymphocytes than with naive CD4+ T cells. Activated memory CD4+ T cells, when used in combination with ICAM-1-bearing HIV-1 particles, were used as a positive control. The data further confirm that HIV-1 particles carrying ICAM-1 display a greater propensity to target memory CD4+ T cells.
Cells of the memory phenotype in a heterogeneous resting CD4+-T-cell population are preferentially targeted by ICAM-1-bearing virions.
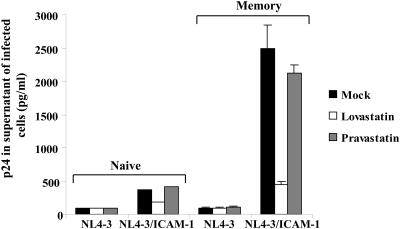
In an attempt to assess if ICAM-1-bearing virions could preferentially target memory CD4+ T cells when infection is performed in unseparated CD4+ T lymphocytes, preparations of negatively selected CD4+ T lymphocytes were exposed to HIV-1 particles either lacking or bearing host ICAM-1. Cells were next separated using anti-CD45RA and anti-CD45RO-coated magnetic beads. Each population was maintained in culture medium containing PHA and IL-2 for 3 days, and supernatants were harvested to quantify the virus content. In some conditions, cells were pretreated either with lovastatin to inhibit interactions between ICAM-1 and LFA-1 interaction or with a control compound (i.e., pravastatin). Results presented in Fig. 6 indicate that virions lacking host ICAM-1 infected both naive and memory CD4+ T cells with a comparable low efficiency. However, memory CD4+ T cells were more efficiently infected with ICAM-1-bearing viruses than cells expressing the naive phenotype, a phenomenon that was abolished upon treatment with lovastatin but not with pravastatin.
FIG. 6.
HIV-1 infection of a heterogeneous CD4+-T-cell population. Unseparated resting CD4+ T cells were either left untreated or treated with lovastatin (50 μM) or pravastatin (50 μM) for 30 min at 37°C before an incubation for 6 h with HIV-1 particles either devoid or bearing ICAM-1. Cells were next washed, and naive and memory CD4+ T cells were separated as described in Materials and Methods. Cells were maintained in culture in the presence of PHA and IL-2 for an additional 72 h, and supernatants were harvested to estimate virus production. Experiments were performed in triplicate, and standard deviations are indicated. Results shown are representative of three different experiments.
DISCUSSION
Identification of intrinsic factors responsible for the preferential infection of memory cells among resting CD4+ T cells is of high importance to achieve a better comprehension of HIV-1 biology. This knowledge is particularly informative since it might help to shed light on mechanisms through which viral reservoirs are established in infected individuals and on the inability of the immune system to better control HIV-1 replication (15, 27, 37, 38, 47). A mechanism that has been proposed to explain the infection of memory CD4-expressing T cells states that HIV-1-infected resting memory CD4+ T lymphocytes derive actually from activated naive CD4+ T cells that become infected and then revert to a resting G0 state (7). However, it has also been postulated that the predominant way by which memory CD4+ T lymphocytes in a quiescent state get infected is through direct infection. The present study is thus in full support of this hypothesis. Indeed, we provide evidence that memory CD4+ T cells are specifically targeted by ICAM-1-bearing HIV-1 particles when infection is carried out in a heterogeneous cell population made of both naive and memory subsets. We demonstrate that the presence of numerous LFA-1 molecules organized in clusters is a key element for a more efficient attachment of HIV-1 particles carrying host ICAM-1 onto memory CD4+ T cells, an event that is also associated with an augmentation of virus entry and a higher level of integrated viral DNA.
Our findings are perfectly in line with a study that has estimated the number of resting naive and memory CD4+ T cells that are infected under in vivo situations in HIV-1-seropositive subjects. In fact, Ostrowski and colleagues have reported that memory CD4+ T cells had a median of fourfold more replication-competent virus and 16-fold more integrated provirus than naive CD4+ T cells in a cohort of 11 patients (39). Another study has also demonstrated that memory T cells from HIV-1-infected patients contain an average of 10 times more copies of HIV-1 DNA than naive T cells (10). In the present study, we found that memory CD4+ T cells carry 10 to 22 times more integrated viral DNA upon infection with ICAM-1-bearing virions than resting naive CD4+ T cells or than these two cellular subsets infected with isogenic viruses lacking ICAM-1. Furthermore, our data reveal that virus production is around 3.4 times greater in quiescent memory CD4+ T cells infected with ICAM-1-bearing HIV-1 particles compared to naive CD4+ T lymphocytes. Overall, our results indicate that memory CD4+ T cells are more permissive to productive infection with HIV-1 particles that bear host-encoded ICAM-1. The physiological relevance of this observation should not be underestimated considering that ICAM-1 has been reported to be acquired by all studied clinical and laboratory variants of HIV-1 independently of the tropism, which were amplified in primary human cells including histocultures of lymphoid tissue (2, 9, 12, 14, 23, 36).
