Abstract
During mRNA synthesis, the polymerase of vesicular stomatitis virus (VSV) copies the genomic RNA to produce five capped and polyadenylated mRNAs with the 5′-terminal structure 7mGpppAmpApCpApGpNpNpApUpCp. The 5′ mRNA processing events are poorly understood but presumably require triphosphatase, guanylyltransferase, [guanine-N-7]- and [ribose-2′-O]-methyltransferase (MTase) activities. Consistent with a role in mRNA methylation, conserved domain VI of the 241-kDa large (L) polymerase protein shares sequence homology with a bacterial [ribose-2′-O]-MTase, FtsJ/RrmJ. In this report, we generated six L gene mutations to test this homology. Individual substitutions to the predicted MTase active-site residues K1651, D1762, K1795, and E1833 yielded viruses with pinpoint plaque morphologies and 10- to 1,000-fold replication defects in single-step growth assays. Consistent with these defects, viral RNA and protein synthesis was diminished. In contrast, alteration of residue G1674 predicted to bind the methyl donor S-adenosylmethionine did not significantly perturb viral growth and gene expression. Analysis of the mRNA cap structure revealed that alterations to the predicted active site residues decreased [guanine-N-7]- and [ribose-2′-O]-MTase activity below the limit of detection of our assay. In contrast, the alanine substitution at G1674 had no apparent consequence. These data show that the predicted MTase active-site residues K1651, D1762, K1795, and E1833 within domain VI of the VSV L protein are essential for mRNA cap methylation. A model of mRNA processing consistent with these data is presented.
Vesicular stomatitis virus (VSV), the prototypic Rhabdovirus, has a nonsegmented negative-sense (nsNS) RNA genome of 11,161 nucleotides comprising a 50-nucleotide 3′ leader region (Le); five genes that encode the viral nucleocapsid (N) protein, phosphoprotein (P), matrix (M) protein, attachment glycoprotein (G) and large polymerase subunit (L), and a 59-nucleotide trailer region (Tr), arranged in the order 3′-Le-N-P-M-G-L-Tr-5′ (1, 3, 4). The viral genomic RNA is encapsidated by N protein to form a RNase-resistant ribonucleoprotein (RNP) complex that acts as a template for the RNA-dependent RNA polymerase (RdRP). The viral components of the RdRP are a monomer of the 241-kDa L protein and a trimer of the 29-kDa P protein (19). During RNA synthesis, the RdRP uses the encapsidated genomic RNA as a template in two distinct reactions: (i) transcription of five mRNAs that encode the N, P, M, G, and L proteins and (ii) replication to yield full-length antigenomic and then genomic strands (reviewed in reference 66).
During transcription the RdRP sequentially synthesizes five capped and polyadenylated mRNAs (1, 3, 4). These mRNAs are not produced in equimolar amounts; rather, their abundance decreases with distance from the 3′ end of the template such that N > P > M > G > L (63). This polarity gradient reflects a localized transcriptional attenuation at each gene junction, where 30% of RdRP molecules fail to transcribe the downstream gene (34). The widely accepted model for mRNA synthesis is the stop-start model of sequential transcription. In the original version of this model, polymerase initiates at a single site on the genome, yielding a leader RNA and, by sequential reinitiation, the five viral mRNAs. Access of polymerase to downstream genes is therefore entirely dependent upon termination of transcription of the upstream gene (hence, stop-start). Recent experiments with VSV indicate that the polymerase molecule that transcribes the leader region does not proceed to transcribe the N mRNA (12, 46, 67). Other than this refinement, the stop-start model is well supported by much experimental evidence (reviewed in reference 66).
The cap structure of nsNS viral mRNAs is formed by a mechanism that appears unique. For VSV (2), respiratory syncytial virus (7), and spring viremia of carp virus (26), the two italicized phosphates of the 5′Gppp5′NpNpN triphosphate bridge have been shown to be derived from a GDP donor. In contrast, cellular and all other known viral capping reactions involve GMP transfer (reviewed in reference 24). This difference, combined with the cytoplasmic location of viral RNA synthesis, suggested that a viral protein, possibly the L protein subunit of polymerase, possesses guanylyltransferase activity, although direct evidence for this is lacking. After capping, the 5′ terminus of the nascent transcript is methylated by [guanine-N-7]- and [ribose-2′-O]-MTases (30, 35, 40, 41, 48-50, 62). These activities have been mapped to the L gene (30). Recent work has shown that alteration of amino acid residue D1671 which resides within a predicted S-adenosylmethionine (SAM)-binding region of L protein inhibited mRNA cap methylation (25). However, the catalytic residues within the polymerase, the substrate requirements for the reactions, and the order in which the mRNA processing reactions occur remain poorly understood.
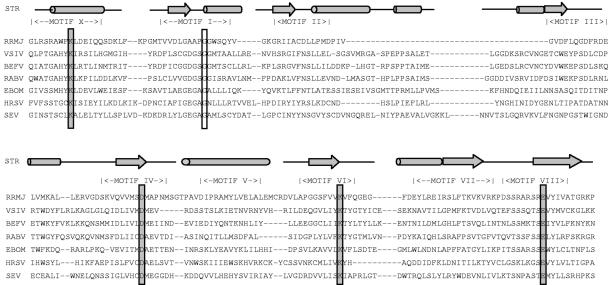
The nucleotide sequence of 39 nsNS RNA virus genomes have been determined (http://www.ncbi.nlm.nih.gov/genomes/VIRUSES/viruses.html). Amino acid sequence alignments between the L proteins of representative members of each family, identified six conserved domains numbered I to VI (45). X-ray crystal structures of representative members of each class of template-dependent polynucleotide polymerase have been determined. Each contains a catalytic core resembling a cupped right hand. Within the palm region are the A-B-C-D motifs found in all polymerases and the E motif, found in RdRPs and reverse transcriptase. Domain III of the nsNS virus L proteins contains these A-B-C-D motifs. Consistent with this, domain III of VSV L was shown to be critical for polymerase activity (58). Functions have yet to be assigned to the other conserved domains, although sequence comparisons to FtsJ/RrmJ (Fig. 1), a heat shock methyltransferase of Escherichia coli, suggest that a region spanning domain VI might function as a [ribose-2′-O]-methyltransferase (9, 22).
FIG. 1.
Amino acid sequence alignments of a region encompassing domain VI of nsNS RNA virus L proteins with the RrmJ heat shock 2′-O-methyltransferase of E. coli. The primary amino acid sequences are shown. The conserved motifs (X and I to VIII) correspond to the SAM-dependent MTase superfamily (53). Residues modified in the present study are boxed as follows: catalytic (shaded) and SAM binding (unshaded). Predicted or known alpha-helical regions are shown by the cylinders and the β-sheet regions by the arrows. STR, structure of RrmJ and predicted structure for the nsNS RNA viruses; EBOM, Ebola virus; BEFV, bovine ephemeral fever virus; VSIV, VSV (Indiana); RABV, rabies virus; HRSV, human RS virus; SEV, Sendai virus; RRMJ, E. coli heat shock methyltransferase.
A comprehensive genetic and biochemical analysis of the conserved domains of the VSV L protein has not been performed. However, studies with the paramyxovirus, Sendai (SeV), showed that genetic alterations introduced throughout each of the conserved domains of L protein revealed multiple defects in a reconstituted RNA synthesis assay (11, 20, 21, 32, 59, 60). These studies did not permit the assignment of specific functions to conserved domains of L protein. Rather, these experiments indicated that the global architecture of the SeV L protein was essential for all polymerase functions. More recently, domains V and VI of the SeV L protein were expressed independently and shown to retain the ability to methylate short RNAs that correspond to the 5′ end of SeV mRNA (43). The ability to functionally separate a domain of the SeV L is consistent with studies of measles virus (MV), in which the coding sequence of green fluorescent protein was inserted at two positions within L protein (17). The resulting polymerase was functional, suggesting that the MV L protein folds and functions as a series of independent globular domains (17).
