Abstract
Vesicular stomatitis virus (VSV) is a negative-strand RNA virus with intrinsic oncolytic specificity due to substantially attenuated antiviral responses in many tumors. We have recently reported that recombinant VSV vector can be used as an effective oncolytic agent to safely treat multifocal hepatocellular carcinoma (HCC) in the livers of immune-competent rats via hepatic artery infusion. When administered at doses above the maximum tolerated dose (MTD), however, the animals suffered from neurotoxicity and/or acute lethal hepatotoxicity. Since VSV is extremely sensitive to the antiviral actions of alpha/beta interferon (IFN-α/β) in normal cells, we tested if prophylactic treatment with rat IFN-α would enhance VSV safety without compromising treatment efficacy in tumor-bearing rats. We found that VSV retained its replication potential in human and rat HCC cells after preincubation with relatively high doses of rat and human IFN-α in vitro, and its MTD in tumor-bearing rats treated systemically with rat IFN-α at 66 IU/g body weight (BW), equivalent to a human IFN-α dose that is currently prescribed for patients with viral hepatitis, was elevated by more than 1/2 log unit. Furthermore, we demonstrate that intratumoral replication of VSV was not attenuated by administration of 66 IU/g BW rat IFN-α, as tumor response and survival advantage in VSV-treated rats in the presence or absence of rat IFN-α were equivalent. The results suggest that prophylactic rat IFN-α treatment elevates the therapeutic index of hepatic arterial VSV therapy for multifocal HCC in rats. Since human IFN-α is currently in clinical use, its prophylactic application should be considered in future clinical translational protocols for VSV-mediated oncolytic virotherapy as a novel therapeutic modality in patients with advanced HCC, as well as other types of cancer.
Oncolytic viruses provide an attractive new tool for treatment of solid cancers because of their abilities to replicate selectively within the tumor and kill neighboring cancer cells upon tumor lysis and secondary infection (20). Vesicular stomatitis virus (VSV) is a negative-strand RNA virus with potent oncolytic properties that is exquisitely sensitive to the antiviral actions of alpha/beta interferons (IFN-α/β) in normal but not in cancer cells (3, 35). This finding has been postulated to be due to the fact that IFN-responsive antiviral pathways, e.g., double-stranded RNA-dependent protein kinase and upstream mediators of eIF2α, are defective in many types of tumors (3, 4, 5, 35). This is in contrast to normal cells, which have an intact IFN system and therefore are able to induce a robust antiviral response (9). Most wild-type strains of VSV, however, are relatively poor inducers of IFN-α/β (21). This is related to the VSV matrix (M) protein, which is capable of inhibiting host gene expression at the level of transcription (1, 13), as well as nuclear-cytoplasmic transport of host mRNAs and protein (28, 38). This inhibition suppresses the production of IFN-α/β and other antiviral proteins in infected cells (1, 36).
IFN-α/β represent an important line of defense against viral infections. This has been exemplified by the high susceptibility to viral infection of mice genetically deficient in some critical components of the IFN system, such as IFNAR (IFN-α receptor) or STAT1 knockout mice (7, 9, 15, 18, 23, 25, 32). Specifically, STAT1−/− mice, lacking a key transcription factor required for IFN signaling, were found to be highly sensitive to infection and lethality by VSV (9, 23). This sensitivity correlated with high VSV titers in the livers of infected STAT1−/− mice, leading to hepatocyte necrosis due to unchecked replication of the virus in the absence of an IFN response (9). Earlier studies demonstrated the feasibility of isolating VSV mutants with strong IFN-inducing phenotypes (14), with many of these mutants containing point mutations in their M proteins, including the M51R mutation originally found in the ts082 and T1026R1 mutant viruses (8). Recently, VSV M mutants with single-amino-acid (M51R) or two-amino-acid (V221F and S226R) substitutions were reported to be significantly attenuated in mice because of their potent induction of IFN-α/β, which can establish an antiviral state in the recipient animal that protects against toxicities associated with infection of healthy cells but retains antitumor activity in several animal tumor models (36). In an alternative approach, Obuchi et al. created a recombinant VSV vector expressing the IFN-β gene with enhanced selectivity for tumor cells (26).
Our group previously described the effective use of recombinant VSV vector as an oncolytic agent to treat hepatocellular carcinoma (HCC) in the livers of immune-competent rats (10). We demonstrated that VSV, administered at the maximum tolerated dose (MTD) via the hepatic artery, could gain access to and selectively replicate in multifocal HCC tumors of various sizes, leading to massive tumor necrosis and significantly prolonged survival (33). To improve treatment outcome, we recently showed that repeated administrations of recombinant VSV-F (rVSV-F) (11), which is an engineered VSV expressing a fusogenic membrane glycoprotein from a heterologous virus through a permanent cannula surgically implanted into the hepatic artery, led to sustained tumor-selective virus replication and substantially enhanced its oncolytic potential in the treatment of advanced multifocal HCC in the livers of rats (34). Here, we demonstrate that VSV, when administered at doses above the MTD, caused neurotoxicity as manifested by limb paralysis and/or acute lethal hepatotoxicity in immune-competent rats. While VSV infections are well known to be lethal in nude mice (17), prophylactic IFN treatment can rescue immune-compromised animals (35). In the study, we tested the hypothesis that the MTD of rVSV-F could be elevated in immune-competent rats by exogenous administration of rat IFN-α at a clinically relevant dose while treatment efficacy against multifocal HCC would not be compromised in these animals.
