Abstract
West Nile virus (WNV) encodes two envelope proteins, premembrane (prM) and envelope (E). While the prM protein of all WNV strains contains a single N-linked glycosylation site, not all strains contain an N-linked site in the E protein. The presence of N-linked glycosylation on flavivirus E proteins has been linked to virus production, pH sensitivity, and neuroinvasiveness. Therefore, we examined the impact of prM and E glycosylation on WNV assembly and infectivity. Similar to other flaviviruses, expression of WNV prM and E resulted in the release of subviral particles (SVPs). Removing the prM glycosylation site in a lineage I or II strain decreased SVP release, as did removal of the glycosylation site in a lineage I E protein. Addition of the E protein glycosylation site in a lineage II strain that lacked this site increased SVP production. Similar results were obtained in the context of either reporter virus particles (RVPs) or infectious lineage II WNV. RVPs or virions bearing combinations of glycosylated and nonglycosylated forms of prM and E could infect mammalian, avian, and mosquito cells (BHK-21, QT6, and C6/36, respectively). Those particles lacking glycosylation on the E protein were modestly more infectious per genome copy on BHK-21 and QT6 cells, while this absence greatly enhanced the infection of C6/36 cells. Thus, glycosylation of WNV prM and E proteins can affect the efficiency of virus release and infection in a manner that is cell type and perhaps species dependent. This suggests a multifaceted role for envelope N-linked glycosylation in WNV biology and tropism.
West Nile virus (WNV) is an arthropod-borne virus classified in the Japanese encephalitis antigenic complex of the family Flaviviridae (9, 20, 32). The natural transmission cycle of WNV involves mosquitoes and birds, with humans and other mammals as incidental hosts (8, 27). Phylogenetic analysis of WNV strains reveals the presence of two closely related but nonetheless distinct virus groups termed lineage I and lineage II (6). Lineage I strains (which include Kunjin viruses) are distributed worldwide (10, 33) and are responsible for all major human outbreaks to date, including the current WNV epidemic in North America (35). In contrast, lineage II WNV strains are restricted to central and southern Africa and do not appear to be as pathogenic as lineage I isolates (6, 7, 35).
WNV contains a single-stranded, plus-sense RNA genome that is translated as a single polyprotein (9). Cleavage of the polyprotein by viral and cellular proteases liberates the viral integral membrane proteins premembrane (prM) and envelope (E), as well as the capsid and seven nonstructural proteins (51). Flavivirus prM and E proteins form heterodimers in the endoplasmic reticulum (ER), where they facilitate virus budding into the ER (2, 40), although one report suggests that the WNV Sarafend strain buds at the plasma membrane (47). As particles travel through the secretory pathway, the bulk of the prM ectodomain is removed by endoproteolysis during transit through the trans-Golgi network (63). Cleavage of prM enables E protein to form head-to-tail homodimers, which form a lattice-like structure covering the surface of the mature, 50 nM diameter virus particle (44). During the process of viral entry, the E protein interacts with an unidentified cell surface receptor(s), followed by uptake into endosomes where the E protein undergoes conformational changes at mildly acid pH, resulting in fusion between the viral and cellular membranes (16, 26).
All WNV isolates contain a highly conserved N-linked glycosylation site within the ectodomain region of prM that is released during the final stages of particle maturation. In contrast, an N-linked glycosylation site in the E protein at residue 154 is present in many but not all lineage I strains. This site is also present in some lineage II strains, though others contain a 4-amino-acid deletion that ablates this N-linked glycosylation site (1, 6). Interestingly, many of the WNV isolates associated with significant human outbreaks, including the current North American epidemic, contain the N-linked glycosylation site in E (22, 35, 54). In addition, N-linked glycosylation of the WNV E protein may be associated with altered viral growth in vitro (56) and neuroinvasiveness in a murine model (3, 5, 61). N-linked glycosylation of the E protein in WNV and other flaviviruses has been linked to alterations in pH sensitivity (5, 36) and virus yield (65) and likely plays a significant role in the interaction between dengue virus and DC-SIGN on the surface of immature dendritic cells in vitro (46, 64). Whether glycosylation of the WNV E glycoprotein plays a significant role in virus assembly, cell surface attachment, and pathogenesis is not yet clear.
In this study, we confirmed that the WNV prM and E proteins from a lineage I (NY99-6480) and II (956 D117 3B) strain localize to the ER and that virus assembly occurs within this organelle. In addition, expression of prM and E in the absence of other viral proteins resulted in the production and secretion of subviral particles (SVPs), as has been described for other flaviviruses (2, 28, 30, 31). Removal of the N-linked glycosylation site in prM or E significantly reduced the level of SVP and virus release from transfected or infected cells. However, reporter particles capable of a single round of infection and recombinant virus particles containing glycosylation-deficient prM were still infectious, albeit slightly less than the wild type. The effects of E protein glycosylation on viral infectivity were more interesting. While the absence of N-linked glycosylation on lineage I or II E proteins was associated with a modest increase in the infectivity of mammalian or avian cells, there was a markedly enhanced infection of C6/36 mosquito cells by these E glycosylation-null viruses. Thus, N-linked carbohydrate structures can significantly impact particle assembly and viral infectivity in a manner that is cell type and perhaps even species dependent.
MATERIALS AND METHODS
Plasmids. (i) prM-E expression vectors.
WNV lineage I envelope proteins were expressed as a polyprotein using plasmid pCBWN (13) and as individual envelope proteins, prM and E protein, from pcDNA 6.2 (Invitrogen, Carlsbad, CA). WNV lineage II envelope proteins, subcloned from molecular clone SP6WN/Xba (68), were expressed individually and also as a polyprotein from pcDNA6.2. Glycosylation mutants of lineage I envelope proteins and lineage II prM were introduced using QuikChange site-directed mutagenesis (Invitrogen, Carlsbad, CA) by creating two nucleotide substitutions resulting in an amino acid change (N to Q), thereby removing the glycosylation site while introducing a chemically conservative mutation. Using overlap extension PCR methodology, the consensus WNV glycosylation site (NYST) was added to the lineage II E protein starting at amino acid 154.
(ii) WNV lineage II.
Infectious molecular clones of WNV encoding point mutations that add or delete N-linked glycosylation sites were constructed by modification of a DNA-launched molecular clone of a lineage II strain of WNV that encodes GFP (WNII-IF) (48). WNII-IF was modified to introduce silent restriction sites that flank the sequences encoding prM and E, simplifying the introduction of different envelope protein gene cassettes. This was accomplished with two sequential overlap extension PCR procedures. First, a silent AflII site (underlined below) was introduced downstream of E by engineering five mutations into an oligonucleotide primer (ACCCTCTCG to ACCTTAAGT) for use in overlap extension PCR. Flanking primers were used to generate a fragment containing this silent mutation into novel BglII and NsiI sites in WNII-IF. In addition to insertion of the silent AflII site, this cloning step removed the majority of sequence encoding prM and E proteins. Next, a silent AgeI site was introduced using a similar approach (ACAGGC to ACCGGT), generating pWNII(ΔME)-IF. A variant of this prM-E recipient vector lacking the IRES-GFP reporter gene cassette was also constructed by restricting the plasmid with NotI followed by religation [pWNII(ΔME)-Not]. Introduction of prM-E genes from WNV lineage II was accomplished by generating PCR fragments flanked by AgeI and AflII sites and introducing them into either pWNII(ΔME)-IF or pWNII(ΔME)-Not using standard techniques. Sequences of primers and infectious clones are available upon request.
Virus production.
WNV lineage I strain 3000.0259 NY 2000 (NY2000) was propagated in C6/36 cells, collected 72 h postinfection (p.i.), and clarified through a 0.2 μM syringe filter (14). Lineage II viruses (strain 956 D117 3B) were produced by transfection of HEK-293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with pWNII-Not (48) or envelope glycosylation mutants as described above. Six hours after transfection, culture medium was removed and replaced with Dulbecco's modified Eagle medium (DMEM)-low glucose containing 10% fetal bovine serum (FBS) and 1% penicillin-streptamycin. Virus containing supernatant was collected 30 h postinfection, clarified through a 0.2 μM syringe filter, and used to infect BHK-21 or C6/36 cells. Virus was collected 48 h postinfection and clarified as described above. For storage, all viral supernatants were mixed with a 30% volume of freezing medium (100% FBS, 30 mM HEPES, pH 8.0) and stored at −80°C.
Antibodies.