Data from virus capture tests performed with NL4-3 particles that were produced in transiently transfected 293T cells and more natural cell targets (i.e., PBMCs and human lymphoid tissue cultured ex vivo) indicate that viruses from 293T cells are more efficiently captured than virions amplified in primary human cells (data not shown). This might suggest that virions produced by 293T cells incorporate larger amounts of host ICAM-1 than viruses that are shed by primary human cells. Although such findings question the significance of the current experiments, it is important to emphasize that previous work carried out with progeny virus produced in PBMCs provided evidence of the dominant role played by interactions between virus-anchored ICAM-1 and cell surface LFA-1 in HIV-1 attachment, entry, and infectivity through the use of blocking agents such as neutralizing antibodies (i.e., MEM25, MEM30, and RR1/1.1.1) and lovastatin (3, 9, 24, 55). Moreover, it was recently demonstrated that the lateral mobility and clustering of LFA-1 are crucial in the early events of HIV-1 life cycle for viruses produced in both 293T cells and PBMCs (56). Altogether, these published observations provide credence to the present work.
Our data differ from those of a previous report showing that the greater virus production in memory CD4+ T cells compared to cells bearing a naive phenotype was not associated with a difference in cell susceptibility to HIV-1 infection (49). These investigators argue that the difference in viral replication between the two cellular subsets is most likely associated with some specific cellular factors that are induced upon T-cell receptor/CD3-mediated signal transduction events. It is important to emphasize that the implication of virus-anchored cell surface membrane constituents in the preferential HIV-1 replication in the memory subtype was not assessed in this work. Moreover, our results are consistent with a previous publication demonstrating a preferential attachment of HIV-1 particles to memory CD4+ T cells (6). These investigators produced their viruses in ICAM-1-expressing chronically infected T cells but were unable to demonstrate the involvement of LFA-1 in this process, which is in sharp contrast to our findings. The explanation for this discrepancy is currently unknown but could relate to the use of a different anti-LFA-1 antibody that is specific for CD18 (β2-chain of LFA-1, clone 685A5) while we have used a blocking anti-CD11a antibody (α-chain of LFA-1). Despite this dissimilarity, both studies demonstrate that the inability of ICAM-1-bearing virions to productively infect naive CD4+ T cells can be reversed by the activation of this cell subset with PHA/IL-2, a process which is known to induce the expression of LFA-1 molecules and their redistribution in clusters.
It has been shown that reorganization of LFA-1 molecules in clusters represents the major mechanism regulating the association between ICAM-1 and LFA-1 (18, 31, 59-61). It is well known that LFA-1 is maintained in a low avidity state in naive CD4+ T cells by the tethering of the integrin tail to the actin cytoskeleton (45, 48). Cellular activation leads to the release of LFA-1 molecules from its cytoskeleton constraint, favoring lateral diffusion and the formation of LFA-1 clusters (18, 28, 52, 64). As memory CD4+ T cells originate from activated naive CD4+ T cells, where LFA-1 molecules are distributed in clusters, it was not surprising to discover LFA-1 clusters on resting memory CD4+ T cells. The presence of these clusters may be independent of cellular activation, since resting memory CD4+ T cells do not express activation markers such as CD25 and CD69 (data not shown). To the best of our knowledge, it represents the first demonstration that LFA-1 molecules are patched on the surface of memory CD4+ T cells.
Given that biochemical events can be transduced in target cells by HIV-1-anchored host proteins (8), it can be postulated that attachment of ICAM-1-bearing virions to the surface of LFA-1-expressing cells might trigger outside-in signaling. This could, in turn, as suggested previously (55), favor a firmer docking of the viral entity onto the target cell surface due to LFA-1 activation and a possible mobilization of LFA-1 to the lipid raft and thus move it closer to CD4. Moreover, it can be proposed that LFA-1-mediated signal transduction events due to engagement with virus-anchored host ICAM-1 can lead to a remodeling of the cytoskeleton, a process that might lead to a destabilization of the plasma membrane and a higher probability to interact with the appropriate chemokine coreceptor CXCR4. The previous demonstration that the F-actin cytoskeleton is remodeled by ICAM-1-dependent signaling through LFA-1 fully supports this scenario (40).
