Abstract
Herpes simplex virus type 1 (HSV-1) glycoprotein E (gE) promotes cell-to-cell spread at basolateral surfaces of epithelial cells, but its activity in neurons is less clear. We used the mouse retina infection model and neuronal cell cultures to define the spread phenotype of gE mutant viruses. Wild-type (WT) and gE-null (NS-gEnull) viruses both infected retina ganglion cell neurons; however, NS-gEnull viral antigens failed to reach the optic nerve, which indicates a defect in axonal localization. We evaluated two Fc receptor-negative gE mutant viruses containing four amino acid inserts in the gE ectodomain. One mutant virus failed to spread from the retina into the optic nerve, while the other spread normally. Therefore, the gE ectodomain is involved in axonal localization, and the Fc receptor and neuronal spread are mediated by overlapping but distinct gE domains. In the retina infection model, virus can travel to the brain via the optic nerve from presynaptic to postsynaptic neurons (anterograde direction) or via nerves that innervate the iris and ciliary body from postsynaptic to presynaptic neurons (retrograde direction). WT virus infected the brain by anterograde and retrograde routes, whereas NS-gEnull virus failed to travel by either pathway. The site of the defect in retrograde spread remains to be determined; however, infection of rat superior cervical ganglia neurons in vitro indicates that gE is required to target virion components to the axon initial segment. The requirement for gE in axonal targeting and retrograde spread highlights intriguing similarities and differences between HSV-1 and pseudorabies virus gE.
Herpes simplex virus type 1 (HSV-1) infects skin, oral, ocular, and genital epithelial cells and spreads to axon fibers that innervate these tissues. The viral DNA, surrounded by capsid and tegument proteins, is transported to the neuronal cell body (3), where the DNA becomes latent in the nucleus. Transport of virus or virion components from epithelial cells to the neuron nucleus involves spread from epithelial cells to the axon terminus (cell-to-cell spread) and transport from the axon terminus to the nucleus (retrograde direction) (53). When the viral DNA reactivates from latency, virion components are produced in the neuron cell body which localize to the axon (axonal localization) and then travel along axon fibers to the axon terminus (anterograde direction) (53). The site for virion assembly in the neuron during reactivation infection remains controversial for alphaherpesviruses and is proposed to occur in the cell body (17), in axon shafts (31, 51), or at the axon terminus (26, 39). Finally, mature virions or virion subunits spread from the axon terminus to epithelial cells (cell-to-cell spread) to produce recurrent infections.
Glycoprotein E (gE) is required for efficient HSV-1 spread from cell-to-cell within epithelial cells in vitro and in vivo, since gE-null virus produces smaller plaques than wild-type (WT) virus (18, 43), and causes smaller skin lesions at the inoculation site in the murine flank model (37). The nature of the spread defect for gE-null virus in neuronal tissues is less clear. A defect in cell-to-cell spread between neurons was suggested based on a reduction in infected cell foci compared to WT virus (19). In vivo experiments using a rat retina infection model in which virus was inoculated into the vitreous body of the eye also indicated that gE-null virus is defective in neuronal cell-to-cell spread based on reduced staining of viral antigens in the retina and brain (19). However, these experiments did not address the possibility that the gE-null virus defect may be in targeting virion components from the neuronal cell body to the axon initial segment or in transporting virion components along axon fibers to the axon terminus rather than from one neuron to another.
The retina infection model can be used to address the site of the spread defect for gE-null virus. An advantage of this model is that virus directly infects the ganglion cell neurons that comprise the innermost cell layer of the retina without first infecting epithelial cells (18, 31). Virion components, including nucleocapsids, tegument, and membrane glycoproteins, move from the cell bodies of the retina ganglion cells into the axon fibers of these same neurons that form the optic nerve and optic tract (30, 31), which represents spread in the anterograde direction. If virion components fail to reach the optic nerve, the spread defect can be localized to a site proximal to the axon terminus rather than implicating cell-to-cell spread. The mature virus or virion components then travel from presynaptic to postsynaptic neurons in an anterograde direction to the brain. During infection of the mouse or rat eye, virus can also infect the endplates of autonomic neurons that innervate the ciliary body and iris to control pupil size (Fig. 1A) (30). Virion components travel from postsynaptic to presynaptic neurons in a retrograde direction to infect regions of the brain that are distinct from those infected by anterograde spread in the optic nerve (9, 40). Therefore, the retina eye model can be used to assess HSV-1 anterograde and retrograde spread to the brain.
FIG. 1.
Mouse retina infection model. (A) Model of the mouse eye. The needle penetrates all layers of the retina before entering the vitreous body (arrow). Virus infects retina cells (red) and can spread anterograde into the optic nerve and to the brain. Virus also infects the iris and ciliary body and travels in a retrograde direction along the autonomic nerves that innervate these structures. (B) Mouse retina. Virus in the vitreous body infects ganglion cell neurons (innermost layer) and spreads cell-to-cell to neuronal and non-neuronal cells in the retina. Viral proteins that are transported along the ganglion cell axon fibers move anterograde in the nerve fiber layer into the optic nerve.
The spread phenotype of a related alphaherpesvirus, pseudorabies (PRV) gE-null virus, has been extensively studied in vivo and in vitro (9, 12, 27, 49, 52). In the rat retina infection model, PRV gE-null virus failed to reach second-order neurons in the brain that synapse with the optic nerve, indicating a defect in anterograde spread from presynaptic to postsynaptic neurons (9). Of note, the PRV gE-null virus had no defect in spread to nuclei in the brain, which indicates intact spread from postsynaptic to presynaptic neurons (retrograde spread) (9, 27). In vitro studies using rat superior cervical ganglia (SCG) neurons demonstrated that few gE-null viral proteins were detected in axon fibers, despite abundant expression in the neuron cell body, suggesting a defect in axonal localization (12).