In the present study we tested the significance of the homology between known [ribose 2′-O]-MTases and a region spanning domain VI of L protein (9, 22). Six L gene mutations were introduced into an infectious cDNA clone of VSV, and recombinant viruses were recovered. These viruses exhibited defects in viral replication as judged by single-step growth curves. By analysis of the viral mRNA cap structure we demonstrate that domain VI of the VSV L protein functions as an mRNA cap methyltransferase and that alteration of residues within the predicted active-site region diminished both [guanine-N-7] and [ribose-2′-O] methylation to undetectable levels.
MATERIALS AND METHODS
Plasmid construction and transfection of mammalian cells.
The pL plasmid containing a functional cDNA clone of the VSV L gene was described previously (56). The coding sequence was modified by site-directed mutagenesis using the QuikChange methodology (Stratagene, La Jolla, CA). The presence of the desired mutation was confirmed by sequence analysis of a 2-kb region of pL that spanned from an FseI site at position 9017 to an AgeI site at position 11004 (numbering refers to the complete VSV [Indiana] genome sequence). After digestion of each pL variant with these restriction enzymes, the resulting 2-kb fragment was subcloned back into pL digested with FseI and AgeI. This approach ensured that no other sequence alterations introduced during the mutagenesis reaction were present within the final L gene clone. Using this method, six L gene mutations were generated (Table 1 and Fig. 1). Plasmids designed to express the viral N and P proteins, and an infectious cDNA clone of the viral genome, pVSV1(+), were as described previously (65). Transfection of baby hamster kidney (BHK-21) cells was performed essentially as described, except that Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used as the lipid transfection reagent.
TABLE 1.
Amino acid changes introduced into domain VI
| Alteration | Rationalea |
|---|---|
| K1651A | Invariant catalytic residue of motif X; essential in RrmJ |
| G1674A | Critical residue of the signature GxGxG SAM-binding motif |
| D1762A | Invariant catalytic residue of motif IV |
| K1795A | Invariant catalytic residue of motif VI; essential in Rrm J |
| E1833A | Invariant catalytic residue of motif VIII; minor role in Rrm J |
| E1833Q | Maintain size |
Recovery and purification of recombinant VSV.
Selected L gene mutations were introduced into pVSV1(+) in two steps. First, a 2.5-kb fragment spanning from the FseI site at 9014 to the HindIII site at 10645 was excised from pL and inserted into FseI/HindIII-digested pVSV1(+). Second, a 0.8-kb HindIII fragment from pVSV1(+) encoding the 5′ terminus of the L gene, the trailer region, and the hepatitis delta virus ribozyme sequence was then inserted at the unique HindIII site to generate pVSV1(+) variants designed to have single amino acid changes within domain VI of L protein. Recombinant VSV was recovered from cDNA by transfection of BHK-21 cells infected with a recombinant vaccinia virus (vTF7-3) that expressed T7 RNA polymerase as described previously (23, 65). Cell culture fluids were collected at 48 to 96 h posttransfection, and recombinant virus was amplified once in BHK-21 cells. Individual plaques were isolated on Vero cells, and seed stocks generated by amplification on BHK-21 cells. Large stocks were then generated by inoculation of 8 to 10 confluent T150 flask BHK-21 cells at a multiplicity of infection (MOI) of 0.01 in a volume of 1 ml of Dulbecco modified Eagle medium (DMEM). At 1 h postadsorption, 15 ml of DMEM (supplemented with 2% fetal bovine serum) was added to the cultures, and infected cells were incubated at 31°C for 24 to 72 h. When extensive cytopathic effect (CPE) was observed, cell culture fluids were clarified by centrifugation at 3,000 × g for 5 min. Virus was concentrated by centrifugation at 40,000 × g for 90 min at 4°C in a Ty 50.2 rotor. The pellet was resuspended in NTE buffer (100 mM NaCl, 10 mM Tris, 1 mM EDTA [pH 7.4]) and further purified through 10% sucrose NTE by centrifugation at 150,000 × g for 1 h at 4°C in an SW50.1 rotor. The final pellet was resuspended in 0.1 to 0.3 ml of NTE buffer. The virus titer was determined by plaque assay on Vero cells, and the protein content was measured by Bradford reagent (Sigma Chemical Co., St. Louis, MO). The L genes of the purified viruses were sequenced again, and these stocks used for in vitro transcription reactions.
Single-cycle growth curves.
Confluent BHK-21 cells were infected with individual viruses at an MOI of 3. After 1 h of adsorption, the inoculum was removed, the cells were washed with DMEM, fresh DMEM (supplemented with 2% fetal bovine serum) was added, and infected cells were incubated at 37°C. Aliquots of the cell culture fluid were removed at the indicated intervals, and virus titers were determined by plaque assay on Vero cells.
Analysis of protein synthesis.
At the indicated time postinfection, cells were washed with methionine- and cysteine-free (M−C−) media and incubated with fresh M−C− medium supplemented with actinomycin D (10 μg/ml). After a 1-h incubation, the medium was replaced with M−C− medium supplemented with EasyTag [35S]-Express (40 μCi/ml) (Perkin-Elmer, Wellesley, MA). Cytoplasmic extracts were prepared and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously (65). Labeled proteins were detected either by autoradiography or by using a phosphorimager.
Analysis of RNA synthesis in cells.
At the indicated time postinfection, cells were incubated with DMEM containing actinomycin D (10 μg/ml). After a 1-h incubation, the medium was replaced with fresh medium containing actinomycin D and [3H]uridine (30 μCi/ml; Moravek Biochemicals, Brea, CA). At the indicated time postlabeling, a cytoplasmic extract was prepared, and RNA was purified after phenol and chloroform extraction essentially as described previously (44). Purified RNA was analyzed by electrophoresis on acid-agarose gels (36) and detected by fluorography.
Transcription of viral RNA in vitro.
Viral RNA was synthesized in vitro essentially as described previously (5) with minor modifications (67). Purified recombinant VSV (10 μg) was activated by incubation with detergent for 5 min at room temperature. RNA synthesis reactions were performed in the presence of nucleoside triphosphates (1 mM ATP and 0.5 mM each of CTP, GTP, and UTP). Where indicated, reactions were supplemented with 1 mM SAM or S-adenosylhomocysteine (SAH) or with 15 μCi of [α-32P]GTP (3,000 Ci/mmol) or 15 μCi of [3H]SAM (85 Ci/mmol; Perkin-Elmer, Wellesley, MA).
Cap methyltransferase assay.
To examine the extent of cap methylation, purified RNAs were digested with RNase T2 (Invitrogen) and/or tobacco acid pyrophosphatase (TAP; Epicenter, Madison, WI), and the products were analyzed by thin-layer chromatography (TLC) on PEI-F cellulose sheets (EM Biosciences). For examination of guanine-N-7 methylation, in vitro transcription reactions were performed in the presence of [α-32P]GTP and 1 mM SAM or SAH. For examination of ribose-2′-O methylation, in vitro transcription reactions were performed in the presence of [3H]SAM. Products of RNA synthesis were purified, and approximately one-fifth of the reaction was incubated with 2 U of TAP and/or 10 U of RNase T2 according to the manufacturer's instructions. After incubation, one-tenth of this reaction was spotted onto a TLC plate, which was developed using 1.2 M LiCl2. Plates were dried, and the spots visualized by using a phosphorimager. Markers 7mGpppA and GpppA (New England Biolabs, Beverly, MA), and their TAP digestion products were visualized by UV shadowing at 254 nm.
Quantitative analysis.
Quantitative analysis was performed by either densitometric scanning of autoradiographs or by using a phosphorimager (GE Healthcare, Typhoon) and ImageQuant TL software (GE Healthcare, Piscataway, NJ). Statistical analysis was performed on three to five separate experiments, and the calculated means are shown in each figure, along with the standard deviation. The significance of the values was determined by a paired Student’s t test.
