Abstract
Human immunodeficiency virus type 1 viral protein R (Vpr) is required for viral pathogenesis and has been implicated in T-cell apoptosis through its activation of caspase 3 and caspase 9 and perturbation of mitochondrial membrane potential. To understand better Vpr-mitochondria interaction, we report here the identification of antiapoptotic mitochondrial protein HAX-1 as a novel Vpr target. We show that Vpr and HAX-1 physically associate with each other. Overexpression of Vpr in cells dislocates HAX-1 from its normal residence in mitochondria and creates mitochondrion instability and cell death. Conversely, overexpression of HAX-1 suppressed the proapoptotic activity of Vpr.
Apoptosis or programmed cell death contributes to the elimination of damaged, aged, or virus-infected cells (13). Apoptosis can be initiated by an extrinsic pathway in which death receptors expressed at the cell surface trigger receptor-activation of caspases leading to mitochondrial membrane permeabilization (MMP) (6, 18, 42). Alternatively, a cell-intrinsic apoptotic pathway can act directly on mitochondria, leading first to mitochondrial membrane permeabilization and then activation of execution caspases (6, 18, 30, 42). MMP is tightly regulated by Bcl2 family proteins which contain both pro- and antiapoptotic members (2, 5, 29). Once triggered, MMP marks the “point of no return” for the apoptotic process (29, 58), whether it be a caspase-dependent or caspase-independent death (26, 39, 50). Because apoptosis is used as a means by the host to defend against invading pathogens, viruses understandably have evolved strategies that target the intrinsic and extrinsic apoptotic pathways. Increasingly, examples illustrate that many viruses, including human immunodeficiency virus type 1 (HIV-1), hepatitis B virus, Sindbis virus, and baculovirus, encode proteins that modulate cell death (reviewed in reference 4).
Human immunodeficiency virus (HIV) principally infects T helper (TH) cells and cells of the monocyte-macrophage lineage, which express the CD4 cell surface protein. The gradual and selective loss of the CD4 subset of T-lymphocytes is a central feature of the pathogenesis of HIV which correlates with the progression from asymptomatic HIV infection to AIDS. Several mechanisms have been proposed to explain this decline, including the rapid turnover and death of infected host cells, as well as “bystander” cell death via indirect means (17). Moreover, several HIV-1 proteins, including Nef, Vif, Vpr, Vpu, Tat, and Rev, have been implicated in apoptosis induction.
HIV-1 Vpr, a 96-amino-acid, 14-kDa protein, is critically involved in HIV-1 pathogenesis in vivo (10, 15, 16). Several functions have been attributed to Vpr including (i) interaction with and translocation of the HIV-1 preintegration complex through the nuclear pore, (ii) induction of apoptosis, (iii) induction of host cell cycle arrest during G2-to-M transition, and (iv) stimulation of viral gene expression (1, 7, 9, 12, 14, 19-21, 25, 32, 40, 41, 43, 46, 48, 53, 55, 60, 61). Studies have documented Vpr-induced apoptosis in human fibroblasts, T-cell lines, and primary cells, including lymphocytes and monocytes (1, 23, 38, 44, 47). Indeed, death of uninfected bystander T cells has also been attributed to secreted Vpr protein. Among several explanations, a leading mechanistic model suggests that Vpr induces cellular apoptosis through dysregulation of MMP. Using isolated mitochondria, others have found that Vpr can target the mitochondrial permeability transition pore complex and promote permeabilization of mitochondrial membranes (23). Whether Vpr's mitochondrion effect can be entirely explained through its binding of inner mitochondria membrane protein, adenine nucleotide translocator (ANT), remains to be clarified (52).
Apoptosis of infected cells may mute the host's immune response to the virus (54). In this regard, we wanted to further understand the details of Vpr's interaction with mitochondria and its apoptotic consequences. Here, we report the identification of HAX-1 (for HS1-associated protein X-1) as a new mitochondrial target for Vpr. HS-1 (for hematopoietic lineage cell-specific protein 1) is a B-cell signaling protein that is a substrate for intracellular protein tyrosine kinases involved in the immune response to extracellular stimuli and in cell differentiation induced by cytokines. HAX-1 was initially reported as an HS-1-binding protein; HAX-1 is a 279-amino-acid (35-kDa) protein with homology to Bcl2. HAX-1 has been shown by others to be an antiapoptotic factor (51). We now document that (i) Vpr binds HAX-1 directly, (ii) overexpression of Vpr causes the egress of HAX-1 from the mitochondria into the cytoplasm, and (iii) overexpression of HAX-1 counters the proapoptotic effect of Vpr in cells.
MATERIALS AND METHODS
Plasmids, cell culture, and transfections.
HeLa cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. Full-length and deletion mutants were generated by PCR amplification and cloning. Plasmids pCDNA-Vpr, pCDNA-FLAG Vpr, and pEYFP-Vpr were constructed by PCR amplification of pNL4-3 Vpr and cloning of PCR products into pCDNA3.1 and pEYFP vectors, respectively. pCDNA-HAX-1 and pCDNA-HA-HAX-1 were constructed by cloning HAX-1 ORF into pCDNA3.1 vector (Invitrogen). HeLa cells were transfected with Lipofectamine Plus and Lipofectamine according to the manufacturer's instructions (Invitrogen).
Antibodies.
Mouse monoclonal anti-HA and anti-FLAG M2 antibodies were commercially purchased (Sigma-Aldrich); rabbit polyclonal 46-amino-acid antibody was from the AIDS Reference and Reagent Program. Mouse monoclonal anti-HAX-1 antibody was from BD Pharmingen.
Western blotting, immunoprecipitation, and confocal imaging.