We used the mouse retina infection model to further define the site of the spread defect for HSV-1 gE-null virus. We demonstrated that gE-null viral proteins are not detected in the optic nerve, despite extensive infection of the retina. In vitro studies with rat SCG neurons demonstrated that virion proteins failed to enter the axon, indicating a defect in axonal localization, which is similar to the defect noted for PRV gE-null virus (12). HSV-1 gE-null virus was also defective in spread to the brain in the retrograde direction, which differs from PRV gE-null virus, and highlights interesting similarities and differences between HSV-1 and PRV gE (9).
MATERIALS AND METHODS
Virus strains.
HSV-1 strain NS is a low-passage clinical isolate that was used as the WT virus (23), and all mutant strains were prepared in the NS background. NS-gEnull virus has a deletion of gE amino acids 124 to 510 in the gE ectodomain (37), whereas rNS-gEnull virus rescues the gE-null defect (37). NS-gE380 virus has four amino acids (XhoI linker) inserted after gE position 380 (43). NS-gE264 virus was prepared as described for NS-gE380 virus (2, 43) by inserting the same four amino acids after gE position 264. The locations of the gE mutations are based on the published sequence of HSV-1 strain 17 (35); however, the NS gE sequence has two additional amino acids (Gly and Glu) at positions 186 and 187 (33). Therefore, the inserts in NS-gE380 and NS-gE264 viruses are at gE positions 382 and 266, and the deletion in the gE-null virus involves amino acids 124 to 512. HSV-1 KOS-gDβ is a gD-null virus formed by replacing gD sequences with lacZ DNA that is under the control of the gD promoter (16). A gD-null pseudotype virus capable of a single replication cycle was prepared by growing this virus on gD complementing (VD60) cells (15, 32). Virus pools were prepared on Vero cells and purified on 5 to 70% sucrose gradients (24). Virus titers were determined by plaque assay on Vero cells (23).
Eye injections.
Mice were anesthetized intraperitoneally with 2.5 mg of ketamine and 0.63 mg of xylazine. A cut in the sclera was made with a 30-gauge needle, and this needle was then used to penetrate into the vitreous body of the right eye. The puncture hole was entered by using a Hamilton syringe and 32-gauge needle containing 1 μl of 4 × 102 to 4 × 105 PFU of virus, which was injected into the vitreous body (Fig. 1A). The needle was held in place for 30 s to minimize loss of fluid from the vitreous body.
Harvesting eyes and brains.
Tissues were harvested 3 to 8 days postinfection (dpi) (47). Mice were anesthetized and perfused by intracardiac injection with 10 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer. Eyes were dissected leaving the optic nerve attached to the retina. Brains were removed, and eyes and brains were fixed separately in 4% paraformaldehyde overnight and then in 30% sucrose overnight at 4°C. Samples were embedded in tissue freezing medium (Polysciences, Inc.) and frozen in an alcohol and dry ice bath. Eyes were cut into 10-μm sagittal sections and brains were cut into 40-μm coronal sections by using a cryostat at −22°C. Eye sections for immunofluorescence were placed on Tissue Tack slides (Polysciences, Inc.) and allowed to dry overnight at room temperature. Brain sections for immunohistochemistry were placed in cryoprotectant solution (30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol in 0.1 M phosphate-buffered saline [PBS]; pH 7.2) and stored at −20°C until staining (9).
Rat superior cervical ganglion cell neuron cultures.
Sympathetic motor neurons were harvested from rat SCG of rat embryos age 15.5 days (Hilltop Labs, Inc.) (14). Glass coverslips were coated with 100 μg of poly-dl-ornithine (Sigma-Aldrich) and 10 μg of laminin (Invitrogen)/ml and placed in 35-mm dishes. Neurons were cultured in equal volumes of Dulbecco modified Eagle medium and Ham F-12 supplemented with 10 mg of bovine serum albumin (BSA)/ml, 4.6 mg of glucose/ml, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 100 mg of holotransferrin/ml, 16 μg of putrescine/ml, 10 μg of insulin/ml, 30 nM selenium, 20 nM progesterone, and 100 ng of nerve growth factor/ml (reagents from Gibco, Sigma-Aldrich, and Invitrogen). Non-neuronal cells were eliminated by adding 1 μM cytosine-β-d-arabinofuranoside (Sigma-Aldrich). At 3 weeks after plating, the neuron cultures were infected with 105 PFU of WT or gE mutant virus for 1 h, the inoculum was removed, and the infection was allowed to proceed for 20 h (12).
Immunofluorescence.
Retinas were stained with a rabbit polyclonal HSV-1 antibody (Dako), a VP5 capsid antibody (provided by Gary Cohen and Roselyn Eisenberg), a VP22 tegument antibody (provided by Gillian Elliott), and nonimmune immunoglobulin G (IgG; Sigma-Aldrich). Rat anti-mouse Thy 1.2 (Pharmingen) was used to stain mouse axon fibers (1), and mouse anti-rat CD90 (Thy 1.1) (Antigenix America) was used to stain rat SCG neurons. Secondary antibodies included donkey anti-rabbit F(ab′)2 Red-X, anti-rat F(ab′)2 Cy2, and anti-mouse F(ab′)2 Cy2 (Jackson Immunoresearch). Nonspecific staining of mouse retina sections was reduced by incubating tissues in PBS with 1% horse serum, 1% BSA, and 0.05% Triton X-100 for 30 min. Primary antibodies were diluted 1:500 and secondary antibodies were diluted 1:200 to 1:250 in PBS with 0.3% Triton X-100. Primary antibodies were added for 2 h, and secondary antibodies were added for 1 h at room temperature. DAPI (4′,6′-diamidino-2-phenylindole) was added at 0.1 μg/ml (Molecular Probes) to stain nuclei.
Neuron cultures were fixed for 10 min with 3.2% paraformaldehyde, incubated with PBS and 3% BSA for 2 h at room temperature to reduce background staining, and then permeabilized for 10 min by using PBS with 3% BSA and 1% saponin. Primary and secondary antibodies were diluted in PBS-BSA-saponin buffer and added for 1 h. Washes were performed with PBS-BSA-saponin buffer (11). Slides were mounted with Fluoromount-G (Southern Biotech) and viewed on a Nikon Eclipse E1000 deconvolution microscope. Images were processed by using software from Phase 3 Imaging Systems.