MATERIALS AND METHODS
Cell culture.
Hep3B, HepG2, McA-RH7777, and BHK-21 cell lines were obtained from the American Type Culture Collection (Manassas, VA). The human HCC cell lines Hep3B and HepG2 were maintained in minimal essential medium-Eagle (Mediatech, Herndon, VA) and supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO). The rat HCC cell line McA-RH7777 and BHK-21 cells were maintained in 10% FBS-Dulbecco modified Eagle medium (DMEM) (Mediatech). All media used in this study contained 100 U/ml penicillin-streptomycin (Mediatech).
Virus generation and replication assays.
rVSV vectors expressing green fluorescent protein (rVSV-GFP) or a mutant form (L289A) of the F protein from Newcastle disease virus that is fusogenic without the viral H protein (rVSV-F) have been described previously (10, 11). Viral titers of working stocks were determined on BHK-21 cells by using standard plaque assays. The resulting titers for VSV-F and VSV-GFP were 2.3 × 108 PFU/ml and 8.4 × 108 PFU/ml, respectively.
Human and rat HCC cells were plated in six-well plates at 105 cells/well, preincubated with various concentrations of recombinant human or rat IFN-α (PBL, New Brunswick, NJ) overnight, and infected with rVSV-GFP (multiplicity of infection [MOI], 0.01) the following day. After infection at room temperature for 30 min, the cells were washed twice with PBS to remove any unabsorbed virus, and complete medium was added. At the indicated time points after infection, 150 μl of supernatant was removed and assayed for viral RNA genome by real-time reverse transcription (RT)-PCR using specific primers as described previously (10).
Animal studies.
Inbred male Buffalo rats (7 to 8 weeks old) were purchased from Harlan (Indianapolis, IN) and housed in a specific-pathogen-free environment under standard conditions. All procedures involving animals were approved by and performed according to guidelines of the Institutional Animal Care and Use Committee of the Mount Sinai School of Medicine. In order to establish multifocal HCC lesions within the liver, the rats were anesthetized with 100 mg/kg of body weight (BW) ketamine (intraperitoneal), 1 mg/kg xylazine (intraperitoneal), and isoflurane using an inhalation anesthesia system (VetEquip, Pleasanton, CA) and subsequently injected with 107 syngeneic McA-RH7777 rat HCC cells in 1 ml of DMEM via the portal vein. Twenty-one days after tumor cell infusion, the animals were anesthetized and underwent laparotomy to assess the presence of multiple tumor lesions macroscopically visible on the liver surface.
The Preclinical Mini-Port implantable access device (Deltec, St. Paul, MN) was used to administer the vector repeatedly via the hepatic artery as described previously (34). Rats were infused either with rVSV-F every other day for 4 days (three injections total) in 1 ml of phosphate-buffered saline (PBS) or with an equivalent volume of buffer over 15 seconds with blocking of the common hepatic artery to prevent backflow into the aorta. Recombinant rat IFN-α at 66 IU/g BW per dose was administered intravenously at the indicated time points. To evaluate the kinetics of viral replication within the tumor lesions, sets of animals were sacrificed at various time points after hepatic arterial infusion of the vector. Tissue samples were obtained using an operating microscope and subjected to plaque assays to determine the viral yield (10). Finally, groups of tumor-bearing animals infused with either rVSV-F alone, rVSV-F plus IFN-α combination, or PBS buffer control were followed for survival, which was checked daily in all animals.
Histology and immunohistochemical staining.
At the indicated time points after vector infusion into the hepatic artery, animals were sacrificed and various major organs (brain, spinal cord, liver, heart, kidneys, and lungs) were fixed in 4% paraformaldehyde overnight and then paraffin embedded. Thin sections (5 μm) were subjected to either hematoxylin and eosin (HE) staining for histological analysis or immunohistochemistry using a monoclonal antibody against the VSV glycoprotein (G) (VSV11-M; Alpha Diagnostic, San Antonio, TX). Immunohistochemistry sections were counterstained with hematoxylin.
Assessment of serum transaminases.
Blood samples were collected from the inferior vena cava at the time of euthanization or before virus injection, and the levels of alanine transferase (ALT) were determined at the Chemistry Laboratory at Mount Sinai School of Medicine.
Assessment of serum cytokine levels.