Although it cannot be excluded that the naive cell subpopulation is totally free of cells expressing a nonnaive phenotype, it should be noted that nonnaive CD45RA CD4+ T cells represent a very small fraction of effector CD4+ T lymphocytes which usually express the CD45RO isoform. To confirm that our purified CD45RA population was truly naive, we performed infectivity experiments with R5-tropic virions based on the notion that the chemokine receptor CCR5 is not expressed on naive CD4+ T cells but is expressed by the effector subset (44). Virus replication was not detected in the CD45RA cell fraction (data not shown), thus confirming that purified CD45RA CD4+ T cells are virtually free of nonnaive CD45RA cells.
Beside phenotypic differences between naive and memory CD4+ T cells, these two distinct cellular subpopulations also exhibit differences with respect to their functional properties and metabolism. Indeed, memory T cells seem to be metabolically more active than naive T cells (53). As memory T cells contain higher levels of RNA and proteins than naive T cells, it has been suggested that the memory subset may be resting in the G1 rather than the G0 state (53). These properties probably also partly explain the greater susceptibility of these cells to HIV-1 infection. Conversely, naive and memory T cells react differently to cellular activation (4, 5). Several differences in the two T-cell subsets have been noted that could affect signaling pathways within these cells, which may explain different susceptibility to viral replication (20, 25, 33). Preferential HIV-1 replication in the memory cell subpopulation appears to be related to a greater stimulus-mediated activation of the nuclear factor of activated T cells in this cell type than what is seen in naive T cells (42). Nuclear factor of activated T cells has been demonstrated to act in synergy with the nuclear factor kappa B for promoting HIV-1 transcription in both T-cell lines and primary human T cells (1, 17, 29, 30). This is not surprising because HIV-1 gene expression is known to rely on a complex interplay between a series of cellular transcription factors and the viral encoded transactivator of transcription Tat. This might help to explain that, even though comparable amounts of ICAM-1-bearing HIV-1 particles entered within activated naive and memory CD4+ T cells, we found that virus production is superior in cells displaying the memory phenotype.
In conclusion, our findings propose a possible mechanistic framework explaining the higher susceptibility of memory CD4+ T cells to HIV-1 infection. The role played by virus-associated host ICAM-1 and cell surface LFA-1 under a cluster formation might be considered a determining factor in the initial events of HIV-1 life cycle since we demonstrate that it can influence the permissiveness of quiescent memory CD4+ T cells to a productive virus infection. The propensity of HIV-1 to preferentially target memory CD4+ T cells that contain LFA-1 clusters might simply reflect a biological adaptation the virus has evolved to establish a sanctuary of long-lived cells that carry integrated viral DNA.
Acknowledgments
We are grateful to S. Méthot for editorial assistance and M. Imbeault for his assistance with real-time PCR assays. We thank M. Dufour for performing flow cytometric analyses.
This work was financially supported by an operating grant to M.J.T. from the Canadian Institute of Health Research (CIHR) HIV/AIDS Research Program (grant HOP-14438). M.R.T. holds a CIHR Doctoral Award, and M.J.T. is the recipient of the Canada Research Chair in Human Immuno-Retrovirology (senior level).
