Abstract
Both endocytic uptake and viral fusion can lead to human immunodeficiency virus type 1 (HIV-1) transfer to CD4+ lymphocytes, either through directional regurgitation (infectious transfer in trans [I-IT]) or through de novo viral production in dendritic cells (DCs) resulting in a second-phase transfer to CD4+ lymphocytes (infectious second-phase transfer [I-SPT]). We have evaluated in immature monocyte-derived DCs both pathways of transfer with regard to their susceptibilities to being blocked by potential microbicidal compounds, including cyanovirin (CNV); the plant lectins Hippeastrum hybrid agglutinin, Galanthus nivalis agglutinin, Urtica dioica agglutinin, and Cymbidium hybrid agglutinin; and the glycan mannan. I-IT was a relatively inefficient means of viral transfer compared to I-SPT at both high and low levels of the viral inoculum. CNV was able to completely block I-IT at 15 μg/ml. All other compounds except mannan could inhibit I-IT by at least 90% when used at doses of 15 μg/ml. In contrast, efficient inhibition of I-SPT was remarkably harder to achieve, as 50% effective concentration levels for plant lectins and CNV to suppress this mode of HIV-1 transfer increased significantly. Thus, our findings indicate that I-SPT may be more elusive to targeting by antiviral drugs and stress the need for drugs affecting the pronounced inhibition of the infection of DCs by HIV-1.
The necessity to lower or halt the spread of human immunodeficiency virus (HIV) infection in the world and particularly in developing countries is an urgent problem that needs to be addressed. Scientific progress has been made in HIV therapy and vaccine research, although it may not significantly impact the areas of highest HIV prevalence. Educational programs and the promotion of condom use have already shown a visible effect on the epidemic (12, 35), but there is need for alternative solutions that can take the infection rate down further. The development of efficient microbicides represents one important tool, and the choice of which compounds need to be used is likely to rely on the basic mechanisms of HIV transmission in vivo.
Although opposing views exist on which cell(s) and which receptor(s) may be most important for HIV transmission, observations in simian immunodeficiency virus macaque models and in in vitro human cellular and mucosal explant models indicate that HIV transmission can occur through multiple pathways at the cellular and molecular levels (12, 19, 21, 22, 25, 32, 34, 35, 44, 45). For instance, at the squamous mucosa, HIV virions may first make infectious contact with intraepithelial CD4+ lymphocytes, with intraepithelial Langerhans cells (LCs) and, in the case of the cervical mucosa and/or areas of breached epithelium, with macrophages, CD4+ lymphocytes, and the lamina propria dendritic cells (DCs).
DCs in this mixture of target cells represent a peculiar leukocyte population with regard to HIV transmission. Not only are DCs uniquely located in mucosal tissue, they are also highly motile in order to communicate immunological knowledge both locally and in primary lymphoid tissues. They also may cause an explosive HIV infection when previously exposed to HIV and subsequently mixed with CD4+ lymphocytes. It is this unique motility and catalytic feature that has led to the hypothesis that DCs may have the ability to transport and transfer HIV locally or distantly to responding CD4+ lymphocytes, thereby establishing infection and seeding the virus effectively in vivo (10, 11, 30, 31). Targeting the HIV virion-DC interaction may be elusive and complicated. For instance, multiple levels of viral attachment and transfer exist in DCs (15, 18, 22, 31, 32, 38-40). DCs can bind and take up virus through CD4; C-type lectin receptors (CLRs) CD206, CD207, and CD209; and other as-yet-unidentified mechanisms (15, 18, 26, 39, 40). Transfer of virus by DCs can occur via a process of endocytic regurgitation (commonly known as infection in trans [15], although herein referred to as infectious transfer in trans [I-IT]) when CD4+ lymphocytes make extracellular contact with HIV-exposed DCs (27, 40). CCR5-using HIV isolates can also enter DCs via CD4 and CCR5 and infect the DCs at low levels. This infection of DCs can lead to a later scenario of viral transfer and amplification of HIV in CD4+ lymphocytes (this later transfer will be referred to as infectious second-phase transfer [I-SPT]) (7, 24, 25, 31, 32, 40). In both settings, HIV virion transfer may occur effectively away from where the microbicide will be initially applied in healthy intact tissues (but may be accessed in damaged/inflamed tissues). Thus, the design and study of relevant compounds must take these issues into account, and these potential compounds should be evaluated in cell models that accommodate the capacity for a DC to effectively deliver virus to CD4+ lymphocytes.
Given its complexity, research focused on blocking HIV attachment and transfer to and from DCs may provide a broader strategy for investigating potential microbicides. Therefore, we have evaluated the efficiencies of viral transfer mechanisms of immature DCs (i.e., the functional phenotypic form of DCs found in mucosa and peripheral tissue) to CD4+ lymphocytes and investigated the blocking capacities of five sugar-recognizing compounds that have previously been shown to inhibit HIV infection of T lymphocytes: three mannose-specific plant lectins, Galanthus nivalis agglutinin (GNA), Hippeastrum hybrid agglutinin (HHA), and Cymbidium hybrid agglutinin (CA) (2-5); the N-acetylglucosamine-specific plant lectin Urtica dioica agglutinin (UDA) (3); the mannose-specific cyanovirin (CNV), derived from the green blue alga Nostoc ellipsosporum (9); and the broad C-type lectin inhibitor mannan.
MATERIALS AND METHODS
Test compounds.
The mannose-specific plant lectins from Galanthus nivalis (GNA), Hippeastrum hybrid (HHA), and Cymbidium hybrid (CA) and the N-acetylglucosamine-specific lectin from Urtica dioica (UDA) were derived and purified from the bulbs of these plants as described before (3, 23, 41-43). CNV was a kind gift of Michael Boyd (National Cancer Institute, Frederick, MD), and its preparation has been previously described (9). Mannan was commercially obtained through Sigma (St. Louis, MO).
Isolation of primary leukocytes.