RESULTS
Amino acid changes to a predicted methyltransferase domain within the VSV L protein.
The SAM-dependent MTase superfamily contains a series of conserved motifs (X and I to VIII) (53). By comparing the amino acid sequence of the Escherichia coli heat shock-induced methyltransferase RrmJ/FtsJ with conserved domain VI of the L protein of nsNS RNA viruses, it was suggested that this region of L protein might function as a [ribose-2′-O]-MTase (9, 22). These alignments indicate that residues G1670, G1672, G1674, and D1735 and residues K1651, D1762, K1795, and E1833 of the VSV L protein correspond to a SAM-binding motif and catalytic KDKE tetrad, respectively (Fig. 1).
As a first step to test this prediction, we engineered the L gene of an infectious cDNA clone of VSV to introduce alanine substitutions at each of the proposed MTase catalytic residues K1651, D1762, K1795, and E1833, as well as to one of the proposed SAM-binding residues, G1674 (Table 1). Based on the postulated reaction mechanism of RrmJ (8, 27, 28) and VP39 of vaccinia virus (9, 31), we anticipated that these mutations in the L gene would prevent RNA methylation. Mutational analysis of RrmJ had indicated that the aligned position equivalent to E1833 played only a minor role in RNA methylation (8, 27, 28). Consequently, we chose to include a second substitution at this position E1833Q, in which the size of the residue was maintained.
Recovery of recombinant viruses with L gene mutations.
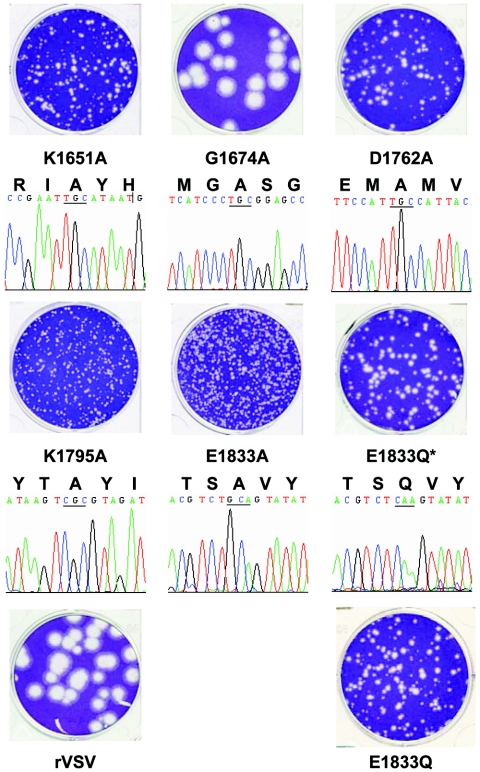
Each of the L gene mutations yielded viable recombinant virus; however, many of these viruses had clear defects in growth (Fig. 2). After 24 h of incubation, virus G1674A which contained an alteration in the predicted SAM-binding domain formed plaques that were 4.1 ± 0.8 mm in diameter, and this was indistinguishable from the plaque morphology of rVSV (4.0 ± 0.5 mm). In contrast, alterations to the proposed active-site residues K1651, D1762, K1795, and E1833 resulted in clear defects in plaque formation, since each of the viruses formed only pinpoint plaques. After 48 h of incubation, the average plaque diameter was 0.9 ± 0.2 mm for K1651A, 1.1 ± 0.2 mm for D1762A, 0.7 ± 0.1 mm for K1795A, 0.8 ± 0.2 mm for E1833A, 1.2 ± 0.2 mm for E1833Q*, and 1.3 ± 0.2 mm for E1833Q (Fig. 2). These data indicate that the proposed MTase active site residues are required for efficient viral replication.
FIG. 2.
Recombinant VSV with mutations in the L gene. The plaque morphology of each of the recombinant viruses is shown compared to rVSV. Note that plaques of K1651A, D1762A, K1795A, E1833A, E1833Q*, and E1833Q were developed after 48 h of incubation compared to rVSV and G1674A, which were developed after 24 h. Differing dilutions of the small plaque viruses were plated to emphasize the plaque morphology. The sequence of the modified region for each mutant virus is shown. Note that the sequence trace shown is negative sense for K1651A, G1674A, D1762A, and K1795A and positive sense for E1833A and E1833Q.
The entire L gene of each recombinant virus was amplified by reverse transcription-PCR, and sequence analysis confirmed the presence of the desired mutation (Fig. 2 and data not shown). Viruses G1674A, D1762A, K1795A, and E1833A contained no additional nucleotide changes within the L gene. Virus K1651A contained an additional change in the complete VSV genome sequence, A5539G, which was noncoding. In contrast, virus E1833Q contained four additional nucleotide changes: G10699A, U10720C, A10739G, and U10850C, of which A10739G resulted in coding change I2002V. Consequently, we renamed E1833Q to E1833Q* to reflect these sequence changes and isolated a fresh E1833Q from an independent transfection (Fig. 2). Sequence analysis of this second isolate of E1833Q confirmed that no additional changes were present within the L gene. The sequence differences in E1833Q* were detected after completion of the experiments shown in Fig. 3, as well as Fig. 7 and 8. However, E1833Q behaved indistinguishably from E1833Q* in its ability to replicate in BHK-21 cells as judged by endpoint titers and in its ability to plaque on Vero cells (Fig. 2 and Table 2).
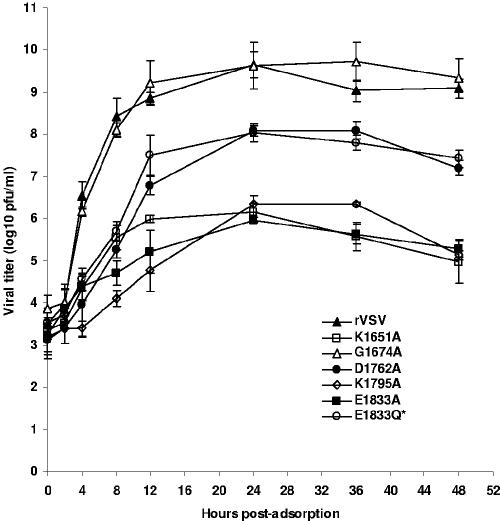
FIG. 3.
Single-step growth assay of recombinant VSV in BHK-21 cells. Confluent BHK-21 cells were infected with individual viruses at an MOI of 3. After a 1-h adsorption, the inoculum was removed, the cells were washed with DMEM, and fresh medium (containing 2% fetal bovine serum) was added, followed by incubation at 37°C. Samples of supernatant were harvested at the indicated intervals over a 48-h time period, and the virus titer was determined by plaque assay on Vero cells. Titers are reported as the mean ± the standard deviation among three independent single-step growth experiments.
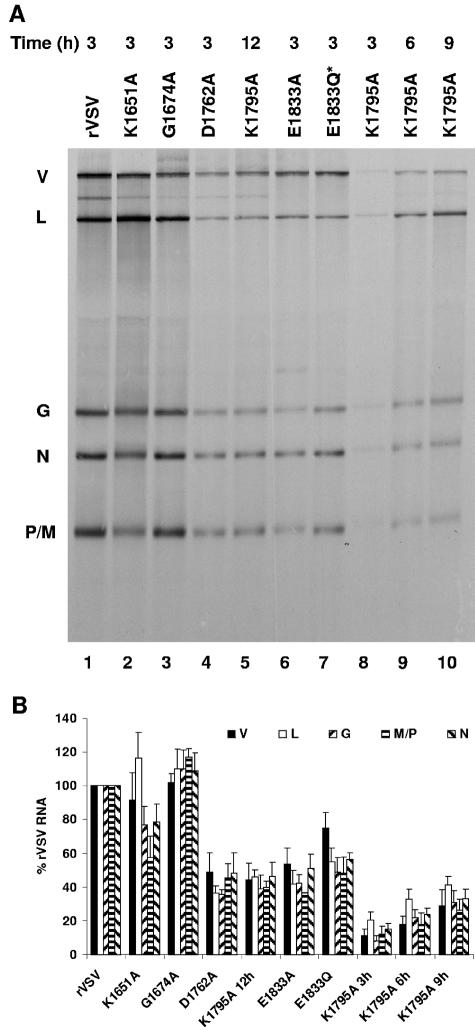
FIG. 7.