REFERENCES
- 1.Bassuk, A. G., R. T. Anandappa, and J. M. Leiden. 1997. Physical interactions between Ets and NF-κB/NFAT proteins play an important role in their cooperative activation of the human immunodeficiency virus enhancer in T cells. J. Virol. 71:3563-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastiani, L., S. Laal, M. Kim, and S. Zolla-Pazner. 1997. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J. Virol. 71:3444-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beausejour, Y., and M. J. Tremblay. 2004. Susceptibility of HIV type 1 to the fusion inhibitor T-20 is reduced on insertion of host intercellular adhesion molecule 1 in the virus membrane. J. Infect. Dis. 190:894-902. [DOI] [PubMed] [Google Scholar]
- 4.Berard, M., and D. F. Tough. 2002. Qualitative differences between naive and memory T cells. Immunology 106:127-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beverley, P. 1991. Immunological memory in T cells. Curr. Opin. Immunol. 3:355-360. [DOI] [PubMed] [Google Scholar]
- 6.Blanco, J., J. Barretina, A. Gutierrez, M. Armand-Ugon, C. Cabrera, B. Clotet, and J. A. Este. 2002. Preferential attachment of HIV particles to activated and CD45RO+CD4+ T cells. AIDS Res. Hum. Retrovir. 18:27-38. [DOI] [PubMed] [Google Scholar]
- 7.Blankson, J. N., D. Persaud, and R. F. Siliciano. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53:557-593. [DOI] [PubMed] [Google Scholar]
- 8.Bounou, S., N. Dumais, and M. J. Tremblay. 2001. Attachment of human immunodeficiency virus-1 (HIV-1) particles bearing host-encoded B7-2 proteins leads to nuclear factor-kappa B- and nuclear factor of activated T cells-dependent activation of HIV-1 long terminal repeat transcription. J. Biol. Chem. 276:6359-6369. [DOI] [PubMed] [Google Scholar]
- 9.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenchley, J. M., B. J. Hill, D. R. Ambrozak, D. A. Price, F. J. Guenaga, J. P. Casazza, J. Kuruppu, J. Yazdani, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 78:1160-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell, J. J., and E. C. Butcher. 2000. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12:336-341. [DOI] [PubMed] [Google Scholar]
- 12.Cantin, R., J. F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. [DOI] [PubMed] [Google Scholar]
- 13.Cantin, R., G. Martin, and M. J. Tremblay. 2001. A novel virus capture assay reveals a differential acquisition of host HLA-DR by clinical isolates of human immunodeficiency virus type 1 expanded in primary human cells depending on the nature of producing cells and the donor source. J. Gen. Virol. 82:2979-2987. [DOI] [PubMed] [Google Scholar]
- 14.Capobianchi, M. R., S. Fais, C. Castilletti, M. Gentile, F. Ameglio, and F. Dianzani. 1994. A simple and reliable method to detect cell membrane proteins on infectious human immunodeficiency virus type 1 particles. J. Infect. Dis. 169:886-889. [DOI] [PubMed] [Google Scholar]
- 15.Cayota, A., F. Vuillier, D. Scott-Algara, V. Feuillie, and G. Dighiero. 1992. Impaired proliferative capacity and abnormal cytokine profile of naive and memory CD4 T cells from HIV-seropositive patients. Clin. Exp. Immunol. 88:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 17.Cron, R. Q., S. R. Bartz, A. Clausell, S. J. Bort, S. J. Klebanoff, and D. B. Lewis. 2000. NFAT1 enhances HIV-1 gene expression in primary human CD4 T cells. Clin. Immunol. 94:179-191. [DOI] [PubMed] [Google Scholar]
- 18.Dustin, M. L., T. G. Bivona, and M. R. Philips. 2004. Membranes as messengers in T cell adhesion signaling. Nat. Immunol. 5:363-372. [DOI] [PubMed] [Google Scholar]
- 19.Engelman, A., G. Englund, J. M. Orenstein, M. A. Martin, and R. Craigie. 1995. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J. Virol. 69:2729-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farber, D. L. 2003. Remembrance of antigens past: new insights into memory T cells. Scand. J. Immunol. 58:145-154. [DOI] [PubMed] [Google Scholar]
- 21.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortin, J. F., R. Cantin, and M. J. Tremblay. 1998. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J. Virol. 72:2105-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank, I., H. Stoiber, S. Godar, H. Stockinger, F. Steindl, H. W. Katinger, and M. P. Dierich. 1996. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS 10:1611-1620. [DOI] [PubMed] [Google Scholar]
