Abstract
Reactivation of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic replication is mediated by the viral RTA transcription factor, but little is known about the physiological processes controlling its expression or activity. Links between autonomic nervous system activity and AIDS-associated Kaposi's sarcoma led us to examine the potential influence of catecholamine neurotransmitters. Physiological concentrations of epinephrine and norepinephrine efficiently reactivated lytic replication of KSHV in latently infected primary effusion lymphoma cells via β-adrenergic activation of the cellular cyclic AMP/protein kinase A (PKA) signaling pathway. Effects were blocked by PKA antagonists and mimicked by pharmacological and physiological PKA activators (prostaglandin E2 and histamine) or overexpression of the PKA catalytic subunit. PKA up-regulated RTA gene expression, enhanced activity of the RTA promoter, and posttranslationally enhanced RTA's trans-activating capacity for its own promoter and heterologous lytic promoters (e.g., the viral PAN gene). Mutation of predicted phosphorylation targets at RTA serines 525 and 526 inhibited PKA-mediated enhancement of RTA trans-activating capacity. Given the high catecholamine levels at sites of KSHV latency such as the vasculature and lymphoid organs, these data suggest that β-adrenergic control of RTA might constitute a significant physiological regulator of KSHV lytic replication. These findings also suggest novel therapeutic strategies for controlling the activity of this oncogenic gammaherpesvirus in vivo.
Kaposi's sarcoma-associated herpesvirus (KSHV; also known as human herpesvirus 8 [HHV-8]) is a lymphotropic gammaherpesvirus originally identified in the context of Kaposi's sarcoma (8) and subsequently implicated in primary effusion lymphoma and multicentric Castleman's disease (6, 66). The KSHV genome most closely resembles herpesvirus saimiri (60), but the virus also bears more distant structural and functional similarities to Epstein-Barr virus (48, 49). Like all herpesviruses, KSHV displays a bifurcating gene expression program that allows it to defer lytic replication and enter a protracted state of latency during which only a small minority of viral genes are expressed (19, 21, 71, 78). KSHV establishes latency in B lymphocytes and vascular endothelial cells, but it must resume lytic replication to disseminate or colonize a new host. Discovery of the physiological signals that control KSHV reactivation is thus key to controlling its pathogenic potential. Suppression of such signals could block the spread of infection, and pharmacological induction of such signals might flush latently infected cells into lytic replication for elimination by nucleoside analogue drugs (31).
Impaired cellular immunity plays a critical role in allowing KSHV replication, but the physiological stimuli that positively induce lytic gene expression are poorly understood. Chemical agents such as phorbol esters or N-butyrate can reactivate KSHV in vitro (45, 50, 57), and proinflammatory cytokines have similar, though weaker effects (7, 44, 46). At the level of the viral genome, lytic reactivation is mediated by the KSHV-encoded transcription factor RTA. Overexpression of the RTA gene is sufficient to trigger lytic replication in latently infected B-cell lines (37, 70), and RTA mutations can block viral reactivation in vitro (36). RTA protein can also autoactivate the RTA promoter (16, 25, 62), but the cellular signals that initiate RTA expression are not well understood.
Several recent studies have shown that high levels of autonomic nervous system activity can accelerate the onset of AIDS-defining conditions during human immunodeficiency virus type 1 infection (11, 12, 14). These effects have been attributed to autonomic nervous system regulation of human immunodeficiency virus type 1 replication (10, 13, 14), but it is also conceivable that autonomic nervous system activity might directly activate opportunistic pathogens such as KSHV. The present studies examined that hypothesis, with an emphasis on the cellular signal transduction pathways that might allow catecholamine neurotransmitters from the autonomic nervous system to regulate key molecular events in KSHV reactivation. Results show that physiological concentrations of epinephrine and norepinephrine can induce lytic replication of KSHV in latently infected lymphoid cells via β-adrenergic activation of the cellular protein kinase A (PKA) signaling pathway. These effects are mediated by increased expression of RTA and posttranslational enhancement of its trans-activating capacity. Results provide an endocrinological perspective on the control of KSHV replication and suggest novel strategies for therapeutic intervention.
MATERIALS AND METHODS
Cell culture.
KSHV reactivation was analyzed in the primary effusion lymphoma (PEL) cell lines KS-1, BC3, and BCBL-1. DG75 and Ramos served as B lymphoid cells uninfected with KSHV or Epstein-Barr virus. Cells were cultured under standard conditions in RPMI plus 10% fetal bovine serum or in serum-free X-VIVO 15 (Cambrex, East Rutherford, NJ) supplemented by a defined mix of growth factors (MITO+, BD Biosciences, Bedford, MA). Reactivation experiments were performed as described (70), using the PKC inducer phorbol-12-myristate-13-acetate (PMA, also known as TPA; Calbiochem, San Diego CA), the PKA inducer dibutyrl cyclic AMP (db-cAMP), norepinephrine ([−]-arterenol; Sigma-Aldrich, St. Louis, MO), or other indicated agents acting on the β-adrenoreceptor (βAR)/cAMP/PKA signaling pathway (all from Sigma-Aldrich or Calbiochem). Norepinephrine and PKA modulating agents were diluted in phosphate-buffered saline and added to cultures in volumes ≤1% of total culture volume. Unstimulated controls received an equivalent volume of vehicle.
Lytic protein expression.
Intracellular expression of KSHV ORF59 was assayed by flow cytometric quantification of indirect immunofluorescence in permeabilized KS1, BC3, or BCBL1 cells; 2 × 106 cells were stained for 30 min at 4°C with 1 μg of mouse-anti KSHV ORF59 monoclonal antibody (Advanced Biotechnology Incorporated, Colombia, MD) in 50 μl of BD Cytofix/Cytoperm (BD Immunocytometry Systems, San Jose, CA), washed in 450 μl of permeabilization buffer, stained for 30 min with 0.5 μg of fluorescein isothiocyanate-conjugated rat anti-mouse immunoglobulin G2b Ab (BD Immunocytometry Systems), and washed again in 450 μl of permeabilization buffer. Fluorescence intensity data were acquired on a FACScan flow cytometer (BD Immunocytometry) with dead cells and debris excluded on the basis of forward- versus side-scatter gating using CellQuest software. KSHV lytic proteins and cellular β-actin were also assayed by Western blot with enhanced chemiluminescence using KSHV patient serum, as previously described (70).
Lytic gene expression.
mRNA for KSHV gene products and human β-adrenoreceptor subtypes were quantified relative to cellular housekeeping genes using real-time reverse transcription (RT)-PCR. Reactions utilized 1/10 of the total DNase-treated (QIAGEN, Valencia, CA) RNA extracted from 106 cells in a one-step thermal cycling protocol (QIAGEN One-Step RT-PCR) with 30 min of reverse transcription at 50°C, 15 min. of RT denaturation at 95°C, and 40 cycles of DNA amplification (15 s at 95°C, 60 s at 60°C). Reactions utilized established primers and fluorescent detection probes for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and KSHV K8.1, ORF29, ORF50, ORF72, and ORF57 (21). Primers for human β-adrenoreceptor subtypes were: β1 (forward: TCG GAA TCC AAG GTG TAG GG, reverse:TGG CTT TTC TCT TTG CCT CG), β2 (forward: CAT GTC TCT CAT CGT CCT GGC CA, reverse:CAC GAT GGA AGA GGC AAT GGC A), and β3 (forward: GGC TTC TTG GGG AGT TTC TTA GG, reverse: TTC TGG AGG GTA GAG TGT CAC AGC), derived from GenBank sequences ADRB1: J03019, ADRB2: M15169, and ADRB3: X70811, respectively. mRNA expression was normalized to GAPDH by subtraction of threshold cycles (Ct; normalized target Ct = target Ct − GAPDH Ct) and quantified as a fold change relative to an unstimulated baseline (fold change = 2[stimulated normalized target Ct - baseline normalized target Ct]).
DNA replication.