Five flavivirus antibodies were used in this study: WNV hyperimmune ascites fluid (ATCC, Manassas, VA); dengue E monoclonal antibody (MAb) 4G2 (15); and WNV E MAbs 4E1 (53), E1 (kindly provided by Mike Diamond, Washington University, St. Louis), and 5H10 (BioReliance Corp., Rockville, MD). A polyclonal rabbit serum raised against calnexin (kindly provided by A. Helenius) was used for immunofluorescence detection of the endoplasmic reticulum. Secondary antibodies goat anti-mouse Alexafluor 594 (for WNV) and goat anti-rabbit Alexafluor 488 (for ER) (Molecular Probes, Eugene, OR) were used for all immunofluorescence studies. A sheep anti-mouse immunoglobulin G (IgG) horseradish peroxidase (HRP) secondary antibody (Amersham Biosciences, Buckinghamshire, England) was used for all Western blots and plastic capture enzyme-linked immunosorbent assays (ELISAs). A streptavidin-HRP conjugate (Sigma) was used in the antigen capture ELISA. MAb 4E1 was directly conjugated to Alexafluor 647 for use in flow cytometry using an antibody conjugation kit (Molecular Probes, Eugene, OR).
Immunofluorescence microscopy.
HeLa cells were plated at 7 × 104 cells per well in 24-well plates containing glass coverslips. The following day, cells were transfected with 1.7 μg of DNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Twenty-four hours posttransfection, all cells were fixed using 2% paraformaldehyde for 20 min at room temperature. Cells were then washed and permeabilized using 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 5 min at room temperature. Prior to incubation with antibody, coverslips were blocked with 5% FBS in PBS for 1 h (room temperature) to overnight (4°C). WNV prM and E proteins were detected using WNV hyperimmune ascites fluid (1:500 dilution; ATCC, Manassas, VA), and the endoplasmic reticulum was detected with a polyclonal rabbit antibody to the ER protein, calnexin (1:250 dilution). All antibodies were diluted in PBS containing 5% FBS. Secondary antibodies used at a 1:500 dilution were goat anti-mouse Alexafluor 594 for WNV ascites fluid and goat anti-rabbit Alexafluor 488 for the calnexin antibody (both from Molecular Probes, Eugene, OR). In addition, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was included with the secondary antibodies at a 1:500 dilution to stain the nucleus. Coverslips were mounted using ProLong (Molecular Probes, Eugene, OR) and imaged at 100× with a Nikon E600 epifluorescence microscope.
Electron microscopy.
Vero cells were plated at 7 × 106 cells in a 10-cm3 dish and infected with lineage II West Nile virus at a multiplicity of infection of 1.0. Twenty-four hours p.i., cells were washed and fixed with 4% glutaraldehyde and placed at 4°C. Cells were then dehydrated, placed in an Epon resin, thin sectioned, placed on a copper grid, poststained, and examined by transmission electron microscopy.
Subviral particle isolation.
HEK-293T cells were transfected with viral envelope protein expression plasmids as described above and the supernatant collected 48 h posttransfection. Supernatants were cleared with a low-speed spin at 500 × g for 10 min and then filtered through a 0.2-μm filter disk. Clarified supernatants were then layered over a 20% sucrose cushion (HEPES buffered, pH 8.0) and ultracentrifuged at 4°C in an SW55 rotor at 40,000 rpm for 1 h or in an SW41Ti rotor at 38,000 rpm for 2 h to pellet the particles. Pelleted SVPs were resuspended overnight at 4°C in 60 μl resuspension buffer (150 mM NaCl-50 mM HEPES [pH 8.0] with protease inhibitors [Roche Molecular Biochemicals, Indianapolis, IN]).
Equilibrium gradient centrifugation.
Subviral and viral particles were separated based on density using a continuous 20 to 60% sucrose gradient. Resuspended SVPs were layered on the top of the gradient and centrifuged in an SW41Ti rotor at 38,000 rpm for 14 h at 4°C. Fractions were collected and the refractive index determined for each. Aliquots from each fraction were run on a 10 to 20% reducing sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel and transferred to a polyvinylidene difluoride membrane. WNV prM and E proteins were detected using WNV HIAF (1:500; ATCC), visualized using a sheep anti-mouse IgG HRP secondary antibody, and imaged with SuperSignal West Femto maximum sensitivity substrate (Pierce Biotechnology, Inc., Rockford, IL).
Plastic capture ELISA.
SVPs were bound to Immulon 2HB ELISA plates (Thermo Lab Systems Inc., Franklin, MA) in binding buffer (0.15 M Na carbonate, 0.35 M Na bicarbonate, pH 9.6) at 4°C overnight. Plates were washed with PBS three times and wells blocked with Blotto (1× PBS, 5% powdered milk, 0.1% Tween 20, 0.1% NaAzide) for a minimum of 1 h. Blotto was removed, and WNV hyperimmune ascites fluid (1:500 diluted in Blotto) was added and allowed to bind for 1 h at 37°C or overnight at 4°C. Unbound antibody was removed by three washes with PBS and sheep anti-mouse IgG HRP added (1:10,000 diluted in Blotto without NaAzide). Plates were incubated at 37°C with the secondary antibody for 45 min, washed three times, and developed by adding TMB peroxidase substrate (KPL, Inc., Gaithersburg, MD). Plates were read in a MRX Revelation plate reader at a wavelength of 450 nm.
Glycosylation assays.
HEK-293T cells were plated in a six-well plate at 1 × 106 cells/well and transfected the following day with WNV glycoprotein expression plasmids. Forty-eight hours posttransfection, both supernatants and cells were collected. SVPs were harvested from the supernatant by pelleting through a 20% sucrose cushion (HEPES buffered, pH 8.0) and cells solubilized in lysis buffer (50 mM Tris-150 mM NaCl-2 mM EDTA-1% Triton X-100 [pH 7.5] with protease inhibitors [Roche Molecular Biochemicals, Indianapolis, IN]). Lysates were centrifuged at 10,000 × g for 10 min to clear cellular debris. Virus and virally infected cells were treated similarly with the exception that frozen viral stocks were thawed and used directly. Both SVPs/virus and lysates were denatured in sample preparation buffer (2% SDS-50 mM Tris [pH 6.8]-10% glycerol) at 55°C for 20 min. Aliquots from each sample were then treated with 50 U of peptide N-glycosidase F (PNGase F) or endoglycosidase H (Endo H) according to the manufacturer's instructions (New England BioLabs, Inc., Beverly, MA) or with PNGase F buffer without enzyme as a control. Digestions were carried out for 3 h at 37°C. Samples were then analyzed under denaturing conditions by SDS-PAGE (7.5% or 10 to 20% gradient) and Western blotting using E MAb 5H10 (1:500, BioReliance Corp., Rockville, MD) or WNV hyperimmune ascites fluid (1:500) and an appropriate HRP-conjugated murine secondary antibody. Blots were imaged using SuperSignal West Femto maximum sensitivity substrate (Pierce Biotechnology, Inc., Rockford, IL) on a FujiFilm LAS-1000 camera.
Antigen capture ELISA.
Flavivirus E-specific antibody 4G2 (15) was bound to Immulon 2HB ELISA plates at a concentration of 100 ng/well diluted in binding buffer at 4°C overnight. Plates were washed with washing buffer (PBS, 0.05% Tween 20) three times and then incubated in blocking buffer (1× PBS, 1% bovine serum albumin, 0.05% Tween 20) for 1 h with shaking at room temperature. Buffer was removed, and WNV particles in blocking buffer containing 1% Triton X-100 were diluted 1:2 across the plate in dilution buffer (blocking buffer with 0.01% Triton X-100). A truncated, soluble form of WNV E protein was produced from a recombinant vaccinia virus (53), purified, bicinchoninic acid quantified, and used as a standard in this assay. Twenty-five ng of soluble E was added to one well on the plate in duplicate and then diluted 1:2 as specified above to generate a standard curve. Particles and standards were allowed to bind to 4G2 for 2 h with shaking at room temperature. Plates were washed three times, 0.2 μg per well of biotinylated MAb E1 was added to each well, and then plates were incubated at room temperature on the shaker for 1 h. Plates were washed as described before, and 100 μl of a 1:2,500 dilution of streptavidin conjugated to HRP (Sigma) was added to each well and incubated on the shaker at room temperature for 1 h. Plates were then washed six times and visualized using a peroxidase substrate (KPL, Inc., Gaithersburg, MD). Plates were read in an MRX Revelation plate reader at a wavelength of 450 nm.
Production of reporter virus particles.
WNV reporter virus particles (RVPs) were produced by complementation of a DNA-launched WNV replicon with structural gene products provided in trans (48a). Briefly, HEK-293T cells were plated at 2 × 106 cells per 10-cm3 dish. The following day, cells were transfected with a total of 20 μg of three different plasmids {3.3 μg replicon plasmid [pWIIrep-GFP], 10 μg WNII capsid [pWcap(s)], and 6.7 μg polyprotein plasmid encoding envelope proteins} using a calcium phosphate transfection kit (Invitrogen, Carlsbad, CA). Medium was replaced on the following day with a low glucose formulation of DMEM (Life Technologies) containing 10% FBS (HyClone) and 1% penicillin-streptomycin solution (Life Technologies). RVP containing medium was collected 48 h posttransfection, cleared with a low-speed spin at 1,600 rpm (Sorvall Legend RT, Newtown, CT) for 10 min, and then filtered through a 0.2-μm filter disk (Corning Incorporated, Corning, NY). Clarified medium was then used in antigen capture ELISAs, quantitative PCR (qPCR), and infection assays as described previously.