Immunohistochemistry.
Brains were processed for immunohistochemistry by removing the cryoprotectant solution with 0.1 M PBS. The cryostat sections were incubated with 0.5% sodium borohydride for 10 min, washed with 0.1 M PBS, treated with 30% methanol and 0.5% hydrogen peroxide for 10 min, and washed with 0.1 and 0.01 M PBS. Polyclonal rabbit anti-HSV-1 antibody (1:2,500) was added for 36 h at room temperature, followed by biotinylated goat anti-rabbit IgG (1:200) and avidin-biotin/peroxidase (1:100; Vector Laboratories, Inc.) each added for 90 min at room temperature. The color was developed by using diaminobenzidine and hydrogen peroxide, and sections were mounted onto gelatin-coated slides (8).
Western blots.
To determine whether the polyclonal HSV-1 antibody (Dako) detects viral glycoproteins, Vero cells were mock-infected or infected with WT virus at a multiplicity of infection (MOI) of 2.5. The cells were lysed (20 mM Tris-Cl [pH 7.5], 50 mM NaCl, 0.5% deoxycholic acid, 0.5% NP-40, and protease inhibitors) at 21 h postinfection (hpi). Viral glycoproteins B, C, D (provided by Gary Cohen and Roselyn Eisenberg), and E that were truncated prior to their transmembrane domains were purified from supernatant fluids of baculovirus-infected cells (4, 33, 44, 48). Cell lysates and purified glycoproteins were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on a 4 to 15% gel, transferred to an Immobilon-P membrane, and probed with polyclonal HSV-1 antibody (1:5,000; Dako).
To detect viral antigens in optic nerve tissue, the optic nerve, optic chiasm, and optic tract were harvested as a unit and separated from the retina. Tissues were homogenized in lysis buffer (0.2 M Tris-Cl [pH 7.2], 10 mM EDTA, 0.3 M NaCl, 0.1% SDS, 0.05% Tween 20, and protease inhibitors; Roche Diagnostics), samples were sonicated on ice and centrifuged at 9,500 × g for 10 min at 4°C, and supernatant fluids were collected. Protein content was determined by the BCA assay (Pierce), and equal concentrations were loaded onto a 4 to 15% SDS-PAGE gel. Proteins were transferred to Immobilon-P membranes and probed with murine monoclonal antibody (MAb) 1D3 (1:1,000) to detect gD (22), murine MAb ICP5 (1:100; Biodesign) to detect VP5, and rabbit anti-VP22 (1:10,000) to detect VP22. Rabbit anti-human actin (1:2,000), which cross-reacts with mouse actin (Novus Biologicals), was used to detect actin as a loading control. Membranes were developed by using ECL (Amersham Corp.). Densitometry analysis was performed to compare the relative concentrations of VP5 antigen after WT or NS-gEnull virus infection of the retina.
Harvesting the retinas for determination of virus titers.
Mice were anesthetized, perfused with PBS, and euthanatized, and the lenses and corneas were removed. The retinas were washed with PBS and frozen in an ethanol and dry ice bath. The retinas were thawed, homogenized in PBS, sonicated, and centrifuged at 1,500 × g at 4°C for 5 min, and the virus titers of the supernatant fluids were determined on Vero cells.
IgG rosetting assays.
IgG Fc receptor expression on HSV-1-infected cells was detected by using IgG-coated erythrocytes. COS cells were infected with WT or gE mutant viruses at an MOI of 2, and at 18 hpi the cells were dissociated and incubated with IgG-coated erythrocytes at a ratio of 100 erythrocytes per infected COS cell. Rosettes were counted by using an inverted light microscope. Infected cells that bound ≥4 erythrocytes were considered positive (43).
Statistics.
P values were determined by using the Student t test. The results were considered significant at a probability (P) of <0.05.
RESULTS
HSV-1 wild-type and gE-null viruses infect the mouse retina.
We investigated the role of HSV-1 gE in spread by using the mouse retina infection model (Fig. 1). Retinas were stained with polyclonal HSV-1 antibody, which detects many viral proteins, including the ectodomains of gB, gC, gD, and gE (Fig. 2A). WT and NS-gEnull viruses infected the mouse retina after injection into the vitreous body. Infection was slightly more extensive with WT than NS-gEnull virus, based on immunofluorescence staining of retinas at 3 and 5 dpi (Fig. 2B). Studies were performed with a gD-null pseudotype virus to verify that the increase in infection by WT and gE-null viruses on day 5 represented virus spread in the retina. gD is required for HSV-1 entry as cell-free virus but is also essential for spread of virion components from one cell to another (32, 41). The gD-null pseudotype virus is capable of entering cells since gD protein is present in the virion envelope, but spread from one cell to another does not occur since no gD protein is produced. When gD-null pseudotype virus was injected into the vitreous body, very little antigen was detected on day 3 and no change was noted on day 5 (Fig. 2B), indicating that the increase in viral antigen after WT and NS-gEnull virus infection represented spread in the retina. Virus titers in the retina were somewhat higher after WT than NS-gEnull virus infection (Fig. 3A), although differences were not statistically significant. Western blot of retinas at 5 dpi showed more VP5 capsid antigen after WT virus infection (Fig. 3B and C), which is consistent with the immunofluorescence and virus titer results.