Buffy coat preparations from healthy donors were obtained from the Blood Bank in Leuven, Belgium. Monocytes were isolated from peripheral blood by positive selection using CD14 magnetic beads (Miltenyi Biotech GmbH, Gladbach, Germany) according to the manufacturer's instructions, with the exception of the use of cold (4°C) phosphate-buffered saline supplemented with 1% human serum and 2 mM EDTA (Sigma). Direct sorting yielded purities of ≥98% CD14+ monocytes as assessed by flow cytometry (CD3 and CD14 staining). CD4+ lymphocytes were sorted by use of a CD4+ lymphocyte isolation kit or by the depletion of HLA-DR+ CD8+ CD11b+ cells (Miltenyi Biotech) (≥98% purity; CD3, CD4, CD8, and HLA-DR staining).
Primary cell and cell line cultures.
Monocytes were converted to immature monocyte-derived DCs (MDDCs) by upright culturing in T-25 or T-75 culture flasks (Becton Dickinson, San Jose, CA) at concentrations of 1 × 106 cells/ml in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (BioWhittaker, Walkersville, MD), 400 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF), and 500 U/ml interleukin-4 (IL-4) (both from PeproTech, Rocky Hill, NJ). After 6 days of culturing without additional feeding, DC populations have the characteristic surface phenotype of high levels of CD206, CD209, and CD1a and negligible CD25, CD80, CD83 and CD14. At this point, half of the culture medium was replaced with fresh IL-4-GM-CSF medium, and MDDCs were used only within a time frame of 3 days after this time point. Peripheral blood mononuclear cells (PBMCs) were activated with phytohemagglutinin (PHA) (2 μg/ml; Sigma) for 2 days, washed, and cultured in the presence of 1 ng/ml IL-2 (Roche Molecular Biochemicals, Indianapolis, IN). The PM1 cell line was obtained from the National Institute of Allergy and Infectious Diseases AIDS Reagent Program (Bethesda, MD) and maintained in RPMI 1640 supplemented with 10% fetal bovine serum. The MT-4 T-cell line was a kind gift of L. Montagnier (at that time at the Pasteur Institute, Paris, France).
Viruses.
HIV type 1 (HIV-1) NL4.3 and BaL were obtained through the AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH) (1, 14). HIV-1 BaL was propagated in PHA-activated PBMCs, whereas NL4.3 was propagated in the MT-4 cell line. Viral input into assays was a function of p24 antigen (Ag) concentrations. Viral titration curves were used to assay infectivity within each system as a function of the amount of p24 Ag.
Quantitation of viral transfer and assessment of viral inhibition from MDDCs to CD4+ lymphocytes.
A quantitative assay measuring early DC-to-T-cell viral transfer events was performed by exposing immature MDDCs to HIV-1 at 10 to 20 ng/ml p24 (1 ng/ml is equivalent to 50 50% tissue culture infective doses [TCID50] for HIV-1 BaL and 250 TCID50 for NL4.3) for 2 h. In the case of the inhibition studies, 2.5 × 105 DCs per 150 μl of IL-4-GM-CSF medium were preincubated with 50 μl of the test compounds (50-μl samples contained five times the final compound concentration) in plates with “V”-shaped bottoms for 30 min under normal culture conditions at 37°C. DCs were then incubated with 50 μl of the viral inoculum with a final concentration of 20 ng/ml p24 for 2 h under normal culture conditions. In each experiment involving the use of inhibitors, positive-control titrations were set up in parallel with 40 ng/ml, 20 ng/ml, 4 ng/ml, 0.8 ng/ml, 0.16 ng/ml and 0.032 ng/ml p24 viral inputs for the DC exposure. After the 2-h exposure, MDDCs were thoroughly washed three times in 250 μl of RMPI 1640 medium supplemented with 10% fetal calf serum and subsequently resuspended in IL-4-GM-CSF medium as described above. MDDCs (5 × 104 in 50 μl of IL-4-GM-CSF medium) were transferred into 96-well plates containing 2 × 105 PHA-activated allogeneic CD4+ lymphocytes (in 150 μl of culture medium containing 10 U/ml IL-2) or 5 × 104 PM1 cells (in 200 μl of RPMI 1640 with 10% fetal calf serum). DC cultures were maintained in parallel to assess the inhibitions of DC infection and to establish the levels of viral DNA input by DCs in the cocultures. The contribution of DCs to R5 HIV-1 DNA in cocultures was consistently observed to be 1 log lower than that observed with CD4+ lymphocytes (see Fig. 2 and Results). DC and DC/T-cell cocultures were harvested 84 h after virus exposure to the DC culture by washing cells in phosphate-buffered saline and later pelleting and storing cells at −80°C until quantitative PCR (Q-PCR) analysis.
FIG. 2.
Measuring the efficiencies of I-IT and I-SPT from MDDCs to recipient cells. Viral inocula of BaL (a to c) and NL4.3 (d) were titrated either directly on recipient T-lymphocyte cells (open triangles), on MDDCs alone (open squares in panels a and b), or on MDDCs which were subsequently cocultured with recipients immediately after the viral pulsing for I-IT (closed circles in panels a through d) or were cocultured after 72 h for I-SPT (open circles). Recipient cells were either activated PBMCs (panel a) or PM1 cells (panels b to d). Virion production efficiency is measured as the accumulation of HIV LTR-gag viral DNA in the coculture as a function of cell number (albumin copy number). Both HIV LTR-gag DNA and albumin DNA were quantitated by Q-PCR as outlined in Materials and Methods. Results are representative of four independent experiments, and error bars represent standard deviations (SD) (n = 4).
The inhibition data were analyzed by linear regression to calculate the concentrations of compounds that afforded 50 and 90% reductions in HIV full-length long terminal repeat (LTR)-gag DNA formation in cocultures. These were designated the 50 and 90% effective concentrations (EC50 and EC90), respectively. We analyzed the different variables with Student's t test to check statistical significance (P < 0.05).
Quantitation of viral transfer and assessment of viral inhibition during I-IT and I-SPT.