Metabolic labeling.
HEK-293T cells were transfected with a plasmid encoding WNV envelope polyprotein for SVPs or with replicon, capsid, and envelope polyprotein for RVPs, as described above. Medium was removed from cells 12 h posttransfection, cells were starved for 30 min in Met/Cys-free DMEM, and then 0.25 mCi [35S]Met-Cys was added to each well. SVPs or RVPs were isolated 36 h posttransfection as described above and analyzed under denaturing conditions by SDS-PAGE and the WNV E, prM, or M protein band for each sample quantified with a Storm-860 phosphorimaging system (Molecular Dynamics, Sunnyvale, CA).
Quantitative PCR.
Viral RNA was isolated from 140 μl clarified RVP or virus containing medium by using the QIAmp viral RNA kit (QIAGEN, Valencia, CA) with the addition of two DNase treatments. Prior to the addition of lysis buffer, particle containing medium was incubated for 30 min with 100 U RNase-free DNase I (Roche Molecular Biochemicals, Indianapolis, IN) at room temperature. In addition, prior to elution, an on-column digestion of DNA was performed for 30 min at room temperature using 30 U RNase-free DNase using a modification of the manufacturer's protocol (QIAGEN, Valencia, CA). RNA was eluted in 50 μl of elution buffer and stored at −20°C until used. A primer was designed to bind the 3′ untranslated region (UTR) of lineage II (5′-CTTACAGCTTCAGCCAAGTTG-3′) for use in cDNA synthesis. Reverse transcriptase-PCR was performed using the Superscript III First-Strand synthesis system according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). The WNV TaqMan primers and probe were designed using a modification of the approach described by Lanciotti et al. (34) (forward, 5′-CAGTGTCAGACCACACTTTAATGT-3′; reverse, 5′-CATAGCCAGGGTTATGGCCGCGTGGG-3′; and probe, 5′-FAM-TCTGCGGAGAGTGCAGTCTGCGAT-TAMRA-3′; Applied Biosystems Inc., Foster City, CA).
For the TaqMan assay, 5 μl of RVP/viral cDNA was combined with 50 pmol of each primer, 10 pmol of the TaqMan probe, and 2× Universal PCR Master Mix (Roche, Branchburg, NJ) and brought to the final 50-μl volume with distilled water. The samples were subjected to amplification (94°C for 1 min and then 50 cycles at 94°C for 15 s, 55°C for 15 s, and 68°C for 15 s) on the Opticon2 system (MJ Research Inc., Waltham, MA). The rate of TaqMan assay positivity was calculated by using 99.9% confidence level settings in the Opticon2 software. Baseline signal value subtracted from each sample was determined by calculating the average fluorescence signal in each well for cycles 1 through 15. Quantitation of the relative number of genomic RNA copies present in RVP and virus preparations was calculated by generating a standard curve (correlation coefficient averaging 0.994) with a quantified DNA plasmid (pWNII-Not). This plasmid encoded a DNA-launched lineage II molecular clone with an identical 3′ UTR to the viral genome contained in both RVPs and replication-competent lineage II WNV used in this study. Plasmid copies were calculated using the following equation: [(Avagadro's number × plasmid concentration)/plasmid molecular weight.] RVP concentrations within the sample preparations used in this study varied between 2 × 104 and 5 × 105 copies/ml. This sensitive and quantitative assay provides a measure of the relative number of viral genome copies but not an absolute number. Reasons for this include the fact that we do not know the efficiency with which the viral RNA was amplified relative to our DNA standard. In addition, the concentration of our DNA standard is based on UV absorbance, which can be affected by variations in plasmid purity.
RVP infection assays.
RVP preparations were normalized by qPCR analysis and used to infect 4 × 104 cells (BHK-21, QT6, C6/36) in a total volume of under 500 μl for 1 h at 37°C in suspension. Cells were then placed in 24-well plates in a volume of 2 ml per well and allowed to adhere overnight. Cells were harvested, fixed in 2% paraformaldehyde in PBS, and analyzed by flow cytometry 40 h postinfection for BHK-21 and QT6 cells and 72 h postinfection for C6/36 cells. The percentage of infected cells was determined by measuring green fluorescent protein (GFP)-expressing cells using a FACScan flow cytometer.
Virus infection assays.
Cells were plated at 3.5 × 104 per well in a 24-well plate the day prior to infection. Viral stocks were thawed and the inoculum normalized by qPCR. Cells were infected with the indicated amount of WNV in a 500-μl volume for 1 h at 37°C. Virus inoculum was then removed and the cells washed five times with PBS to remove any free virus. Cells were harvested by trypsinization at the indicated times, washed with cold fluorescence-activated cell sorter (FACS) buffer (PBS, 2% FBS), and fixed in 1.5% paraformaldehyde for 1 h at 4°C. The cells were then pelleted, washed with FACS buffer with 0.1% saponin, and stained with a MAb 4E1 (53) conjugated to Alexafluor 647 (Molecular Probes, Eugene, OR). Cells were analyzed for WNV E protein expression using a FACSCalibur flow cytometer.
Viral PFU assay.
BHK-21 clone 15 cells were seeded at 3.5 × 105 per well in a 12-well plate 2 to 3 h prior to infection. Frozen (−80°C) aliquots of virus were diluted 1:10 in a total volume of 300 μl of α-minimal essential media (MEM)-2% FBS. These stocks were then diluted 1:5 in the same medium for a total of 11 dilutions. Next, 150 μl of each dilution was used to infect one well each in a 12-well plate. Inoculum was left in the wells for 1 h and removed, and then wells were overlaid with a 1:1 mix of 2× α-MEM-8% FBS with melted 2% low-melting-point SeaPlaque agarose (Cambrex Bio Science Rockland, Inc.). After the agar overlay solidified, the plates were returned to 5% CO2 at 37°C. Three days after infection, the plates were fixed with 10% formaldehyde for at least 2 h and then stained with 1% crystal violet-20% ethanol. The number of plaques was counted in each well and used to calculate the PFU.
RESULTS
Intracellular localization of WNV lineage I and II envelope proteins.
Most studies on flavivirus assembly indicate that these viruses bud into the lumen of the ER (40, 41, 60). However, the Sarafend strain of WNV, a lineage II strain, has been reported to bud at the plasma membrane (47). We examined the intracellular distribution of prM and E in HeLa cells infected with a lineage I (NY99-6480) and a lineage II (956 D117 3B) strain of WNV and cells transfected with plasmids encoding lineage I and II envelope polyproteins. Detection by indirect immunofluorescence showed that both prM and E colocalized with calnexin, a molecular chaperone that resides within the lumen of the ER. Consistent with our light microscopic results, and as noted below, intracellular glycosylated forms of WNV prM and E remained Endo H sensitive, supporting localization in the ER. Finally, transmission electron microscopy of Vero cells infected with lineage II strain 956 D117 3B of WNV clearly showed virus particles within the rough ER and contained in membrane vesicles within cells (Fig. S1, http://www.med.upenn.edu/micro/domslab/hannajvi.pdf). Thus, consistent with the results of others (40, 41, 60), we conclude that in HeLa and Vero cells, the WNV strains used in this study assemble by budding into the ER and not at the plasma membrane.
Secretion of subviral particles by expression of envelope proteins.
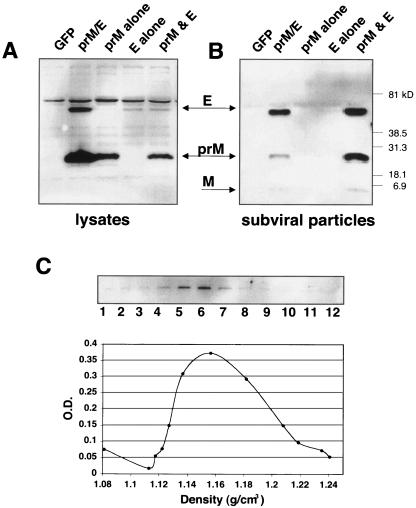
Expression of the prM and E glycoproteins of several flaviviruses in the absence of other viral proteins or the viral genome results in the production and secretion of recombinant SVPs (2, 28, 30, 31). SVPs are also a natural by-product of flavivirus infection (52) and, for those studied thus far, are structurally (albeit smaller) and antigenically similar to infectious virus (11, 23, 55). Since WNV is classified as a BSL-3 agent, WNV SVPs represent a useful tool to study envelope protein structure and function. It has already been shown that expression of WNV prM and E results in the release of viral antigen into the medium (13). To further characterize this soluble antigen, we transfected human HEK-293T cells with plasmids expressing lineage I prM and E singly, in combination, or as part of the polyprotein to determine if viral envelope release into the medium is dependent on the expression of both envelope proteins and whether they must be expressed in cis. The presence of prM and E in both cell lysates and pelleted media collected from transfected cells was determined by Western blot analysis. Lineage I WNV E, prM, and the mature M protein were recovered only from pelleted cell media when both proteins were expressed (Fig. 1A and 1B), independent of whether they were expressed from one plasmid or in trans. The same experiments were carried out with lineage II envelope proteins with similar results (data not shown). When analyzed by equilibrium density gradient ultracentrifugation, the SVPs sedimented as a discrete population with an apparent density of 1.16 g/cm3 (Fig. 1C), which corresponds to the relative density for tick-borne encephalitis virus SVPs (55).