Western blotting and immunoprecipitation were performed as previously described (28, 56). For confocal microscopy, HeLa cells were cultured on 25-mm coverslips (Thomas Scientific, Swedesboro, NJ) and transfected with plasmid DNA. One day later, cells were fixed with 3.7% formaldehyde, permeabilized with phosphate-buffered saline containing 0.1% Triton X-100, and incubated with primary antibodies followed with anti-mouse/anti-rabbit antibodies conjugated to Alexa Fluor 488/594 (Molecular Probes, Eugene, OR). Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes). Coverslips were mounted onto glass slides with ProLong Antifade Kit (Invitrogen) and examined with a Leica laser-scanning microscope. For staining of cells with Mitotracker (Invitrogen), the cultured cells were incubated with Mitotracker dye for 15 min in the incubator before washing and fixation for staining.
Coimmunoprecipitation.
MT4/HeLa cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (Tris-buffered saline [pH 8.0] containing 1% Triton X-100 or NP-40, 1 mg of bovine serum albumin/ml, and 1 mM EDTA) with protease inhibitor (phenylmethylsulfonyl fluoride and aprotinin [10 μg/ml]), 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS). Cell lysates were prepared by sonication and precleared first with anti-mouse/anti-rabbit immunoglobulin G-agarose before incubation with either anti-HAX or Anti-Vpr antibody. Immune complexes were captured by using protein A- or G-agarose beads, followed by analysis by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
GST pull-down assay.
Expression and purification of glutathione S-transferase (GST) fusion proteins were performed as described previously (3). 35S-labeled HAX-1 proteins were made with TNT reticulocyte lysate system (Promega). 35S-labeled proteins were incubated with 2 μg of GST-Vpr fusion or GST protein in 0.2 ml of binding buffer (10 mM HEPES [pH 7.5], 50 mM NaCl, 0.1% Nonidet P-40, 0.5 mM dithiothreitol, 0.5 mM EDTA) for 1 to 2 h, washed four times, and analyzed by SDS-PAGE, followed by autoradiography. A portion of the reaction mixture was also analyzed by Coomassie blue staining to visualize GST fusion proteins.
Yeast two-hybrid screen and assays.
The complete coding sequence of HIV-1 pNL4-3 vpr was amplified by PCR and ligated into the BamHI and PstI sites of the yeast pBTM116 vector to produce LexA-Vpr as bait for yeast two-hybrid screening. Yeast two-hybrid library screening was performed as described previously (24, 33). Briefly, the yeast reporter strain L40 was transformed with pBTM-Vpr bait plasmid, followed by 250 μg of human bone marrow cDNA library (Clontech). Approximately 107 transformants were screened on medium containing 2 mM 3-amino-(1,2,4) triazole (Sigma) lacking leucine, tryptophan, and histidine. After 3 days of growth, His+ colonies were assayed for β-galactosidase activity by using colony lift filter assays. Positive cDNA clones were recovered and tested for interaction specificity by retransformation into yeast either with Vpr bait or with a nonspecific bait, lamin.
RESULTS
Identification of HAX-1 as a Vpr-interacting protein.
To search for Vpr-associated factor(s), we performed a yeast two-hybrid library screen using as bait a fusion protein comprised of full-length Vpr fused to the LexA protein (LexA-Vpr). HAX-1 was initially identified from an open-ended screening of the library. The specificity of the interactions between Vpr and HAX-1 was next verified by directly transforming yeast cells with isolated plasmids of LexA-Vpr and GalAD-HAX-1 (HAX-1 fused with the Gal4 activation domain); positive interaction between the two proteins was indicated by blue colonies on an X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plate (Fig. 1A). Control transformation of yeast with LexA-lamin plus GalAD-HAX-1 produced no blue colonies, supporting the specificity of Vpr-HAX-1 interaction.
FIG. 1.
Interaction between Vpr and HAX-1. (A) Vpr and HAX-1 interact in yeast two-hybrid assay. The L40 yeast expressing both LexA BD and GAL4AD hybrid proteins was analyzed on selective plates containing 2 mM concentration of 3-amino-(1,2,4) triazole. Growth in the absence of His with a positive β-galactosidase signal are indications of the interaction between the hybrid proteins. LexA-lamin fusion protein was used as a negative control. (B) HAX-1 and Vpr interact in vitro. Bacterially expressed purified GST-Vpr was used for in vitro binding assay with [35S]methionine-labeled in vitro-translated HAX-1 protein. HAX-1 bound GST-Vpr (lane 3) but not GST-alone (lane 2). (C) Interaction between Vpr and HAX-1 in human cells. HAX-1 interacts with Vpr expressed from HIV-1 in MT4 cells infected with infectious HIV-1 NL4-3. MT4 cells were harvested 4 days postinfection by centrifugation and were lysed in RIPA buffer. Cell lysates were subjected to sonication and coimmunoprecipitation assays with anti-Vpr or with irrelevant control antibody (isotype control). The immunoprecipitates were resolved by SDS-PAGE, transferred to membrane, and probed with mouse monoclonal anti-HAX-1 antibody. The top panel shows coimmunoprecipitation of HAX-1 with Vpr. The middle panel shows Western blotting of endogenous HAX-1. The bottom panel shows expression of Vpr from pNL4-3.
Vpr binds to HAX-1 in vitro and inside cells.
To check further the interaction between Vpr and HAX-1, GST pull-down assays were performed with recombinant GST-Vpr fusion protein and in vitro-synthesized 35S-labeled HAX-1 protein. Proteins captured by the beads were washed, solubilized, and resolved by SDS-PAGE, followed by autoradiography. As shown in Fig. 1B, GST-Vpr fusion protein but not GST alone captured HAX-1 (Fig. 1B, compare lanes 2 and 3); this result is fully consistent with a physical protein-protein interaction between Vpr and HAX-1.
To examine Vpr-HAX-1 interaction inside cells, we next attempted coimmunoprecipitate cell-endogenous HAX-1 with Vpr expressed from an infecting HIV-1 molecular clone, NL4-3. MT4 cells were infected with HIV-1 molecular clone NL4-3 and harvested 4 days postinfection. Cells lysed in RIPA buffer were sonicated and immunoprecipitated with anti-Vpr antibody. The immunoprecipitates were resolved by SDS-PAGE and analyzed by Western blotting with anti-HAX-1 antibody. As shown in Fig. 1C (lane 4), HAX-1 was found to coprecipitate with Vpr, a finding consistent with the results from yeast two-hybrid (Fig. 1A) and GST pull down (Fig. 1B) assays. Taken together, the results suggest that the Vpr interacts directly with cell endogenous HAX-1 in HIV-1-infected cells.