FIG. 2.
gE-null virus infects the retina. (A) Western blot performed on Vero cell extracts of mock-infected (lane 1) and WT virus-infected (lane 2) cells and truncated purified baculovirus proteins gD, gE, gC, and gB (lanes 3 to 6, respectively) (200 ng each) probed with polyclonal HSV-1 antibody (Dako). (B) Mouse eyes were infected with 4 × 104 PFU of WT, NS-gEnull, or KOS-gDβ (gD-null pseudotype) virus, and retinas were stained for viral antigens by immunofluorescence at 3 and 5 dpi. The gD-null pseudotype virus infects very few cells (white arrow). Abbreviations: RPE, retinal pigmented epithelium; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Colors: red, viral antigens (polyclonal HSV-1 antibody); green, ganglion cell axons (anti-Thy1.2); blue, nuclei (DAPI). Magnification, ×200. Photomicrographs of infection with WT and NS-gEnull viruses are representative of three retinas per virus examined on day 3 and 10 retinas per virus examined on day 5. The results shown with KOS-gDβ virus are representative of eight retinas evaluated on day 3 and nine retinas on day 5.
FIG. 3.
Virus titers and detection of viral antigens in infected retinas. (A) Virus titers in mouse retinas 1, 3, and 5 dpi with WT or NS-gEnull virus. The results are the mean ± the standard deviation of two to three retinas per time point. (B) Western blot of retinas 5 dpi with WT or NS-gEnull virus (three pooled retinas per virus). Actin was used as a loading control. (C) Densitometry was used to measure the relative intensity of VP5 staining by Western blot, which is reduced for NS-gEnull virus compared to WT virus.
gE is required for viral spread from the retina to the optic nerve.
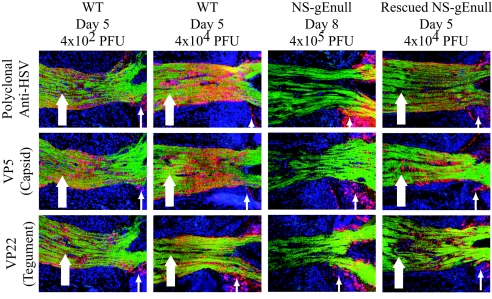
We examined the optic nerve to evaluate whether virion proteins travel into the axon fibers of neurons that originate in the retina. Mouse eyes were injected with 4 × 102 or 4 × 104 PFU of WT virus and stained with polyclonal anti-HSV antibody, antibody to VP5 capsid protein, or antibody to VP22 tegument protein at 5 dpi. At both WT virus inoculation titers, viral antigens were readily detected in the optic nerve with all three antibodies (Fig. 4). In contrast, no viral proteins, including gB, gC, gD, VP5, or VP22 antigens, were detected in the optic nerve by immunofluorescence after infection with NS-gEnull virus at 4 × 105 PFU (1,000-fold-higher dose than WT virus) either at day 5 (result not shown) or day 8, despite infection in the retina (Fig. 4). Rescued gE-null virus produced infection in the optic nerve comparable to WT virus, indicating that deletion of the gE DNA accounted for the defect in viral proteins reaching the optic nerve.
FIG. 4.
NS-gEnull virus antigens are not detected in the optic nerves of infected eyes. Mouse eyes were infected with 4 × 102 or 4 × 104 PFU of WT virus, 4 × 105 PFU of NS-gEnull virus, or 4 × 104 PFU of rescued NS-gEnull virus. Optic nerves were harvested at 5 or 8 dpi and stained by immunofluorescence with polyclonal antibody to HSV-1, VP5, and VP22. Optic nerves are shown at the exit site from the retina (thin arrows). WT and rescued NS-gEnull antigens were detected in the retinas and optic nerves (thick arrows). NS-gEnull virus antigens were detected in the retinas but not in the optic nerves. Viral antigens are red, optic nerve fibers are green (anti-Thy1.2), and nuclei are blue (DAPI). Magnification, ×200.
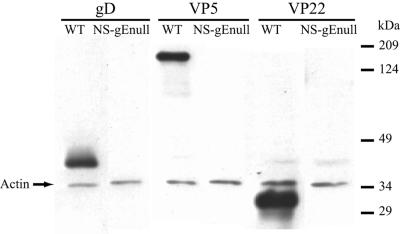
Western blots were performed as an additional approach to detect viral antigens in the optic nerve. Antibodies to capsid VP5 protein and tegument VP22 protein were evaluated; however, instead of polyclonal anti-HSV antibody we used an antibody to gD, which is the most abundant HSV-1 membrane glycoprotein detected in strain NS (25). All three proteins were detected 5 dpi with 4 × 104 PFU of WT virus, whereas no viral antigen was evident after infection with a 10-fold-higher dose of NS-gEnull virus examined 8 dpi (Fig. 5). Therefore, after gE-null virus infection no viral proteins were detected in the optic nerve by immunofluorescence and Western blotting, despite extensive infection of the mouse retina.
FIG. 5.
Western blot of viral proteins in the optic nerves. Mouse eyes were infected with 4 × 104 PFU of WT virus and the optic nerves were harvested at 5 dpi or with 4 × 105 PFU of NS-gEnull virus and the optic nerves were harvested at 8 dpi. Optic nerves from five mice were pooled and probed for gD, VP5, and VP22. Actin was used as a loading control.
Optic nerve infection and IgG Fc-receptor activities involve overlapping but distinct gE domains.
We evaluated two HSV-1 gE linker insertion mutant viruses to determine whether spread of viral antigens into the optic nerve and Fc receptor activity are mediated by distinct gE domains. NS-gE264 and NS-gE380 have four amino acids inserted in the gE ectodomain at NS gE positions 266 and 382, respectively. We previously reported that NS-gEnull and NS-gE380 viruses are defective in IgG Fc receptor activity (43). We evaluated NS-gE264 virus for IgG Fc receptor activity by counting the percentage of infected cells that formed rosettes with IgG-coated erythrocytes. NS-gE264 virus failed to form rosettes (IgG Fc receptor negative) and was as impaired as NS-gE380 or NS-gEnull virus (Fig. 6A).
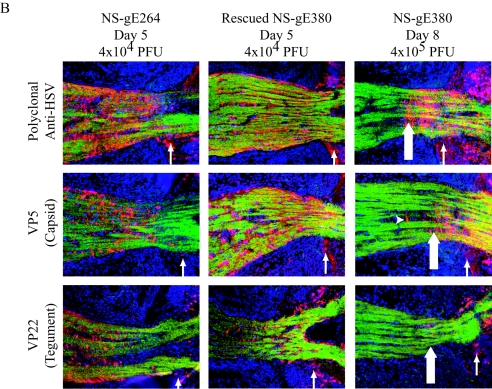
FIG. 6.