FIG. 1.
Coexpression of viral envelope proteins prM and E together produce SVPs. HEK-293T cells were transfected with plasmids encoding a lineage I prM and E polyprotein (strain NY99-6480) or each envelope protein individually, as indicated. Supernatants and cell lysates were collected 48 h posttransfection and SVPs isolated by ultracentrifugation of supernatant through a 20% sucrose cushion. Both lysates and pelleted SVPs were analyzed by Western blotting using antibodies that recognized the two WNV membrane proteins (αprM/αE). (A) Shown is a representative Western blot of lysates from cells transfected with an irrelevant protein (GFP), the envelope polyprotein (prM/E), prM protein alone, E protein alone, and prM and E expressed from different plasmids. (B) Western blot of the accompanying pelleted SVPs released in the supernatant from each transfection. (C) SVPs produced by transfection of HEK-293T cells with WNV prM/E were pelleted and then layered on the top of a 20 to 60% sucrose gradient and centrifuged for 17 h to allow particles to reach equilibrium. Fractions of equal volume were collected and the refractive index measured for each. Shown is a representative Western blot of fractions collected and probed with a WNV E-specific antibody. Additionally, a plastic capture ELISA was used to measure the WNV antigen quantity in each fraction. Refractive index numbers were converted to density values (g/cm3) and plotted against optical density (O.D.) readings in the ELISA to identify the density at which the majority of particles migrated in the total sample.
Glycosylation profile of envelope proteins within cells and incorporation into particles.
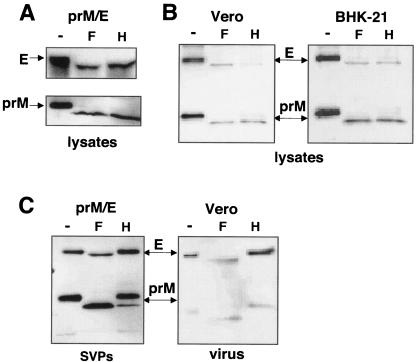
N-linked glycosylation plays an important role in both the assembly and the infectivity of many viruses (12, 17, 37, 49, 57, 58, 64, 66, 67). All strains of WNV examined thus far encode a potential N-linked glycosylation site in the ectodomain of the prM protein at amino acid 15. In contrast, the presence of a potential N-linked glycosylation site in E is variable in both WNV lineages, although only lineage II isolates contain a 4-amino-acid deletion at this position that ablates the N-linked glycosylation site. To determine if these glycosylation sites are utilized, HEK-293T cells were transfected with a plasmid expressing a lineage I prM/E polyprotein (NY99-6480) containing potential glycosylation sites in both prM and E. Cell lysates were prepared and analyzed by Western blotting, with aliquots digested with either PNGase F (removes all N-linked carbohydrate structures) or Endo H (removes only immature carbohydrate structures). Two different types of SDS-PAGE gels were used to resolve the E and prM proteins obtained from HEK-293T cells (Fig. 2A). In cell lysates, both prM and E migrated more quickly following PNGase F digestion, indicating that the single N-linked sites in prM and E were utilized. Similar results were obtained with Endo H, indicating that in cells, WNV glycoproteins were predominantly in high mannose form, consistent with their localization to the ER (Fig. 2A). Identical results were obtained with lysates from either BHK-21 or Vero cells infected with lineage II virus (Fig. 2B).
FIG. 2.
Glycosylation status of WNV envelope proteins in lysates and released particles. Lysates from HEK-293T cells transfected with a prM/E plasmid (A) or Vero and BHK-21 cells infected with virus (B) were collected 48 h after transfection or infection and treated with glycosidases PNGase F (F), Endo H (H), or buffer only (−) as a control. (C) SVPs pelleted from the HEK-293T cells transfected as shown in panel A or virus produced by the infected Vero cells shown in part B were similarly digested. Shown are Western blots of treated lysates (A and B), SVPs, or virus (C) probed with antibodies that recognize WNV membrane proteins (αprM/αE).
In contrast to their cell-associated forms, prM and E released from cells either as SVPs or as virus were largely or completely Endo H resistant (Fig. 2C), consistent with modification of the carbohydrate side chains as the envelope proteins were transported through the Golgi apparatus. These data show that when present, the glycosylation sites on prM and E are utilized and suggest that the rate-limiting step of particle assembly is folding and association in the ER (39), while transport and processing through the Golgi network and secretory pathway occurs quickly, resulting in the majority of Golgi-processed particles being released into the supernatant.
Effects of glycosylation on SVP formation.
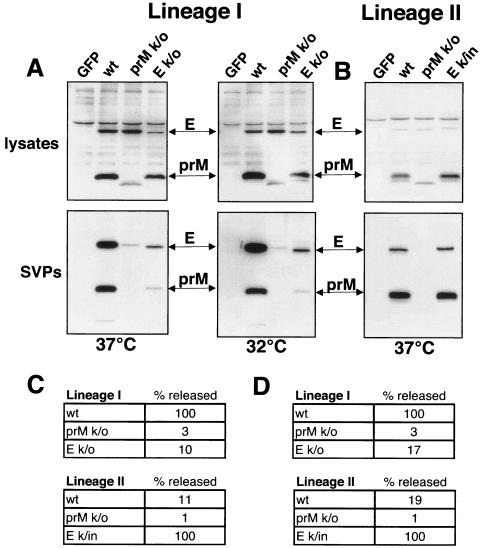
To assess the effects of N-linked glycosylation on WNV SVP assembly and release, we individually removed or added the glycosylation sites in prM and E of lineage I (NY99-6480) and II (956 D117 3B) envelope polyproteins. The lineage I construct contained glycosylation sites in both prM and E that were individually removed by changing the asparagines in each N-linked site to a glutamine. The lineage II polyprotein encoded a glycosylation site in prM but contained a 4-amino-acid deletion in E found only in lineage II strains, resulting in the loss of the E glycosylation site. Therefore, we made one construct removing the glycosylation site in prM and another in which we introduced the N-linked site commonly found in lineage I strains (NYST) into the lineage II E protein. Western blots of cell lysates and media from HEK-293T cells transfected with lineage I constructs showed that the loss of either the prM or E protein glycosylation sites resulted in a marked decrease in the secretion of SVPs (Fig. 3A). Changes in amino acid sequence or polypeptide modifications can adversely affect protein folding; therefore, we repeated the experiment at a lower temperature, 32°C, which can sometimes rescue a misfolding phenotype, but obtained similar results (Fig. 3A). In agreement with results obtained with the envelope proteins from the lineage I strain, when lineage II proteins were expressed, we found that the loss of glycosylation in prM resulted in a decrease in SVP release. However, the introduction of an N-linked glycosylation site in lineage II E protein had no obvious effect on SVP release, as judged by nonquantitative Western blot analysis (Fig. 3B).
FIG. 3.
Glycosylation influences SVP production. HEK-293T cells were transfected with polyprotein plasmids encoding prM and E from lineage I or lineage II WNV wild type (wt), glycosylation-mutated prM or E, or an irrelevant protein (GFP) as a control. Mutants used were: prM knockout (k/o), which lacked the glycosylation site in prM; E k/o, which lacked the glycosylation site in the E protein of lineage I WNV; and E k/in, which contained a 4-amino-acid insert (NYST) reestablishing the N-linked glycosylation site in the lineage II E protein. Cell lysates and released SVPs were collected 48 h posttransfection. (A) Western blot of lineage I lysates and SVPs from a standard transfection at 37°C probed with antibodies that recognize the WNV membrane proteins (αprM/αE). Repeating the experiment at 32°C did not alter the phenotype of either mutant. (B) Western blot of lineage II lysates and SVPs probed with WNV HIAF. (C) SVP quantified using a WNV E antigen capture ELISA. Data represent three separate experiments normalized by setting the sample from each lineage with the highest release of antigen to 100% release. (D) SVP release was also quantified by metabolically labeling cells expressing the indicated constructs with [35S]methionine-cysteine and measuring the density of the resulting WNV E bands using a phosphorimager.