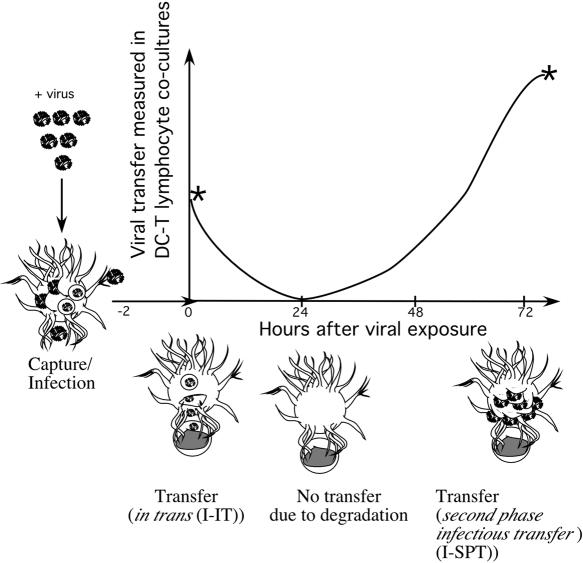
DCs after HIV exposure have previously been shown to transfer virus by two distinct mechanisms: I-IT and I-SPT (this is summarized in the scheme presented in Fig. 1). In the case of I-IT, virus transfer is not dependent on DC infection, and virus can be transferred in the short term only (less than 24 h after exposure). Virus transfer in I-SPT is dependent on infection and occurs in the long term (more than 48 h) (Fig. 1). To distinguish between the transfers in I-IT and I-SPT, we pulsed virus in the presence of inhibitors and either (i) cultured DCs immediately with lymphocytes for I-IT or (ii) cultured DCs alone for 72 h, after which they were cultured with lymphocytes for I-SPT. Cocultures in both I-IT and I-SPT were then cultured for 84 h for viral amplification to take place, after which cocultures were washed, harvested, and analyzed for viral DNA via Q-PCR. For both I-IT and I-SPT, DC cultures were maintained in parallel to subtract the input of DC viral DNA. The shortcoming of the I-IT measurement method is that background virus transfer via I-SPT may occur when using R5 HIV isolates; therefore, the X4 virus NL4.3 was included as a more representative measurement for I-IT in this assay, and subsequent comparisons were made with R5 HIV-1 BaL I-SPT.
FIG. 1.
Schematic diagram highlighting the phases of transfer from immature DCs to CD4+ T lymphocytes after viral pulsing. At time point −2 h, DCs are pulsed with virus. Time point 0 h is when DCs are cocultured with CD4+ lymphocytes for I-IT. Time point 72 h is when DCs are to be cocultured with CD4+ lymphocytes to measure I-SPT. The peaks for viral transfer for I-IT (time point 0 h) and for I-SPT (time point 72 h) are marked by asterisks on the curve. Stylized DCs at several time points highlight the major means of transfer at different time points in the curve. The diagram is based on the observations outlined in reference 40 and on data obtained from 10 different blood donors.
Quantitative PCR.
For the lysis of cells stored dry at −80°C, pellets were resuspended at 10 × 106 cells/ml in DNA lysis buffer (10 mM Tris [pH 8.3], 2.5 mM MgCl2, 50 mM KCl, 0.45% Tween 20, 0.45% Igepal-630 [all chemicals from Sigma]) and 1 mg/ml proteinase K (Roche Biochemicals, Indianapolis, IN). Quantitative PCR for HIV-1 LTR-gag DNA and an estimation of cell numbers using albumin DNA copy numbers were performed using primers and molecular probes as previously described (13).
RESULTS
MDDC-CD4+ lymphocyte coculture assay development.
In order to evaluate the effectiveness of the test compounds in blocking HIV transfer from DCs to CD4+ lymphocytes, it was necessary to develop an assay which was highly reproducible, scalable for the parallel evaluation of multiple compounds, and sensitive to low-dose virus input. Therefore, we initially used a previously described assay that was developed to measure viral half-life infectivity within MDDCs (40). The shortcoming of this assay was that for the DC exposure to HIV-1, a high viral inoculum level was needed for the detection of HIV LTR-gag DNA in DC-CD4+ T lymphocyte cocultures (24-h cocultivation). Limiting HIV doses to a range from 0.01 ng/ml to 25 ng/ml p24 Ag (1 ng/ml p24 Ag corresponds to 50 TCID50 when titrating on PHA-activated PBMCs) did not result in levels of LTR-gag DNA detectable by Q-PCR either in HIV-exposed DCs alone or in cocultures of HIV-exposed DC and PHA-stimulated PBMCs (data not shown). However, by increasing the time of cocultivation from 24 to 84 h, we could obtain reproducible and detectable differences in proviral DNA production levels at viral titers from 10 to 25 ng/ml p24 in both DC cultures and DC-PBMC cocultures (Fig. 2a). In DC-PBMC cocultures, the vast majority of detectable DNA appeared in the PBMCs, which was confirmed by measuring viral DNA in DCs alone and subtracting this value from that for the viral DNA measured in the DC-PBMC cocultures. We consistently observed HIV DNA levels in the cocultures that were at least 1 log higher than the DNA level observed in DCs cultured alone. Also, the use of viral laboratory strains (e.g., HIV NL4.3) that inefficiently infect DCs resulted in undetectable levels of HIV DNA in DC cultures (data not shown) and confirmed the values for viral DNA in cocultures to be a quantitative reflection of virus transfer from DCs to CD4+ lymphocytes (Fig. 2) rather than of HIV DNA directly generated by DCs alone. Although the initial assay was set up with virus-exposed DCs in cocultivation with PHA-activated PBMCs at a ratio of 5 × 104 DCs/2 × 105 PBMCs, we observed variability in the level of HIV LTR-gag DNA when using different donors. In order to find a more reproducible donor of CD4+ T-cell populations, we used the PM1 cell line, which is preferable to other R5 HIV-1-permissive cell lines since it represents a subclonal population rather than a CCR5-transfected cell line and is more representative of a CD4+ lymphocyte population with respect to low CCR5 surface levels (data not shown). Due to a high level of cell division of this cell line resulting in the progressive dilution of the number of cells that were initially infected by HIV at the start of the experiment, we cocultured the DCs with PM1 cells at a ratio of 1:1.
Efficiency of I-IT and I-SPT in immature DCs.