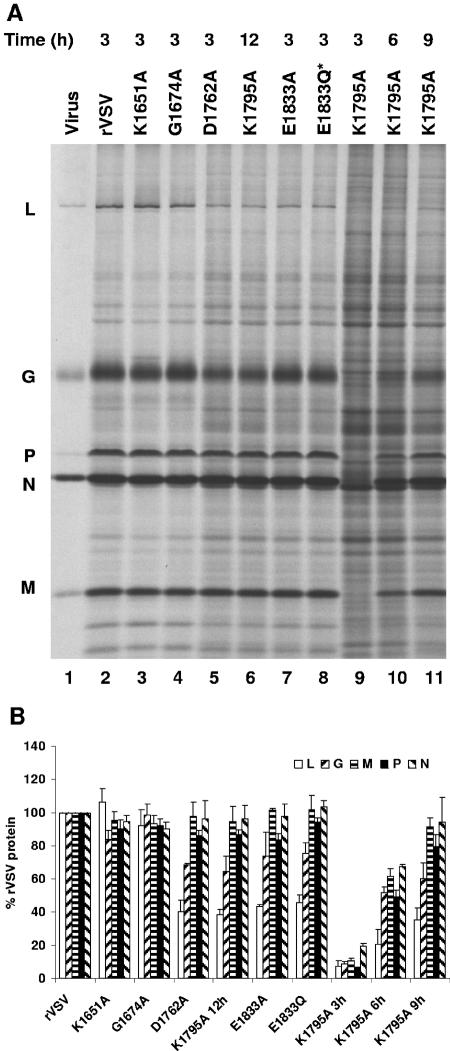
Effect of L gene mutations on viral RNA synthesis in BHK-21 cells. (A) BHK-21 cells were infected with the wild-type and mutant viruses at an MOI of 3. Viral RNAs were labeled with [3H]uridine as described in Materials and Methods, resolved by electrophoresis on acid-agarose gels, and visualized by fluorography. RNA extracted from an equivalent number of cells was loaded in each lane. For K1795A, viral RNA was labeled with [3H]uridine at 3, 6, 9, and 12 h postinfection. The infecting virus is indicated above the lanes, along with the time postinfection at which the labeling commenced. The identity of the RNAs is shown on the left. V, genomic and antigenomic replication products; L, G, N, and P/M, mRNA. (B) Autoradiographs of five independent experiments were scanned and analyzed as described in Materials and Methods. For each of the resolved RNA products, the mean ± the standard deviation was expressed as a percentage of that observed for rVSV.
FIG. 8.
Effect of L gene mutations on viral protein synthesis in BHK-21 cells. (A) BHK-21 cells were infected with the wild-type and mutant viruses at an MOI of 3. Proteins were labeled by incorporation of [35S]methionine-cysteine in the presence of actinomycin D as described in Materials and Methods. Cytoplasmic extracts were prepared, and proteins were analyzed by SDS-PAGE and detected by using a phosphorimager. Extract from equivalent numbers of cells was loaded in each lane. For K1795A, viral proteins were labeled either at 3, 6, 9, or 12 h postinfection. The infecting virus is indicated above the lanes, along with the time postinfection at which the labeling commenced. The identity of the proteins is shown on the left. (B) Three independent experiments were used to generate the quantitative analysis shown. For each protein the mean ± the standard deviation was expressed as a percentage of that observed for rVSV.
TABLE 2.
Summary of phenotypic properties of VSV L gene mutants
| Mutant | Mean plaque size (mm) ± SDa | Titer at 24 h (log10 PFU/ml) | RNA synthesis (% rVSV)
|
Protein (% rVSV)c | % 7mG | % Am | |
|---|---|---|---|---|---|---|---|
| Cells | In vitro | ||||||
| rVSV | 4.0 ± 0.5* | 9.7 ± 0.3 | 100 | 100 | 100 | 97 | 100 |
| K1651A | 0.9 ± 0.2† | 6.2 ± 0.2 | 60-120b | 75 | 100 | <1 | <1 |
| G1674A | 4.1 ± 0.8* | 9.6 ± 0.6 | >100 | 100 | 100 | 96 | 96 |
| D1762A | 1.1 ± 0.2† | 8.1 ± 0.1 | 50 | 75 | 40-100* | <1 | <1 |
| K1795A | 0.7 ± 0.2† | 6.3 ± 0.2 | 10 | 40 | 10† | <1 | <1 |
| E1833A | 0.8 ± 0.2† | 6.0 ± 0.1 | 50 | 60 | 40-100* | 11 | 5 |
| E1833Q* | 1.2 ± 0.2† | 7.8 ± 0.2 | 50 | 100 | 40-100* | <1 | <1 |
| E1833Q | 1.3 ± 0.2† | 7.9 ± 0.2 | NDd | 100 | ND | <1 | <1 |
*,Plaque diameter was measured at 24 h postinoculation; †,plaque diameter was measured at 48 h postinoculation.
The percentage of each mRNA varied: N, 80%; P/M, 60%; G, 80%; and L, 120%.
*, N, P, and M proteins were 100% of rVSV levels (G, 70%; L, 40%). †, At later times postinfection, the levels of L and G protein were specifically reduced.
ND, not determined.
To examine the effect of these L gene mutations on viral growth more specifically, we monitored the kinetics of release of infectious virus by single-step growth assay. Briefly, BHK-21 cells were infected with each of the indicated recombinants at an MOI of 3, and viral replication was assessed at time points from 0 to 48 h postinfection as described in Materials and Methods. The experiment was performed three independent times, and the average titer at each time point was plotted to generate the graph shown (Fig. 3). Recombinant G1674A replicated with almost indistinguishable kinetics to rVSV. At 24 h postinfection virus titer was 9.6 ± 0.6 and 9.7 ± 0.3 log10 PFU ml−1 for G1674A and rVSV, respectively. In contrast, viruses D1762A, E1833Q, and E1833Q* showed a delay in replication and reached titers of 8.1 ± 0.1, 7.9 ± 0.2, and 7.8 ± 0.2 log10 PFU ml−1, respectively, at 24 h postinfection. Recombinants K1651A, K1795A, and E1833A showed the most significant defect in replication, reaching titers of 6.2 ± 0.2, 6.3 ± 0.2, and 6.0 ± 0.1 log10 PFU ml−1, respectively, at 24 h postinfection. These data show that changes to the predicted MTase active-site residues compromised virus replication resulting in a 10- to 1,000-fold reduction in virus titer at 24 h postinfection, whereas alteration of the predicted SAM-binding residue had no detectable effect. These findings correlate well with the plaque diameter for each of the recombinant viruses (Fig. 2).
Effect of L gene mutations on mRNA cap methylation.