Viral antigen spread into the optic nerve and IgG Fc receptor activity are separable gE functions. (A) COS cells were infected at an MOI of 2 with WT, NS-gE380, NS-gE264, or NS-gEnull virus and tested for rosetting of IgG-coated erythrocytes at 18 hpi. The three gE mutant viruses all have reduced IgG Fc receptor activity compared to WT virus (P < 0.001). (B) Mice were infected with 4 × 104 PFU of NS-gE264 virus, 4 × 104 PFU of rescued NS-gE380 virus, or 4 × 105 PFU of NS-gE380 virus. The optic nerves were harvested at 5 dpi for NS-gE264 and rescued NS-gE380 viruses and at 8 dpi for NS-gE380 virus. NS-gE264 virus infected the retina (thin arrow) and spread into the optic nerve similar to rescued NS-gE380 virus, whereas NS-gE380 virus infected the retina (thin arrow) but was defective in spread into the optic nerve (thick arrow). The arrowhead shows a supporting glial cell infected in the optic nerve and stained with VP5 antibody. Viral antigens are red, optic nerve axon fibers are green (anti-Thy1.2), and nuclei are blue (DAPI). Magnification, ×200. The results shown are representative of optic nerves obtained from three mice for NS-gE264 virus, four mice for rescued NS-gE380 virus, and ten mice for NS-gE380 virus.
We previously reported that rescued NS-gE380 virus restores Fc receptor activity and virulence in the murine flank model (43). We postulated that the reduced virulence of NS-gE380 virus likely reflected a defect in spread rather than Fc receptor activity, since murine IgG does not bind to the HSV-1 Fc receptor (28). We demonstrated the importance of the HSV-1 IgG Fc receptor in mice by passive transfer of human IgG, which is capable of binding to the HSV-1 Fc receptor (37).
Retina eye injection studies were performed with NS-gE264, NS-gE380, and rescued NS-gE380 viruses to further characterize the spread defect of NS-gE380 virus and to determine whether gE uses distinct domains for spread in neurons and Fc binding. All three viruses produced extensive retina infection. Viral antigens were readily detected in the optic nerve after infection with rescued NS-gE380 virus (Fc receptor positive) and NS-gE264 virus (Fc receptor negative) at 5 dpi, whereas little antigen was noted in the optic nerve after infection with NS-gE380 virus (Fc receptor negative) at 5 dpi (results not shown) or 8 dpi (Fig. 6B). Therefore, viral antigen spread into the optic nerve and Fc receptor activities are overlapping but distinct gE functions, and the gE ectodomain contributes to both processes. Of note, the NS-gE380 and NS-gEnull viruses differ in their infection phenotypes since some NS-gE380 antigens were detected in the proximal segment of the optic nerve, whereas none were detected after NS-gEnull virus infection.
HSV-1 spread to the brain.
We evaluated the transport of WT, NS-gEnull, and NS-gE380 viruses to the mouse brain after retina infection. WT viral antigens were detected in the optic tract, dorsal and ventral lateral geniculate nuclei, and superior colliculus, indicating anterograde spread. Viral antigen was also detected in the intergeniculate leaflet of the lateral geniculate nucleus and in the oculomotor and Edinger-Westphal nuclei, representing retrograde spread (Fig. 7). After infection with NS-gEnull or NS-gE380 virus at 10-fold-higher doses than used for WT virus, no viral antigen was detected in regions indicative of anterograde spread at 5 dpi (results not shown) or 8 dpi (Fig. 7). However, NS-gE380 antigens were detected in the oculomotor and Edinger-Westphal nuclei, supporting retrograde spread, although staining was less intense than with WT virus and antigens were not present until 8 dpi (Fig. 7C). Therefore, the NS-gEnull mutant virus is defective in both anterograde and retrograde spread, whereas the NS-gE380 mutant virus is defective in anterograde spread with a reduced capability for retrograde spread.
FIG. 7.
NS-gEnull viral antigens do not reach the brain by anterograde or retrograde spread. Mouse eyes were infected with 4 × 104 PFU of WT virus and 4 × 105 PFU of NS-gEnull or NS-gE380 viruses. Brains were stained by immunohistochemistry using polyclonal HSV-1 antibody at 5 dpi for WT virus or at 8 dpi for NS-gEnull and NS-gE380 viruses. (A) Antigens from WT virus were found in the optic tract (arrow) and the dorsal lateral geniculate nucleus (arrowhead). NS-gEnull and NS-gE380 viruses did not infect these nuclei. (B) Antigens from WT virus were detected in the dorsal lateral geniculate nucleus (arrowhead), the ventral lateral geniculate nucleus (hollow arrowhead), and the intergeniculate leaflet of the lateral geniculate nucleus (arrow). NS-gEnull and NS-gE380 viruses did not infect theses nuclei. (C) Antigens from WT virus were detected in the superior colliculus (arrowhead) and in the oculomotor and Edinger-Westphal nuclei (arrow). No viral antigen was detected in these nuclei after infection with NS-gEnull virus. Antigens from NS-gE380 virus were present in the oculomotor and Edinger-Westphal nuclei (arrow). The results shown are representative of four brains infected with WT virus and three brains infected with NS-gEnull and NS-gE380 viruses.
Infection of rat SCG neurons in vitro.
We infected primary neuronal cells to further define the site of the block in axonal infection for NS-gEnull virus. Embryonic rat SCG neurons were infected with 105 PFU of WT or gE mutant viruses. At 20 hpi, cells were permeabilized and stained with polyclonal HSV, VP5, or VP22 antibodies (Fig. 8A). WT viral antigens were apparent in the neuron cell bodies and axon fibers. NS-gEnull viral antigens were detected in the cell bodies but not in the axon fibers. Therefore, the neuronal cell spread defect for the gE mutant viruses is prior to or at the axon initial segment.