Previous observations have highlighted the ability of DCs to “trans enhance” HIV infection. That is, DCs have the capacity to take up virus at low levels and thereafter to expose these virions to neighboring lymphocytes. Whether this is predominantly from I-IT or I-SPT in immature DCs is not known. Therefore, virus was titrated to cover 4 logs of HIV p24 Ag concentrations (i.e., from 0.064 to 80 ng p24 Ag/ml) in order to study I-IT and I-SPT efficiencies in comparison to that of cell-free infection of PM1 cells. Given that I-SPT may be starting in BaL cocultures and that this may partially contribute to the levels attributed to I-IT, we also used NL4.3 in our studies, since it is inefficient in infecting DCs (16) and thus would predominantly, if not exclusively, reflect I-IT.
For BaL, the level of HIV LTR/gag DNA formation in I-IT cocultures was significantly lower than that in direct virus-inoculated PM1 cells (P < 0.05) at viral inoculum concentrations of greater than 4 ng/ml of p24. In contrast, there was a significant enhancement observed in the number of HIV DNA copies/cell in I-SPT compared to that for direct viral inoculation of PM1 cells over the same range of inoculum concentrations (P < 0.05) (Fig. 2c). For NL4.3, although the viral infection level in I-IT cocultures was lower than that in direct inoculation, there was no significant difference (Fig. 2d). Given that I-IT values of BaL and NL4.3 were similar and not significantly different, comparisons were also made between NL4.3 I-IT and BaL I-SPT (given that the former clearly lacked any significant levels of I-SPT and thus may be more representative of true I-IT). At viral inoculum concentrations of 20 ng/ml to 0.8 ng/ml, there was a significantly higher level of HIV LTR-gag copies/cell for BaL in I-SPT than for NL4.3 in I-IT (P < 0.05) (Fig. 2c and d).
Ability of mannose-specific plant lectins and CNV to inhibit DC I-IT.
The ability of HIV to infect target cells is not restricted to the surface of the mucosa, where microbicides can be applied. Indeed, DCs can capture virus and migrate subsequently to regions away from the site of topically applied microbicides. In order to simulate a scenario whereby DCs capture virus and transfer virus to CD4+ T cells away from the microbicide in peripheral tissue, we exposed DCs to several test compounds for a brief time period prior to the addition of the viral inoculum. Subsequent washing and cocultivation with CD4+ lymphocytes provided a situation where the DCs were not exposed to the microbicide and the viral inoculum anymore but were in contact with the responding CD4+ lymphocytes. The first set of compounds consisted of CNV, three mannose-specific plant lectins (i.e., GNA, HHA, and CA), and the N-acetylglucosamine-specific plant lectin UDA, all which have been previously shown to inhibit HIV infection in T lymphocytes and monocytes (2-5).
At a BaL inoculation level of 20 ng/ml of p24 to the DCs (Fig. 3a), all compounds evaluated in this cell model exerted marked antiviral activities, with EC50 values ranging from 0.01 for HHA to 0.07 μg/ml for CA. When the X4 virus NL4.3 was used, there was an increase in EC50 values ranging from 0.07 μg/ml for CNV to 0.50 μg/ml for the lectin CA (Table 1 and Fig. 3b).
FIG. 3.
Antiviral activity of plant lectins and CNV against HIV-1 BaL (a) and HIV-1 NL4.3 (b) prior to I-IT. Briefly, MDDCs were exposed to increasing levels of inhibitors and preincubated for 30 min prior to addition of the viral inoculum to give final concentrations of 20 ng/ml for both BaL and NL4.3. After incubation with inhibitor and virus for 2 h, MDDCs were washed three times and resuspended in IL-4/GM-CSF culture medium. MDDCs were then mixed with recipient PM1 cells at a ratio of 1:1 and cocultured for 4 days. After 4 days, cells from cocultures were harvested and DNA quantified for both HIV LTR-gag DNA and cellular albumin DNA as outlined in the legend to Fig. 2. The percent infectivity was calculated by dividing the value for HIV LTR-gag per cell of the sample with that for the positive control. Results are representative of at least four independent experiments, and the error bars represent SD (n = 4).
TABLE 1.
Summary of EC50 values of evaluated lectins for I-IT of HIV-1 BaL and NL4.3
| Lectin | EC50 for I-IT (μg/ml) ofa:
|
|
|---|---|---|
| BaL | NL4.3 | |
| CNV | 0.016 ± 0.009b | 0.074 ± 0.065 |
| UDA | 0.050 ± 0.060 | 0.102 ± 0.061 |
| HHA | 0.007 ± 0.001 | 0.308 ± 0.275 |
| GNA | 0.017 ± 0.006 | 0.169 ± 0.210 |
| CA | 0.065 ± 0.050 | 0.495 ± 0.478 |
EC50 values calculated from two independent donors.
Mean ± SD (n = 2).
Ability of sugar-binding proteins to inhibit DC I-IT with various concentrations of virus.
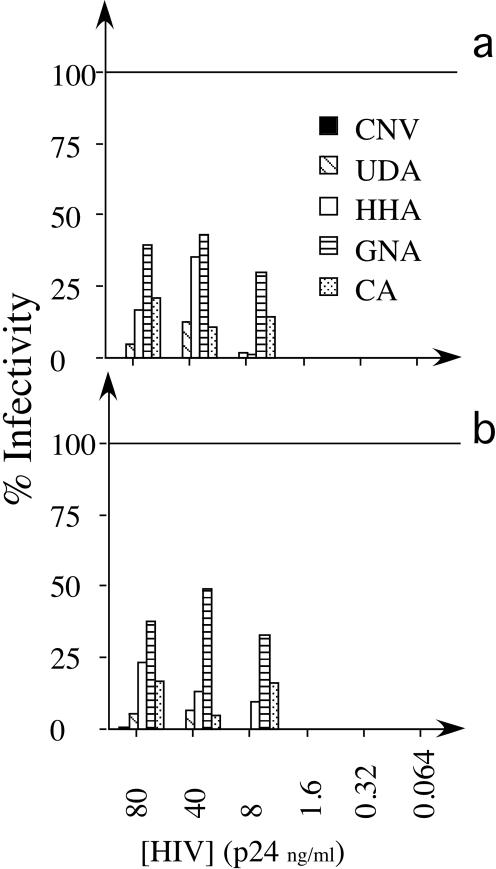
As one cannot reliably predict the viral inoculation per sexual encounter, we choose to vary viral dose at fixed inhibitor concentrations (i.e., 15 μg/ml, which is approximately the EC100 for all inhibitors used). As expected, virus infections at low levels of BaL and NL4.3 (≤1.6 ng/ml p24 Ag) were effectively inhibited below the detection limit for all compounds (Fig. 4).