Expression of DN HAX-1 mutant induces apoptosis.
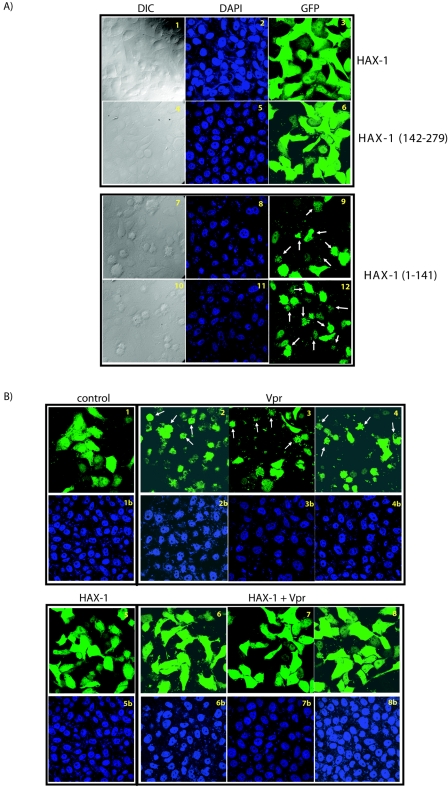
Previously, it was reported that HAX-1 contained limited homology with the BH1 and BH2 domains of Bcl-2 family members (45, 51). Elsewhere, in other experimental systems, HAX-1 was found to be a mitochondria protein (51), which when overexpressed blocked Bax-induced apoptosis (45). Formally, it has not been fully clarified whether HAX-1 serves a direct role in preventing cellular apoptosis or only plays an indirect role in influencing the expression, stability, and/or function of Bax. To address HAX-1 function in our experimental setting, we constructed several deletion mutants. We reasoned that some or all of the mutants might be dominant negative (DN) for HAX-1 function. One prediction is that if HAX-1 were to play a constitutive role in suppressing cellular apoptosis, then expression of DN HAX-1 should produce cell death. To check this prediction, we introduced wild-type HAX-1, HAX-1(142-279) mutant, or HAX-1(1-141) mutant separately into HeLa cells, along with a green fluorescent protein (GFP) plasmid that marked the transfected cells. After 36 to 48 h, cells were fixed and stained with DAPI. We scored GFP-positive cells for apoptosis based on the visualization of condensed nuclei with nuclear fragmentation. We found that neither full-length HAX-1 nor HAX-1(142-279) (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5 A1 through 6) induced apoptosis. However, overexpression of HAX-1 (1-141) produced frequent nuclear condensation and fragmentation consistent with apoptosis (Fig. 2A7 to A12). An interpretation of these results is that HAX-1(142-279) is a loss-of-function mutant, whereas HAX-1(1-141) is a DN mutant. The finding that DN HAX-1(1-141) induced apoptosis is consistent with an intrinsically protective role served by cell-endogenous HAX-1 against cell death.
FIG. 2.
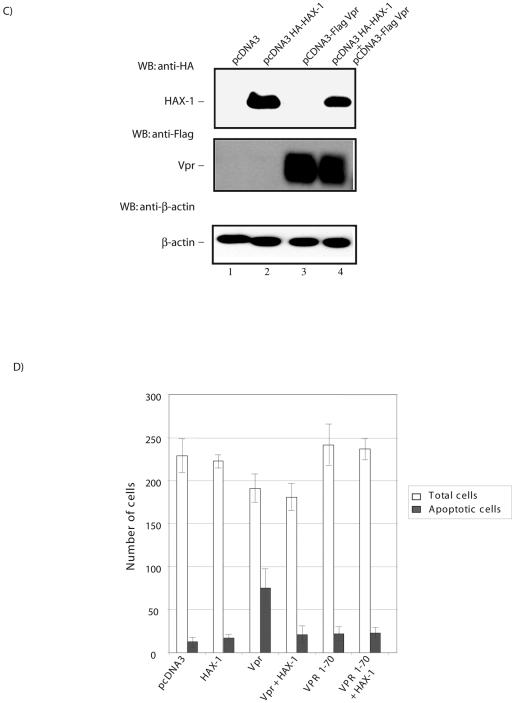
Vpr-induced cell death is suppressed by HAX-1 overexpression. (A) HAX-1 influences cellular apoptosis. Expression of DN HAX-1 induces cell death in HeLa cells. pcDNA HAX-1 WT, pcDNA HAX-1(1-141), and pcDNA HAX-1(142-279) were cotransfected with pCMV-GFP into HeLa cells. At 36 to 48 h posttransfection, cells were fixed and stained with the nuclear stain DAPI. The transfected GFP-expressing cells were examined by microscopy for differential interference contrast (DIC), DAPI, and green fluorescence. pcDNA HAX-1(1-141) expression in transfected cells resulted in significant cell death (compare subpanels 9 and 12 with subpanels 3 and 6). Apoptotic cells were detected as GFP-positive cells with nuclear condensation and fragmentation. (B) HAX-1 suppressed Vpr-induced cell death. HeLa cells were transfected with HAX-1 and Vpr as indicated along with cytomegalovirus (CMV)-GFP as a marker for transfected cells. Overexpression of HAX-1 suppressed Vpr-induced cell death (compare subpanels 2 to 4 with subpanels 6 to 8). (C) Western blot analysis of the expression of Vpr and HAX-1 in transfected HeLa cells. Cell lysates prepared from transfections above (Fig. 2B) were analyzed by blotting for expression of the indicated proteins from transiently transfected plasmids. The top row shows detection of transfected HAX-1 with a light exposure of film. The middle row shows detection of Flag-tagged transfected Vpr. The bottom row shows β-actin signals as loading controls. (D) Graphic representation of suppression cell death induced by HIV-1 Vpr. Cell viability of transfected cells was observed 36 to 48 h posttransfection by counting the number of live green (total cells) and apoptotic green (apoptotic cells) fluorescent cells under the microscope. Values are representative of three independent assays.