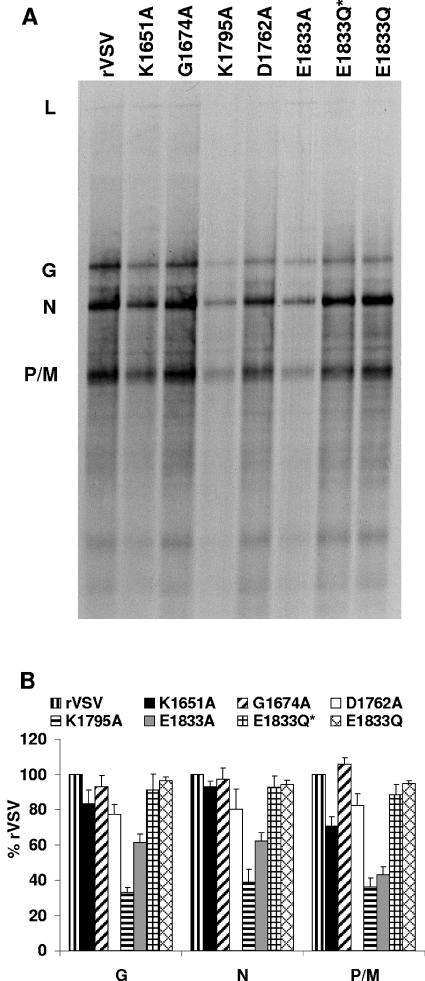
To directly examine whether the alterations in domain VI of L protein affected mRNA cap methylation, transcription reactions were performed in vitro. Briefly, 10 μg of virus was activated with detergent and incubated with nucleoside triphosphates supplemented with [α-32P]GTP as described above. Total RNA was extracted, purified, and analyzed by acid-agarose gel electrophoresis as described previously (Fig. 4). With the exception of K1795A and E1833A, the levels of mRNA synthesis were similar for each virus. Quantitative analysis showed that K1795A and E1833A synthesized approximately 40 and 60% of the levels of rVSV mRNA. To compensate for these defects in mRNA synthesis, the amount of virus used in the in vitro transcription reactions in subsequent experiments was increased 2.5-fold.
FIG. 4.
Transcription of viral mRNAs in vitro. (A) Transcription reactions were performed in vitro in the presence of [α-32P]GTP, the RNA was purified and analyzed by electrophoresis on acid-agarose gels as described in Materials and Methods. The products were detected by using a phosphorimager. The source of the virus used in the in vitro transcription reactions is indicated above the gel, and the identity of the mRNAs is shown on the left. (B) Three independent experiments were used to generate the quantitative analysis shown. For each mRNA the mean ± the standard deviation was expressed as a percentage of that observed for rVSV.
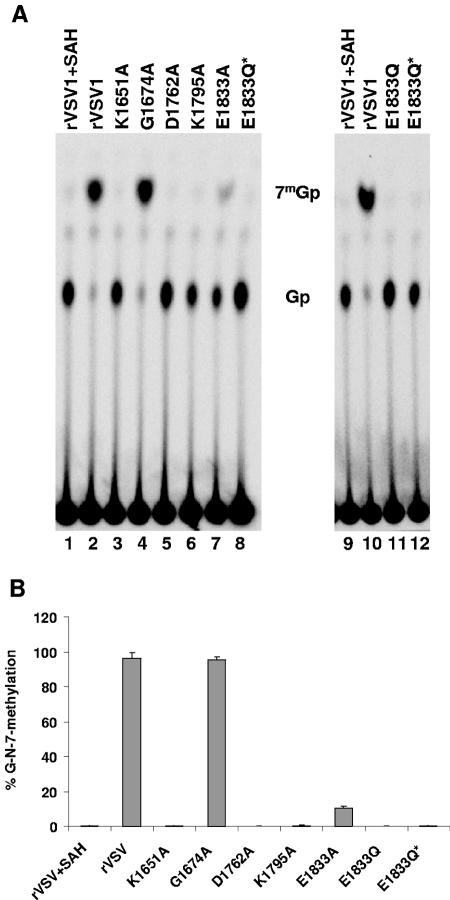
TAP specifically cleaves the pyrophosphate bond of the GpppN cap but does not degrade the mRNA (57). Consequently, cleavage of VSV mRNAs with TAP should yield Gp or, if the cap structure is methylated, 7mGp. To examine the extent of [guanine-N-7] methylation of viral mRNA, in vitro transcription reactions were performed in the presence of [α-32P]GTP. RNA was extracted, purified, and incubated with TAP, and the products of cleavage resolved by TLC on PEI-F cellulose as described in Materials and Methods. For rVSV when transcription reactions were performed in the presence of SAH, the by-product formed upon methyl group transfer from SAM during cap methylation, a single product of TAP cleavage was detected (Fig. 5A, lane 1). This comigrated with the Gp marker and not the 7mGp marker generated by TAP cleavage of GpppA and 7mGpppA, indicating that for rVSV the cap structure was not methylated in the presence of SAH. In contrast, TAP cleavage of rVSV mRNA synthesized in the presence of SAM yielded a major product that comigrated with the 7mGp marker (Fig. 5A, lane 2).
FIG. 5.
Effect of L gene mutations on cap methyltransferase activity. (A) Viral mRNA was synthesized in vitro as described in the text in the presence of either 1 mM SAM or SAH and 15 μCi of [α-32P]GTP. Purified mRNAs were digested with 2 U of TAP, and the products were analyzed by TLC on PEI-F cellulose sheets. The plates were dried, and the spots were visualized by using a phosphorimager. The identity of the virus is shown at the top of the plate, and the migration of the markers 7mGp and Gp is shown in the center. (B) Quantitative analysis was performed on five independent experiments. For each virus the released 7mGp (mean ± the standard deviation) was expressed as a percentage of the total released cap structure.
Quantitative analysis of three independent experiments showed that 7mGp accounted for 96% of the released cap structure for G1674A (Fig. 5A, lane 4) and this was essentially indistinguishable to the observed 97% for rVSV, suggesting that this predicted SAM-binding residue was not critical for [guanine-N-7]-MTase activity. In contrast, TAP digestion of the RNAs synthesized by E1833A showed that approximately 11% of the released cap was of the form 7mGp (Fig. 5A, lane 7). Viruses K1651A, D1762A, K1795A, E1833Q, and E1833Q* showed essentially no cap methylation, with TAP digestion yielding >99% Gp (Fig. 5A). These data clearly demonstrate that each of the alterations to the proposed MTase active-site residues diminished [guanine-N-7] methylation below the limits of detection of our assay.
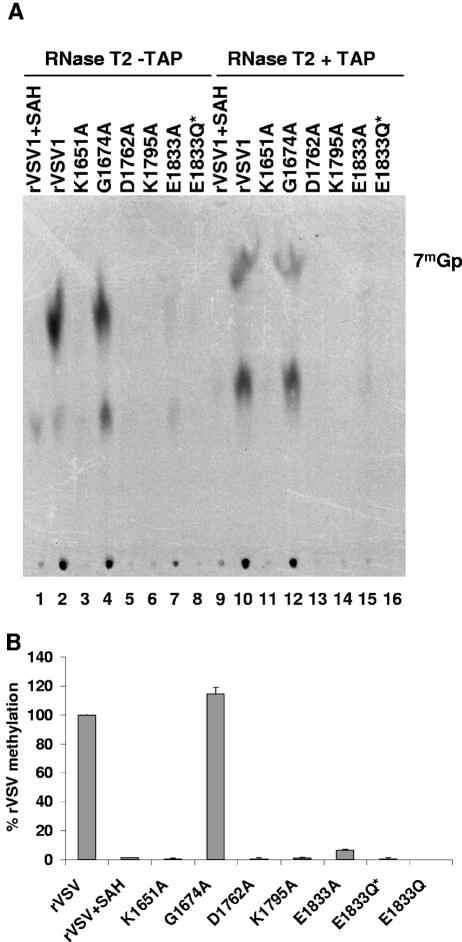
RNase T2 is an endoribonuclease that exhibits a preference for cleavage of phosphodiester bonds on the 3′ side of A residues. Consequently, complete digestion of the VSV mRNA cap structure 7mGpppAmpApCpApGp with RNase T2 should yield 7mGpppAm if the cap structure was both [guanine-N-7] and [ribose-2′-O] methylated. TAP digestion of this product would yield 7mG and Am. To examine whether the mutations in the L gene affected [ribose-2′-O] and/or [guanine-N-7] methylation, transcription reactions were performed in vitro in the presence of [3H]SAM as a methyl donor as described in Materials and Methods. RNA was extracted, purified, and incubated with TAP and/or RNase T2, and the products of cleavage were resolved by TLC on PEI-F cellulose as described earlier. For rVSV when transcription reactions were performed in the presence of [3H]SAM, a single major product of RNase T2 cleavage was detected (Fig. 6A, lane 2), and this product was not observed when reactions were supplemented with SAH (Fig. 6A, lane 1). Digestion with TAP and RNase T2 resolved this product into two species, 7mGp and Am (Fig. 6A, lane 10), which were absent when transcription reactions were performed in the presence of SAH demonstrating that they were methylated (Fig. 6A, lane 9).