Blood samples were collected from the retro-orbital vein on day 1 after vector injection, and the levels of rat serum cytokines (tumor necrosis factor alpha [TNF-α], interleukin-6 [IL-6], IFN-γ, and IL-1β) were determined by enzyme-linked immunosorbent assays (Biosource, Camarillo, CA).
Statistical analyses.
Survival curves of animals were plotted according to the Kaplan-Meier method. Statistical significances in different treatment groups were compared using the log-rank test. Results and graphs were obtained using the GraphPad Prism 3.0 program (GraphPad Software, San Diego, CA).
RESULTS
VSV administered via the hepatic artery at doses above the MTD cause neurotoxicity and acute lethal hepatotoxicity in immune-competent rats.
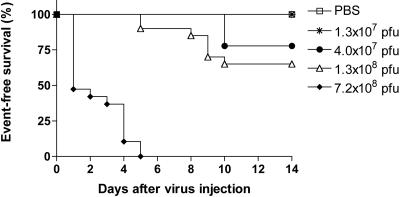
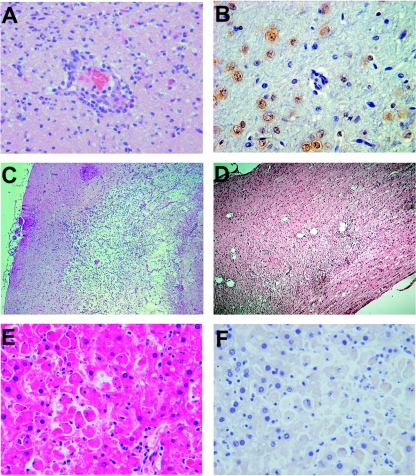
First, the MTD of VSV administered into the hepatic artery of normal Buffalo rats was determined. Recombinant VSV-GFP in half-log dose increments was infused into healthy animals. It was noted that when VSV was administered at doses that were 1/2 to 1 log unit above the MTD (1.3 × 107 PFU), animals showed clinical signs of neurotoxicity, including altered consciousness, excitability, and limb paralysis, which occurred four and more days after virus infusion (Fig. 1). The rats with signs of neurotoxicity were sacrificed, and the brain and spinal cord were subjected to histopathological analysis (Fig. 2A to D). Neuronal necrosis and inflammatory mononuclear cell infiltrates primarily located around the brain vessels (perivascular cuffing) were clearly evident within the central nervous system (Fig. 2A), and some animals also showed histological signs of meningitis. Immunohistochemistry for VSV-G protein confirmed the presence of positive foci within the choroid plexus, glial cells, and neurons (Fig. 2B). Animals that were sacrificed at 10 days post-virus infusion showed histological signs of neuronophagia and inflammatory demyelination within the brain (Fig. 2C), as well as the spinal cord (Fig. 2D). These findings confirmed that VSV, administered via the hepatic artery at doses above the MTD, was able to reach, infect, and replicate to a certain degree within the central nervous system, causing the histological picture of viral encephalomyelitis.
FIG. 1.
Dose escalation study and determination of MTD in healthy Buffalo rats after hepatic artery infusion of VSV. The animals were infused with rVSV-GFP at the indicated vector doses and monitored for the occurrence of toxic events and survival over a 14-day period. Animals showing toxic events were sacrificed, and the major organs, including brain, spinal cord, and liver, were subjected to histopathological analysis.
FIG. 2.
Neuro- and hepatotoxicity in healthy Buffalo rats treated with rVSV-GFP above the MTD (1.3 × 107 PFU). Histopathological analysis and immunohistochemistry for VSV G protein of representative sections of the brain (A to C), spinal cord (D), and liver (E and F) obtained from animals infused with rVSV-GFP at doses above the MTD (original magnifications, ×10 [C and D] and ×40 [A, B, E, and F]).
After infusion of 7.2 × 108 PFU of VSV (>50 times the MTD), a significant fraction of rats died within the first 3 days (Fig. 1), and clinically these animals appeared icteric but showed no signs of hepatomegaly or ascites. HE analysis of the liver showed patchy multifocal hepatocyte necrosis with minimal inflammatory-cell infiltrations, which was apparent throughout 50 to 80% of the liver (Fig. 2E). There were no remarkable abnormalities in the heart, kidneys, and lungs (data not shown). These findings suggest that the animals died of acute liver failure. Interestingly, VSV-G immunostaining in the liver was negative (Fig. 2F), suggesting that hepatotoxicity might not be a direct result of viral replication in the liver.