To quantify the release of SVPs, we utilized an antigen capture ELISA where pelleted medium from transfected cells was bound to wells coated with a flavivirus E antibody (4G2) and the amount of bound SVPs detected by another noncompeting E monoclonal antibody (MAb) (E1). Quantitation based on ELISA data confirmed a dramatic decrease in SVP release with the removal of either the prM or E glycosylation sites in lineage I (3% and 10% of wild-type levels, respectively) (Fig. 3C). Removal of the N-linked glycosylation site in lineage II prM also resulted in a dramatic decrease in SVP release. However, SVP release was greatly enhanced with the introduction of an N-linked glycosylation site in the lineage II E protein (approximately 1 log). Since these mutations could potentially alter the reactivity of these proteins with the antibodies used in the Western blot and ELISA, we also analyzed SVP release by metabolically labeling particles with [35S]methionine-cysteine. SVPs were pelleted from the medium of transfected cells by ultracentrifugation, separated by SDS-PAGE, and quantified by phosphorimage analysis. Quantitation of the prM and E band densities confirmed the effects of removing or adding carbohydrate sites on particle release seen using the antigen capture ELISA (Fig. 3D).
Effects of glycosylation on virus production.
We have developed methods for the production of WNV particles capable of a single round of replication (RVPs) by complementation of a subgenomic replicon with structural genes in trans (24, 25, 48a, 59). RVPs were produced by transfection of HEK-293T cells with plasmids expressing WNV capsid, a DNA-launched replicon encoding GFP, and either wild-type or glycosylation mutant envelopes from lineage I and lineage II strains of WNV (Fig. 4A). Particle-containing medium was collected 48 h posttransfection and particle release quantified by both WNV antigen capture ELISA and quantitative PCR (qPCR) using primers and a probe specific for the 3′ UTR of the WNV genome. Viral RNA genome copies within a sample were calculated using a DNA plasmid as a standard. This sensitive qPCR assay made it possible to measure the relative number of viral genome copies in a sample rather than an absolute number. Thus, we were able to measure the levels of viral antigen as well as the viral genome number (Fig. 4B). Such an analysis is required because mutations in the envelope proteins that impact folding or overall expression levels could alter the ratio of infectious to noninfectious particles by affecting the levels of SVP production and this shift would not be appreciated using only an antigen ELISA (48a). We found that removal of N-linked glycosylation from either prM or E reduced the production of RVPs, as measured by either viral antigen or WNV genome copies, though not always to the same extent (Fig. 4B). Removal of the N-linked site from either lineage I or II prM had a greater impact on the release of viral antigen than on the release of viral genome copies. Altering the glycosylation status of the E protein, however, had little effect on the ratio of genome-carrying virus particles to subviral particles. Thus, N-linked glycosylation had similar effects on SVP and RVP secretion.
FIG. 4.
Glycosylation affects reporter virus particle release. (A) Schematic of the three plasmids used to produce RVPs: replicon plasmid (pWIIrep-GFP) encoding the WNV nonstructural proteins (NS1 through NS5), GFP in place of the three structural proteins (C, prM, and E), protease cleavage site (FMDV 2a) and small fragments of C and E needed for proper transcription/translation; an envelope polyprotein (prM/E) plasmid; and capsid plasmid [pWcap(s)]. (B) All three plasmids were transfected into HEK-293T cells, and RVP-containing supernatant was collected 48 h posttransfection. Wild-type (wt) and glycosylation mutant envelopes from both lineage I (NY99-6480) and II (956 D117 3B) strains were used to produce RVPs. A comparison between the measurement of particle release based on antigen content (E antigen capture ELISA) or genome copies (qPCR) from the same RVP preparation is given. For comparison purposes, the results from each assay were normalized by setting the construct from each lineage with the highest production of particles, i.e., the E-glycosylated form in each lineage, to 100% particle release. Values presented are the combined results from three separate assays. Error bars indicate 1 standard deviation from the mean. k/o, knockout; k/in, knockin.
To determine if changes in glycosylation affected the overall structure of the particles released, we analyzed lineage I wild-type, prM glycosylation-null, and E glycosylation-null RVPs by equilibrium density gradient ultracentrifugation. All RVP preparations sedimented with the same apparent density (1.16 g/cm3), indicating that the bulk of the particles produced with each envelope construct were similar (data not shown). Additionally, we produced [35S]methionine-cysteine-labeled RVPs and assessed the ratio of E to prM/M and M to prM for each of the lineage I and lineage II envelope constructs. We found that changing the glycosylation status of either lineage I or lineage II E protein had no effect on prM processing or on the ratio of E protein to prM. The very low levels of protein recovered from viruses lacking the glycosylation site in prM precluded an accurate assessment of protein content and processing (Fig. S2, http://www.med.upenn.edu/micro/domslab/hannajvi.pdf). Taken together, these data suggest that the glycosylation status of either E or prM does not significantly alter the overall density or organization of the viral particles.
Effect of glycosylation on reporter virus infectivity.
To assess the ability of prM and E glycosylation mutant RVPs to infect target cells, we produced RVPs in HEK-293T cells, collected the medium, and quantified the number of viral genome copies by qPCR. Based on these data, RVPs for each construct were normalized to each other based on genome copies and used to infect BHK-21 cells, varying the viral input over a broad range. Infection efficiency was determined by measuring the percentage of GFP-expressing cells using flow cytometry 40 h postinfection (Fig. 5A). All envelopes were capable of mediating the infection of target cells. For lineage I, infectivity increased slightly when the glycosylation site in E was removed. In complement, introduction of a glycosylation site into the lineage II E protein reduced infectivity. Removal of the prM glycosylation site in either lineage had a negligible effect on viral infectivity.
FIG. 5.
RVP infectivity over multiple dilutions of inocula. RVP infections were normalized based on WNV genome copies per ml in each particle preparation and used to infect target cells over multiple relative genome copies ranging from 40,000 to 750 depending on the cell type. Each infection was carried out in duplicate and cells collected for FACS analysis 40 h p.i. for BHK (A) and QT6 (B) and 72 h p.i. for C6/36 cells (C). Data were plotted according to virus lineage and cell type. The x axis represents the relative genome copies. Percent infection is shown on the y axis and represents the number of GFP-expressing cells as measured by FACS analysis. wt, wild type; k/o, knockout; k/in, knockin.
The natural infection cycle for West Nile virus occurs between mosquitos and birds, with mammals as incidental hosts. For this reason, we tested RVP infectivity on QT6 (quail) and C6/36 (Aedes albopictus mosquito) cells (Fig. 5B and 5C). Relative infection levels on QT6 cells were consistent with results seen on BHK-21 cells. However, altering glycosylation patterns had a dramatic effect on the infection of C6/36 mosquito cells. For lineage I WNV, the removal of the E protein glycosylation site enhanced RVP infectivity by more than 30-fold. Likewise, introducing a glycosylation site into the lineage II E protein reduced viral infectivity by an equivalent amount (Fig. 5B). Thus, the presence of an N-linked carbohydrate chain at amino acid position 154 on the WNV E protein of both lineages I and II greatly reduced viral infectivity on C6/36 mosquito cells while the presence of a carbohydrate structure on E had only a modest effect on the infection of mammalian or avian cells.
Effect of glycosylation on replication-competent virus infectivity.
To examine the effects of prM and E protein glycosylation on WNV infectivity in the context of replication-competent virus, lineage II (956 D117 3B) glycosylation mutant envelopes were cloned into a DNA-launched infectious molecular clone derived from the same viral strain (48). Virus stocks were produced in BHK-21 cells, viral RNA was quantified by qPCR, and an equivalent number of genome copies for each virus was used to infect BHK-21, QT6, and C6/36 cells across a broad range of viral dilutions. To monitor initial virus infection, intracellular FACS staining was performed 12 h postinfection for BHK-21 and QT6 cells and 24 h postinfection for C6/36 cells. Consistent with the results seen with RVPs, the absence of glycosylation on prM resulted in a decrease in virus release, as measured by viral antigen levels and genome copies (data not shown), but had only modest effects on initial infectivity (Fig. 6A through C). The presence of the E protein glycosylation site was associated with enhanced virus assembly and release (data not shown) but with a threefold decrease in viral infectivity on BHK-21 and QT6 cells (Fig. 6A and 6B) and with a greater than 30-fold decrease in infectivity on C6/36 cells. Thus, these results using replication-competent virus are consistent with the RVP data.
FIG. 6.