FIG. 6.
Effect of L gene mutations on [ribose-2′-O] methylation. (A) Viral mRNA was synthesized in vitro as described in the text in the presence of 15 μCi of [3H]SAM. Purified mRNAs were digested with 10 U of RNase T2 and/or 2 U of TAP, and the products were analyzed by TLC on PEI-F cellulose sheets. The plates were dried, and the spots were visualized by using a phosphorimager. The identity of the virus is shown at the top of the plate, and the migration of the markers 7mGp and Gp is shown on the right. (B) Quantitative analysis was performed on three independent experiments. For each virus the released 7mG and Am (mean ± the standard deviation) was expressed as a percentage of that observed for rVSV.
Quantitative analysis demonstrated that alteration of the predicted SAM-binding residue G1674A did not affect the abundance of either the 7mG or Am, suggesting that this predicted SAM-binding residue was not critical for either [guanine-N-7] or [ribose-2′-O] methylation (Fig. 6A, lanes 4 and 12). In contrast, alterations to the predicted catalytic residues K1651A, D1762A, K1795A, and E1833Q* reduced both [guanine-N-7] and [ribose-2′-O] methylation to the limit of detection of our assay (Fig. 6A, lanes 11, 13, 14, and 16). Recombinant E1833A showed approximately 5% of the activity of rVSV (Fig. 6A, lane 15). These data clearly demonstrate that alterations to the predicted [ribose-2′-O]-MTase domain affected both [guanine-N-7] and [ribose 2′-O] methylation. These data are thus consistent with domain VI of L protein functioning as an mRNA cap MTase and show that the predicted active-site residues are critical for mRNA cap methylation.
Effect of L gene mutations on viral RNA and protein synthesis in infected cells.
These experiments demonstrated that recombinant viruses that contained alterations to the predicted MTase active-site residues were defective in methylation and that these defects correlated with diminished replication in cell culture. We anticipated that these alterations would affect translation of viral mRNAs, which would lead to a decrease in RNA replication thus generating fewer templates for mRNA transcription. To test this, we examined RNA and protein synthesis in infected cells.
Viral RNA synthesis was examined in infected cells at the indicated times postinfection. Briefly, BHK-21 cells were infected with each of the indicated recombinants at an MOI of 3, and RNA was metabolically labeled by incorporation of [3H]uridine for 3 h in the presence of actinomycin D. Total cytoplasmic RNA was then purified and analyzed by electrophoresis on acid-agarose gels (Fig. 7A). Quantitative analysis (Fig. 7B) of five independent experiments showed that the single amino acid change to the predicted SAM-binding residue G1674A had no detectable effect on levels of the viral RNAs (compare Fig. 7A, lanes 1 and 3). In contrast, individual changes to each of the proposed catalytic residues K1651, D1762, K1795, and E1833 affected viral RNA levels (compare Fig. 7A, lanes 1, 2, 4, to 10). For D1762A and E1833A, the observed levels of the N, P/M, G, and L mRNAs and the genomic replication products (Fig. 7A, band V) were approximately 40 to 50% of those for rVSV (Fig. 7B). Similar defects were observed for each of these recombinants when RNA synthesis was examined from 6 to 9 h postinfection (data not shown). For E1833Q*, a similar reduction in mRNA synthesis was observed, but the reduction in genome replication was less dramatic in that approximately 75% of the levels of rVSV replication were observed (Fig. 7B). A more pronounced defect was observed for K1795A, where levels of mRNA and genomic replication products were <10% of those for rVSV (Fig. 7A, lane 8). At later times postinfection (Fig. 7A, lanes 5, 9, and 10), the levels of K1795A RNAs were still <40% of those observed for rVSV at 3 to 6 h postinfection. Whether these reductions reflect a specific defect in mRNA synthesis that results in a reduction in replication or a general defect in all RNA synthesis could not be distinguished by this assay. Recombinant K1651A exhibited a unique phenotype in that the relative abundance of each of the mRNAs differed compared to rVSV (Fig. 7A, lane 2). Specifically, the L mRNA was 120%, G and N mRNAs were 80%, and the P/M mRNAs were 60% of the rVSV levels (Fig. 7B). Statistical analysis of these data using a paired Student’s t test demonstrated that the modest difference in the measured values for G, N, and P/M were indeed significant (P < 0.05). Whether these differences in the relative mRNA abundance reflect an affect of the K1651A alteration on transcriptional attenuation or the differential stability of the shorter mRNAs could not be determined by this assay. However, despite this perturbation in relative mRNA levels, the genomic replication products were present in approximately equivalent amounts to rVSV (Fig. 7A, compare product V lanes 1 and 2). These data demonstrate that changes to the predicted SAM binding residue G1674 had no detectable effect on RNA synthesis, whereas alterations to the predicted MTase active-site residues resulted in a defect in viral RNA synthesis. These defects, however, varied from relatively modest for K1651A, where levels of P and M mRNA were reduced to 60%, to K1795A, which decreased all RNA levels to <10% of those of rVSV.
To examine the effects of these L gene mutations on viral protein synthesis, cells were infected with each of the recombinant viruses, and protein synthesis was examined by metabolic labeling as described. Briefly, BHK-21 cells were infected at an MOI of 3, and at the indicated time postinfection the cells were incubated with [35S]methionine-cysteine for 3 h. After incubation, cytoplasmic extracts were prepared, and total protein was analyzed by SDS-PAGE (Fig. 8A). Quantitative analysis (Fig. 8B) of the levels of viral proteins identified three groups of viruses. Group I included viruses that were similar to the wild type (G1674A and K1651A), a second group (D1762A, E1833A, and E1833Q*) that showed a specific reduction in L protein to approximately 40% and in G protein to approximately 70% of wild-type levels, and virus K1795A, which showed a significant delay in protein synthesis and a specific reduction in L and G protein levels. For each of the viruses the levels of the L protein correlated well with the observed levels of L mRNA (compare Fig. 7B and 8B). In contrast, the levels of N, P, and M protein levels observed for K1651A, D1762A, E1833A, and E1833Q* were approximately equivalent to those of rVSV, despite the observed reductions in the corresponding mRNA levels (Fig. 7B). These data show that the mutations to the predicted MTase domain of VSV L affected viral protein levels in infected cells. However, the RNA synthesis data (Fig. 7B) demonstrate that the major affect of these mutations was on levels of the viral mRNA.
DISCUSSION
We performed a genetic and biochemical analysis of the VSV polymerase to determine whether domain VI of L protein functions as a methyltransferase. We generated six site-directed mutations in the L gene and determined the effect of these changes on viral growth and gene expression. Individual amino acid changes to the proposed MTase catalytic residues decreased levels of viral replication and resulted in defects in mRNA cap methylation in vitro. In contrast, alteration of a predicted SAM-binding residue was individually not sufficient to affect viral replication, or cap methylation (Table 2). These findings identify a function for domain VI of the VSV L protein in mRNA cap methylation, and they map amino acid residues that are important for this activity. Consequently, these findings have implications for the mechanism of mRNA processing in VSV and by extrapolation other nsNS RNA viruses.
Comparison of domain VI of VSV L to other known methyltransferases.