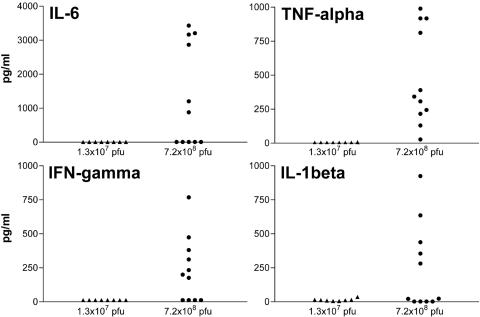
To assess systemic proinflammatory responses elicited by VSV after hepatic arterial administration, the serum levels of proinflammatory cytokines (TNF-α, IFN-γ, IL-6, and IL-1β) were determined 1 day after virus infusion (Fig. 3). When VSV was administered at 7.2 × 108 PFU, there were significant elevations of proinflammatory cytokines. In contrast, the proinflammatory cytokine levels in the VSV-injected animals at the MTD were well below concentrations associated with systemic toxicity in animals and in human clinical trials (29, 33). The results indicated that hepatic arterial administration of VSV at doses much higher than its MTD induced a systemic proinflammatory cytokine response in immune-competent rats, which might be a contributing factor to development of acute lethal hepatotoxicity.
FIG. 3.
Serum proinflammatory cytokine profile in healthy Buffalo rats after hepatic arterial infusion of VSV. The animals were injected with 1.3 × 107 PFU rVSV-GFP (n = 8), maximum tolerated dose, or 7.2 × 108 PFU rVSV-GFP (n = 11), and blood samples were collected from the retro-orbital vein 1 day later. Serum levels of a panel of proinflammatory rat cytokines (IL-6, TNF-α, IL-1β, and IFN-γ) were determined using commercially available enzyme-linked immunosorbent assays.
VSV retains its replication potential in human and rat HCC cells preincubated with IFN-α in vitro.
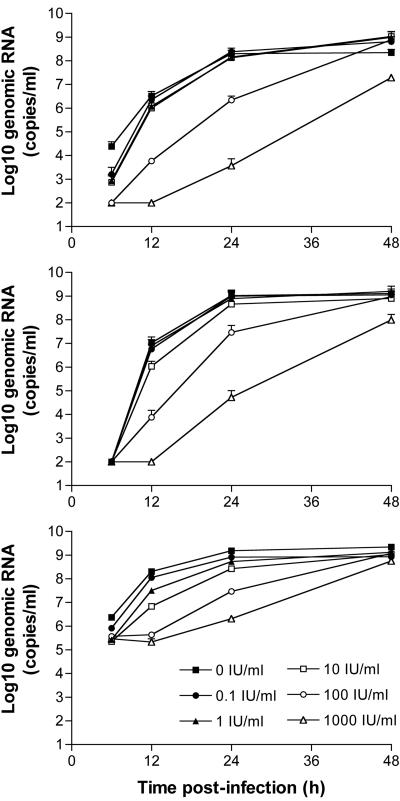
To evaluate the replication potential of VSV in the presence of various concentrations of IFN-α in HCC cells in vitro, the rat (McA-RH7777) and human (Hep3B and HepG2) HCC cell lines were examined in virus replication assays (Fig. 4). Cells were preincubated with rat or human IFN-α overnight and then infected with rVSV-GFP at a low MOI of 0.01. The supernatants were harvested at various time points postinfection, total RNAs from the cell culture supernatants were prepared, and the RNA samples were analyzed for the presence and concentrations of genomic VSV RNA by real-time RT-PCR. The results showed that VSV replication in rat and human HCC cells was not attenuated by IFN-α concentrations of up to 10 IU/ml. The replication kinetics of VSV in HCC cells preincubated with 100 IU/ml IFN-α appeared to be slightly delayed but reached similar titers at 48 h after infection. At 1,000 IU/ml, rat and human IFN-α VSV replication was clearly attenuated in McA-RH7777 and Hep3B cells, respectively, while the virus could still replicate to high levels with rapid kinetics in HepG2 cells, indicating that the latter cell line was unresponsive to the antiviral activity of human IFN-α. Therefore, VSV retained its replication potential in human and rat HCC cells in vitro after preincubation with relatively high doses of IFN-α.
FIG. 4.
Multicycle growth curves of VSV in human and rat HCC cells treated with IFN-α. McA-RH7777 (top), Hep3B (middle), and HepG2 (bottom) cells were preincubated with various concentrations of rat or human IFN-α overnight and then infected with rVSV-GFP at an MOI of 0.01. Aliquots of tissue culture supernatants were collected at the indicated time points, and viral genomic RNA was determined by real-time RT-PCR, which has been shown to be equivalent to PFU titers in BHK-21 cells. Results from two independent experiments performed in triplicate (mean ± standard deviation) are shown. Error bars are included in all three graphs, although some of them are within the symbols and not visible in the semilog plots.
The MTD of rVSV-F administered via the hepatic artery combined with prophylactic rat IFN-α treatment in healthy rats is significantly elevated.