FIG. 3.
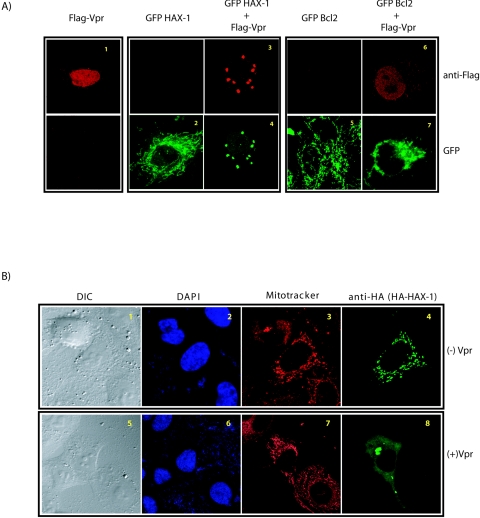
Localization of Vpr and HAX-1 in HeLa cells. Transfected cells were visualized 36 to 48 h after transfection. (A) Flag-Vpr and GFP-HAX-1 colocalize. Flag-Vpr is primarily nuclear (subpanel 1); GFP-HAX-1 protein is mainly mitochondrial (subpanel 2). The cotransfected GFP-HAX-1 and Flag-Vpr colocalize outside of the nucleus and the mitochondria (subpanels 3 and 4). In contrast, Flag-Vpr and GFP-Bcl2 do not colocalize (subpanels 5 to 7). (B) HAX-1 localizes to extra mitochondrial sites in the presence of Vpr. HeLa cells transfected with HAX-1 alone (subpanels 1 to 4) and HAX-1 plus Vpr (subpanels 5 to 8) were stained with Mitotracker before fixation and were probed with anti-HA antibody-anti-mouse Alexa 488. HAX-1 in the absence of Vpr colocalized with Mitotracker-stained mitochondria but in the presence of Vpr formed cytoplasmic bodies that are not stained by Mitotracker.
FIG. 4.
HAX-1 interaction with the C terminus of Vpr correlated with suppression of apoptosis. (A) Confocal examination of distribution of Vpr mutants in HeLa cells in the presence or absence of HAX-1. Staining patterns of YFP-VprS79A and YFP-Vpr(1-70) are shown. (B) Mapping of the Vpr region required for HAX-1 interaction. HeLa cells transiently expressing the indicated protein(s) were immunoprecipitated with mouse monoclonal antibody to HAX-1. The immunoprecipitates were subjected to SDS-PAGE, transferred to membrane, and probed with rabbit polyclonal anti-YFP. The top panel shows that YFP-Vpr WT coimmunoprecipitated with HA-HAX-1, whereas YFP alone, YFP-Vpr(1-70), and YFP-Vpr(1-51) did not. The middle panel shows equal expression levels of all YFP-tagged proteins. The bottom panel verified for equal expression of transfected HA-HAX-1. (C) Schematic representations of Vpr mutants, their interactions with HAX-1, and their apoptosis-inducing capacity.
FIG. 5.
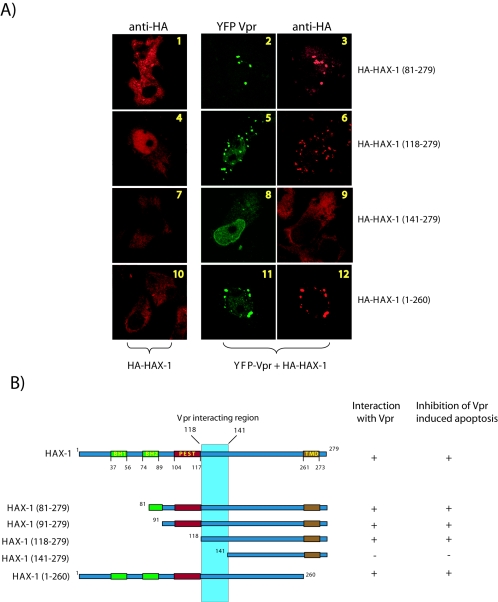
Mapping of amino acids 118 to 141 of HAX-1 as a region of interaction with Vpr. (A) Confocal examination of expression and distribution of HAX-1 in HeLa cells. Fluorescent images indicate that the various HAX-1 deletion mutants are either capable or not capable of sequestering Vpr. (B) Diagrammatic summary of HAX-1 deletion mutant correlating the ability to sequester Vpr with ability to halt Vpr-induced apoptosis. Bcl2 homology domains 1 and 2 (BH1 and -2, green), PEST sequence (PEST, red), and transmembrane domain (TMD, brown) are indicated.
Vpr-induced apoptosis is suppressed by overexpression of HAX-1.
Several studies have shown that expression of Vpr causes apoptotic cell death (22, 23, 38, 44, 47). Because Vpr binds HAX-1 (Fig. 1) and because DN-HAX-1, in itself, promoted apoptosis (Fig. 2A), we wondered whether Vpr's effect on cell death might arise from its physical sequestration of limiting amounts of intracellular HAX-1. To explore this possibility, we revisited Vpr's capacity to induce cell death. In our cultured cells, we observed that nuclear fragmentation rapidly ensued after Vpr expression (Fig. 2B2-4). Provocatively, when HAX-1 was coexpressed with Vpr in cells, mortality of recipient cells normally expected from receiving Vpr-alone disappeared (Fig. 2B, compare panels 6 to 8 to 2 to 4). These results are compatible with Vpr's proapoptotic effect being manifest through its sequestration of cell-endogenous HAX-1 and with such sequestration being competitively muted by overexpression of exogenous HAX-1. In Fig. 2C, we performed control Western blotting to monitor the degree of expression of transfected HAX-1 and Vpr. Indeed, based on Western blotting, it was evident that the level of expression of transfected HAX-1 exceeds the amount detected for cell endogenous HAX-1 (Fig. 2C, compare lanes 1 and 3 to lanes 2 and 4).