Replicating KSHV DNA was assayed by Gardella gel analysis as previously described (23). Briefly, 2 × 107 KS1 cells were lysed during electrophoresis through a 0.75% agarose gel for 3 h at 0.8 V/cm followed by 17 h at 4.5 V/cm. Resolved DNA was transferred to a Hybond N+ membrane (Amersham-Pharmacia, Buckinghamshire England), UV cross-linked, and probed with a 3-kb 32P-labeled PCR product spanning the majority of the KSHV ORF50 locus. Supernatant particle-associated KSHV DNA was assayed by treating 1 ml of 0.45-μm-filtered PEL cell supernatants with 2 μg DNase (Worthington Biochemical Corporation, Lakewood, NJ) and 10 mM MgCl for 15 min, followed by extraction of particle-protected DNA (QIAGEN MiniElute virus spin kit) and real-time PCR amplification of KSHV K8.1 DNA (45 cycles of 95°C for 15 seconds and 60°C for 60 seconds) with resolution of products on an 3% agarose gel.
Infectious virus.
PEL cell production of infectious KSHV was assayed by suspending peripheral blood mononuclear cells (PBMC) from healthy donors in PEL cell supernatants that had been filtered at 0.45 μm and treated with DNase as described above; 2 × 106 PBMC were stimulated with PHA for 72 h, washed, and incubated for 1 h. in 2 ml of cell culture supernatant from PEL cells treated with phorbol myristate acetate (PMA), norepinephrine, db-cAMP, or vehicle for 48 h. After extensive washing, PBMC were cultured for another 24 h and establishment of KSHV genomic DNA in PBMC was assayed by PCR detection of ORF50 (forward: AAC CAG AAG CCT CGG GCG AAG, reverse: GTG CAC GCC ACG GAT GTC) or K8.1 (forward: AAA GCG TCC AGG CCA CCA CAG A, reverse: GGC AGA AAA TGG CAC ACG GTT AC) in cellular DNA (45 cycles of 95°C for 15 seconds and 60°C for 60 seconds, with SYBR green real-time detection). Cellular RNA was also extracted from 8 × 106 PBMC, treated with RNase-free DNase (QIAGEN RNEasy), and assayed for expression of the latent gene product ORF72 by real-time RT-PCR as described (21). Results were normalized to GAPDH DNA amplified in parallel and visualized on a 2% agarose gel.
Overexpression of PKA catalytic subunit.
PEL cells were transduced with a self-inactivating lentiviral vector expressing a constitutively active form of PKA (43) and an enhanced green fluorescent protein (EGFP) reporter gene under control of a recombinant Rh-MLV promoter (34). Both sequences were translated from a single transcript bearing the PS3 internal ribosome entry site (IRES) (73). cDNA for the human immunodeficiency virus type 1 central polypyrimidine tract (77) was introduced into pSIN-18-Rh (34) at the XhoI site upstream of the Rh murine leukemia virus promoter to produce pSIN18RhMLV-E-CPPT. cDNA of the PKA catalytic subunit α (PRKACA: X07767) was amplified with primers bearing AgeI and SalI restriction sites and subcloned into pCR-Blunt TOPO (Invitrogen, Carlsbad, CA), sequenced, released by digestion, purified, and subcloned into AgeI and SalI sites of pSIN18RhMLV-E-CPPT to produce pSIN18RhMLV-E-CPPT-PKA. The PS3 IRES-EGFP sequence was excised from pDF-PS3 (73) and subcloned into the NotI site of a circularized pCRII-TOPO vector (Invitrogen). The EcoRV site upstream of the subcloned fragment was changed into a SalI site, and a SalI/XhoI fragment was subcloned into pSIN18RhMLV-E-CPPT-PKA to produce pSIN18RhMLV-E-CPPT-PKA-PS3-EGFP. The negative control vector pSIN18RhMLV-E-CPPT-EGFP included EGFP sequences in the absence of upstream PRKACA-PS3 sequences. Vectors were constructed by transfection into 293T cells (34), and target cells were transduced by 1 h of incubation in vector-containing supernatants supplemented with 10 μg/ml Polybrene.
RTA promoter activity.
Luciferase reporter assays utilized a 3-kb sequence upstream of the RTA translation start site (pRpluc) (16), and three truncation variants generated by restriction enzyme digestion of pRpluc (pRp1 cut at PstI, pRp2 at NdeI, and pRp8 at KpnI) to delete six potential cAMP response elements (CREs) detected by sequence-based bioinformatics (76); 4 μg of each reporter construct was electroporated along with 50 ng of the control pRLCMV (Renilla luciferase driven by the cytomegalovirus [CMV] promoter) (Promega, Madison, WI) and 6 μg of empty pcDNA3 vector (10 μg total) into 107 KS-1, BC3, BCBL-1, or DG75 cells (240 V, 125 Ω, 950 μF) or 5 × 107 Ramos cells supplemented with 26 μg/ml DEAE dextran (2 cycles of 320 V, 0 Ω, 950 μF), using a BTX ECM 630 pulse generator. Dual luciferase assays (Promega) were performed in triplicate, with firefly luciferase light units normalized to Renilla luciferase light units prior to analysis of log fold change by Student's t test.
RTA trans-activating capacity.
RTA protein was expressed in the context of RTA promoter assays by replacing 4 μg of pcDNA3 with pcDNA3/RTA (a pcDNA3-based vector expressing genomic KSHV RTA under control of the CMV promoter) (16) or pFLAG/RTA (C-terminally FLAG-tagged KSHV RTA) (3, 65). To control for any effect of PKA on the CMV promoter (56), data were normalized to Renilla luciferase levels driven by the CMV promoter (pRLCMV). To assess RTA-mediated trans-activation of a heterologous KSHV promoter, luciferase reporter assays were conducted as above after replacing pRpluc with pLUC/-69, a pGL3-Basic vector expressing firefly luciferase from a 69-nucleotide fragment of the KSHV PAN RNA promoter (64).
Expressed RTA protein levels were assessed in parallel using flow cytometry to detect C-terminal EGFP-tagged ORF50 expressed under control of the same promoter (a kind gift from Joonho Choe, Department of Biological Sciences, Korean Advanced Institute of Science and Technology) (26). Fluorescence intensity data were acquired on a FACScan flow cytometer (BD Immunocytometry) with dead cells and debris excluded on the basis of forward versus side scatter gating using CellQuest software. In each of three replicate experiments, GFP expression was quantified as a percent change above background fluorescence levels, with statistical significance of differences assessed by paired t test.
RTA phosphorylation targets.
The predicted amino acid sequence of RTA (AF091348) was scanned by Phosphobase 2.0 (32) to identify consensus PKA phosphorylation sites (55). Functional significance of predicted PKA and PKC phosphorylation candidates at serines 526 (S525) and 526 (S526) was assessed by converting both codons to alanines through site-directed mutagenesis of pcDNA3/RTA-FLAG, with PCR primers changing RTA nucleotides 1573 to 1574 from GC to AG to produce S525A; and nucleotide 1576 from G to T to produce S526A. Mutations were verified by sequencing and characterized in PAN promoter luciferase assays as above.
Overexpression of β-adrenoreceptors.
To assess the effects of β-adrenoreceptor overexpression in the absence of exogenous ligands, PEL cells were electroporated as described above with 4 μg of mFLAG-β-pcDNA3 (a gift from B. Kobilka, Stanford University) or pcDNA3 (empty vector). At 24 and 48 h later, cells were assayed for lytic protein expression by ORF59 flow cytometry as described above.
RESULTS
Effect of catecholamines on latent KSHV infection.