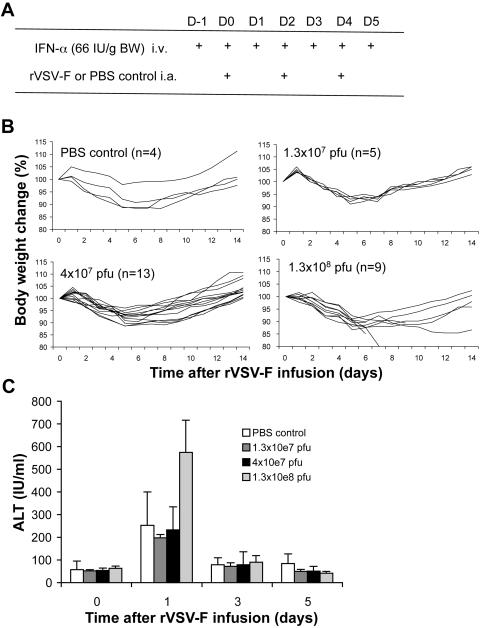
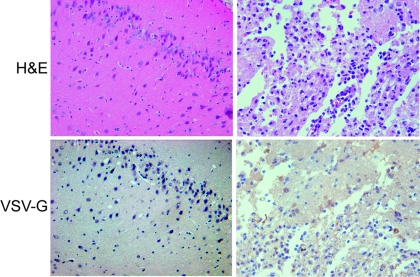
Since VSV is extremely sensitive to the antiviral actions of IFN-α/β in normal cells (6), we tested if the use of exogenous IFN-α would significantly elevate its MTD in rats. We recently reported that repeated (three times) administration of rVSV-F via the hepatic artery led to significantly improved survival in HCC-bearing rats (34), and the MTD of VSV in rats with prophylactic rat IFN-α treatment was determined using this regimen. Healthy Buffalo rats were infused via the hepatic artery with PBS control or rVSV-F in half-log dose increments in the presence of daily intravenous injections of rat IFN-α at 66 IU/g BW, which is equivalent to a dose that is currently prescribed for patients with viral hepatitis (27), and the animals were monitored for the occurrence of toxic events and for survival (Fig. 5A). The highest dose of rVSV-F with rat IFN-α that resulted in no vector-associated toxicities was determined to be 4 × 107 PFU/injection (total virus dose, 1.2 × 108 PFU), while the MTD of a single administration of VSV without prophylactic rat IFN-α treatment was 1.3 × 107 PFU (Fig. 1). Even with prophylactic IFN-α treatment, animals that received (three times) rVSV-F administration at doses above 4 × 107 PFU/injection showed signs of toxicity, including severe body weight loss, limb paralysis and death (Fig. 5B). Histological examination of the brains and spinal cords of animals treated with rVSV-F at 4 × 107 PFU/injection without prophylactic rat IFN-α treatment confirmed the presence of necrotic areas and VSV-G-positive foci by immunohistochemistry, which were not observed in the VSV-injected animals with prophylactic rat IFN-α treatment (Fig. 6). In addition, we evaluated ALT levels in these animals as a sensitive measure of hepatotoxicity. While there were no significant ALT elevations in rats treated with rVSV-F at doses of up to 4 × 107 PFU/injection with prophylactic rat IFN-α treatment compared with PBS control-treated animals (Fig. 5C), rats treated with higher VSV doses (1.3 × 108 PFU/injection) showed hepatotoxicity with ALT levels of up to 750 IU/ml. The results indicated that the MTD of rVSV-F in healthy Buffalo rats treated with 66 IU/g BW/day of rat IFN-α was elevated by at least 1/2 log unit (from 1.3 × 107 PFU/injection to 4 × 107 PFU/injection).
FIG. 5.
Determination of the MTD of repeated hepatic arterial infusions of rVSV-F vector with prophylactic rat IFN-α treatment in healthy Buffalo rats. (A) Treatment schema. Recombinant VSV-F or PBS control was repeatedly (three times) infused into the hepatic artery of rats using the Mini-Port implantable access device in combination with daily intravenous (i.v.) injections of 66 IU/g BW rat IFN-α. i.a., intra-arterial. (B) Rats treated with rat IFN-α were randomly assigned to infusions with PBS (n = 4) or rVSV-F at the following doses: 1.3 × 107 PFU (n = 5), 4 × 107 PFU (n = 13), and 1.3 × 108 PFU (n = 9). The animals were monitored over a period of 14 days for event-free survival and for weight changes. (C) Serum ALT levels (mean ± standard deviation) obtained from the same animals before (time point 0), and 1, 3, and 5 days after infusions with various doses of rVSV-F or PBS. The error bars indicate standard deviations.
FIG. 6.
Representative photomicrographs of rat brains from animals at day 14 after injection with 4 × 107 PFU of rVSV-F with (left) and without (right) prophylactic rat IFN-α treatment. Top panels, H&E staining; bottom panels, VSV-G immunohistochemical staining; magnification, ×40. The left panels show intact brain tissue without evidence of virus-induced necrosis. The right panels show a region of virus-induced necrosis with positive immunohistochemical staining for VSV-G in several cells within the necrotic focus.
Intratumoral replication and treatment efficacy of rVSV-F are not attenuated in multifocal HCC-bearing rats by IFN-α treatment.