We next quantified the ability of HAX-1 to suppress Vpr-induced apoptosis. In Fig. 2D, we counted the number of GFP-positive cells (total cells) and the number of green cells that were apoptotic (apoptotic cells). We found that Vpr-induced apoptosis was reduced by threefold when HAX-1 was coexpressed in trans. Expression of HAX-1-alone, Vpr1-70 mutant alone or of Vpr1-70 mutant plus HAX-1 produced apoptosis similar to the controls cells transfected with pCDNA3 vector.
Mutually altered cellular localizations of HAX-1 and Vpr upon simultaneous overexpression.
Although Vpr is generally located at the nucleus and nuclear membrane, some investigators have found its additional presence in mitochondria (22). On the other hand, HAX-1 is largely mitochondrial and is found rarely in the endoplasmic reticulum (51). To verify the notion that Vpr might sequester HAX-1 and perturb the latter's activity, we investigated whether the two proteins show some level of colocalization inside cells. We transfected HeLa cells with either Flag-Vpr (Fig. 3A1) or GFP-HAX-1 (Fig. 3A2) or both (Fig. 3A3 and 4). Cells were fixed 24 h later and stained with anti-Flag antibody, as well as visualized for GFP. We found that GFP-HAX-1 (Fig. 3A2) showed a speckled pattern that mirrored Mitotracker staining of mitochondria (Fig. 3B3); on the other hand, Flag-Vpr alone was diffusely nuclear (Fig. 3A1). Intriguingly, coexpression of GFP-HAX-1 with Flag-Vpr resulted in a remarkable redistribution of both molecules (Fig. 3A3 and 4). Under such conditions, Flag-Vpr relocated from the nucleus into the cytoplasm, whereas GFP-HAX-1 departed the mitochondria to appear with the cytoplasmic Flag-Vpr. The redistributed Vpr-HAX-1 bodies do not costain with Golgi, endoplasmic reticulum, or mitochondria (data not shown). Currently, it is not clear whether these bodies localize with bona fide cytoplasmic organelles.
As controls, we also visualized mitochondrion-resident GFP-Bcl2 (Fig. 3A5) and GFP-Bcl2 plus Flag-Vpr (Fig. 3A6). Here, simultaneous expression of Bcl2 and Vpr had neither a significantly perturbing nor a colocalizing effect on each other (compare Fig. 3A3, 4). A minor “blurring” by confocal visualization of Bcl2 in the mitochondria may be due to perturbation of mitochondrial membrane potential by Vpr. Overall, our findings suggest that Vpr has a specific interaction with HAX-1 but not Bcl2. Next, to make sure that our results are not idiosyncratic to GFP-tagged HAX-1, we also examined the behavior of an hemagglutinin (HA)-tagged HAX-1 (Fig. 3B). In agreement with GFP-HAX-1, HA-HAX-1 was also dislocated from the mitochondria by overexpressed Flag-Vpr (compare Fig. 3B4). Altogether, our results are compatible with a scenario in which the mitochondrion-intrinsic cell-protective function of HAX-1 is disturbed by Vpr expression.
Physical interaction between Vpr and HAX-1 correlates with apoptosis.
To characterize how HAX-1 is targeted by Vpr, we sought to better understand the domain in Vpr responsible for this function. We tested one point (VprS79A) and two deletion [Vpr(1-70) and Vpr(1-51)] mutants of Vpr (Fig. 4A and B) for their association with HAX-1. As shown in Fig. 4A, two distinct intracellular profiles were observed. VprS79A was entirely nuclear (Fig. 4A1), whereas Vpr(1-70) (Fig. 4A4) and Vpr(1-50) (data not shown) were located in extranuclear speckles. Interestingly, when the Vpr mutants were coexpressed with HAX-1, two different patterns were seen. VprS79A exited the nucleus and colocated with HAX-1 (Fig. 4A3). On the other hand, neither Vpr1-70 (Fig. 4A4) nor Vpr1-51 (data not shown) appeared to colocalize with HAX-1, nor did these two mutants perturb HAX-1's normal mitochondrial location (Fig. 4A6).
We also checked the region of interaction between HAX-1 and Vpr by coimmunoprecipitations. We transfected HA-tagged HAX-1 with YFP alone, YFP-Vpr, YFP-Vpr(1-70) and YFP-Vpr(1-51) (Fig. 4B) into cells. Cell lysates were immunoprecipitated with anti-HA, and immunoprecipitates were Western blotted with anti-YFP. As shown in Fig. 4B, YFP-Vpr coprecipitated with HAX-1, a finding consistent with the results from yeast two-hybrid (Fig. 1A) and GST pull down (Fig. 1B) and earlier coimmunoprecipitation (Fig. 1C) assays. In contrast, Vpr(1-70) and Vpr(1-51) failed to associate with HAX-1 (Fig. 4B, lanes 3 and 4), suggesting that the C terminus of Vpr spanning amino acids 71 to 96 is required for interaction with HAX-1.
We also examined our transfected cells for signs of apoptosis (Fig. 4C). We found agreement with previous data on Vpr-induced apoptosis. Hence, Jacotot et al. (23) had reported that full-length Vpr and a carboxy-Vpr form (Vpr52-95), but not an amino-Vpr form [Vpr(1-51) and Vpr(1-70)], triggered cellular apoptosis. Our data are consistent with those earlier results and further suggest a correlation between Vpr-induced apoptosis and Vpr's ability to associate with HAX-1. Thus, we observed that Vpr proteins competent or incompetent for HAX-1 binding are, respectively, active or inactive for inducing apoptosis (Fig. 4C).
Sequestration of Vpr and suppression of apoptosis maps to amino acids 118 to 141 of HAX-1.