Our interest in domain VI of the VSV L protein stemmed from published sequence alignments and structure predictions which suggested that this region might adopt an MTase fold closely resembling that of FtsJ/RrmJ (9, 22), a heat shock methylase responsible for modification of the 2′-OH of U2552 in E. coli 23S rRNA (8). The 1.5-Å crystal structure of RrmJ in complex with SAM identified a SAM-binding region and suggested that the active site of RrmJ was formed by a catalytic tetrad of residues: K38, D124, K164, and E199 (8). Site-directed mutagenesis of RrmJ is more consistent with a catalytic triad of residues K38, D124, and K164, with E199 playing only a minor role in the methyltransfer reaction in vivo (27). These results are remarkably similar to the findings reported here, in which we show that alterations to the VSV L protein at residues K1651, D1762, and K1795 diminished methylation below the limits of detection of our assay, whereas a change at E1833 retained partial activity (Fig. 5A and 6A).
Well-characterized viral mRNA cap MTase's for which structural information exists include vaccinia virus VP39 (31), and the Dengue virus (DEN) nonstructural protein 5 (18). The structure of the reovirus core demonstrated that the λ2 contains two separate MTase domains, but owing to the difficulty of isolating enzymatically active λ2 protein, these activities have not yet been definitively assigned (10, 47). By extrapolation we suggest that K1651, D1762, K1795, and E1833 of VSV L protein are equivalent to K41, D138, K175, and E207 of vaccinia virus VP39 and to K61, D146, K181, and E217 of DEN NS5. However, it should be cautioned that RrmJ, VP39, and DEN NS5 function as a [ribose 2′-O]-MTase, whereas in the experiments described here we observed defects in both [guanine-N-7] and [ribose 2′-O]-MTase activity.
Does domain VI function as a guanine-N-7 or ribose-2′-O methyltransferase?
The experiments described here show that alterations in the proposed MTase active-site residues affect both [guanine-N-7] and [ribose 2′-O] methylation. However, sequence analysis that guided our mutagenesis suggested that this domain of L functions as a [ribose-2′-O]-MTase (9, 22). How might we account for this apparent discrepancy? One possibility is that domain VI functions as both a [ribose-2′-O]-MTase and a [guanine-N-7]-MTase. We do not favor this explanation, since the chemistry of the two RNA methylation reactions is quite distinct, as shown by studies with vaccinia virus where these reactions are carried out by two separate MTases with different substrate specificities (6, 54). An alternative explanation that is consistent with our data is a sequential model for VSV mRNA cap methylation in which the product of one methyltransferase acts as the substrate for the second. We favor the suggestion that [ribose 2′-O] methylation is essential for [guanine-N-7] methylation. Such an order of methylation would contrast with other mRNA cap methylation reactions in which the capping guanylate is methylated first, followed by the 2′ OH of the ribose. This suggestion is consistent with previous pulse-chase experiments in which 2′-O methylated cap structures of VSV mRNAs were chased into fully methylated cap structures at high SAM concentrations in vitro (62). However, subsequent studies reached different conclusions, suggesting that in fact the order of the VSV methylation reactions was reversed (39) or was not obligatory (29).
Recent studies with SeV support a role for domain VI of L protein as a [guanine-N-7]-MTase (43). In these experiments, a fragment of L protein that included domains V and VI was able to methylate short SeV-specific RNA sequences in vitro at the [guanine-N-7] position. Although these experiments demonstrated that the C terminus of the SeV L protein has [guanine-N-7]-MTase activity, the role of specific amino acid residues was not examined. In addition, the short RNA's were not 2′-O methylated, suggesting that either this “trans-methylation” assay did not recapitulate all facets of mRNA cap methylation or that the 2′-O-MTase activity resides elsewhere within L protein.
Sequence alignments show that domain VI of L protein from Newcastle disease virus (NDV) contains a clearly identifiable SAM binding motif, as well as the proposed catalytic K-D-K-E tetrad. However, NDV mRNAs are not 2′-O methylated (13), raising the possibility that these residues are conserved because they are required for [guanine-N-7]-MTase activity. Close inspection of this region of all nsNS RNA virus polymerases shows a difference in the proposed SAM binding region for the Filoviridae, as well as for the Rubulavirus and Avulavirus genera of the Paramyxoviridae. Each of these viruses contains the sequence AxGxG rather than GxGxG within motif I of the SAM-dependent MTase superfamily (53). It will be of interest to determine the biologic consequences of this difference and whether this change is responsible for the lack of 2′-O methylation in NDV and possibly other nsNS RNA viruses.
Our finding that amino acid alterations within domain VI affect mRNA cap methylation is reminiscent of studies of host range (hr) mutants of VSV. These viruses were competent for growth in BHK cells or chicken embryo fibroblasts, but were severely restricted for their growth in many human cell lines (42). Biochemical characterization of these viruses demonstrated that hr1 was completely defective for mRNA methylation in vitro and that hr8 was defective for [guanine-N-7] methylation (33). To explain the host range of these viruses, it was suggested that permissive cells might contain high levels of a cytoplasmic MTase that could overcome a defect in viral mRNA cap methylation or that the mutations might increase the Km of the viral MTase for SAM and that permissive cells contained higher intracellular levels of SAM. Recently, the sequence of hr1 was determined and shown to contain two amino acid changes within L protein at N505D and D1671V (25). The defect in mRNA cap methylation correlated with the substitution D1671V which resides within the predicted SAM-binding region of domain VI. It will be of interest to determine the role of other residues within this predicted SAM binding region on mRNA methylation and host range.
Viral gene expression.
In the present study, we generated a panel of recombinant viruses with defects in mRNA cap methyltransferase activity in vitro. Although these viruses show defects in growth in cell culture, they indicate that the viral methyltransferase is not essential for VSV replication. At the onset of these studies we anticipated that perturbations to cap methylation would likely be accompanied by alterations in viral protein synthesis, viral RNA synthesis, and virus titers. Remarkably, when protein synthesis was examined in BHK-21 cells from 3 to 6 h postinfection, the levels of most viral proteins were similar to those of rVSV, despite clear defects in viral mRNA synthesis and virus titers observed for several of the recombinants. These findings suggest that the VSV mRNAs are synthesized in excess of their requirements for translation in infected cells and that a twofold reduction in viral mRNA abundance (as observed for D1762A, E1833A, and E1833Q) does not result in a similar reduction in viral protein.
In VSV-infected cells there is a rapid shutoff of host cell protein synthesis (52, 61, 64). It has been suggested that an excess of viral mRNAs outcompete cellular mRNAs for translation (37, 38). However, in subsequent experiments in which VSV DI particles were used to decrease viral mRNA levels 14-fold, host cell translation was efficiently shut off, suggesting that this earlier hypothesis was incorrect (55). Rather, viral infection appears to modulate components of the translation machinery. Host translation can be restored by supplementing infected cell extracts with partially purified initiation factors eIF-2 or eIF-4F (16). Recent work has shown that the eIF-4F complex is altered in VSV-infected cells, in that eIF-4E is dephosphorylated and the 4E binding protein (4E-BP1) is activated (14). Decreasing the available pool of the cap binding complex thus contributes to the shut off of host cell translation in infected cells. In the experiments described here, we found that L gene mutations that compromise mRNA cap methylation in vitro do not result in a corresponding reduction in protein synthesis in infected cells (Fig. 8). This suggests that the effective recruitment of the translational machinery by a VSV mRNA may not be entirely dependent upon a fully methylated mRNA cap structure. However, it should be cautioned that whereas in the present study we show clear defects in cap methylation in vitro, we cannot eliminate the possibility that a cellular MTase promiscuously methylates the viral mRNA in infected cells and that this might lead to their more efficient translation. Previous work demonstrated that the VSV mRNA cap structure 7mGpppAmpApC could be found in the form 7mGpppm6AmpAmpC in infected cells (41). These two additional methylation events are absent on in vitro-synthesized mRNA and were thought to be mediated by cellular MTases. The biologic consequence of these methylations is not understood, but it will be of interest to examine the methylation status of the mRNA in infected cells.