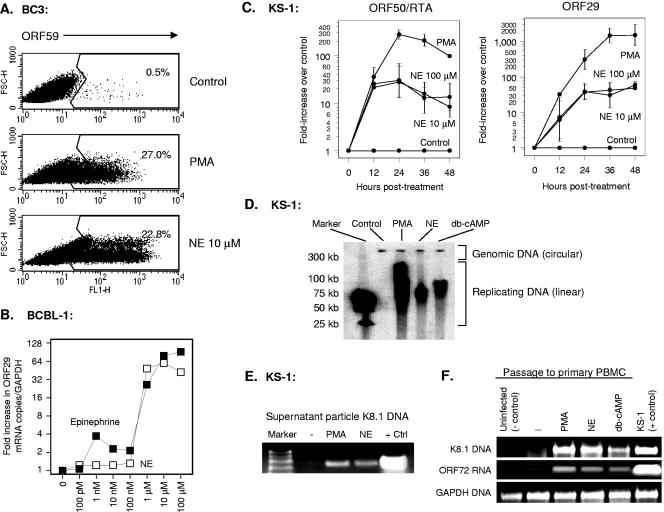
To determine whether autonomic nervous system activity might reactivate lytic replication of KSHV, we treated three latently infected B-cell lines with physiological concentrations of the catecholamine neurotransmitters epinephrine and norepinephrine (18, 39, 58, 61) and assayed lytic protein expression 48 h later. Catecholamine concentrations ranging from 1 nM to 100 μM activated lytic gene expression in KS-1, BC3, and BCBL-1 cells when assessed at the level of protein (Fig. 1A) and mRNA (Fig. 1B). All lytic gene classes were induced, ranging from the immediate-early gene RTA/ORF50 to the late lytic gene ORF29 (Fig. 1C). Similar effects were observed for representative early genes (e.g., ORF59; data not shown). Norepinephrine induced KSHV DNA replication (Fig. 1D), and PCR analysis of cell culture supernatants confirmed an increased concentration of particle-associated KSHV DNA (Fig. 1E). Supernatants from norepinephrine-treated PEL cells contained sufficient concentrations of viral particles to permit de novo infection of primary PBMC (Fig. 1F). Thus, physiological concentrations of norepinephrine can induce the entire lytic replication cycle in lymphoid cells latently infected with KSHV.
FIG. 1.
Effect of norepinephrine (NE) on KSHV reactivation from latency. (A) Expression of the KSHV lytic cycle protein ORF59 was assayed by flow cytometry using indirect immunofluorescence in BC3 cells fixed and permeabilized 24 h after exposure to 20 ng/ml PMA, 10 μM norepinephrine, or an equivalent volume of vehicle. (B) Dose dependence of catecholamine effects on KSHV lytic gene expression were also assessed by quantitative real-time RT-PCR detection of KSHV ORF29 mRNA expression (normalized to GAPDH mRNA) 24 h following exposure of BCBL-1 cells to indicated concentrations of epinephrine or norepinephrine. Similar results were observed for ORF50/RTA mRNA (data not shown). (C) Kinetics of norepinephrine-induced lytic gene expression were assessed for immediate-early ORF50/RTA and late lytic ORF29 at the indicated time points by real-time RT-PCR (data represent mean ± standard error fold change in target mRNA concentration in three replicates after normalization to cellular GAPDH). Norepinephrine induction of ORF50/RTA mRNA was statistically significant at all time points (12 h, P < 0.0001; 24 h, P = 0.0011; 36 h, P = 0.0004; 48 h, P = 0.0027; all by t test on log-transformed induction values). Norepinephrine induction of ORF29 was not significant by 12 h (P = 0.2086), but was highly significant at all subsequent time points (24 h, P = 0.0003; 36 h, P = 0.0006; 48 h, P = 0.0003). Similar kinetics were observed in BCBL-1 cells (data not shown). (D) Gardella gel analysis of replicating KSHV DNA was carried out by electrophoresis of KS-1 cell-associated DNA and hybridization with a 32P-labeled PCR amplicon from the KSHV ORF50 locus to distinguish episomal genomic DNA from linear replicating DNA. Data show cells treated for 48 h with vehicle (Control), PMA, norepinephrine, or the PKA activator db-cAMP. (E) KSHV particle-associated DNA was assayed by PCR amplification of KSHV K8.1 sequences in 20 μl of DNase-treated 0.45-μm-filtered supernatant from KS-1 cell cultures treated with vehicle, PMA, or norepinephrine for 48 h (positive control is KS-1 cell-associated DNA). (F) Presence of infectious KSHV in cell culture supernatants from E was assessed by incubating PHA-stimulated PBMC in filtered/DNased supernatants for 1 h followed by washing. 24 h later, PBMC were assayed for KSHV K8.1 DNA and cellular GAPDH by real-time PCR. PBMC not exposed to PEL cell supernatants served as negative controls, and KS-1 cells served as positive controls.
Mediating receptors.
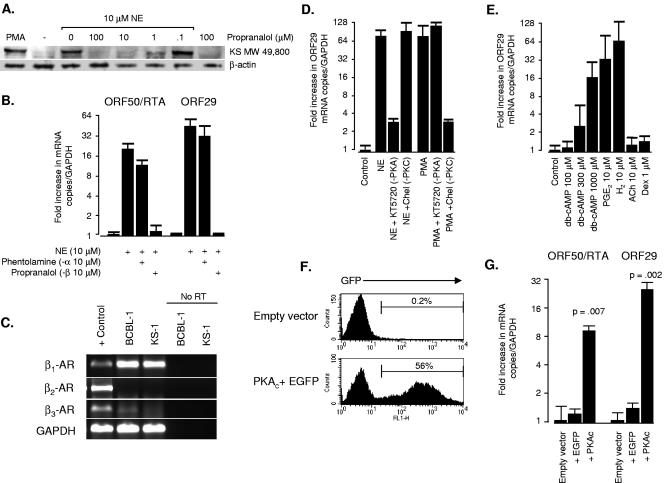
Most catecholamine effects on leukocytes are mediated by β-adrenergic receptors (63) (βAR), but α-adrenoreceptors can also be expressed under certain conditions (30). To identify the specific receptors mediating catecholamine reactivation of KSHV, we pretreated PEL cells with either the β antagonist propranalol or the α antagonist phentolamine for 1 h prior to norepinephrine exposure. β-Blockade efficiently inhibited norepinephrine-mediated reactivation of KSHV lytic protein (Fig. 2A) and mRNA (Fig. 2B). In contrast, α-adrenergic blockade did not affect norepinephrine-mediated reactivation of KSHV lytic gene expression (Fig. 2B). Neither inhibitor had any effect on HHV-8 lytic replication in the absence of exogenous norepinephrine (data not shown).
FIG. 2.