Our results to this point are consistent with a model in which Vpr sequesters and dislocates mitochondrion-protective HAX-1, leading to cellular apoptosis. This model predicts that there should be a HAX-1 mutant which, when overexpressed, would bind Vpr competitively and prevent Vpr's disturbance of wild-type HAX-1 function. To check this prediction, we constructed several overlapping HAX-1 deletion mutants (Fig. 5B). In Fig. 5A, we observed that some HAX-1 deletion mutants showed minor differences from wild-type HAX-1 in intracellular localization (Fig. 3). Interestingly, all mutants, with the exception of HAX-1(141-279), were competent for sequestering Vpr (Fig. 5A and B).
When we examined the functional consequences of HAX-1 mutant overexpression, a one-to-one correlation emerged between HAX-1 mutants capable of binding Vpr and their ability to suppress Vpr-induced apoptosis in cells (Fig. 5B). These results are complementary to the above findings (Fig. 4) and demonstrate that overexpressed HAX-1 mutants can competitively capture Vpr and abrogate Vpr's ability to perturb the limiting apoptosis-protective function of cell-endogenous mitochondria-located HAX-1. We quantified that overexpression of HAX-1 mutants [HAX-1(81-279), HAX-1(91-279), HAX-1(118-279), and HAX-1(1-260)] suppressed Vpr induced apoptosis by ∼2-3-fold (data not shown). On the other hand, HAX-1(141-279) failed entirely to suppress Vpr-induced apoptosis. One interpretation of these results is that HAX-1 is required to stabilize mitochondria and that Vpr can physically bind and dislocate HAX-1 from mitochondria leading to destabilized MMP and apoptosis.
DISCUSSION
Mitochondrial physiology is intimately tied to cellular apoptosis (18, 31, 57) One hypothesis for mitochondrial cell death involves a changes in MMP culminating in the release of apoptogenic factors such as cytochrome c and apoptosis inhibiting factor from the mitochondrial intermembrane space (34, 35, 49, 59) Whether cells live or die is tied to a balance between the actions of antiapoptotic (e.g., Bcl-2/Bcl-XL) and proapoptotic (e.g., Bax, ANT-1, and VDAC) factors. Within this context, HAX-1 is an antiapoptotic factor thought most likely to be located at the outer mitochondrial membrane (8). Overexpression of HAX-1 has been shown previously to prevent Bax-induced MMP and the subsequent apoptosis of cells (45).
Vpr has been found to perturb MMP (23). Previously, Jacotot et al. (23) used a synthetic HIV-1 Vpr peptide to study its interaction with isolated mitochondria in vitro. At micromolar concentrations of Vpr, these authors observed rapid dissipation of MMP; this change in potential correlated with a leakage from purified mitochondria of apoptogenic proteins such as cytochrome c. Similarly, in Jurkat T cells, Vpr expression also produced aberrant MMP with accompanying apoptosis (23, 37). Currently, Vpr's apoptogenic MMP effect has been largely attributed to its interaction with an inner mitochondrial membrane protein, ANT-1 (22, 23). Although Vpr-ANT-1 interaction is consistent with many extant findings, it remains perplexing how an interaction or disturbance at the inner mitochondrial membrane mechanistically provokes perforation of the outer membrane leading to the release of apoptogenic factors normally resident in the intermembranous space. Our new finding that an outer membrane factor, HAX-1, is also targeted by Vpr clarifies how HIV-1 might fully perforate both layers of mitochondria membranes leading to apoptosis. Our Vpr-HAX-1 finding also validates a prediction made by Jacotot et al. based on their Vpr52-96 results that, separate from ANT-1, Vpr must have a second target located in the outer mitochondrial membrane (22). The current finding that overexpression of HAX-1 is sufficient to abolish Vpr-induced apoptosis in cells (Fig. 2) is further consistent with our interpretation and suggests that for purposes of apoptosis, stabilization of the outer mitochondrial membrane is dominant over destabilization of the inner membrane (i.e., via ANT-1).
HAX-1 was originally identified by yeast two-hybrid screen as a protein that associates with HS-1 (for hematopoietic lineage cell-specific protein 1). HS-1 is a B-cell signaling protein and is a substrate for intracellular protein tyrosine kinases involved in the immune response to extracellular stimuli and in cell differentiation induced by cytokines (51). HAX-1 possesses two Bcl2 homologous domains BH1 and BH2 which are conserved among Bcl-2 family proteins. Additional amino acid sequences in HAX-1 retain similarities with apoptosis regulating protein Nip3, which has been found to bind antiapoptotic factor Bcl2 (51). Indeed, recent findings have also shown that HAX-1 can directly bind to Bcl2, which is also predominantly located in the outer mitochondrial membrane (36). It is interesting that two other human viruses, Epstein-Barr virus and Kaposi's sarcoma herpesvirus, have also been found to encode proteins that target HAX-1 (11, 27, 45). Currently, it is not fully understood why disparate viruses would commonly converge on this mitochondrial protein. Potentially, if apoptosis of infected cells is one way for viruses to evade the elicitation of a vigorous host immune response, then it could be reasonable why Epstein-Barr virus, Kaposi's sarcoma herpesvirus, and HIV-1 would share this interaction. If this reasoning is correct, then the discovery of small molecules which might interdict Vpr-HAX-1 interaction could contribute as an adjunct to the better development of an immunity-eliciting AIDS vaccine.
Acknowledgments
This study was supported in part by grants (NSC93-2314-B-002-104 and NSC94-2314-B-002-062) from the National Science Council, Taiwan, and by intramural research funds from the NIAID, NIH.