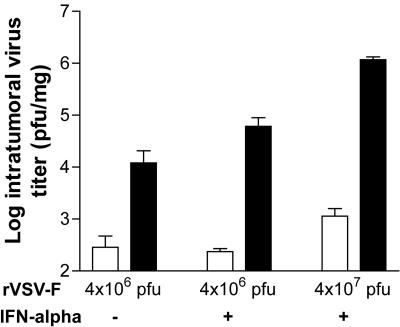
To evaluate the extents of viral replication within the tumors after hepatic artery infusion of rVSV-F in the presence of IFN-α, sets of multifocal HCC-bearing animals were sacrificed at 30 min and 1 day after a single administration of the vector (Fig. 7). When vector was administered at 4 × 106 PFU with or without IFN-α at 66 IU/g BW, infectious intratumoral viral yields similarly increased by at least 100-fold at 1 day versus 30 min post-vector injection. At 4 × 107 PFU rVSV-F in the presence of rat IFN-α, there was more than a 1,000-fold increase in infectious-virus yield in the HCC lesions from 30 min to 1 day post-vector infusion. Therefore, our results clearly demonstrate that intratumoral rVSV-F replication was not attenuated by exogenous administrations of rat IFN-α at 66 IU/g BW.
FIG. 7.
Quantification of intratumoral VSV replication in the presence or absence of IFN-α treatment. Viral titers (PFU/mg) in tumor samples obtained from multifocal HCC-bearing animals at 30 min (open bars) and 1 day (black bars) after a single hepatic arterial infusion of rVSV-F at various doses with or without intravenous injections of rat IFN-α at 66 IU/g BW on days −1 and 0 (mean plus standard deviation; n = 3/time point). Tumor lesions were obtained for infectious-virus extraction, and the samples were analyzed by standard plaque assay (detection limit, 100 PFU/mg tissue [wet weight]).
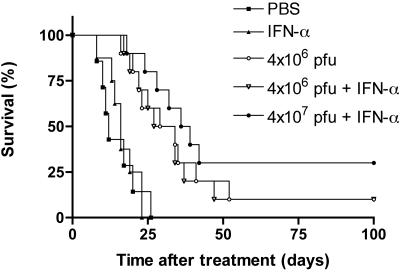
Our final set of experiments was designed to test the effect of IFN-α on the treatment efficacy of rVSV-F in our rat model of multifocal HCC. Rats bearing 5 to 10 visible HCC tumors in their livers with sizes ranging from 1 to 10 mm in diameter were randomly assigned to repeated infusions with rVSV-F via the hepatic artery at 4 × 106 PFU/injection with (n = 10) or without (n = 10) 66 IU/g BW/day IFN-α, 4 × 107 PFU/injection with 66 IU/g BW/day IFN-α (n = 10), 66 IU/g BW/day IFN-α (n = 8), or PBS control (n = 7), and survival was followed (Fig. 8). Buffer- or IFN-α-treated rats started to die of tumor progression at 8 days after initiation of the treatment, with all control animals deceased by 26 days (median survival, 12 and 16 days, respectively). There was no statistically significant difference between PBS- and rat IFN-α-treated animals (P = 0.82). The median survival of rats treated with rVSV-F at 4 × 106 PFU/injection with or without rat IFN-α was extended to 30.5 and 31.5 days, and the differential survival rates compared to PBS- or rat IFN-α-treated control animals were statistically significant by log-rank test analysis (P = 0.001). At this dose level, there was no statistically significant difference between rVSV-F- and rVSV-F plus rat IFN-α-treated rats (P = 0.86). The median survival in the animal group treated with 4 × 107 PFU/injection with IFN-α at 66 IU/g BW/day further increased to 37.5 days (P = 0.0001 versus control), and three out of 10 animals survived for more than 100 days. Although there is a trend toward better efficacy when comparing the survival curves of tumor-bearing animals treated with 4 × 107 PFU/injection versus 4 × 106 PFU/injection of rVSV-F with rat IFN-α, statistically significant difference was not reached and more animals will be needed to determine if that is indeed the case.
FIG. 8.
Kaplan-Meier survival curve of Buffalo rats with multifocal HCC after repeated hepatic arterial infusions of rVSV-F with IFN-α treatment. Tumor-bearing rats were repeatedly infused with 4 × 106 PFU VSV-F with (n = 10) or without 66 IU/g BW/day IFN-α (n = 10), 4 × 107 PFU VSV-F with 66 IU/g BW/day IFN-α (n = 10), 66 IU/g BW/day IFN-α (n = 8), or PBS control (n = 7) via the hepatic artery on day zero and were followed for survival. The results from three consecutive sets of animals were combined, with stratification.
The long-term-surviving rats were sacrificed at 100 days after treatment and evaluated for residual malignancy. No tumor was visible macroscopically on the liver surface or elsewhere. Histologically, there was also no evidence of residual tumor cells or hepatitis. The results indicated that the large multifocal tumors (up to 10 mm in diameter at the time of first virus injection) had undergone complete remission in these animals, which enjoyed long-term and tumor-free survival.