Role of the β-adrenergic receptor and cAMP/PKA signaling pathway in norepinephrine-induced reactivation of KSHV. (A) To define the role of β-adrenergic receptors in norepinephrine-mediated reactivation of lytic gene expression, BCBL-1 cells were treated with indicated concentrations of the β-antagonist propranalol for 1 h before exposure to 10 μM norepinephrine, and then assayed for KSHV lytic protein expression by Western blot 48 h later (primary antibody: Kaposi's sarcoma patient serum). Results show induction of the major KSHV lytic protein species at MW 49,800 which was the only band diagnostic of reactivation by the PMA positive control. (B) Specificity of adrenergic receptor involvement was tested by treating KS-1 cells with the α-antagonist phentolamine or the β-antagonist propranalol for 1 h prior to norepinephrine (NE) exposure; 24 h later, concentrations of mRNA for the immediate-early ORF50/RTA and late ORF29 were assessed by real-time RT-PCR (values normalized to GAPDH and expressed as a ratio relative to vehicle-treated controls). (C) Expression of mRNA for β1, β2, and β3 adrenergic receptors was assessed in untreated KS-1 and BCBL-1 cells by real-time RT-PCR. Products were resolved on a 3.5% agarose gel and compared to positive control PCR products (parallel amplification of genomic DNA for β1 and β3 adrenergic receptors or a PBMC cDNA library for β2 adrenergic receptors and GAPDH). To verify that RT-PCR results were free of contaminating DNA, BCBL-1 and KS-1 RNA samples were amplified in parallel in the absence of reverse transcriptase (No RT). Data are representative of four independent experiments in which β1 adrenergic receptors were consistently detected at high levels, and β2 and β3 receptors were not significantly expressed. (D) To evaluate the role of PKA and PKC in norepinephrine activation of KSHV lytic gene expression, KS-1 cells were pretreated with the PKA antagonist KT5720 (1 μM) or the PKC antagonists chelerythrine chloride (Chel, 1 μM) or bisindolylmaleimide HCl (300 nM) for 1 h prior to norepinephrine exposure. Expression of mRNA for the late lytic gene ORF29 was assayed by real-time RT-PCR 24 h later. PKA blockade inhibited norepinephrine-induced lytic gene expression by >90%, but PKC inhibitors failed to block norepinephrine effects. Chelerythrine and bisindolylmaleimide (data not shown) efficiently blocked PMA induction of ORF29, verifying that inhibitors were capable of blocking known PKC activators. KT5720 failed to block PMA-mediated ORF29 expression, indicating that norepinephrine/PKA and PMA/PKC signaling pathways are functionally independent in PEL cells. (E) To determine whether cAMP activity was sufficient to induce lytic gene expression, KS-1 cells were treated with graded doses of pharmacological db-cAMP or with indicated concentrations of physiological cAMP inducers prostaglandin E2 (PGE2) and histamine (H2). cAMP/PKA specificity was evaluated using hormones that signal through alternative pathways, such as acetylcholine (ACh) or the glucocorticoid dexamethasone (Dex). (F) To determine if PKA activity alone is sufficient to induce lytic gene expression, KS-1 cells were transduced with a lentiviral vector (34) expressing the catalytic subunit of PKA (PKAc) and a downstream EGFP reporter sequence translated from a single mRNA bearing the PS3 synthetic internal ribosome entry site (73). At 48 h following transduction with an empty vector, or vector bearing bicistronic EGFP and PKAc, EGFP-positive cells were quantified by flow cytometry to assess transduction efficiency. Cells transduced with EGFP alone showed transduction efficiencies comparable to comparable to EGFP plus PKAc (data not shown). (G) Also at 48 h posttransduction, concentrations of mRNA for immediate-early ORF50/RTA and late lytic ORF29 were quantified by real-time RT-PCR (data normalized to GAPDH and expressed as a ratio relative to empty vector controls). Data represent the mean (± standard error) of three independent experiments, with statistical significance evaluated by paired t test. Similar effects were observed with BCBL-1 cells (data not shown).
To define the subtype of β receptor responsible for these effects, we assayed mRNA for each of the three known βAR types by RT-PCR and found only β1 adrenoreceptor mRNA to be detectable in appreciable quantities in PEL cells (Fig. 2C). Catecholamine sensitivity profiles matched the known pharmacology of β1ARs, with epinephrine triggering a low-range increase in KSHV lytic gene expression (∼1 nM range) in addition to paralleling norepinephrine-mediated induction over the range of 100 nM to 10 μM (Fig. 1B). β-Adrenoreceptors required exogenous stimulation to activate KSHV lytic genes, as shown by studies in which overexpression of β-adrenoreceptors in the absence of adrenergic ligands failed to induce ORF59 protein expression (data not shown; average 0.4% cells positive by flow cytometry at 24 h versus 0.2% positive for the empty vector control, difference P = 0.625).
Role of the cAMP/PKA signaling pathway.
In lymphoid cells, βARs signal primarily through Gαs-mediated activation of the adenylyl cyclase/cAMP/PKA signaling pathway (29, 63). Other signaling pathways can potentially be activated (15, 38, 79), so we sought to verify PKA's role by treating PEL cells with the PKA antagonist KT5720 prior to catecholamine exposure. KT5720 inhibited norepinephrine induction of ORF29 mRNA by more than 90% (Fig. 2D), and had similar effects on RTA/ORF50 (data not shown). In contrast, the protein kinase C (PKC) antagonists chelerythrine chloride and bisindolylmaleimide hydrochloride failed to block norepinephrine-mediated induction of KSHV lytic genes (results for chelerythrine chloride shown in Fig. 2D, and similar data for bisindolylmaleimide hydrochloride not shown). Both PKC antagonists blocked KSHV reactivation by PMA, but KT5720 had no effect on PMA-mediated lytic gene expression. Thus, PKA and PKC represent functionally independent signaling pathways for KSHV reactivation in PEL cells.
Direct activation of cAMP signaling with the cell-permeable analogue db-cAMP also activated KSHV lytic gene expression, and other physiological cAMP inducers such as prostaglandin E2, and histamine (H2) had similar effects (Fig. 2E). Hormone-induced reactivation was specific to ligands that stimulate Gαs/adenylyl cyclase/cAMP/PKA signaling. Hormones that signal through other pathways failed to induce KSHV lytic gene expression (e.g., acetylcholine or the glucocorticoid receptor agonist dexamethasone) (Fig. 2E).
Activation of PKA is the most common mechanism by which cAMP regulates cell function, but other cAMP targets have been identified (17). To determine whether PKA activity can account for cAMP-induced KSHV reactivation, we expressed a constitutively active form of PKA in KS-1 and BCBL-1 cells using a self-inactivating lentiviral vector. The α catalytic subunit of PKA (PKAc) was expressed from a bicistronic mRNA that included a C-terminal EGFP reporter sequence independently translated via a synthetic internal ribosome entry site. This vector transduced more then 50% of KS-1 and BCBL-1 cells (Fig. 2F). Expression of the reporter gene alone had minimal impact on KSHV lytic gene mRNA levels, but expression of the reporter gene in conjunction with PKAc up-regulated expression of both immediate-early (RTA) and late lytic transcripts (ORF29) (Fig. 2G). Thus, PKA activity alone is sufficient to induce lytic gene expression in lymphoid cells latently infected with KSHV.
Effect of PKA signaling on RTA expression and activity.
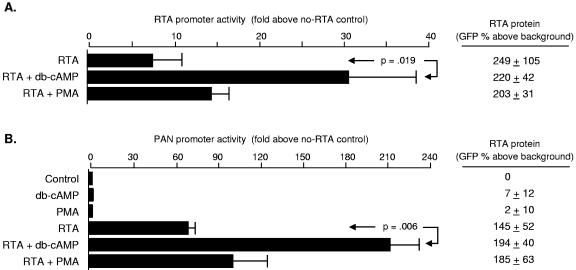
KSHV reactivation from latency is controlled by RTA-mediated trans-activation, and we sought to determine how βAR/PKA signaling might modulate that process. To evaluate direct effects on the RTA promoter in the absence of any contribution from viral gene products, uninfected DG75 or Ramos cells were electroporated with a reporter construct expressing firefly luciferase under the control of ∼3 kb of KSHV genomic DNA upstream of ORF50 (previously described in reference 16). Cells were then stimulated with the PKA activator db-cAMP or the PKC activator PMA, and luciferase reporter activity was assessed 18 h later. Reporter gene activity increased by fivefold following PKA activation in DG75 cells (Fig. 3A) and 14-fold in Ramos cells (not shown), indicating that cellular transcription factors alone are sufficient to mediate PKA induction of the RTA promoter. Similar effects emerged in the context of BC3 and KS-1 PEL cells (Fig. 3A and data not shown, respectively).
FIG. 3.