FIG. 8.
gE is required for HSV-1 anterograde spread in vitro. Embryonic rat SCG neurons were infected with 105 PFU of WT or NS-gEnull virus (A) or NS-gE380, NS-gE264, or KOS-gDβ virus (B). At 20 hpi, neurons were stained with anti-Thy1.2 (green) and polyclonal anti-HSV, anti-VP5, or anti-VP22 (red). Nuclei were stained with DAPI (blue). White arrows indicate viral antigens in axon fibers. Magnification, ×200. Boxed inset areas are shown in the adjacent photomicrograph at ×400 magnification.
We compared the Fc receptor mutant viruses, NS-gE264 and NS-gE380, for infection of SCG neurons. Both viruses infected the neuron cell bodies; however, viral antigens were only detected in axons after infection with the NS-gE264 virus (Fig. 8B). The cells shown were stained with polyclonal HSV antibody (Fig. 8B); however, similar results were obtained with VP5 and VP22 antibodies (results not shown). Viral antigens were also readily detected in axons after infection with the gD-null pseudotype virus, KOS-gDβ (Fig. 8B). Therefore, spread in the retina was reduced for gE-null virus (slight impairment) and KOS-gDβ virus (marked impairment), but only the gE-null mutant virus was defective in antigen expression in axons, which indicates a defect in axonal localization.
DISCUSSION
We demonstrated a defect in transport of major envelope glycoproteins, VP22 tegument and VP5 capsid proteins into the optic nerve after NS-gEnull virus infection of the mouse retina. HSV-1 gE-null viral antigens were readily observed in the retina, including the innermost cell layer consisting of ganglion cell neurons. The axon fibers of these neurons form the optic nerve, and no viral antigens were observed in the optic nerve near its origin from the retina. Viral antigens were undetectable despite using a 1,000-fold higher titer for infection with NS-gEnull (4 × 105 PFU) than WT (4 × 102 PFU) virus and staining for antigens in the optic nerve at 8 dpi, which is 3 days longer than for WT virus. These results place the site of the defect in NS-gEnull virus spread prior to the axon terminus. The in vitro studies with embryonic rat SCG neurons indicate that the defect is in axonal localization, since viral antigens fail to enter the axon. The gE-null virus spread defect in vivo is probably not related to reduced retina infection, since gE-null virus replicates and spreads in the retina to levels that are only minimally different than WT virus, and yet in vitro gE-null virus is markedly defective in axonal localization.
The in vitro results with NS-gEnull virus are similar to findings recently reported by Ch'ng and Enquist that showed a defect in anterograde axonal localization for PRV gE-null virus (12). However, the defect reported with PRV gE-null virus appears to be less complete than with HSV-1 gE-null virus, since some viral proteins were detected in the proximal axon segments after PRV infection, whereas none were detected after HSV-1 infection. Additional studies will be required to further define the magnitude of the defect for HSV-1 gE-null virus, including immunofluorescent staining for additional viral proteins and quantitative cultures using a two-chamber system as used to assess PRV infection (13).
The axonal localization defect after retina infection using HSV-1 and PRV gE-null viruses are similar; however, the viruses are strikingly different in retrograde spread. PRV gE-null virus is not defective in retrograde spread from the rat eye to the oculomotor and Edinger-Westphal nuclei in the brain (9, 27), while HSV-1 gE-null virus fails to spread from the mouse eye to these nuclei. These differences are probably not related to the animal species used (rats for PRV and mice for HSV-1), since PRV gE-null virus spreads retrograde from the skin to dorsal root ganglia and the brain in a murine flank model (8), while HSV-1 gE-null virus fails to spread to dorsal root ganglia in this mouse model (43). In addition, our current results demonstrate that HSV-1 gE-null virus has a similar spread phenotype in rat neurons in vitro and murine neurons in vivo. The results described in the present study are entirely consistent with our prior findings in the murine flank model that showed a total defect in retrograde spread for NS-gEnull virus and a partial reduction for NS-gE380 virus based on viral DNA levels in dorsal root ganglia after skin infection (43).
Our mouse retina infection model results differ from a prior report of HSV-1 gE-null virus in the rat retina infection model (19). We found smaller differences in retina infection comparing WT and gE-null viruses but greater differences in brain infection comparing the two viruses (19). In the prior report, no distinctions were made between anterograde and retrograde spread of gE-null virus to the brain and, importantly, we conclude that the defect in gE-null virus anterograde spread is in axonal localization rather than in cell-to-cell spread.
Our study does not define the site of the block in retrograde spread for gE-null virus. HSV-1 gE is not required for virus entry into epithelial cells (18, 43), and current evidence suggests that transport of virion components from the cell membrane to the nucleus of polarized epithelial cells (retrograde spread) involves nucleocapsid and tegument proteins but not membrane glycoproteins (3, 20, 34, 46). However, mechanisms of transport of virion components to cell nuclei may differ depending on whether the virus enters via the axon terminus or the cell body and whether the virus enters by fusing with cell membranes or by endocytosis (38). Additional studies with in vitro neuronal cell cultures will be required to define the site of the block for gE-null virus in retrograde spread.
The molecular mechanisms accounting for the axonal localization defect of HSV-1 gE-null virus remain to be determined. We are considering several potential mechanisms. One possibility is that gE is required for the mature virus or virion subunits to travel from the trans-Golgi network to the axon. A second consideration is that gE is necessary for the mature virus to penetrate the cytoskeletal network at the axon initial segment. The axon initial segment is rich in F-actin, spectrin, ankyrin, amphiphysin II, and dynamin I (54, 55). These proteins are postulated to form a physical barrier to membrane protein transport into axons (5), and perhaps gE is required to overcome this barrier. A third possibility is that gE may associate with microtubular motor proteins, such as kinesin, either directly or indirectly by interaction with other viral or cellular proteins (45) to mediate axonal localization of intact virions.