FIG. 4.
Antiviral activity of plant lectins and CNV at fixed concentrations against HIV-1 BaL (a) and HIV-1 NL4.3 (b) prior to I-IT with increasing levels of viral inoculum. All inhibitors were used at 15 μg/ml, and viral inoculum levels were increased to cover a range from 0.064 to 80 ng/ml p24 Ag for both BaL and NL4.3. Conditions, detection strategy, and percent infectivity calculations are outlined in the legend to Fig. 3.
UDA had significantly lower I-IT EC50 values for both BaL and NL4.3 than did GNA and CA (P < 0.05) but not HHA or CNV at viral inoculum levels above 8 ng/ml of p24 Ag (Fig. 4). Note that CNV at a concentration of 15 μg/ml consistently inhibited I-IT to levels below the detection limit for all viral doses used, and thus CNV was significantly more potent than GNA, HHA, UDA, and CA (P < 0.05) at viral inoculum levels greater than or equal to 8 ng/ml of p24 Ag for both BaL and NL4.3 (Fig. 4).
Inhibition at the DC surface with mannan, a C-type lectin receptor inhibitor.
If CD209 and/or other lectin receptors are responsible for the I-IT, the inhibition of one or all of these receptors is likely to have a significant impact on virus transfer. Therefore, the antiviral activity of mannan, a well-known inhibitor of the C-type lectin receptor, was evaluated. In contrast to the other inhibitors, high levels of mannan (up to 1 mg/ml) are required, since the number of interacting molecules on a DC is far greater than that on the viral surface. As shown in Fig. 5, mannan was efficient at inhibiting I-IT by approximately 50 to 75% at concentrations from 1 to 1,000 μg/ml for both NL4.3 and BaL infections. For NL4.3, it reached a threshold of about 40 to 50%, after which higher concentrations of mannan could not reduce I-IT further (Fig. 5a). In varying the viral dose in the presence of 1.25 mg/ml mannan, the inhibitory efficiency of mannan decreased markedly. Once the viral inoculum levels of BaL and NL4.3 exceeded 8 ng/ml, there was no significant decrease in I-IT for either BaL or NL4.3 (P > 0.05) compared to the control of medium alone (Fig. 5b).
FIG. 5.
Antiviral activity of mannan against HIV-1 BaL and HIV-1 NL4.3 prior to direct DC infection and I-IT with increasing levels of mannan (a) or set mannan levels with increasing viral inoculum levels (b). In panel a, increasing levels of mannan were preincubated with MDDC under conditions outlined in the legend to Fig. 3, after which infection of DCs and/or I-IT was subsequently detected and the percent infectivity was calculated as described for Fig. 3. In panel b, a fixed level of mannan (1.25 mg/ml) was used, and viral inoculum was added at increasing levels as in the experiment outlined in Fig. 4.
Inhibition of DC infection and I-SPT in immature DCs with the sugar-binding proteins and mannan.
The ability of DCs to transfer virus in I-SPT is not only greater and more efficient than that seen in I-IT but may also represent long-term virus transfer from DCs which have migrated from the periphery to the draining lymph nodes. Initially, we addressed this second-phase virion transfer as a function of DC infection. Indeed, when using virus in the presence of blocking CD4 antibodies (data not shown) and reverse transcriptase inhibitors such as zidovudine (40) or when using virus which inefficiently infects DCs in this study (see NL4.3 second-phase transfer in Fig. 2d), no measurable I-SPT could be observed. When looking at the inhibition of BaL infection of DCs alone (Fig. 6a), all compounds were quite effective (>50% inhibition at their lowest concentrations of 0.0048 μg/ml, except for mannan, which was effective at 1 μg/ml or higher). In fact, at a dose of 0.32 μg/ml, a complete inhibition of DC infection was noted with all compounds tested except for mannan, for which an EC100 value of ≥1 mg/ml was observed (Fig. 6a).
FIG. 6.
Antiviral activities of mannan, plant lectins, and CNV in (a) direct DC inhibition and (b) I-SPT. As described for Fig. 3 and 5, DCs were preincubated with various amounts of plant lectins, CNV, and/or mannan, after which virus was added to give final p24 concentrations of 20 ng/ml. After 2 h of viral pulsing, MDDCs were washed thoroughly and, in contrast to I-IT, were cultured alone in IL-4/GM-CSF culture medium for 3 days. After 3 days, residual I-IT was undetectable, and I-SPT commenced (see also Fig. 2d); therefore, MDDCs were washed once, resuspended in IL-4/GM-CSF medium, and subsequently added to cocultures at a ratio of 1:1 for MDDC cells to PM1 cells and cocultured for 4 days as for I-IT. Detection and percent infectivity calculations are as outlined in the legend for Fig. 3. Results are representative of at least three independent experiments, and error bars indicate SD (n = 3).
However, it must be noted that HIV DNA levels in DCs alone are low at this viral inoculum level (the highest control level was only 0.12 copies per cell), and low EC50 values may be more of a reflection of the limiting levels of detection for viral DNA in DCs.
Comparison of the EC50 values for the lectins obtained in I-IT with those obtained in I-SPT (Table 1 and Table 2) showed that the EC50 values for all compounds significantly increased for I-SPT (P < 0.05 with the exception of the HHA EC50 values for NL4.3 inhibition) (Fig. 6b and Table 2). At higher compound concentrations, EC90 values for NL4.3 I-IT and for BaL I-IT were not significantly different (P > 0.05) from those observed for I-SPT (Fig. 6b). In I-SPT, CNV was the only compound with EC50 values that were significantly lower than those of the plant lectins (P < 0.05) (Fig. 6b and Table 2). In contrast, mannan showed marginal activity in inhibiting I-SPT, with only 63% inhibition at 1 mg/ml (Fig. 6b), and was not significantly different from the positive control of medium alone (P > 0.05).
TABLE 2.