Regulation of RTA promoter activity by PKA. (A) Activity of the RTA promoter was assessed by luciferase reporter assays in which pRpluc (firefly luciferase coding sequence controlled by ∼3 kb of KSHV genomic DNA upstream of ORF50) was electroporated into the Ramos or DG75 B cell lines, which are known to be free of KSHV and Epstein-Barr virus, or into BC3 PEL cells containing latent KSHV. Following electroporation, cells were incubated for 18 h in medium supplemented as indicated with the PKA activator db-cAMP (300 μM) or the PKC activator PMA (20 ng/ml). Firefly luciferase activity was normalized to Renilla luciferase activity generated by pRLCMV (Renilla luciferase under control of the CMV promoter), and the statistical significance of triplicate determinations was evaluated by t test. Results showed significant PKA-mediated induction of RTA promoter activity in both cell types, but db-cAMP up-regulated RTA promoter activity more strongly KSHV-containing BC3 cells (as indicated by a cell type × db-cAMP interaction term from a factorial analysis of variance, P < 0.0001). Similar effects were observed when PEL cell lines were treated with 10 μM norepinephrine (data not shown). (B) Bioinformatic analysis of the RTA promoter revealed six potential cAMP response elements (CREs) within 2 kb of the ORF50 transcription start site. To assess their role, we compared db-cAMP effects on the full-length promoter (pRpluc) with effects on a series of truncation mutants omitting the distal 1.05 kb but retaining all six putative CREs (pRp1), omitting the distal four CREs (pRp2), and omitting all predicted CREs (pRp8). Experiments were carried out KS-1 cells as described above (A), with data represented as the mean (+ standard error) fold enhancement in normalized luciferase activity for db-cAMP-treated cells relative to controls. Deletion of the distal four CREs led to a quantitative reduction in PKA-inducibility, but db-cAMP continued to activate the RTA promoter even in constructs lacking all predicted CRE sites (e.g., pRp8). Similar results emerged in BC3 cells (data not shown).
Several cellular transcription factors respond to PKA, including the CREB/ATF family of basic leucine zipper proteins (5, 40, 47, 53). Bioinformatic analysis of the 3-kb sequence upstream of ORF50 revealed six putative cAMP response elements (CREs) that could potentially support CREB/ATF-mediated activation of the RTA promoter (Fig. 3B). To define the functional significance of these sites, we compared PKA responsiveness of the full-length promoter (−3.09 kb) with that of truncated variants lacking the distal four CREs (between −1.32 and −2.04 kb) or all six CREs (−0.29 kb) (Fig. 3B). Reporter constructs were electroporated into BC3 PEL cells (to assess activity in a the context of KSHV latency) or uninfected Ramos cells (to assess activity in the absence of KSHV gene products) and cells were subsequently treated for 18 h with db-cAMP or PMA. Deletion of the distal four CREs reduced PKA inducibility by approximately 50% in BC3 PEL cells (Fig. 3B), and by a similar amount in uninfected Ramos cells (data not shown). Despite this quantitative reduction in PKA responsiveness, the RTA promoter continued to show significant induction by db-cAMP even when all six putative CRE sites were eliminated (an average 17-fold enhancement over basal activity in the −0.29-kb construct, P < 0.001; Fig. 3B). Thus, CREB/ATF factors may quantitatively enhance PKA effects on the RTA promoter, but other transcription factors also play a significant role.
Reporter studies conducted in KSHV-infected PEL cells suggested that viral gene products might participate in PKA-mediated activation of the RTA promoter. As shown in Fig. 3A, PKA activation up-regulated RTA promoter activity by ∼16-fold in KSHV-infected BC3 cells, compared to ∼5-fold in uninfected DG75 tested in parallel (difference, P = 0.0063 as assessed by the cell type × db-cAMP interaction term from a factorial analysis of variance, F[1, 8] = 13.49). Norepinephrine had similar effects (data not shown), with RTA promoter activity increasing by an average 9.37-fold in BCBL-1 PEL cells (standard error ± 1.6-fold, P = 0.0092 by t test), and significantly less in DG75 cells (difference from BCBL-1, P = 0.0012, F[1, 4] = 56.47).
One of the most powerful inducers of the RTA promoter is the RTA protein itself (16, 25, 62). To determine whether PKA might enhance RTA's trans-activating capacity independently of its effects on RTA protein levels, we expressed RTA in trans from a heterologous promoter and assessed db-cAMP effects on RTA promoter activity. As in previous studies (16, 25, 62), RTA protein significantly enhanced activity of the RTA promoter (Fig. 4A). Addition of db-cAMP increased that effect by fourfold, from an average of sevenfold above basal activity to more than 30-fold (Fig. 4A). This increase in trans-activating capacity did not stem from changes in RTA protein levels, which were measured in parallel using flow cytometry to quantify expression of GFP-tagged ORF50 (right panel Fig. 4A). Similar effects were observed in latently infected KS-1 and BCBL-1 cells, with promoter activity increasing from ∼16-fold above basal activity levels in the presence of RTA alone to ∼125-fold above basal activity following the addition of db-cAMP (difference P = 0.007 by t test; data not shown). Norepinephrine also exerted similar effects, with RTA promoter activity increasing from 15.5- ± 2.9-fold above basal levels with RTA protein alone to 40.8- ± 1.4-fold above basal levels when RTA was supplemented by 10 μM norepinephrine (data not shown). Thus, βAR/PKA signaling appears to posttranslationally enhance the ability of RTA protein to trans-activate the RTA promoter.
FIG. 4.
Posttranslational enhancement of RTA trans-activating capacity by PKA. (A) To determine whether RTA protein might contribute to PKA-mediated activation of RTA promoter activity, FLAG-tagged RTA was expressed in trans (vector: pFLAG/RTA) in uninfected DG-75 cells and supplemented by 1 mM db-cAMP or 20 ng/ml PMA as indicated. Luciferase activity was expressed as the mean fold change above basal promoter activity for triplicate determinations, with statistical significance assessed by t test. Firefly luciferase activity was normalized to Renilla luciferase from pRLCMV, driven by the same CMV immediate-early enhancer-promoter used to express RTA. To ensure that PKA effects were not mediated by altered RTA protein levels, parallel electroporation studies quantified the density of GFP-tagged RTA by flow cytometry. Results of three independent studies are reported as the mean ± standard error of green fluorescence intensity after subtraction of background fluorescence intensity in cells electroporated with vector DNA alone. Neither db-cAMP nor PMA significantly enhanced RTA protein levels. (B) To determine whether PKA-induced enhancement of RTA trans-activating capacity affects heterologous viral promoters, reporter assays were carried out as in A substituting pLUC/-69 (firefly luciferase driven by the KSHV PAN promoter) (64) for the RTA reporter construct. Effects of PKA signaling were tested in uninfected Ramos cells with no ectopic RTA (equivalent quantity of vector DNA) or ectopic RTA as in A. Parallel flow cytometry analyses of cells electroporated with an ORF50-GFP expression vector verified substantial expression of ORF50 protein (P < 0.001 by t test), but db-cAMP and PMA did not significantly alter protein quantity.
To determine whether PKA enhancement of RTA trans-activating capacity might impact other KSHV lytic genes, we also analyzed activity of the promoter driving expression of the KSHV polyadenylated nuclear (PAN) RNA gene (3, 64). In uninfected DG75 or Ramos cells, db-cAMP had a negligible effect on the PAN promoter (Fig. 4B and Fig. 5). Expression of RTA alone significantly increased PAN promoter activity, as previously reported (36, 64, 65, 70). Activation of PKA in conjunction with RTA expression led to a further enhancement of reporter gene activity, rising from ∼60-fold above basal activity to >200-fold (Fig. 4B). Thus, PKA signaling can enhance RTA-mediated induction of KSHV lytic promoters even when the PKA pathway has no direct effect on the promoter via cellular transcription factors. Norepinephrine induced similar dynamics, with RTA-mediated activation of the PAN promoter rising from 150.4- ± 6.0-fold above basal activity to 1,518.0- ± 333.0-fold in the presence of norepinephrine (difference P = 0.0097 by t test; data not shown).
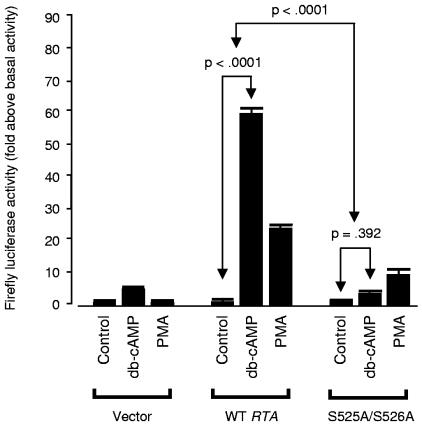
FIG. 5.