Other HSV-1 membrane proteins in addition to gE may be involved in mediating axonal targeting. gE forms a heterodimeric complex with gI (10, 21, 29). HSV-1 gI-null virus was shown to be defective in spread to the brain using a rat retina infection model (19); however, whether the defect was in axonal localization, transport of virion components along axon fibers, or transynaptic spread was not established. A contribution of HSV-1 Us9 to anterograde spread is also possible based on studies with PRV Us9 (6, 7, 50).
We evaluated the spread phenotype of two HSV-1 gE ectodomain mutant viruses, NS-gE264 and NS-gE380. Both mutant viruses are defective in IgG Fc receptor activity. However, NS-gE264 viral antigens were detected in the optic nerve and in neuronal cell axons, whereas NS-gE380 virus was defective in axonal localization of viral proteins. Therefore, distinct gE ectodomains are required for axonal localization of viral proteins and IgG Fc receptor activity. Of note, the NS-gE380 mutant virus was capable of retrograde spread, which suggests that different gE domains may be involved in anterograde and retrograde spread. NS-gE380 viral antigens were detected in the optic nerve, which differed from NS-gEnull virus. The significance of this observation is unclear; however, we cannot attribute the abnormal spread phenotype of the NS-gE380 virus to alterations in gI, since gI expression (H. M. Friedman, unpublished observation) and gE/gI complex formation are comparable to WT virus (2). The optic nerve contains ganglion cell neuron axon fibers and supporting glial cells (astrocytes and oligodendrocytes) (36). A possible interpretation of the NS-gE380 viral antigen seen in the optic nerve is that the supporting glial cells are infected, rather than the ganglion cell axons. Since NS-gE380 virus is relatively intact for retrograde spread, it is possible that the virus infects the supporting cells in the optic nerve by retrograde spread from the retina. This hypothesis is consistent with results shown in Fig. 7 and 8B that demonstrate an anterograde, but not a retrograde spread defect for the NS-gE380 virus.
The retina contains non-neuronal Müller cells that extend from the ganglion cell layer to the retina pigmented epithelial cell layer; therefore, spread in the retina may not require the virus to cross a synapse. However, the lack of NS-gEnull viral antigens in the optic nerve in vivo and failure of antigens to enter axons in vitro indicate that a critical defect occurs in axonal localization. This defect is prior to the axon terminus and likely accounts for most, if not all, of the abnormal anterograde spread phenotype of this mutant virus, which extends previous reports by more clearly defining the site of the anterograde-spread defect for gE-null virus (19).
Studies with PRV have established viral and host factors involved in spread (12, 17); however, important differences exist between HSV-1 and PRV, including homology of only 8% of their genomic sequences and divergent effects on host cell gene expression (42). Our results highlight interesting similarities between HSV-1 and PRV gE in axonal targeting and important differences in retrograde spread of these two distantly related mammalian alphaherpesviruses.
Acknowledgments
This study was supported by Public Health Service grants AI33063 from the National Institute of Allergy and Infectious Disease and HL28220 from the National Heart, Lung, and Blood Institute, NIH training grant T32 GM07229, and the Foundation Fighting Blindness.
We thank Albert McGuire for assistance with the eye injections.
REFERENCES
- 1.Barnstable, C. J., and U. C. Drager. 1984. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience 11:4847-855. [DOI] [PubMed] [Google Scholar]
- 2.Basu, S., G. Dubin, M. Basu, V. Nguyen, and H. M. Friedman. 1995. Characterization of regions of herpes simplex virus type 1 glycoprotein E involved in binding the Fc domain of monomeric IgG and in forming a complex with glycoprotein I. J. Immunol. 154:260-267. [PubMed] [Google Scholar]
- 3.Bearer, E. L., X. O. Breakefield, D. Schuback, T. S. Reese, and J. H. LaVail. 2000. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc. Natl. Acad. Sci. USA 97:8146-8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, F. C., J. C. Whitbeck, M. Ponce de Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 77:9542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boiko, T., and B. Winckler. 2003. Picket and other fences in biological membranes. Dev. Cell 5:191-192. [DOI] [PubMed] [Google Scholar]
- 6.Brideau, A. D., J. P. Card, and L. W. Enquist. 2000. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. J. Virol. 74:834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brideau, A. D., M. G. Eldridge, and L. W. Enquist. 2000. Directional transneuronal infection by pseudorabies virus is dependent on an acidic internalization motif in the Us9 cytoplasmic tail. J. Virol. 74:4549-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brittle, E. E., A. E. Reynolds, and L. W. Enquist. 2004. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J. Virol. 78:12951-12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 66:3032-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman, T. L., I. You, I. M. Joseph, P. J. Bjorkman, S. L. Morrison, and M. Raghavan. 1999. Characterization of the interaction between the herpes simplex virus type I Fc receptor and immunoglobulin G. J. Biol. Chem. 274:6911-6919. [DOI] [PubMed] [Google Scholar]
- 11.Ch'ng, T., E. A. Flood, and L. W. Enquist. 2005. DNA viruses: culturing primary and transformed neuronal cells for studying pseudorabies virus infection, p. 299-315. In P. M. Lieberman (ed.), Methods and protocols: methods in molecular biology. The Humana Press, Inc., New York, N.Y. [DOI] [PubMed]
- 12.Ch'ng, T. H., and L. W. Enquist. 2005. Efficient axonal localization of alphaherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. J. Virol. 79:8835-8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 79:10875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ch'ng, T. H., E. A. Flood, and L. W. Enquist. 2005. Culturing primary and transformed neuronal cells for studying pseudorabies virus infection. Methods Mol. Biol. 292:299-316. [DOI] [PubMed] [Google Scholar]
- 15.Connolly, S. A., D. J. Landsburg, A. Carfi, J. C. Whitbeck, Y. Zuo, D. C. Wiley, G. H. Cohen, and R. J. Eisenberg. 1282. Potential nectin-1 binding site on herpes simplex virus glycoprotein D. J. Virol. 79:1282-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean, H. J., S. S. Terhune, M. T. Shieh, N. Susmarski, and P. G. Spear. 1994. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell-type-dependent alterations in infectivity. Virology 199:67-80. [DOI] [PubMed] [Google Scholar]