Virus infectivity over multiple dilutions of inocula. Lineage II virus infections (strain 956 D117 3B) were normalized based on WNV genome copies per ml in each viral stock and used to infect target cells over multiple relative genome copies ranging from 50,000 to 750 depending on the cell type. Each infection was carried out in duplicate and cells collected for FACS analysis 12 h p.i. for BHK (A) and QT6 (B) and 24 h p.i. for C6/36 cells (C). The x axis shows the relative genome copies. Percent infection is shown on the y axis and represents the number of GFP-expressing cells as measured by flow cytometry analysis. Points outside the linear range for each infection curve were excluded from the presented data.
The dramatic decrease in viral infectivity on C3/36 cells when E protein was glycosylated was somewhat surprising, since this cell line is often used to propagate WNV stocks. We therefore performed viral growth curves on C3/36 cells and other commonly used cell lines, BHK-21 and Vero cells. To do this and to make our work comparable to that of others, we normalized the virus inocula by PFU (multiplicity of infection of 1.0) rather than by genome copy number. Under these conditions, infection with the E-glycosylated mutant produced up to a log more virus, as measured by PFU, than E glycosylation-null wild-type lineage II virus when grown on all cell types tested (Fig. S3, http://www.med.upenn.edu/micro/domslab/hannajvi.pdf). This data is consistent with results previously published (5, 56) indicating that the viruses used in this study exhibit similar growth kinetics when infections are normalized by PFU. The large increase in virus production seen when the E protein is glycosylated (Fig. 3) may partially offset the decrease in viral infectivity (Fig. 6) over the course of a spreading viral infection. Additionally, PFU measures the ability of viruses not only to infect target cells but also to replicate and spread to surrounding cells. Therefore, normalizing virus inocula based on PFU measurement can mask significant differences in virus entry. Not surprisingly, the prM glycosylation-null virus produced viral titers approximately 1 log lower than wild-type virus on BHK-21 and Vero cells and almost 3 logs less when grown on C6/36 cells, consistent with its association with markedly reduced virus production. Sequencing of viral RNA extracted at 48 h postinfection confirmed that the glycosylation status for all viruses was consistent with the genotype of the virus inoculum.
DISCUSSION
Compared to past WNV outbreaks, the current North American WNV epidemic is impressive in both terms of extent and duration (18, 45). While there may be many contributory factors, it is possible that the current WNV strain circulating in North America and those responsible for other significant human outbreaks are more virulent than strains not associated with significant human disease. Thus far, major WNV epidemics have been invariably associated with lineage I strains of WNV while lineage II strains appear to be less virulent. The molecular determinants that are responsible for differential WNV virulence have not been identified, but factors that influence the efficiency of virus production as well as attachment and entry of WNV into cells could play a major role in viral pathogenesis (62).
In comparing E protein sequences between different lineage I and lineage II WNV strains, perhaps the most significant difference is the presence in some strains of a single N-linked glycosylation site in the E glycoprotein, while in other strains, this site is absent. In lineage II strains, a 4-amino-acid deletion is often responsible for the absence of this glycosylation sequence (6). All North American WNV isolates described to date contain the E protein N-linked glycosylation site, as have some other WNV strains associated with significant human outbreaks (22, 35, 54). In general, carbohydrate structures can have significant effects on both virus production (12, 17, 57, 58, 66, 67) and attachment of virus particles to the cell surface (37, 49, 64). Most flaviviruses have one or two conserved N-linked glycosylation sites in their E glycoproteins and a single N-linked site in prM that is not present in the mature M protein (19). The presence or absence of specific glycosylation sites in flavivirus E glycoproteins has been associated with alterations in virus production (56, 65) and pathogenesis, including neuroinvasiveness (4, 5, 42).
We found that, when present, the N-linked glycosylation sites in the prM and E glycoproteins of lineage I strain NY99-6480 (14) and lineage II strain 956 D117 3B (68) of WNV were utilized, thus providing a biochemical marker of protein transport. When the glycoproteins were expressed in the context of either subviral or replication-competent virus particles, cell-associated prM and E were sensitive to digestion with Endo H, which is consistent with their localization to the ER. In contrast, the prM and E glycoproteins present on subviral particles as well as WNV released into the medium were resistant to Endo H digestion, consistent with their passage through the Golgi apparatus. Coupled with our electron microscopic observations that revealed virus particles within the rough ER, we conclude that WNV assembles and buds in the ER and quickly transits the Golgi apparatus en route to the cell surface. Our conclusion is consistent with the work of others on both WNV and other flaviviruses (40, 41, 60).
To obtain a clearer understanding of the role that N-linked glycosylation might play on virus production and infectivity, we developed a quantitative E protein ELISA that was used to measure the release of E protein into the medium of transfected or infected cells and coupled this with a quantitative PCR assay to measure viral RNA. Use of both these assays was important since modifications to structural protein processing can affect the relative ratios between SVPs and genome-bearing virus particles (38, 48a), which would not be obvious by ELISA measurements alone. We found that elimination of the highly conserved N-linked glycosylation site in either lineage I or lineage II prM led to a dramatic decrease in the release of SVPs, RVPs, and lineage II replication-competent virus particles. The glycosylation site in the prM protein is in the region of the protein that is removed by proteolysis as WNV transits the Golgi apparatus (19, 63). Therefore, the fact that this glycosylation site is so highly conserved despite its absence in the mature virus particle suggests that its primary role is to assist protein folding in the ER rather than in virus infection. Large, hydrophilic N-linked carbohydrate structures often play important roles in protein folding, both by positioning the polypeptide chain to which they are attached on the outer surface of the folding molecule and by serving as ligands for molecular chaperones such as calnexin and calreticulin (21). In the case of other flaviviruses, prM is known to play a chaperone role by facilitating the correct folding of E and protecting E from premature conformational changes as it traverses the secretory pathway (29, 39). Studies with conformation-dependent antibodies may help reveal whether the folding of E is dependent in part upon proper prM glycosylation. However, such studies will be complicated by the fact that the block to virus assembly is far from complete, with anywhere from about 1% to 10% normal SVP or virus production occurring in the absence of prM glycosylation.
While the loss of glycosylation in prM had a significant effect on virus production, the virus that was produced was nearly as infectious as wild-type WNV. In our hands, cleavage of prM was not complete and we found some prM associated even with replication-competent WNV. Whether residual prM plays any role in virus infectivity is not known. However, the mature M protein lacks the carbohydrate site, so M proteins derived from either wild-type or glycosylation-defective prM may be indistinguishable on the surface of virus particles. The fact that virus produced with glycosylation-defective prM is nearly as infectious as wild-type virus again suggests that the primary function of the prM carbohydrate structure is to assist protein folding and virus assembly. Once the virus is assembled, this domain, which is largely absent in mature virus, plays little role in modulating viral infectivity on the cell types used in this study.
Alterations in E protein glycosylation also affected virus production and infectivity. When the E protein was not glycosylated, as in our mutant lineage I strain or in a naturally glycosylation-deficient lineage II virus, SVP and virus production were reduced approximately 10-fold relative to viruses containing the E protein glycosylation site. This reduction level is similar to that seen in tick-borne encephalitis SVP release upon removal of the E glycosylation site or treatment with tunicamycin, a glycosylation inhibitor (39, 40). Thus, at the level of particle production, absence of E protein glycosylation had effects that were similar to, although less pronounced than, those observed when the prM glycosylation site was removed. Interestingly, when the infectivity of RVPs bearing these envelopes was analyzed, those particles lacking glycosylation on E in both lineages I and II were somewhat more infectious than wild-type RVPs per genome copy on BHK-21 and QT6 cells. When replication-competent lineage II viruses containing the same envelope glycosylation modifications were used, the absence of E protein glycosylation was associated with approximately threefold enhanced infectivity on BHK-21 and QT6 cells.
Since removal of an N-linked glycosylation site appeared to have a greater effect on SVP release than on the release of genome-bearing virus particles, it was possible that the reduced infectivity simply resulted from an altered particle-to-viral genome ratio, with noninfectious particles competing for virus binding sites on the cell surface. However, this trend remained similar over a broad range of viral inocula. Therefore, while the presence of N-linked carbohydrate on E facilitates more robust particle release, perhaps due to more efficient protein folding, this same sugar moiety resulted in a modest reduction in initial infectivity on BHK-21 and QT6 cells. While the reason for this is not clear, it is worth noting that placement of the E carbohydrate structure on West Nile, tick-borne encephalitis, and Dengue virus particles positions the sugar where it can protect or mask the fusion loop of a partner E protein when in the homodimer conformation found on mature viruses (43, 44, 50). Additionally, studies done with West Nile and dengue viruses have found that mutants lacking the E protein N-linked glycosylation site are more susceptible to irreversible conformational changes when exposed to acidic pH (5, 36). Therefore, E glycosylation likely plays additional roles in modulating the initial fusion events and subtle differences in pH sensitivity could alter initial infectivity.