Role of RTA serines 525 and 526 in PKA-mediated enhancement of trans-activation. To evaluate the functional role of predicted phosphorylation targets at S525 and S526 of RTA, both residues were converted to alanines through PCR-based site-directed mutagenesis. RTA's trans-activating capacity was assayed using a luciferase reporter driven by the KSHV PAN promoter as in Fig. 4B. Reporter constructs were electroporated into uninfected Ramos cells accompanied by either 1 μg of pFLAG/RTA (WT RTA), 1 μg of pFLAG/RTA-S525A-S526A (mutant), or 1 μg of empty pFLAG (Vector). Cells were subsequently cultured in the presence of 300 μM db-cAMP or 20 ng/ml PMA as indicated, and Firefly luciferase activity was measured 18 h later as a fold change above basal activity after normalization to control Renilla luciferase levels. Values represent the mean (± standard error) fold increase in PAN promoter activity relative to the vehicle-treated control for each construct (Vector, WT RTA, S525A-S526A mutant RTA). Similar results emerged when data were normalized to a common basal condition (e.g., vehicle-treated cells electroporated with the empty vector). In similar studies using KS-1 cells and norepinephrine, endogenous regulation of RTA expression complicated analyses but data continued to show greater PKA-induced up-regulation of RTA activity on the PAN promoter for wild-type RTA versus the S525A-S526A mutant (results reported in main text).
Posttranslational effects on RTA.
RTA protein is extensively phosphorylated (36), but it is unclear what role this plays in regulating lytic gene expression. Bioinformatic analysis of the RTA coding sequence identified 10 potential PKA phosphorylation sites (32, 55). One site at serine 526 (S526) fell adjacent to a predicted PKC phosphorylation target at serine 525 (S525), suggesting that this region might represent a generalized target for serine/threonine kinases that modulate KSHV activity. To evaluate that hypothesis, we converted both serine codons to alanines via site-directed mutagenesis and tested the resulting mutant RTA (S525A-S526A) for PKA-mediated enhancement of PAN promoter activity.
The S525A-S526A mutations substantially impaired PKA's ability to enhance the basal trans-activating capacity of RTA (Fig. 5). PAN promoter activity increased >50-fold above basal levels when wild-type RTA was supplemented with PKA signaling, but less than 2-fold when the S525A-S526A mutant was accompanied by PKA activation (difference P < 0.0001, assessed by the interaction term from a factorial analysis of variance, F[1, 8] = 55.77). Thus, PKA-induced up-regulation of RTA activity requires the S525/S526 tandem serines. PKC also enhanced the trans-activating capacity of wild-type RTA (PMA effects in Fig. 5), although this effect was less pronounced than that of PKA. PKC's effects were also abrogated by the S525A-S526A mutations, suggesting that this region of RTA might represent a generalized target for modulation by serine/threonine kinases. Although the S525A-S526A mutant was resistant to trans-activational enhancement by both PKA and PKC, it remained a functional transcription factor as shown by its capacity to induce the PAN promoter by an average of 20-fold above its basal activity level (P < 0.0001 by t test).
DISCUSSION
The present data show that physiological concentrations of catecholamine neurotransmitters can efficiently reactivate KSHV lytic replication in latently infected lymphoid cells. These effects are mediated by β1 adrenoreceptors and subsequent activation of the cAMP/PKA signaling pathway. Other activators of the PKA signaling pathway have similar effects (e.g., db-cAMP, prostaglandin E2, histamine), and overexpression of the PKA catalytic subunit alone is sufficient to induce KSHV lytic gene expression. β-Adrenergic signaling induces expression of KSHV genes from all stages of the lytic replication cycle and is accompanied by genome replication and production of infectious viral particles. These effects appear to be mediated by PKA regulation of the viral RTA gene-the primary “molecular switch” controlling KSHV lytic replication (36, 70). Both norepinephrine and PKA induce RTA gene expression via cellular transcription control pathways, and both signals can also enhance the capacity of existing RTA protein to trans-activate viral lytic cycle promoters (including the RTA promoter). These results suggest that autonomic nervous system activity could constitute an important determinant of KSHV reactivation dynamics in vivo. Αdrenergic reactivity also suggests several potential therapeutic strategies, including β-blockade to limit reactivation or use of β-agonists to flush latent virus into lytic replication for eradication by nucleoside analogues (31).
PKA signaling alone appears to be sufficient to reactivate KSHV, as shown by the parallel effects of physiological PKA inducers (e.g., catecholamines, prostaglandin E2, and histamine), pharmacological PKA activators (e.g., db-cAMP), and genetic overexpression of the PKA catalytic subunit. Complementary findings have recently emerged from a genome-wide overexpression scan that identified the PKA catalytic subunit as one of the most powerful inducers of RTA-mediated trans-activation (J. Harada, Genomics Institute of the Novartis Foundation). These data suggest that KSHV might reactivate in response to a broad spectrum of extracellular signals that converge on the cAMP/PKA second messenger system. Most previous studies of KSHV have relied on PKC activators to induce lytic replication (e.g., PMA/tetradecanoyl phorbol acetate) (36). Like PKA, PKC is a ubiquitous serine/threonine kinase mediating transcriptional response to a diverse array of extracellular stimuli (51, 52). Although the biological effects of these two kinases are often antagonistic, PKA and PKC target similar amino acid motifs (52, 55). The PKA-mediated pathway for KSHV reactivation is independent of the PKC-mediated pathway, as shown by the fact that PKC inhibitors failed to block norepinephrine induction of lytic gene expression. However, the parallel effects of PKA and PKC on KSHV lytic gene expression suggest a generalized role for cellular serine/threonine kinases in regulating the balance between viral latency and lytic replication.
The present studies identify two distinct mechanisms by which the βAR/cAMP/PKA signaling pathway can modulate the activity of RTA. Cellular transcription control pathways can directly activate the RTA promoter in the absence of KSHV gene products (e.g., in uninfected B lymphoid cells), and PKA can posttranslationally enhance the trans-activating capacity of RTA protein. PKA control of RTA gene expression appears to be mediated in part by CREB/ATF transcription factors, as shown by a 50% decline in PKA-mediated induction of RTA promoter constructs that lack any identifiable CRE. However, PKA can still enhance RTA promoter activity by >15-fold in the absence of CRE sites, suggesting that other transcription factors also play a significant role. Identifying those factors and mapping their mechanisms of action will provide further information about the host cell processes regulating KSHV replication. As in the present analysis of the βAR/cAMP/PKA pathway, such studies could also identify new systemic regulators of KSHV activity and suggest further targets for therapeutic intervention.
Alternative strategies for controlling KSHV reactivation may also come from the discovery that PKA and PKC share a common capacity to regulate the RTA protein's activity as a transcription factor. Kinase-mediated posttranslational modification of RTA could explain why PKA activates KSHV lytic promoters more strongly in PEL cells than in similar B lymphoid cells lacking endogenous viral gene products. Given the parallel effects of PKA and PKC on RTA activity, and the functional blockade of those effects by phoshpho-resistant mutations at serines 525 and 526, it is tempting to speculate that kinase-induced phosphorylation mediates this posttranslational effect. The presence of these serines within a nuclear localization signal (33) also suggests a potential mechanism for functional modulation. However, we have not been able to directly verify changes in the phosphorylation of either wild-type serine due to the high background levels of phosphorylation previously noted (36). In addition, mutation of either serine alone failed to block PKA's effect. This could indicate a requirement for coordinated activity of PKA and PKC, or the need for more complex secondary structural alterations to completely block the effects of either kinase alone.
Posttranslational enhancement of RTA trans-activating capacity requires more detailed analysis, but the present data clearly show that cellular kinases can regulate the activity of this key viral switch protein. Similar results have emerged in analyses of PKC-mediated control of the Epstein-Barr virus BZLF transcription factor (1, 20, 22), suggesting that gammaherpesvirus lytic regulators may have evolved a functional sensitivity to cellular kinases as a general mechanism for synchronizing reactivation with favorable cellular conditions. If so, pharmacological manipulation of cellular kinases could provide potential strategies for controlling gammaherpesvirus replication.