- 17.del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 79:3903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dingwell, K. S., C. R. Brunetti, R. L. Hendricks, Q. Tang, M. Tang, A. J. Rainbow, and D. C. Johnson. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68:834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dingwell, K. S., L. C. Doering, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 69:7087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohner, K., A. Wolfstein, U. Prank, C. Echeverri, D. Dujardin, R. Vallee, and B. Sodeik. 2002. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell 13:2795-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubin, G., I. Frank, and H. M. Friedman. 1990. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J. Virol. 64:2725-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309:633-635. [DOI] [PubMed] [Google Scholar]
- 23.Friedman, H. M., E. J. Macarak, R. R. MacGregor, J. Wolfe, and N. A. Kefalides. 1981. Virus infection of endothelial cells. J. Infect. Dis. 143:266-273. [DOI] [PubMed] [Google Scholar]
- 24.Friedman, H. M., L. Wang, N. O. Fishman, J. D. Lambris, R. J. Eisenberg, G. H. Cohen, and J. Lubinski. 1996. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J. Virol. 70:4253-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handler, C. G., R. J. Eisenberg, and G. H. Cohen. 1996. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J. Virol. 70:6067-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husak, P. J., T. Kuo, and L. W. Enquist. 2000. Pseudorabies virus membrane proteins gI and gE facilitate anterograde spread of infection in projection-specific neurons in the rat. J. Virol. 74:10975-10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson, P. J., E. B. Myhre, and J. Blomberg. 1985. Specificity of Fc receptors induced by herpes simplex virus type 1: comparison of immunoglobulin G from different animal species. J. Virol. 56:489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, D. C., and V. Feenstra. 1987. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J. Virol. 61:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labetoulle, M., P. Kucera, G. Ugolini, F. Lafay, E. Frau, H. Offret, and A. Flamand. 2000. Neuronal pathways for the propagation of herpes simplex virus type 1 from one retina to the other in a murine model. J. Gen. Virol. 81:1201-1210. [DOI] [PubMed] [Google Scholar]
- 31.LaVail, J. H., A. N. Tauscher, E. Aghaian, O. Harrabi, and S. S. Sidhu. 2003. Axonal transport and sorting of herpes simplex virus components in a mature mouse visual system. J. Virol. 77:6117-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, X., J. M. Lubinski, and H. M. Friedman. 2004. Immunization strategies to block the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 78:2562-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luxton, G. W. G., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 102:5832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGeoch, D. J., A. Dolan, S. Donald, and F. J. Rixon. 1985. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 181:1-13. [DOI] [PubMed] [Google Scholar]
- 36.Miller, R. H., S. David, R. Patel, E. R. Abney, and M. C. Raff. 1985. A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve: in vivo evidence for two distinct astrocyte lineages. Dev. Biol. 111:35-41. [DOI] [PubMed] [Google Scholar]
- 37.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicola, A. V., and S. E. Straus. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 78:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickard, G. E., C. A. Smeraski, C. C. Tomlinson, B. W. Banfield, J. Kaufman, C. L. Wilcox, L. W. Enquist, and P. J. Sollars. 2002. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J. Neurosci. 22:2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauch, D. A., N. Rodriguez, and R. J. Roller. 2000. Mutations in herpes simplex virus glycoprotein D distinguish entry of free virus from cell-cell spread. J. Virol. 74:11437-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray, N., and L. W. Enquist. 2004. Transcriptional response of a common permissive cell type to infection by two diverse alphaherpesviruses. J. Virol. 78:3489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saldanha, C. E., J. Lubinski, C. Martin, T. Nagashunmugam, L. Wang, H. van Der Keyl, R. Tal-Singer, and H. M. Friedman. 2000. Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease. J. Virol. 74:6712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sisk, W. P., J. D. Bradley, R. J. Leipold, A. M. Stoltzfus, M. Ponce de Leon, M. Hilf, C. Peng, G. H. Cohen, and R. J. Eisenberg. 1994. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 68:766-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, G. A., L. Pomeranz, S. P. Gross, and L. W. Enquist. 2004. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proc. Natl. Acad. Sci. USA 101:16034-16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surace, E. M., A. Auricchio, S. J. Reich, T. Rex, E. Glover, S. Pineles, W. Tang, E. O'Connor, A. Lyubarsky, A. Savchenko, E. N. Pugh, Jr., A. M. Maguire, J. M. Wilson, and J. Bennett. 2003. Delivery of adeno-associated virus vectors to the fetal retina: impact of viral capsid proteins on retinal neuronal progenitor transduction. J. Virol. 77:7957-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tal-Singer, R., C. Peng, M. Ponce De Leon, W. R. Abrams, B. W. Banfield, F. Tufaro, G. H. Cohen, and R. J. Eisenberg. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69:4471-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tirabassi, R. S., R. A. Townley, M. G. Eldridge, and L. W. Enquist. 1997. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J. Virol. 71:6455-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomishima, M. J., and L. W. Enquist. 2001. A conserved alpha-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomishima, M. J., and L. W. Enquist. 2002. In vivo egress of an alphaherpesvirus from axons. J. Virol. 76:8310-8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whealy, M. E., J. P. Card, A. K. Robbins, J. R. Dubin, H. J. Rziha, and L. W. Enquist. 1993. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J. Virol. 67:3786-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitley, R. J., D. W. Kimberlin, and B. Roizman. 1998. Herpes simplex viruses. Clin. Infect. Dis. 26:541-553. [DOI] [PubMed] [Google Scholar]
- 54.Winckler, B., P. Forscher, and I. Mellman. 1999. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397:698-701. [DOI] [PubMed] [Google Scholar]
- 55.Winckler, B., and I. Mellman. 1999. Neuronal polarity: controlling the sorting and diffusion of membrane components. Neuron 23:637-640. [DOI] [PubMed] [Google Scholar]