- 24.Giguere, J. F., and M. J. Tremblay. 2004. Statin compounds reduce human immunodeficiency virus type 1 replication by preventing the interaction between virion-associated host intercellular adhesion molecule 1 and its natural cell surface ligand LFA-1. J. Virol. 78:12062-12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain, S. F., C. F. Anderson, and D. L. Farber. 2002. Differential SLP-76 expression and TCR-mediated signaling in effector and memory CD4 T cells. J. Immunol. 168:1557-1565. [DOI] [PubMed] [Google Scholar]
- 26.Iezzi, G., K. Karjalainen, and A. Lanzavecchia. 1998. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8:89-95. [DOI] [PubMed] [Google Scholar]
- 27.Janossy, G., N. Borthwick, R. Lomnitzer, E. Medina, S. B. Squire, A. N. Phillips, M. Lipman, M. A. Johnson, C. Lee, and M. Bofill. 1993. Lymphocyte activation in HIV-1 infection. I. Predominant proliferative defects among CD45R0+ cells of the CD4 and CD8 lineages. AIDS 7:613-624. [DOI] [PubMed] [Google Scholar]
- 28.Kim, M., C. V. Carman, and T. A. Springer. 2003. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301:1720-1725. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita, S., B. K. Chen, H. Kaneshima, and G. P. Nolan. 1998. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell 95:595-604. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita, S., L. Su, M. Amano, L. A. Timmerman, H. Kaneshima, and G. P. Nolan. 1997. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity 6:235-244. [DOI] [PubMed] [Google Scholar]
- 31.Krauss, K., and P. Altevogt. 1999. Integrin leukocyte function-associated antigen-1-mediated cell binding can be activated by clustering of membrane rafts. J. Biol. Chem. 274:36921-36927. [DOI] [PubMed] [Google Scholar]
- 32.Kuiper, H., M. Brouwer, M. de Boer, P. Parren, and R. A. van Lier. 1994. Differences in responsiveness to CD3 stimulation between naive and memory CD4+ T cells cannot be overcome by CD28 costimulation. Eur. J. Immunol. 24:1956-1960. [DOI] [PubMed] [Google Scholar]
- 33.Leitenberg, D., T. J. Novak, D. Farber, B. R. Smith, and K. Bottomly. 1996. The extracellular domain of CD45 controls association with the CD4-T cell receptor complex and the response to antigen-specific stimulation. J. Exp. Med. 183:249-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.List, J., and A. T. Haase. 1997. Integration of visna virus DNA occurs and may be necessary for productive infection. Virology 237:189-197. [DOI] [PubMed] [Google Scholar]
- 35.Margolick, J. B., D. J. Volkman, T. M. Folks, and A. S. Fauci. 1987. Amplification of HTLV-III/LAV infection by antigen-induced activation of T cells and direct suppression by virus of lymphocyte blastogenic responses. J. Immunol. 138:1719-1723. [PubMed] [Google Scholar]
- 36.Martin, G., and M. J. Tremblay. 2004. HLA-DR, ICAM-1, CD40, CD40L, and CD86 are incorporated to a similar degree into clinical human immunodeficiency virus type 1 variants expanded in natural reservoirs such as peripheral blood mononuclear cells and human lymphoid tissue cultured ex vivo. Clin. Immunol. 111:275-285. [DOI] [PubMed] [Google Scholar]
- 37.Medina, E., N. Borthwick, M. A. Johnson, S. Miller, and M. Bofill. 1994. Flow cytometric analysis of the stimulatory response of T cell subsets from normal and HIV-1+ individuals to various mitogenic stimuli in vitro. Clin. Exp. Immunol. 97:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miedema, F. 1992. Immunological abnormalities in the natural history of HIV infection: mechanisms and clinical relevance. Immunodefic. Rev. 3:173-193. [PubMed] [Google Scholar]
- 39.Ostrowski, M. A., T. W. Chun, S. J. Justement, I. Motola, M. A. Spinelli, J. Adelsberger, L. A. Ehler, S. B. Mizell, C. W. Hallahan, and A. S. Fauci. 1999. Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J. Virol. 73:6430-6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter, J. C., M. Bracke, A. Smith, D. Davies, and N. Hogg. 2002. Signaling through integrin LFA-1 leads to filamentous actin polymerization and remodeling, resulting in enhanced T cell adhesion. J. Immunol. 168:6330-6335. [DOI] [PubMed] [Google Scholar]
- 41.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 71:4847-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robichaud, G. A., B. Barbeau, J. F. Fortin, D. M. Rothstein, and M. J. Tremblay. 2002. Nuclear factor of activated T cells is a driving force for preferential productive HIV-1 infection of CD45RO-expressing CD4+ T cells. J. Biol. Chem. 277:23733-23741. [DOI] [PubMed] [Google Scholar]
- 43.Roederer, M., P. A. Raju, D. K. Mitra, and L. A. Herzenberg. 1997. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J. Clin. Investig. 99:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763. [DOI] [PubMed] [Google Scholar]