Summary of EC50 values of evaluated lectins for I-SPT of HIV BaL
| Lectin | EC50 for I-SPT (μg/ml) of BaLa |
P value forb:
|
|
|---|---|---|---|
| I-IT of BaL | I-IT of NL4.3 | ||
| CNV | 0.789 ± 0.019c | 0.0081 | 0.0145 |
| UDA | 2.905 ± 0.177 | 0.0187 | 0.0190 |
| HHA | 2.604 ± 0.368 | 0.0288 | 0.0561 |
| GNA | 2.530 ± 0.481 | 0.0433 | 0.0493 |
| CA | 3.265 ± 0.134 | 0.0130 | 0.0258 |
EC50 values calculated from two independent donors.
P values are calculated from comparison with results of I-SPT of BaL (two-tailed paired t test).
Mean ± SD (n = 2).
DISCUSSION
DCs may represent one of several pathways that HIV uses to gain access to its target cells in lymphoid tissue. With a specific emphasis on HIV-blocking compounds, we have observed the mechanisms by which immature DCs can transfer virus to CD4+ lymphocytes and evaluated their efficiencies. Immature DCs were chosen, as they represent the “sentinel” population of DCs in peripheral tissue (17, 33). In addition, immature DCs have the capacity to transfer virus upon infection or without being infected, in contrast to mature DCs, in which the former characteristic tends to predominate (16, 27, 40). The in vitro-derived DCs were chosen, since they currently represent the only suitable in vitro-derived model in which these events could be observed. Although in vivo-derived DCs could be used, the screening of several compounds simultaneously with various concentrations of compound and/or viral dose would not be feasible with current DC isolation methods (i.e., the relatively low absolute numbers of viable cells is the limiting factor for the use of such cells in this assay). With respect to infectious virus transfer, I-SPT represents the most sensitive and efficient form of viral transfer, compared directly to I-IT. The degree to which HIV could be inhibited by test compounds also relied on this feature, as the more efficient the virion transfer was, the higher were the EC50 values. The mode of action of each compound also played a key role. Indeed, compounds that targeted only one pathway at the cell surface (e.g., mannan binding to mannose-binding lectins) often did not work well, presumably since multiple paths of virus transmission are clearly involved in the infection process. In contrast, attacking the virus at the level of the viral envelope proved to be a more attractive target. Indeed, plant lectins and in particular CNV could block I-IT but also subsequent I-SPT. Thus, the sugar-binding proteins are likely to represent more-multivalent compounds with respect to blocking the multiple mechanisms of transfer at the mucosal epithelium.
The marked ability of I-SPT to outscore I-IT is consistent with several features with respect to the role of immature DCs in the transmission of HIV and the quality and overall quantity of newly produced virions over time. For the former point, it has been previously shown that immature DCs are hostile towards internalized virions (29, 40). Indeed, the location of the virus in a nonlysosomal compartment has no impact on its ability to survive. In addition, the virus that is exposed to the recipient cell by the DC in I-IT is essentially “old” virus. Thus, unless a mechanism is present to counteract these effects on half-lives, the level of viral infection by I-IT in a recipient CD4+ lymphocyte would be expected to be lower than that seen in recipient cells that have just recently received cell-free virus. McDonald and colleagues have visualized a mechanism that may counter the negative factors in DC I-IT mentioned above (27), resulting in an enhancement of recipient CD4+ T-cell infection compared to cell-free virus infection of CD4+ T cells (referred to by others as trans enhancement). This enhancing mechanism is seen in polarized viral regurgitation by mature DCs and concomitant polarized CD4 and CCR5 on the opposing contacting surface of the recipient CD4+ T cell (27). Although similar polarized budding has been seen in immature DC I-IT, it is not observed to the extent that is seen in mature DCs. Thus, trans enhancement with regard to I-IT may be restricted only to the mature DC phenotype.
In contrast, the concept of I-SPT has received little, if any, support. The underlying factor of I-SPT is the ability of HIV to infect DCs. The level of DC infection may indeed be undetectable compared to that of the infection of a macrophage and/or a CD4+ T cell. For instance, in certain viral titrations and blocking experiments, viral DNA in DC cultures was undetectable, but there was a readily detectable I-SPT when DCs were brought into coculture (Fig. 6). The ways whereby this occurs and becomes ultimately efficient compared to those of I-IT may be twofold. First, newly produced virus that is likely to bud from a polarized point towards CD4+ T cells (20) would be far more efficient than the regurgitation of older (earlier-caught) virus. Second, the probability of virion transfer by any infected DC is likely to be a function of its cellular half-life (assuming the viral production continues over this time and the cellular phenotype is constant), and one would expect that the half-life of an infected DC would be far greater than that for a cell-free virion or for one that is internalized into a digestive compartment.
Although I-SPT outscores I-IT, the latter still may exist in vivo. Thus, the approach of concomitantly blocking both HIV transfer mechanisms would be advisable in this situation. It has been known for some time that mannose- and N-acetylglucosamine-specific plant lectins which can bind the HIV envelope block HIV infection in vitro (3, 4). However, their therapeutic use in infected individuals may be limited, as they are not expected to have oral bioavailability (4), and several levels of resistance to these compounds are also observed in vitro (5). The mannose-binding CNV is an anti-HIV agent that was originally isolated from cultures of the cyanobacterium Nostoc ellipsosporum (9). The common feature of both plant lectins and CNV is that they bind to the HIV envelope protein in a carbohydrate-dependent manner. Specificity for carbohydrate structures varies, with GNA and HHA recognizing α-(1-3)-linked mannose residues and α-(1-3)- and α-(1-6)-linked mannose residues, respectively, CA binding to mannose sugars (while their preferred conformation is not known), CNV targeting α-(1-2)-linked mannose residues on high-mannose-level glycan structures (6, 8), and UDA recognizing α-(1-4)-linked oligomers of N-acetyl-d-glucosamine (GlcNAc). The capacity of CNV and plant lectins to bind directly to the virus envelope is advantageous for the prevention of both pathways of virus transfer. In I-IT, the inhibitors are effective even after the endocytosis of virions and later regurgitation. They are also effective at blocking the HIV infection of DCs to levels suitable to afford inhibition in I-SPT. CNV was usually more potent than the plant lectins and mannan. However, it must be noted that CNV is active predominantly in its wild-type monomeric form (molecular weight, 8,700), whereas the lectins GNA and HHA are functional in their tetrameric states (molecular weight, 50,000) and CA and UDA are active in their dimeric and monomeric states, respectively. Indeed, if one looks at molarity on this functional basis, then the inhibitory activities of the plant lectins (in tetrameric and dimeric forms) would come much closer to that of CNV.