REFERENCES
- 1.Ayyavoo, V., A. Mahboubi, S. Mahalingam, R. Ramalingam, S. Kudchodkar, W. V. Williams, D. R. Green, and D. B. Weiner. 1997. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor κB. Nat. Med. 3:1117-1123. [DOI] [PubMed] [Google Scholar]
- 2.Belzacq, A. S., H. L. Vieira, F. Verrier, G. Vandecasteele, I. Cohen, M. C. Prevost, E. Larquet, F. Pariselli, P. X. Petit, A. Kahn, R. Rizzuto, C. Brenner, and G. Kroemer. 2003. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 63:541-546. [PubMed] [Google Scholar]
- 3.Bouhamdan, M., S. Benichou, F. Rey, J. M. Navarro, I. Agostini, B. Spire, J. Camonis, G. Slupphaug, R. Vigne, R. Benarous, and J. Sire. 1996. Human immunodeficiency virus type 1 Vpr protein binds to the uracil DNA glycosylase DNA repair enzyme. J. Virol. 70:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boya, P., A. L. Pauleau, D. Poncet, R. A. Gonzalez-Polo, N. Zamzami, and G. Kroemer. 2004. Viral proteins targeting mitochondria: controlling cell death. Biochim. Biophys. Acta 1659:178-189. [DOI] [PubMed] [Google Scholar]
- 5.Brenner, C., H. Cadiou, H. L. Vieira, N. Zamzami, I. Marzo, Z. Xie, B. Leber, D. Andrews, H. Duclohier, J. C. Reed, and G. Kroemer. 2000. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene 19:329-336. [DOI] [PubMed] [Google Scholar]
- 6.Brenner, C., and G. Kroemer. 2000. Apoptosis. Mitochondria-the death signal integrators. Science 289:1150-1151. [DOI] [PubMed] [Google Scholar]
- 7.Chen, R., E. Le Rouzic, J. A. Kearney, L. M. Mansky, and S. Benichou. 2004. Vpr-mediated incorporation of UNG2 into HIV-1 particles is required to modulate the virus mutation rate and for replication in macrophages. J. Biol. Chem. 279:28419-28425. [DOI] [PubMed] [Google Scholar]
- 8.Cilenti, L., M. M. Soundarapandian, G. A. Kyriazis, V. Stratico, S. Singh, S. Gupta, J. V. Bonventre, E. S. Alnemri, and A. S. Zervos. 2004. Regulation of HAX-1 antiapoptotic protein by Omi/HtrA2 protease during cell death. J. Biol. Chem. 279:50295-50301. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, E. A., R. A. Subbramanian, and H. G. Gottlinger. 1996. Role of auxiliary proteins in retroviral morphogenesis. Curr. Top. Microbiol. Immunol. 214:219-235. [DOI] [PubMed] [Google Scholar]
- 10.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 11.Dufva, M., M. Olsson, and L. Rymo. 2001. Epstein-Barr virus nuclear antigen 5 interacts with HAX-1, a possible component of the B-cell receptor signalling pathway. J. Gen. Virol. 82:1581-1587. [DOI] [PubMed] [Google Scholar]
- 12.Emerman, M. 1996. HIV-1, Vpr and the cell cycle. Curr. Biol. 6:1096-1103. [DOI] [PubMed] [Google Scholar]
- 13.Ferri, K. F., and G. Kroemer. 2001. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3:E255-E263. [DOI] [PubMed] [Google Scholar]
- 14.Forget, J., X. J. Yao, J. Mercier, and E. A. Cohen. 1998. Human immunodeficiency virus type 1 Vpr protein transactivation function: mechanism and identification of domains involved. J. Mol. Biol. 284:915-923. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 17.Gougeon, M. L., and L. Montagnier. 1999. Programmed cell death as a mechanism of CD4 and CD8 T cell deletion in AIDS: molecular control and effect of highly active anti-retroviral therapy. Ann. N. Y. Acad. Sci. 887:199-212. [DOI] [PubMed] [Google Scholar]
- 18.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 19.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hrimech, M., X. J. Yao, F. Bachand, N. Rougeau, and E. A. Cohen. 1999. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J. Virol. 73:4101-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacotot, E., K. F. Ferri, C. El Hamel, C. Brenner, S. Druillennec, J. Hoebeke, P. Rustin, D. Metivier, C. Lenoir, M. Geuskens, H. L. Vieira, M. Loeffler, A. S. Belzacq, J. P. Briand, N. Zamzami, L. Edelman, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2001. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J. Exp. Med. 193:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacotot, E., L. Ravagnan, M. Loeffler, K. F. Ferri, H. L. Vieira, N. Zamzami, P. Costantini, S. Druillennec, J. Hoebeke, J. P. Briand, T. Irinopoulou, E. Daugas, S. A. Susin, D. Cointe, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2000. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin, D. Y., F. Spencer, and K. T. Jeang. 1998. Human T-cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93:81-91. [DOI] [PubMed] [Google Scholar]
- 25.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joza, N., S. A. Susin, E. Daugas, W. L. Stanford, S. K. Cho, C. Y. Li, T. Sasaki, A. J. Elia, H. Y. Cheng, L. Ravagnan, K. F. Ferri, N. Zamzami, A. Wakeham, R. Hakem, H. Yoshida, Y. Y. Kong, T. W. Mak, J. C. Zuniga-Pflucker, G. Kroemer, and J. M. Penninger. 2001. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549-554. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi, Y., K. Nakajima, M. Igarashi, T. Morita, M. Tanaka, M. Suzuki, A. Yokoyama, G. Matsuda, K. Kato, M. Kanamori, and K. Hirai. 2000. Interaction of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP) with HS1-associated protein X-1: implication of cytoplasmic function of EBNA-LP. J. Virol. 74:10104-10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kibler, K. V., and K. T. Jeang. 2001. CREB/ATF-dependent repression of cyclin a by human T-cell leukemia virus type 1 Tax protein. J. Virol. 75:2161-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroemer, G. 2003. Mitochondrial control of apoptosis: an introduction. Biochem. Biophys. Res. Commun. 304:433-435. [DOI] [PubMed] [Google Scholar]
- 30.Kroemer, G., B. Dallaporta, and M. Resche-Rigon. 1998. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60:619-642. [DOI] [PubMed] [Google Scholar]
- 31.Kroemer, G., and J. C. Reed. 2000. Mitochondrial control of cell death. Nat. Med. 6:513-519. [DOI] [PubMed] [Google Scholar]