- 45.Sampath, R., P. J. Gallagher, and F. M. Pavalko. 1998. Cytoskeletal interactions with the leukocyte integrin beta2 cytoplasmic tail. Activation-dependent regulation of associations with talin and alpha-actinin. J. Biol. Chem. 273:33588-33594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders, M. E., M. W. Makgoba, S. O. Sharrow, D. Stephany, T. A. Springer, H. A. Young, and S. Shaw. 1988. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J. Immunol. 140:1401-1407. [PubMed] [Google Scholar]
- 47.Schellekens, P. T., M. T. Roos, F. De Wolf, J. M. Lange, and F. Miedema. 1990. Low T-cell responsiveness to activation via CD3/TCR is a prognostic marker for acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus-1 (HIV-1)-infected men. J. Clin. Immunol. 10:121-127. [DOI] [PubMed] [Google Scholar]
- 48.Smith, C. W., S. D. Marlin, R. Rothlein, C. Toman, and D. C. Anderson. 1989. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J. Clin. Investig. 83:2008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spina, C. A., H. E. Prince, and D. D. Richman. 1997. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J. Clin. Investig. 99:1774-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevenson, M. 1997. Molecular mechanisms for the regulation of HIV replication, persistence and latency. AIDS 11(Suppl. A):S25-S33. [PubMed] [Google Scholar]
- 51.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart, M. P., A. McDowall, and N. Hogg. 1998. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J. Cell Biol. 140:699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stout, R. D., and J. Suttles. 1992. T cells bearing the CD44hi “memory” phenotype display characteristics of activated cells in G1 stage of cell cycle. Cell. Immunol. 141:433-443. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki, Y., N. Misawa, C. Sato, H. Ebina, T. Masuda, N. Yamamoto, and Y. Koyanagi. 2003. Quantitative analysis of human immunodeficiency virus type 1 DNA dynamics by real-time PCR: integration efficiency in stimulated and unstimulated peripheral blood mononuclear cells. Virus Genes 27:177-188. [DOI] [PubMed] [Google Scholar]
- 55.Tardif, M. R., and M. J. Tremblay. 2003. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J. Virol. 77:12299-12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tardif, M. R., and M. J. Tremblay. 2005. Regulation of LFA-1 activity through cytoskeleton remodeling and signaling components modulates the efficiency of HIV type-1 entry in activated CD4+ T lymphocytes. J. Immunol. 176:926-935. [DOI] [PubMed] [Google Scholar]
- 57.Tremblay, M. J., J. F. Fortin, and R. Cantin. 1998. The acquisition of host-encoded proteins by nascent HIV-1. Immunol. Today 19:346-351. [DOI] [PubMed] [Google Scholar]
- 58.Tsurutani, N., M. Kubo, Y. Maeda, T. Ohashi, N. Yamamoto, M. Kannagi, and T. Masuda. 2000. Identification of critical amino acid residues in human immunodeficiency virus type 1 IN required for efficient proviral DNA formation at steps prior to integration in dividing and nondividing cells. J. Virol. 74:4795-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Kooyk, Y., S. J. van Vliet, and C. G. Figdor. 1999. The actin cytoskeleton regulates LFA-1 ligand binding through avidity rather than affinity changes. J. Biol. Chem. 274:26869-26877. [DOI] [PubMed] [Google Scholar]
- 60.van Kooyk, Y., P. Weder, K. Heije, and C. G. Figdor. 1994. Extracellular Ca2+ modulates leukocyte function-associated antigen-1 cell surface distribution on T lymphocytes and consequently affects cell adhesion. J. Cell Biol. 124:1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Kooyk, Y., P. Weder, F. Hogervorst, A. J. Verhoeven, G. van Seventer, A. A. te Velde, J. Borst, G. D. Keizer, and C. G. Figdor. 1991. Activation of LFA-1 through a Ca2(+)-dependent epitope stimulates lymphocyte adhesion. J. Cell Biol. 112:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiskerchen, M., and M. A. Muesing. 1995. Human immunodeficiency virus type 1 integrase: effects of mutations on viral ability to integrate, direct viral gene expression from unintegrated viral DNA templates, and sustain viral propagation in primary cells. J. Virol. 69:376-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu, Y., and J. W. Marsh. 2003. Early transcription from nonintegrated DNA in human immunodeficiency virus infection. J. Virol. 77:10376-10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan, B., D. A. Calderwood, B. Yaspan, and M. H. Ginsberg. 2001. Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J. Biol. Chem. 276:28164-28170. [DOI] [PubMed] [Google Scholar]