DISCUSSION
Laboratory strains of VSV have little if any pathogenicity for healthy adult humans (30). However, the potential for VSV to cause disease in cancer patients who may be immune compromised has not been explored. Previous studies testing the effectiveness of VSV as an oncolytic virus have shown that immune-compromised mice are highly susceptible to lethal infections with VSV (35). Other studies also suggested that VSV, when inoculated intranasally in immune-competent mice and rats, can infect the brain and cause lethal encephalitis (2, 17, 24, 37). Other routes of infection in mice, such as the intraperitoneal or intravenous routes, have previously been reported to induce antiviral immunity without significant pathogenesis, even when high doses of virus were inoculated (16, 31).
Previous data suggested that recombinant VSV vectors are much more attenuated than the wild-type virus (12, 26). When we administered recombinant VSV via the hepatic artery, we were surprised by toxicities in our immune-competent rat model. At doses above the MTD, the animals suffered from severe neurotoxicity, as manifested by altered consciousness, excitability, limb paralysis, and death. Histologically, focal areas of neuronal necrosis were found within the brain and spinal cord that were positive for VSV-G by immunohistochemistry, confirming the diagnosis of viral encephalomyelitis. In addition, when administered at the highest dose level, VSV also induced acute lethal hepatotoxicity. Since no VSV antigen could be detected in the normal liver, the results suggest that the observed hepatotoxicity was not a direct result of intrahepatic VSV replication, which is consistent with our previous results demonstrating that normal rat and human hepatocytes are nonpermissive for VSV growth in vitro (10). However, we also observed a systemic proinflammatory cytokine response in the rats treated with high-dose VSV, which could subsequently lead to hepatocyte necrosis in these animals. As a consequence, patients treated with VSV in future clinical trials should be closely monitored not only for neurotoxic events, but also for elevations of proinflammatory cytokines that could lead to liver damage or fulminant liver failure in extreme cases.
To improve the safety of VSV-based oncolytic virotherapy, we explored the potential usefulness of prophylactic IFN-α treatment. It is well known that VSV is extremely sensitive to the antiviral actions of IFN-α/β in normal cells (6), potentially providing an effective tool to suppress unwanted replication in normal tissues. In addition, recombinant human IFN-α is an approved drug that would be readily applicable in the clinic. Indeed, we found that exogenous IFN-α administration, at an equivalent dose that that is currently prescribed for patients with viral hepatitis (27), elevates the MTD of VSV in rats by at least 1/2 log unit. We further demonstrated that VSV can replicate to high levels in human and rat HCC cells in vitro after preincubation with relatively high doses of IFN-α. These findings confirm previous studies showing that several human HCC cell lines have an intrinsically poor ability to produce and respond to IFN-α/β (19, 22). Intratumoral replication of VSV was not attenuated in multifocal-HCC-bearing rats, as tumor response and survival advantage in the treated animals were essentially identical in the presence or absence of IFN-α. Taken together, the results suggest that there was a significant enhancement of the therapeutic index in tumor-bearing rats by prophylactic administration of IFN-α. Since neurotoxicity is a common feature among other RNA viruses that are being developed as oncolytic agents (39), prophylactic IFN-α treatment might be applicable for those vectors as well. In conclusion, this treatment regimen should be considered in future clinical translational protocols for VSV-mediated virotherapy as a novel therapeutic modality for patients with advanced HCC, as well as other types of cancer.
Acknowledgments
We thank Tian-Gui Huang for discussions and Sonal Harbaran and Jing Xu for their excellent technical assistance.
Support was received from the National Institutes of Health (Grant CA100830) (S. L. C. Woo) and Deutsche Forschungsgemeinschaft (Grant EB 234/1-1) (O. Ebert).