Our data on blocking the viral attachment and uptake of virus into DCs (endocytosis as opposed to infection[s]) indicated that there is no or only a weak inhibition observed for all lectins and CNV. The mechanism of blocking may be the attachment of inhibitors prior to uptake and/or in the endosome itself (assuming the inhibitors are taken into the same compartment). Thus, the important feature of both plant lectins and CNV is that HIV remains noninfectious while it is passaged through the DC endosome and subsequently recycled to the surface for transfer to CD4+ lymphocytes.
Although mannan could inhibit both I-IT and I-SPT, it could not do so completely. This is observed in I-IT, for which inhibition is often reached only at a threshold of 50 to 60% when mannan is used at concentrations as high as 1 mg/ml. The lack of efficient blocking of I-IT may be due to several mechanisms. First, mannan is a biological ligand for DCs, and it is likely that the compound may be taken up and degraded. Thus, the compound concentration needed for efficient inhibition would therefore be higher than for other compounds that may be more stable and efficiently persist either in the medium and/or on the receptor being investigated. However, this effect should be attributable only at lower compound concentrations and not at concentrations of up to 1 mg/ml. Second, the presence of mannan may stimulate other processes in DCs. Indeed, it recently has been observed in fresh ex vivo LCs that the presence of mannan actually increases HIV uptake (F. Hladik, personal communication). For instance, micropinocytosis may increase in the presence of ligands that trigger innate immune receptors, such as CLRs. Moreover, other virion uptake and attachment processes may exist independently of CLRs, as uptake of virus can occur on ex vivo blood DC subsets in the absence of CLR expression (33, 38, 40). The recent hypothesis that virions are essentially modified exosomes (28) may suggest that mechanisms of exosomal handling of virions by DCs may also be in operation with HIV. This latter point is intriguing, as recent observations of DCs have shown that exosome uptake is quite active in DCs (28) and may give us an indication of the CLR/CD4-independent pathway at which DCs can take up virions.
A common observation in this study is that the inhibition of I-SPT is not as easy as that of I-IT. This concept is consistent with the higher efficiency of I-SPT relative to that of I-IT in transmitting HIV. However, it also highlights the importance of affording potent viral inhibition in the genital mucosa. For instance, an effective threshold level of viral inoculum not inhibited by a microbicidal drug will need to be close to zero. In the majority of cases, we could not detect DC infection in populations of 30,000 cells via Q-PCR. However, there was an infected population that represented less than 0.01% (under the assumption of one copy of HIV per DC), which is, in absolute terms, fewer than 5 DCs that need to be infected in a population of 50,000 DCs in order to establish a robust and readily detectable level of infection in a coculture. One cannot rule out other cellular mechanisms existing either alone or in parallel with DC-mediated or DC-catalyzed transmission events.
Thus, a microbicidal strategy developing a compound that is not only a potent antiviral but also able to show concomitant activity in several cell models would be imperative. Our studies revealed that CNV and several plant lectins may have the ability to afford this. The successful blocking of simian immunodeficiency virus/HIV-1 transmission by the use of CNV in microbicide formulations in macaques provides further support that the mechanism of action of this class of compounds through the direct binding of the viral envelope is indeed successful in vivo (36, 37) and therefore that they have the potential to be effective agents as future topical anti-HIV microbicides.
Acknowledgments
The research was supported by the European Commission (René Descartes Prize—2001 Krediet no. HPAW-CT-2002-90001), the 6th Framework Programme Project EMPRO (no. 503558), the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (G.0267.04), and a grant from the ANRS.
We thank Sandra Claes and Eric Fonteyn for excellent technical assistance. Stuart G. Turville and Kurt Vermeire have postdoctoral fellowships of the “Onderzoeksfonds Leuven.”
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini, J., S. Hatse, K. Vermeire, K. Princen, S. Aquaro, C. F. Perno, E. De Clercq, H. Egberink, G. Vanden Mooter, W. Peumans, E. Van Damme, and D. Schols. 2004. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 48:3858-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzarini, J., J. Neyts, D. Schols, M. Hosoya, E. Van Damme, W. Peumans, and E. De Clercq. 1992. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antivir. Res. 18:191-207. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J., D. Schols, J. Neyts, E. Van Damme, W. Peumans, and E. De Clercq. 1991. α-(1-3)- and α-(1-6)-d-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob. Agents Chemother. 35:410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini, J., K. Van Laethem, S. Hatse, K. Vermeire, E. De Clercq, W. Peumans, E. Van Damme, A. M. Vandamme, A. Bolmstedt, and D. Schols. 2004. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J. Virol. 78:10617-10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bewley, C. A., and S. Otero-Quintero. 2001. The potent anti-HIV protein cyanovirin-N contains two novel carbohydrate binding sites that selectively bind to Man(8) D1D3 and Man(9) with nanomolar affinity: implications for binding to the HIV envelope protein gp120. J. Am. Chem. Soc. 123:3892-3902. [DOI] [PubMed] [Google Scholar]
- 7.Blauvelt, A., H. Asada, M. W. Saville, V. Klaus-Kovtun, D. J. Altman, R. Yarchoan, and S. I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Investig. 100:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botos, I., B. R. O'Keefe, S. R. Shenoy, L. K. Cartner, D. M. Ratner, P. H. Seeberger, M. R. Boyd, and A. Wlodawer. 2002. Structures of the complexes of a potent anti-HIV protein cyanovirin-N and high mannose oligosaccharides. J. Biol. Chem. 277:34336-34342. [DOI] [PubMed] [Google Scholar]