Our most surprising results were obtained when we performed infection studies with mosquito C6/36 cells. WNV naturally cycles between avian and mosquito hosts, providing the rationale for examining the consequences of glycosylation on viral infectivity with different cell types. The presence or absence of the E protein N-linked glycosylation site on WNV had a profound effect on the ability of RVPs and replication-competent lineage II virus to infect C6/36 cells. The absence of E protein glycosylation in both the lineage I and II envelopes tested was associated with the robust initial infection of C6/36 cells over a wide range of inocula. In contrast, the presence of E protein glycosylation decreased infectivity on these cells more than 30-fold. This effect of E glycosylation on viral infectivity was far greater than that seen on BHK-21 or QT6 cells, indicating a strong host cell dependence. Together, our results indicate that the presence or absence of the E protein glycosylation site can affect WNV assembly and release as well as infectivity, with infectivity in turn being dependent upon the cell type examined. In mammalian cells, the modest decrease in viral infectivity associated with E protein glycosylation may be more than offset by efficient virus production, while in mosquito cells, the absence of glycosylation may be more significant. These cell-type-dependent effects of glycosylation on WNV infectivity may begin to explain the absence of E protein N-linked glycosylation in many clinical isolates. When nonglycosylated Kunjin isolates are passaged in Vero cells, the E protein glycosylation site is often acquired by three to four passages (56), suggesting that there are as yet unidentified selective pressures for the absence of E glycosylation that are absent from current culture systems.
In summary, our results indicate that the highly conserved N-linked glycosylation site present in WNV prM plays a major role in modulating virus assembly and release but has a minimal effect on virus infectivity. Likewise, virus assembly is enhanced when the WNV E protein is glycosylated. In both cases, the presence of N-linked carbohydrate structures may enable prM and E to interact with the molecular chaperones calnexin and calreticulin, which could improve the kinetics and efficiency of protein folding. In addition to virus assembly, E protein glycosylation can influence virus infectivity, sometimes in dramatic ways. The reason for this is not clear, but the deleterious effect of E protein glycosylation on the infection of mosquito C6/36 cells could be related to the presence of different attachment factors or receptors on insect cells relative to those present on the mammalian or avian cell lines studied here. Clearly, further understanding the role of WNV glycosylation in viral tropism and pathogenesis will require the use of multiple cell types, both for the production of virus as well as for virus infection studies.
Acknowledgments
This research was supported by NIH U54 AI57168 and by a grant provided by the University of Pennsylvania. Sheri L. Hanna received funding support from both Public Health Service Training grants T32-GM-007229 and T32-AI-07324-13. Melissa Sanchez was supported by NIH F31 RR05074.
We thank Michael Diamond for E1 MAb to the WNV E protein, Gwon-Jen J. Chang for the pCBWN plasmid, and Vladimir Yamshchikov for the SP6WN/Xba WN lineage II molecular clone; Neelima Shah for electron microscopy; Indira Akula for technical support; and Carl Davis, Angela Ciuffi, and Richard Milne for helpful discussions.
REFERENCES
- 1.Adams, S. C., A. K. Broom, L. M. Sammels, A. C. Hartnett, M. J. Howard, R. J. Coelen, J. S. Mackenzie, and R. A. Hall. 1995. Glycosylation and antigenic variation among Kunjin virus isolates. Virology 206:49-56. [DOI] [PubMed] [Google Scholar]
- 2.Allison, S. L., K. Stadler, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J. Virol. 69:5816-5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley, D. W., C. T. Davis, M. Whiteman, B. Granwehr, R. M. Kinney, and A. D. Barrett. 2004. Molecular determinants of virulence of West Nile virus in North America. Arch. Virol. Suppl. 2004:35-41. [DOI] [PubMed] [Google Scholar]
- 4.Beasley, D. W., L. Li, M. T. Suderman, and A. D. Barrett. 2001. West Nile virus strains differ in mouse neurovirulence and binding to mouse or human brain membrane receptor preparations. Ann. N. Y. Acad. Sci. 951:332-335. [DOI] [PubMed] [Google Scholar]
- 5.Beasley, D. W., M. C. Whiteman, S. Zhang, C. Y. Huang, B. S. Schneider, D. R. Smith, G. D. Gromowski, S. Higgs, R. M. Kinney, and A. D. Barrett. 2005. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J. Virol. 79:8339-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthet, F. X., H. G. Zeller, M. T. Drouet, J. Rauzier, J. P. Digoutte, and V. Deubel. 1997. Extensive nucleotide changes and deletions within the envelope glycoprotein gene of Euro-African West Nile viruses. J. Gen. Virol. 78(Pt 9):2293-2297. [DOI] [PubMed] [Google Scholar]
- 7.Burt, F. J., A. A. Grobbelaar, P. A. Leman, F. S. Anthony, G. V. Gibson, and R. Swanepoel. 2002. Phylogenetic relationships of southern African West Nile virus isolates. Emerg. Infect. Dis. 8:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell, G. L., A. A. Marfin, R. S. Lanciotti, and D. J. Gubler. 2002. West Nile virus. Lancet Infect. Dis. 2:519-529. [DOI] [PubMed] [Google Scholar]
- 9.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44:649-688. [DOI] [PubMed] [Google Scholar]
- 10.Charrel, R. N., A. C. Brault, P. Gallian, J. J. Lemasson, B. Murgue, S. Murri, B. Pastorino, H. Zeller, R. de Chesse, P. de Micco, and X. de Lamballerie. 2003. Evolutionary relationship between Old World West Nile virus strains. Evidence for viral gene flow between Africa, the Middle East, and Europe. Virology 315:381-388. [DOI] [PubMed] [Google Scholar]
- 11.Corver, J., A. Ortiz, S. L. Allison, J. Schalich, F. X. Heinz, and J. Wilschut. 2000. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269:37-46. [DOI] [PubMed] [Google Scholar]
- 12.Courageot, M.-P., M.-P. Frenkiel, C. D. Dos Santos, V. Deubel, and P. Desprès. 2000. α-Glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum. J. Virol. 74:564-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis, B. S., G. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebel, G. D., A. P. Dupuis II, K. Ngo, D. Nicholas, E. Kauffman, S. A. Jones, D. Young, J. Maffei, P. Y. Shi, K. Bernard, and L. D. Kramer. 2001. Partial genetic characterization of West Nile virus strains, New York State, 2000. Emerg. Infect. Dis. 7:650-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falconar, A. K. 1999. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch. Virol. 144:2313-2330. [DOI] [PubMed] [Google Scholar]
- 16.Gollins, S. W., and J. S. Porterfield. 1986. pH-dependent fusion between the flavivirus West Nile and liposomal model membranes. J. Gen. Virol. 67(Pt 1):157-166. [DOI] [PubMed] [Google Scholar]
- 17.Gruters, R. A., J. J. Neefjes, M. Tersmette, R. E. de Goede, A. Tulp, H. G. Huisman, F. Miedema, and H. L. Ploegh. 1987. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature 330:74-77. [DOI] [PubMed] [Google Scholar]
- 18.Hayes, C. G. 2001. West Nile virus: Uganda, 1937, to New York City, 1999. Ann. N. Y. Acad. Sci. 951:25-37. [DOI] [PubMed] [Google Scholar]
- 19.Heinz, F. X., and S. L. Allison. 2000. Structures and mechanisms in flavivirus fusion. Adv. Virus Res. 55:231-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinz, F. X., M. S. Collett, R. H. Purcell, E. A. Gould, C. R. Howard, M. Houghton, R. J. M. Moorman, C. M. Rice, and H.-J. Thiel. 2000. Family Flaviviridae, p. 859-878. In M. H. V. van Regenmortel, C. M. Fauquet, and D. H. L. Bishop (ed.), Virus taxonomy: classification and nomenclature of viruses. Academic Press, San Diego, Calif.