REFERENCES
- 1.Ahmed, M., M. O. McKenzie, S. Puckett, et al. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, T., A. K. Mohammed, B. G. Henriksson, et al. 1993. Immunohistochemical and behaviour pharmacological analysis of rats inoculated intranasally with vesicular stomatitis virus. J Chem. Neuroanat. 6:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balachandran, S., and G. N. Barber. 2000. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 50:135-138. [DOI] [PubMed] [Google Scholar]
- 4.Balachandran, S., M. Porosnicu, and G. N. Barber. 2001. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J. Virol. 75:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balachandran, S., and G. N. Barber. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5:51-65. [DOI] [PubMed] [Google Scholar]
- 6.Belkowski, L. S., and G. C. Sen. 1987. Inhibition of vesicular stomatitis viral mRNA synthesis by interferons. J. Virol. 61:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray, M. 2001. The role of the type I interferon response in the resistance of mice to filovirus infection. J. Gen. Virol. 82:1365-1373. [DOI] [PubMed] [Google Scholar]
- 8.Desforges, M., J. Charron, S. Berard, et al. 2001. Different host-cell shutoff strategies related to the matrix protein lead to persistence of vesicular stomatitis virus mutants on fibroblast cells. Virus Res. 76:87-102. [DOI] [PubMed] [Google Scholar]
- 9.Durbin, J. E., R. Hackenmiller, M. C. Simon, et al. 1996. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84:443-450. [DOI] [PubMed] [Google Scholar]
- 10.Ebert, O., K. Shinozaki, T. G. Huang, et al. 2003. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 63:3605-3611. [PubMed] [Google Scholar]
- 11.Ebert, O., K. Shinozaki, C. Kournioti, et al. 2004. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 64:3265-3270. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez, M., M. Porosnicu, D. Markovic, et al. 2002. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J. Virol. 76:895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferran, M. C., and J. M. Lucas-Lenard. 1997. The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. J. Virol. 71:371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francoeur, A. M., L. Poliquin, and C. P. Stanners. 1987. The isolation of interferon-inducing mutants of vesicular stomatitis virus with altered viral P function for the inhibition of total protein synthesis. Virology 160:236-245. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Sastre, A., R. K. Durbin, H. Zheng, et al. 1998. The role of interferon in influenza virus tissue tropism. J. Virol. 72:8550-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gobet, R., A. Cerny, E. Ruedi, et al. 1988. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp. Cell Biol. 56:175-180. [DOI] [PubMed] [Google Scholar]
- 17.Huneycutt, B. S., Z. Bi, C. J. Aoki, et al. 1993. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J. Virol. 67:6698-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, A. J., and J. T. Roehrig. 1999. New mouse model for dengue virus vaccine testing. J. Virol. 73:783-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keskinen, P., M. Nyqvist, T. Sareneva, et al. 1999. Impaired antiviral response in human hepatoma cells. Virology 263:364-375. [DOI] [PubMed] [Google Scholar]
- 20.Kirn, D., R. L. Martuza, and J. Zwiebel. 2001. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat. Med. 7:781-787. [DOI] [PubMed] [Google Scholar]
- 21.Marcus, P. I., L. L. Rodriguez, and M. J. Sekellick. 1998. Interferon induction as a quasispecies marker of vesicular stomatitis virus populations. J. Virol. 72:542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melen, K., P. Keskinen, A. Lehtonen, et al. 2000. Interferon-induced gene expression and signaling in human hepatoma cell lines. J. Hepatol. 33:764-772. [DOI] [PubMed] [Google Scholar]
- 23.Meraz, M. A., J. M. White, K. C. Sheehan, et al. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84:431-442. [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi, K., D. H. Harter, and K. C. Hsu 1971. Neuropathological and immunofluorescence studies of experimental vesicular stomatitis virus encephalitis in mice. J. Neuropathol. Exp. Neurol. 30:266-277. [DOI] [PubMed] [Google Scholar]
- 25.Mrkic, B., J. Pavlovic, T. Rulicke, et al. 1998. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 72:7420-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obuchi, M., M. Fernandez, and G. N. Barber. 2003. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 77:8843-8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrillo, R. P. 2004. Overview of treatment of hepatitis B: key approaches and clinical challenges. Semin. Liver Dis. 24:23-29. [DOI] [PubMed] [Google Scholar]
- 28.Petersen, J. M., L. S. Her, V. Varvel, et al. 2000. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol. 20:8590-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid, T., E. Galanis, J. Abbruzzese, et al. 2002. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 62:6070-6079. [PubMed] [Google Scholar]
- 30.Richmond, J. Y., and S. L. Nesby-O'Dell. 2002. Laboratory security and emergency response guidance for laboratories working with select agents. Morb. Mortal. Wkly. Rep. Recomm. Rep. 51:1-6. [PubMed] [Google Scholar]
- 31.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1242. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 32.Ryman, K. D., W. B. Klimstra, K. B. Nguyen, et al. 2000. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 74:3366-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinozaki, K., O. Ebert, C. Kournioti, et al. 2004. Oncolysis of multifocal hepatocellular carcinoma in the rat liver by hepatic artery infusion of vesicular stomatitis virus. Mol. Ther. 9:368-376. [DOI] [PubMed] [Google Scholar]
- 34.Shinozaki, K., O. Ebert, and S. L. Woo. 2004. Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV. Hepatology 41:196-203. [DOI] [PubMed] [Google Scholar]
- 35.Stojdl, D. F., B. Lichty, S. Knowles, et al. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821-825. [DOI] [PubMed] [Google Scholar]
- 36.Stojdl, D. F., B. D. Lichty, B. R. tenOever, et al. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263-275. [DOI] [PubMed] [Google Scholar]
- 37.van den Pol, A. N., K. P. Dalton, and J. K. Rose. 2002. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J. Virol. 76:1309-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Kobbe, C., J. M. van Deursen, J. P. Rodrigues, et al. 2000. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell 6:1243-1252. [DOI] [PubMed] [Google Scholar]
- 39.Yang, W. Q., X. Lun, C. A. Palmer, et al. 2004. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin. Cancer Res. 10:8561-8576. [DOI] [PubMed] [Google Scholar]