- 9.Boyd, M. R., K. R. Gustafson, J. B. McMahon, R. H. Shoemaker, B. R. O'Keefe, T. Mori, R. J. Gulakowski, L. Wu, M. I. Rivera, C. M. Laurencot, M. J. Currens, J. H. Cardellina II, R. W. Buckheit, Jr., P. L. Nara, L. K. Pannell, R. C. Sowder II, and L. E. Henderson. 1997. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41:1521-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron, P., M. Pope, A. Granelli-Piperno, and R. M. Steinman. 1996. Dendritic cells and the replication of HIV-1. J. Leukoc. Biol. 59:158-171. [DOI] [PubMed] [Google Scholar]
- 11.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 12.DiClemente, R. J., G. M. Wingood, K. F. Harrington, D. L. Lang, S. L. Davies, E. W. Hook III, M. K. Oh, R. A. Crosby, V. S. Hertzberg, A. B. Gordon, J. W. Hardin, S. Parker, and A. Robillard. 2004. Efficacy of an HIV prevention intervention for African American adolescent girls: a randomized controlled trial. JAMA 292:171-179. [DOI] [PubMed] [Google Scholar]
- 13.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 14.Gartner, S., P. Markovits, D. M. Markovitz, M. H. Kaplan, R. C. Gallo, and M. Popovic. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215-219. [DOI] [PubMed] [Google Scholar]
- 15.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 16.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grassi, F., C. Dezutter-Dambuyant, D. McIlroy, C. Jacquet, K. Yoneda, S. Imamura, L. Boumsell, D. Schmitt, B. Autran, P. Debre, and A. Hosmalin. 1998. Monocyte-derived dendritic cells have a phenotype comparable to that of dermal dendritic cells and display ultrastructural granules distinct from Birbeck granules. J. Leukoc. Biol. 64:484-493. [DOI] [PubMed] [Google Scholar]
- 18.Gummuluru, S., M. Rogel, L. Stamatatos, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 77:12865-12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch, V. M., M. E. Sharkey, C. R. Brown, B. Brichacek, S. Goldstein, J. Wakefield, R. Byrum, W. R. Elkins, B. H. Hahn, J. D. Lifson, and M. Stevenson. 1998. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: evidence of macrophage-dependent viral amplification. Nat. Med. 4:1401-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hladik, F., G. Lentz, R. E. Akridge, G. Peterson, H. Kelley, A. McElroy, and M. J. McElrath. 1999. Dendritic cell-T-cell interactions support coreceptor-independent human immunodeficiency virus type 1 transmission in the human genital tract. J. Virol. 73:5833-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, Q., I. Frank, V. Williams, J. J. Santos, P. Watts, G. E. Griffin, J. P. Moore, M. Pope, and R. J. Shattock. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaku, H., E. J. Van Damme, W. J. Peumans, and I. J. Goldstein. 1990. Carbohydrate-binding specificity of the daffodil (Narcissus pseudonarcissus) and amaryllis (Hippeastrum hybr.) bulb lectins. Arch. Biochem. Biophys. 279:298-304. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura, T., S. S. Cohen, D. L. Borris, E. A. Aquilino, S. Glushakova, L. B. Margolis, J. M. Orenstein, R. E. Offord, A. R. Neurath, and A. Blauvelt. 2000. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J. Exp. Med. 192:1491-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura, T., F. O. Gulden, M. Sugaya, D. T. McNamara, D. L. Borris, M. M. Lederman, J. M. Orenstein, P. A. Zimmerman, and A. Blauvelt. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. USA 100:8401-8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 27.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 28.Morelli, A. E., A. T. Larregina, W. J. Shufesky, M. L. Sullivan, D. B. Stolz, G. D. Papworth, A. F. Zahorchak, A. J. Logar, Z. Wang, S. C. Watkins, L. D. Falo, Jr., and A. W. Thomson. 2004. Endocytosis, intracellular sorting and processing of exosomes by dendritic cells. Blood 104:3257-3266. [DOI] [PubMed] [Google Scholar]
- 29.Moris, A., C. Nobile, F. Buseyne, F. Porrot, J. P. Abastado, and O. Schwartz. 2004. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood 103:2648-2654. [DOI] [PubMed] [Google Scholar]
- 30.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 31.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reece, J. C., A. J. Handley, E. J. Anstee, W. A. Morrison, S. M. Crowe, and P. U. Cameron. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoneburner, R. L., and D. Low-Beer. 2004. Population-level HIV declines and behavioral risk avoidance in Uganda. Science 304:714-718. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, C. C., P. Emau, Y. Jiang, M. B. Agy, R. J. Shattock, A. Schmidt, W. R. Morton, K. R. Gustafson, and M. R. Boyd. 2004. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res. Hum. Retrovir. 20:11-18. [DOI] [PubMed] [Google Scholar]
- 37.Tsai, C. C., P. Emau, Y. Jiang, B. Tian, W. R. Morton, K. R. Gustafson, and M. R. Boyd. 2003. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retrovir. 19:535-541. [DOI] [PubMed] [Google Scholar]
- 38.Turville, S. G., J. Arthos, K. M. Donald, G. Lynch, H. Naif, G. Clark, D. Hart, and A. L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood 98:2482-2488. [DOI] [PubMed] [Google Scholar]
- 39.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 40.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 41.Van Damme, E. J., A. K. Allen, and W. J. Peumans. 1987. Isolation and characterization of a lectin with exclusive specificity towards mannose from snowdrop (Galanthus nivalis) bulbs. FEBS Lett. 215:140-144. [Google Scholar]
- 42.Van Damme, E. J., A. K. Allen, and W. J. Peumans. 1998. Related mannose-specific lectins from different species of the family Amaryllidaceae. Plant Physiol. 73:52-57. [Google Scholar]
- 43.Van Damme, E. J., H. Kaku, F. Perini, I. J. Goldstein, B. Peeters, F. Yagi, B. Decock, and W. J. Peumans. 1991. Biosynthesis, primary structure and molecular cloning of snowdrop (Galanthus nivalis L.) lectin. Eur. J. Biochem. 202:23-30. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, Z. Q., S. W. Wietgrefe, Q. Li, M. D. Shore, L. Duan, C. Reilly, J. D. Lifson, and A. T. Haase. 2004. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc. Natl. Acad. Sci. USA 101:5640-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]