- 32.Le Rouzic, E., and S. Benichou. 2005. The Vpr protein from HIV-1: distinct roles along the viral life cycle. Retrovirology 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, D. Y., and H. M. Shih. 2002. Essential role of the 58-kDa microspherule protein in the modulation of Daxx-dependent transcriptional repression as revealed by nucleolar sequestration. J. Biol. Chem. 277:25446-25456. [DOI] [PubMed] [Google Scholar]
- 34.Liu, X., C. N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147-157. [DOI] [PubMed] [Google Scholar]
- 35.Mancini, M., D. W. Nicholson, S. Roy, N. A. Thornberry, E. P. Peterson, L. A. Casciola-Rosen, and A. Rosen. 1998. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J. Cell Biol. 140:1485-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuda, G., K. Nakajima, Y. Kawaguchi, Y. Yamanashi, and K. Hirai. 2003. Epstein-Barr virus (EBV) nuclear antigen leader protein (EBNA-LP) forms complexes with a cellular anti-apoptosis protein Bcl-2 or its EBV counterpart BHRF1 through HS1-associated protein X-1. Microbiol. Immunol. 47:91-99. [DOI] [PubMed] [Google Scholar]
- 37.Muthumani, K., A. Y. Choo, D. S. Hwang, M. A. Chattergoon, N. N. Dayes, D. Zhang, M. D. Lee, U. Duvvuri, and D. B. Weiner. 2003. Mechanism of HIV-1 viral protein R-induced apoptosis. Biochem. Biophys. Res. Commun. 304:583-592. [DOI] [PubMed] [Google Scholar]
- 38.Muthumani, K., D. S. Hwang, B. M. Desai, D. Zhang, N. Dayes, D. R. Green, and D. B. Weiner. 2002. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J. Biol. Chem. 277:37820-37831. [DOI] [PubMed] [Google Scholar]
- 39.Penninger, J. M., and G. Kroemer. 2003. Mitochondria, AIF and caspases-rivaling for cell death execution. Nat. Cell Biol. 5:97-99. [DOI] [PubMed] [Google Scholar]
- 40.Popov, S., M. Rexach, G. Zybarth, N. Reiling, M. A. Lee, L. Ratner, C. M. Lane, M. S. Moore, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed, J. C., and G. Kroemer. 2000. Mechanisms of mitochondrial membrane permeabilization. Cell Death Differ. 7:1145. [DOI] [PubMed] [Google Scholar]
- 43.Rogel, M. E., L. I. Wu, and M. Emerman. 1995. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J. Virol. 69:882-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roumier, T., H. L. Vieira, M. Castedo, K. F. Ferri, P. Boya, K. Andreau, S. Druillennec, N. Joza, J. M. Penninger, B. Roques, and G. Kroemer. 2002. The C-terminal moiety of HIV-1 Vpr induces cell death via a caspase-independent mitochondrial pathway. Cell Death Differ. 9:1212-1219. [DOI] [PubMed] [Google Scholar]
- 45.Sharp, T. V., H. W. Wang, A. Koumi, D. Hollyman, Y. Endo, H. Ye, M. Q. Du, and C. Boshoff. 2002. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 76:802-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart, S. A., B. Poon, J. B. Jowett, Y. Xie, and I. S. Chen. 1999. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc. Natl. Acad. Sci. USA 96:12039-12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Chen. 2000. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subbramanian, R. A., A. Kessous-Elbaz, R. Lodge, J. Forget, X. J. Yao, D. Bergeron, and E. A. Cohen. 1998. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J. Exp. Med. 187:1103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Susin, S. A., H. K. Lorenzo, N. Zamzami, I. Marzo, B. E. Snow, G. M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, N. Larochette, D. R. Goodlett, R. Aebersold, D. P. Siderovski, J. M. Penninger, and G. Kroemer. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441-446. [DOI] [PubMed] [Google Scholar]
- 50.Susin, S. A., N. Zamzami, and G. Kroemer. 1998. Mitochondria as regulators of apoptosis: doubt no more. Biochim. Biophys. Acta 1366:151-165. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki, Y., C. Demoliere, D. Kitamura, H. Takeshita, U. Deuschle, and T. Watanabe. 1997. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J. Immunol. 158:2736-2744. [PubMed] [Google Scholar]
- 52.Vieira, H. L., D. Haouzi, C. El Hamel, E. Jacotot, A. S. Belzacq, C. Brenner, and G. Kroemer. 2000. Permeabilization of the mitochondrial inner membrane during apoptosis: impact of the adenine nucleotide translocator. Cell Death Differ. 7:1146-1154. [DOI] [PubMed] [Google Scholar]
- 53.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welsh, R. M., K. Bahl, and X. Z. Wang. 2004. Apoptosis and loss of virus-specific CD8+ T-cell memory. Curr. Opin. Immunol. 16:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao, X. J., A. J. Mouland, R. A. Subbramanian, J. Forget, N. Rougeau, D. Bergeron, and E. A. Cohen. 1998. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72:4686-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yedavalli, V. S., C. Neuveut, Y. H. Chi, L. Kleiman, and K. T. Jeang. 2004. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119:381-392. [DOI] [PubMed] [Google Scholar]
- 57.Zamzami, N., and G. Kroemer. 2001. The mitochondrion in apoptosis: how Pandora's box opens. Nat. Rev. Mol. Cell. Biol. 2:67-71. [DOI] [PubMed] [Google Scholar]
- 58.Zamzami, N., and G. Kroemer. 2003. Apoptosis: mitochondrial membrane permeabilization—the (w)hole story? Curr. Biol. 13:R71-R73. [DOI] [PubMed] [Google Scholar]
- 59.Zamzami, N., S. A. Susin, P. Marchetti, T. Hirsch, I. Gomez-Monterrey, M. Castedo, and G. Kroemer. 1996. Mitochondrial control of nuclear apoptosis. J. Exp. Med. 183:1533-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao, R. Y., and R. T. Elder. 2005. Viral infections and cell cycle G2/M regulation. Cell Res. 15:143-149. [DOI] [PubMed] [Google Scholar]
- 61.Zhou, Y., Y. Lu, and L. Ratner. 1998. Arginine residues in the C terminus of HIV-1 Vpr are important for nuclear localization and cell cycle arrest. Virology 242:414-424. [DOI] [PubMed] [Google Scholar]