Αdrenergic reactivity is a common characteristic of α- and β-herpesviruses (2, 56, 59, 74, 75) and may explain the propensity of those viruses to reactivate in response to environmental stress (27, 42, 54, 59, 74, 75). The gammaherpesvirus Epstein-Barr virus is sensitive to stress-induced glucocorticoids (4, 24, 35, 41, 67, 68), and the present studies suggest that KSHV might also reactivate during stress-induced autonomic nervous system activity. The emergence of stress-reactive biology in all major classes of Herpesviridae suggests a potential evolutionary advantage for latent viruses to monitor organismal stress levels, which could be diagnostic of reduced cellular immune threat (28, 54, 72) or lowered host-transmission hurdles (9, 69). Reactivation of KSHV in response to catecholamines may thus represent one example in which herpesviruses monitor their host to detect ecological conditions favoring their survival in much the same way they seek to create those conditions by manipulating the cellular microenvironment (48).
Acknowledgments
This work was supported by the National Institutes of Health (S.C.: AI49135, AI52737; R.S.: CA91791 and DE14153; J.Z.: AI36059 and AI 36554), the Norman Cousins Center at UCLA, and the James L. Pendelton Charitable Trust.
REFERENCES
- 1.Baumann, M., H. Mischak, S. Dammeier, W. Kolch, O. Gires, D. Pich, R. Zeidler, H. J. Delecluse, and W. Hammerschmidt. 1998. Activation of the Epstein-Barr virus transcription factor BZLF1 by 12-O-tetradecanoylphorbol-13-acetate-induced phosphorylation. J. Virol. 72:8105-8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom, D. C., J. G. Stevens, J. M. Hill, and R. K. Tran. 1997. Mutagenesis of a cAMP response element within the latency-associated transcript promoter of HSV-1 reduces adrenergic reactivation. Virology 236:202-207. [DOI] [PubMed] [Google Scholar]
- 3.Brown, H. J., M. J. Song, H. Deng, T. T. Wu, G. Cheng, and R. Sun. 2003. NF-κB inhibits gammaherpesvirus lytic replication. J. Virol. 77:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacioppo, J. T., J. K. Kiecolt-Glaser, W. B. Malarkey, B. F. Laskowski, L. A. Rozlog, K. M. Poehlmann, M. H. Burleson, and R. Glaser. 2002. Autonomic and glucocorticoid associations with the steady-state expression of latent Epstein-Barr virus. Horm. Behav. 42:32-41. [DOI] [PubMed] [Google Scholar]
- 5.Carey, M., and S. T. Smale. 2000. Transcriptional regulation in eukaryotes: concepts, strategies, and techniques. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 6.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 7.Chang, J., R. Renne, D. Dittmer, and D. Ganem. 2000. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology 266:17-25. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 9.Cole, S. W. 2005. The complexity of dynamic host networks, p. 605-628. In T. S. Deisboeck and S. Kauffman (ed.), Complex systems science in biomedicine. Kluwer Academic-Plenum Publishers, New York, N.Y.
- 10.Cole, S. W., B. D. Jamieson, and J. A. Zack. 1999. cAMP externalizes lymphocyte CXCR4: implications for chemotaxis and HIV infection. J. Immunol. 162:1392-1400. [PubMed] [Google Scholar]
- 11.Cole, S. W., M. E. Kemeny, J. L. Fahey, J. A. Zack, and B. D. Naliboff. 2003. Psychological risk factors for HIV pathogenesis: mediation by the autonomic nervous system. Biol. Psychiatry 54:1444-1456. [DOI] [PubMed] [Google Scholar]
- 12.Cole, S. W., M. E. Kemeny, S. E. Taylor, B. R. Visscher, and J. L. Fahey. 1996. Accelerated course of human immunodeficiency virus infection in gay men who conceal their homosexual identity. Psychosomatic Med. 58:219-231. [DOI] [PubMed] [Google Scholar]
- 13.Cole, S. W., Y. D. Korin, J. L. Fahey, and J. A. Zack. 1998. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J. Immunol. 161:610-616. [PubMed] [Google Scholar]
- 14.Cole, S. W., B. D. Naliboff, M. E. Kemeny, M. P. Griswold, J. L. Fahey, and J. A. Zack. 2001. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. Proceedings of the National Academy of Sciences of the USA. 98:12695-12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davare, M. A., V. Avdonin, D. D. Hall, E. M. Peden, A. Burette, R. J. Weinberg, M. C. Horne, T. Hoshi, and J. W. Hell. 2001. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293:98-101. [DOI] [PubMed] [Google Scholar]
- 16.Deng, H., A. Young, and R. Sun. 2000. Auto-activation of the rta gene of human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus. J Gen. Virol. 81:3043-3048. [DOI] [PubMed] [Google Scholar]
- 17.de Rooij, J., F. J. Zwartkruis, M. H. Verheijen, R. H. Cool, S. M. Nijman, A. Wittinghofer, and J. L. Bos. 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474-477. [DOI] [PubMed] [Google Scholar]
- 18.Dimsdale, J. E., and J. Moss. 1980. Plasma catecholamines in stress and exercise. JAMA 243:340-342. [PubMed] [Google Scholar]
- 19.Dittmer, D., M. Lagunoff, R. Renne, K. Staskus, A. Haase, and D. Ganem. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 72:8309-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Guindy, A. S., L. Heston, Y. Endo, M. S. Cho, and G. Miller. 2002. Disruption of Epstein-Barr virus latency in the absence of phosphorylation of ZEBRA by protein kinase C. J. Virol. 76:11199-11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fakhari, F. D., and D. P. Dittmer. 2002. Charting latency transcripts in Kaposi's sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol. 76:6213-6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis, A., T. Ragoczy, L. Gradoville, L. Heston, A. El-Guindy, Y. Endo, and G. Miller. 1999. Amino acid substitutions reveal distinct functions of serine 186 of the ZEBRA protein in activation of early lytic cycle genes and synergy with the Epstein-Barr virus R transactivator. J. Virol. 73:4543-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardella, T., P. Medveczky, T. Sairenji, and C. Mulder. 1984. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J. Virol. 50:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser, R., L. A. Kutz, R. C. MacCallum, and W. B. Malarkey. 1995. Hormonal modulation of Epstein-Barr virus replication. Neuroendocrinology 62:356-361. [DOI] [PubMed] [Google Scholar]
- 25.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gwack, Y., H. Byun, S. Hwang, C. Lim, and J. Choe. 2001. CREB-binding protein and histone deacetylase regulate the transcriptional activity of Kaposi's sarcoma-associated herpesvirus open reading frame 50. J. Virol. 75:1909-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins, F. J., and A. Baum. 1995. Stress and reactivation of latent herpes simplex virus: a fusion of behavioral medicine and molecular biology. Ann. Behav. Med. 17:116-123. [DOI] [PubMed] [Google Scholar]
- 28.Kalinichenko, V. V., M. B. Mokyr, L. H. Graf, Jr., R. L. Cohen, and D. A. Chambers. 1999. Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a beta-adrenergic receptor mechanism and decreased TNF-alpha gene expression. J. Immunol. 163:2492-2499. [PubMed] [Google Scholar]
- 29.Kammer, G. M. 1988. The adenylate cyclase-cAMP-protein kinase A pathway and the regulation of the immune response. Immunol. Today 9:222. [DOI] [PubMed] [Google Scholar]
- 30.Kavelaars, A. 2002. Regulated expression of alpha-1 adrenergic receptors in the immune system. Brain Behav. Immunol. 16:799-807. [DOI] [PubMed] [Google Scholar]
- 31.Kedes, D. H., and D. Ganem. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J. Clin. Investig. 99:2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreegipuu, A., N. Blom, and S. Brunak. 1999. PhosphoBase, a database of phosphorylation sites: release 2.0. Nucleic Acids Res. 27:237-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnan, R., A. Kavirayani, K. Driscoll, W. Bu, D. Palmeri, and D. M. Lukac. 2004. Nuclear/cytoplasmic localization of the KSHV ORF50/RTA protein can be manipulated to regulate its activity. Abstract from the Seventh international workshop on KSHV and related agents, University of California, Santa Cruz, 21 August 2004.