- 21.Helenius, A., E. S. Trombetta, D. N. Hebert, and J. F. Simons. 1997. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 7:193-200. [DOI] [PubMed] [Google Scholar]
- 22.Hindiyeh, M., L. M. Shulman, E. Mendelson, L. Weiss, Z. Grossman, and H. Bin. 2001. Isolation and characterization of West Nile virus from the blood of viremic patients during the 2000 outbreak in Israel. Emerg. Infect. Dis. 7:748-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt, A. R., C. B. Cropp, and G. J. Chang. 2001. A recombinant particulate antigen of Japanese encephalitis virus produced in stably-transformed cells is an effective noninfectious antigen and subunit immunogen. J. Virol. Methods 97:133-149. [DOI] [PubMed] [Google Scholar]
- 24.Jones, C. T., C. G. Patkar, and R. J. Kuhn. 2005. Construction and applications of yellow fever virus replicons. Virology 331:247-259. [DOI] [PubMed] [Google Scholar]
- 25.Khromykh, A. A., A. N. Varnavski, and E. G. Westaway. 1998. Encapsidation of the flavivirus kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J. Virol. 72:5967-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura, T., S. W. Gollins, and J. S. Porterfield. 1986. The effect of pH on the early interaction of West Nile virus with P388D1 cells. J. Gen. Virol. 67(Pt 11):2423-2433. [DOI] [PubMed] [Google Scholar]
- 27.Komar, N., S. Langevin, S. Hinten, N. Nemeth, E. Edwards, D. Hettler, B. Davis, R. Bowen, and M. Bunning. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 9:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konishi, E., and A. Fujii. 2002. Dengue type 2 virus subviral extracellular particles produced by a stably transfected mammalian cell line and their evaluation for a subunit vaccine. Vaccine 20:1058-1067. [DOI] [PubMed] [Google Scholar]
- 29.Konishi, E., and P. W. Mason. 1993. Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. J. Virol. 67:1672-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konishi, E., S. Pincus, E. Paoletti, R. E. Shope, T. Burrage, and P. W. Mason. 1992. Mice immunized with a subviral particle containing the Japanese encephalitis virus prM/M and E proteins are protected from lethal JEV infection. Virology 188:714-720. [DOI] [PubMed] [Google Scholar]
- 31.Kroeger, M. A., and P. C. McMinn. 2002. Murray Valley encephalitis virus recombinant subviral particles protect mice from lethal challenge with virulent wild-type virus. Arch. Virol. 147:1155-1172. [DOI] [PubMed] [Google Scholar]
- 32.Kuno, G., G. J. Chang, K. R. Tsuchiya, N. Karabatsos, and C. B. Cropp. 1998. Phylogeny of the genus Flavivirus. J. Virol. 72:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanciotti, R. S., G. D. Ebel, V. Deubel, A. J. Kerst, S. Murri, R. Meyer, M. Bowen, N. McKinney, W. E. Morrill, M. B. Crabtree, L. D. Kramer, and J. T. Roehrig. 2002. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology 298:96-105. [DOI] [PubMed] [Google Scholar]
- 34.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. MacKenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 36.Lee, E., R. C. Weir, and L. Dalgarno. 1997. Changes in the dengue virus major envelope protein on passaging and their localization on the three-dimensional structure of the protein. Virology 232:281-290. [DOI] [PubMed] [Google Scholar]
- 37.Lin, G., G. Simmons, S. Pohlmann, F. Baribaud, H. Ni, G. J. Leslie, B. S. Haggarty, P. Bates, D. Weissman, J. A. Hoxie, and R. W. Doms. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lobigs, M., and E. Lee. 2004. Inefficient signalase cleavage promotes efficient nucleocapsid incorporation into budding flavivirus membranes. J. Virol. 78:178-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenz, I. C., S. L. Allison, F. X. Heinz, and A. Helenius. 2002. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J. Virol. 76:5480-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenz, I. C., J. Kartenbeck, A. Mezzacasa, S. L. Allison, F. X. Heinz, and A. Helenius. 2003. Intracellular assembly and secretion of recombinant subviral particles from tick-borne encephalitis virus. J. Virol. 77:4370-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackenzie, J. M., and E. G. Westaway. 2001. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J. Virol. 75:10787-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMinn, P. C. 1997. The molecular basis of virulence of the encephalitogenic flaviviruses. J. Gen. Virol. 78(Pt 11):2711-2722. [DOI] [PubMed] [Google Scholar]
- 43.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313-319. [DOI] [PubMed] [Google Scholar]
- 44.Mukhopadhyay, S., B. S. Kim, P. R. Chipman, M. G. Rossmann, and R. J. Kuhn. 2003. Structure of West Nile virus. Science 302:248. [DOI] [PubMed] [Google Scholar]
- 45.Murgue, B., H. Zeller, and V. Deubel. 2002. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr. Top. Microbiol. Immunol. 267:195-221. [DOI] [PubMed] [Google Scholar]
- 46.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng, M. L., J. Howe, V. Sreenivasan, and J. J. Mulders. 1994. Flavivirus West Nile (Sarafend) egress at the plasma membrane. Arch. Virol. 137:303-313. [DOI] [PubMed] [Google Scholar]
- 48.Pierson, T. C., M. S. Diamond, A. A. Ahmed, L. E. Valentine, C. W. Davis, M. A. Samuel, S. L. Hanna, B. A. Puffer, and R. W. Doms. 2005. An infectious West Nile virus that expresses a GFP reporter gene. Virology 334:28-40. [DOI] [PubMed] [Google Scholar]
- 48a.Pierson, T. C., M. D. Sanchez, B. A. Puffer, A. A. Ahmed, B. J. Geiss, L. E. Valentine, L. A. Altamura, M. S. Diamond, and R. W. Doms. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology, in press. [DOI] [PubMed]
- 49.Pohlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77:4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 51.Rice, C. M. 1996. Flaviviridae: the viruses and their replication, p. 931-959. In B. N. Fields, P. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 52.Russell, P. K., W. E. Brandt, and J. M. Dalrymple. 1980. The togviruses. Academic Press, New York, N.Y.
- 53.Sanchez, M. D., T. C. Pierson, D. McAllister, S. L. Hanna, B. A. Puffer, L. E. Valentine, M. M. Murtadha, J. A. Hoxie, and R. W. Doms. 2005. Characterization of neutralizing antibodies to West Nile virus. Virology 336:70-82. [DOI] [PubMed] [Google Scholar]
- 54.Savage, H. M., C. Ceianu, G. Nicolescu, N. Karabatsos, R. Lanciotti, A. Vladimirescu, L. Laiv, A. Ungureanu, C. Romanca, and T. F. Tsai. 1999. Entomologic and avian investigations of an epidemic of West Nile fever in Romania in 1996, with serologic and molecular characterization of a virus isolate from mosquitoes. Am. J. Trop. Med. Hyg. 61:600-611. [DOI] [PubMed] [Google Scholar]
- 55.Schalich, J., S. L. Allison, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1996. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J. Virol. 70:4549-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scherret, J. H., J. S. Mackenzie, A. A. Khromykh, and R. A. Hall. 2001. Biological significance of glycosylation of the envelope protein of Kunjin virus. Ann. N. Y. Acad. Sci. 951:361-363. [DOI] [PubMed] [Google Scholar]
- 57.Schlesinger, S., A. H. Koyama, C. Malfer, S. L. Gee, and M. J. Schlesinger. 1985. The effects of inhibitors of glucosidase I on the formation of Sindbis virus. Virus Res. 2:139-149. [DOI] [PubMed] [Google Scholar]
- 58.Schlesinger, S., C. Malfer, and M. J. Schlesinger. 1984. The formation of vesicular stomatitis virus (San Juan strain) becomes temperature-sensitive when glucose residues are retained on the oligosaccharides of the glycoprotein. J. Biol. Chem. 259:7597-7601. [PubMed] [Google Scholar]
- 59.Scholle, F., Y. A. Girard, Q. Zhao, S. Higgs, and P. W. Mason. 2004. trans-Packaged West Nile virus-like particles: infectious properties in vitro and in infected mosquito vectors. J. Virol. 78:11605-11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serafino, A., M. B. Valli, A. Alessandrini, A. Ponzetto, G. Carloni, and L. Bertolini. 1997. Ultrastructural observations of viral particles within hepatitis C virus-infected human B lymphoblastoid cell line. Res. Virol. 148:153-159. [DOI] [PubMed] [Google Scholar]
- 61.Shirato, K., H. Miyoshi, A. Goto, Y. Ako, T. Ueki, H. Kariwa, and I. Takashima. 2004. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J. Gen. Virol. 85:3637-3645. [DOI] [PubMed] [Google Scholar]
- 62.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304:237-242. [DOI] [PubMed] [Google Scholar]
- 63.Stadler, K., S. L. Allison, J. Schalich, and F. X. Heinz. 1997. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 71:8475-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vorndam, V., J. H. Mathews, A. D. Barrett, J. T. Roehrig, and D. W. Trent. 1993. Molecular and biological characterization of a non-glycosylated isolate of St Louis encephalitis virus. J Gen. Virol. 74(Pt 12):2653-2660. [DOI] [PubMed] [Google Scholar]
- 66.Walker, B. D., M. Kowalski, W. C. Goh, K. Kozarsky, M. Krieger, C. Rosen, L. Rohrschneider, W. A. Haseltine, and J. Sodroski. 1987. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc. Natl. Acad. Sci. USA 84:8120-8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu, S.-F., C.-J. Lee, C.-L. Liao, R. A. Dwek, N. Zitzmann, and Y.-L. Lin. 2002. Antiviral effects of an iminosugar derivative on flavivirus infections. J. Virol. 76:3596-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamshchikov, V. F., G. Wengler, A. A. Perelygin, M. A. Brinton, and R. W. Compans. 2001. An infectious clone of the West Nile flavivirus. Virology 281:294-304. [DOI] [PubMed] [Google Scholar]