- 34.Kung, S. K., S. S. An, and I. S. Chen. 2000. A murine leukemia virus (MuLV) long terminal repeat derived from rhesus macaques in the context of a lentivirus vector and MuLV gag sequence results in high-level gene expression in human T lymphocytes. J. Virol. 74:3668-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kupfer, S. R., and W. C. Summers. 1990. Identification of a glucocorticoid-responsive element in Epstein-Barr virus. J. Virol. 64:1984-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 38.Luttrell, L. M., S. S. Ferguson, Y. Daaka, W. E. Miller, S. Maudsley, G. J. Della Rocca, F. Lin, H. Kawakatsu, K. Owada, D. K. Luttrell, M. G. Caron, and R. J. Lefkowitz. 1999. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283:655-661. [DOI] [PubMed] [Google Scholar]
- 39.Madden, K. S., V. M. Sanders, and D. L. Felten. 1995. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu. Rev. Pharmacol. Toxicol. 35:417-448. [DOI] [PubMed] [Google Scholar]
- 40.Mayr, B., and M. Montminy. 2001. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell. Biol. 2:599-609. [DOI] [PubMed] [Google Scholar]
- 41.Mehta, S. K., D. L. Pierson, H. Cooley, R. Dubow, and D. Lugg. 2000. Epstein-Barr virus reactivation associated with diminished cell-mediated immunity in Antarctic expeditioners. J. Med. Virol. 61:235-240. [DOI] [PubMed] [Google Scholar]
- 42.Mehta, S. K., R. P. Stowe, A. H. Feiveson, S. K. Tyring, and D. L. Pierson. 2000. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J. Infect. Dis. 182:1761-1764. [DOI] [PubMed] [Google Scholar]
- 43.Mellon, P. L., C. H. Clegg, L. A. Correll, and G. S. McKnight. 1989. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 86:4887-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mercader, M., B. Taddeo, J. R. Panella, B. Chandran, B. J. Nickoloff, and K. E. Foreman. 2000. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am. J. Pathol. 156:1961-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, G., M. O. Rigsby, L. Heston, E. Grogan, R. Sun, C. Metroka, J. A. Levy, S. J. Gao, Y. Chang, and P. Moore. 1996. Antibodies to butyrate-inducible antigens of Kaposi's sarcoma-associated herpesvirus in patients with HIV-1 infection. N. Engl. J. Med. 334:1292-1297. [DOI] [PubMed] [Google Scholar]
- 46.Monini, P., S. Colombini, M. Sturzl, D. Goletti, A. Cafaro, C. Sgadari, S. Butto, M. Franco, P. Leone, S. Fais, G. Melucci-Vigo, C. Chiozzini, F. Carlini, G. Ascherl, E. Cornali, C. Zietz, E. Ramazzotti, F. Ensoli, M. Andreoni, P. Pezzotti, G. Rezza, R. Yarchoan, R. C. Gallo, and B. Ensoli. 1999. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood 93:4044-4058. [PubMed] [Google Scholar]
- 47.Montminy, M. 1997. Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem. 66:807-822. [DOI] [PubMed] [Google Scholar]
- 48.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. M. Knipe and P. M. Howley (ed.), Fields' virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 49.Moore, P. S., and Y. Chang. 2001. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Phil. Trans. R. Soc. Lond. B Biol. Sci. 356:499-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore, P. S., S. J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishizuka, Y. 1992. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258:607-614. [DOI] [PubMed] [Google Scholar]
- 52.Nishizuka, Y. 1986. Studies and perspectives of protein kinase C. Science 233:305-312. [DOI] [PubMed] [Google Scholar]
- 53.Pabo, C. O., and R. T. Sauer. 1992. Transcription factors: structural families and principles of DNA recognition. Annu. Rev. Biochem. 61:1053-1095. [DOI] [PubMed] [Google Scholar]
- 54.Padgett, D. A., J. F. Sheridan, J. Dorne, G. G. Berntson, J. Candelora, and R. Glaser. 1998. Social stress and the reactivation of latent herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 95:7231-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearson, R. B., and B. E. Kemp. 1991. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 200:62-81. [DOI] [PubMed] [Google Scholar]
- 56.Prosch, S., C. E. C. Wendt, P. Reinke, C. Priemer, M. Pooert, D. H. Kruger, H.-D. Volk, and W.-D. Docke. 2000. A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology 272:357-365. [DOI] [PubMed] [Google Scholar]
- 57.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 58.Richter, S. D., T. H. Schurmeyer, M. Schedlowski, A. Hadicke, U. Tewes, R. E. Schmidt, and T. O. Wagner. 1996. Time kinetics of the endocrine response to acute psychological stress. J. Clin. Endocrinol. Metab. 81:1956-1960. [DOI] [PubMed] [Google Scholar]
- 59.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 60.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saitoh, M., T. Yanagawa, T. Kondoh, H. Miyakoda, H. Kotake, and H. Mashiba. 1995. Neurohumoral factor responses to mental (arithmetic) stress and dynamic exercise in normal subjects. Intern. Med. 34:618-622. [DOI] [PubMed] [Google Scholar]
- 62.Sakakibara, S., K. Ueda, J. Chen, T. Okuno, and K. Yamanishi. 2001. Octamer-binding sequence is a key element for the autoregulation of Kaposi's sarcoma-associated herpesvirus ORF50/lytA gene expression. J. Virol. 75:6894-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanders, V. M., and R. H. Straub. 2002. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav. Immun. 16:290-332. [DOI] [PubMed] [Google Scholar]
- 64.Song, M. J., H. J. Brown, T. T. Wu, and R. Sun. 2001. Transcription activation of polyadenylated nuclear RNA by RTA in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:3129-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song, M. J., X. Li, H. J. Brown, and R. Sun. 2002. Characterization of interactions between RTA and the promoter of polyadenylated nuclear RNA in Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 76:5000-5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 67.Stowe, R. P., S. K. Mehta, A. A. Ferrando, D. L. Feeback, and D. L. Pierson. 2001. Immune responses and latent herpesvirus reactivation in spaceflight. Aviat. Space Environ. Med. 72:884-891. [PubMed] [Google Scholar]
- 68.Stowe, R. P., D. L. Pierson, D. L. Feeback, and A. D. Barrett. 2000. Stress-induced reactivation of Epstein-Barr virus in astronauts. Neuroimmunomodulation 8:51-58. [DOI] [PubMed] [Google Scholar]
- 69.Stumpf, M. P. H., Z. Laidlaw, and V. A. A. Jansen. 2002. Herpesviruses hedge their bets. Proc. Natl. Acad. Sci. USA 99:15234-15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun, R., S. F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valitutti, S., M. Dessing, and A. Lanzavecchia. 1993. Role of cAMP in regulating cytotoxic T lymphocyte adhesion and motility. Eur. J. Immunol. 23:790-795. [DOI] [PubMed] [Google Scholar]
- 73.Venkatesan, A., and A. Dasgupta. 2001. Novel fluorescence-based screen to identify small synthetic internal ribosome entry site elements. Mol. Cell. Biol. 21:2826-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner, E. K., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitley, R. J. 2001. Herpes simplex viruses, p. 2461-2509. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 76.Wingender, E., P. Dietze, H. Karas, and R. Knuppel. 1996. TRANSFAC: a database on transcription factors and their DNA binding sites. Nucleic Acids Res. 24:238-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]
- 78.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zou, Y., I. Komuro, T. Yamazaki, S. Kudoh, H. Uozumi, T. Kadowaki, and Y. Yazaki. 1999. Both Gs and Gi proteins are critically involved in isoproterenol-induced cardiomyocyte hypertrophy. J. Biol. Chem. 274:9760-9770. [DOI] [PubMed] [Google Scholar]