Viral mRNA abundance.
Recombinant K1651A displays an unusual phenotype in that the levels of each mRNA differed modestly relative to rVSV: L (120%), G and N (80%), and P/M (60%). One possible explanation for these data are that alteration K1651A affects the process of transcriptional attenuation; however, the relative abundance of each of the mRNAs was not altered when the levels of RNA synthesized in vitro were examined, arguing against this explanation (Fig. 4 and 7). A second possibility is that the viral mRNAs were less stable because they were not properly methylated; however, one would expect to see a similar effect for each of the other viruses with defects in methylation. Previous experiments with VSV demonstrated that in vitro transcription reactions performed in the presence of SAH led to the formation of giant heterogeneous poly(A) tails (51). It seems possible that perturbing methylation might affect mRNA polyadenylation and that this could differentially affect the stability of the transcripts in infected cells. Perhaps alteration of K1651 results in a subtle conformational change within L protein such that a domain of L involved in polyadenylation is impacted. In any event, further experiments will be necessary to determine the mechanism by which the relative levels of each of the mRNAs of K1651A are altered. Such studies might also explain why K1651A exhibits a 1,000-fold defect in viral growth (Fig. 3) and yet has only a modest defect in viral gene expression.
In summary, we have shown that amino acid changes to a predicted MTase motif in domain VI of the VSV L protein disrupt mRNA cap methylation and affect viral replication. The lack of effective vaccines for many nsNS RNA viruses combined with the emergence of new nsNS RNA viruses underscores the need to develop effective therapeutics against this order of viruses. The methyltransferase activities of these viruses are suggested as attractive targets for the development of antiviral drugs (15). These studies contribute to such an objective by defining a region within the VSV L protein against which such inhibitors might be targeted.
Acknowledgments
We thank David Cureton, Max Nibert, David Knipe, and Steve Buratowski for critical reviews of the manuscript.
This study was supported by grant AI059371 from the National Institutes of Health to S.P.J.W.
REFERENCES
- 1.Abraham, G., and A. K. Banerjee. 1976. Sequential transcription of the genes of vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 73:1504-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham, G., D. P. Rhodes, and A. K. Banerjee. 1975. The 5′ terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell 5:51-58. [DOI] [PubMed] [Google Scholar]
- 3.Ball, L. A. 1977. Transcriptional mapping of vesicular stomatitis virus in vivo. J. Virol. 21:411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball, L. A., and C. N. White. 1976. Order of transcription of genes of vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 73:442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltimore, D., A. S. Huang, and M. Stampfer. 1970. Ribonucleic acid synthesis of vesicular stomatitis virus. II. An RNA polymerase in the virion. Proc. Natl. Acad. Sci. USA 66:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbosa, E., and B. Moss. 1978. mRNA(nucleoside-2′-)-methyltransferase from vaccinia virus: characteristics and substrate specificity. J. Biol. Chem. 253:7698-7702. [PubMed] [Google Scholar]
- 7.Barik, S. 1993. The structure of the 5′ terminal cap of the respiratory syncytial virus mRNA. J. Gen. Virol. 74(Pt. 3):485-490. [DOI] [PubMed] [Google Scholar]
- 8.Bugl, H., E. B. Fauman, B. L. Staker, F. Zheng, S. R. Kushner, M. A. Saper, J. C. Bardwell, and U. Jakob. 2000. RNA methylation under heat shock control. Mol. Cell 6:349-360. [DOI] [PubMed] [Google Scholar]
- 9.Bujnicki, J. M., and L. Rychlewski. 2002. In silico identification, structure prediction and phylogenetic analysis of the 2′-O-ribose (cap 1) methyltransferase domain in the large structural protein of ssRNA negative-strand viruses. Protein Eng. 15:101-108. [DOI] [PubMed] [Google Scholar]
- 10.Bujnicki, J. M., and L. Rychlewski. 2001. Reassignment of specificities of two cap methyltransferase domains in the reovirus lambda 2 protein. Genome Biol. 2:RESEARCH0038. [DOI] [PMC free article] [PubMed]
- 11.Chandrika, R., S. M. Horikami, S. Smallwood, and S. A. Moyer. 1995. Mutations in conserved domain I of the Sendai virus L polymerase protein uncouple transcription and replication. Virology 213:352-363. [DOI] [PubMed] [Google Scholar]
- 12.Chuang, J. L., and J. Perrault. 1997. Initiation of vesicular stomatitis virus mutant polR1 transcription internally at the N gene in vitro. J. Virol. 71:1466-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colonno, R. J., and H. O. Stone. 1976. Newcastle disease virus mRNA lacks 2′-O-methylated nucleotides. Nature 261:611-614. [DOI] [PubMed] [Google Scholar]
- 14.Connor, J. H., and D. S. Lyles. 2002. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 76:10177-10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Clercq, E. 2004. Antivirals and antiviral strategies. Nat. Rev. Microbiol. 2:704-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dratewka-Kos, E., I. Kiss, J. Lucas-Lenard, H. B. Mehta, C. L. Woodley, and A. J. Wahba. 1984. Catalytic utilization of eIF-2 and mRNA binding proteins are limiting in lysates from vesicular stomatitis virus-infected L cells. Biochemistry 23:6184-6190. [DOI] [PubMed] [Google Scholar]
- 17.Duprex, W. P., F. M. Collins, and B. K. Rima. 2002. Modulating the function of the measles virus RNA-dependent RNA polymerase by insertion of green fluorescent protein into the open reading frame. J. Virol. 76:7322-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egloff, M. P., D. Benarroch, B. Selisko, J. L. Romette, and B. Canard. 2002. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J. 21:2757-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emerson, S. U., and R. R. Wagner. 1972. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J. Virol. 10:297-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feller, J. A., S. Smallwood, S. M. Horikami, and S. A. Moyer. 2000. Mutations in conserved domains IV and VI of the large (L) subunit of the Sendai virus RNA polymerase give a spectrum of defective RNA synthesis phenotypes. Virology 269:426-439. [DOI] [PubMed] [Google Scholar]
- 21.Feller, J. A., S. Smallwood, M. H. Skiadopoulos, B. R. Murphy, and S. A. Moyer. 2000. Comparison of identical temperature-sensitive mutations in the L polymerase proteins of Sendai and parainfluenza 3 viruses. Virology 276:190-201. [DOI] [PubMed] [Google Scholar]
- 22.Ferron, F., S. Longhi, B. Henrissat, and B. Canard. 2002. Viral RNA-polymerases: a predicted 2′-O-ribose methyltransferase domain shared by all Mononegavirales. Trends Biochem. Sci. 27:222-224. [DOI] [PubMed] [Google Scholar]
- 23.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuichi, Y., and A. J. Shatkin. 2000. Viral and cellular mRNA capping: past and prospects. Adv. Virus Res. 55:135-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grdzelishvili, V. Z., S. Smallwood, D. Tower, R. L. Hall, D. M. Hunt, and S. A. Moyer. 2005. A single amino acid change in the L-polymerase protein of vesicular stomatitis virus completely abolishes viral mRNA cap methylation. J. Virol. 79:7327-7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta, K. C., and P. Roy. 1980. Alternate capping mechanisms for transcription of Spring Viremia of Carp Virus: evidence for independent mRNA initiation. J. Virol. 33:292-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hager, J., B. L. Staker, H. Bugl, and U. Jakob. 2002. Active site in RrmJ, a heat shock-induced methyltransferase. J. Biol. Chem. 277:41978-41986. [DOI] [PubMed] [Google Scholar]
- 28.Hager, J., B. L. Staker, and U. Jakob. 2004. Substrate binding analysis of the 23S rRNA methyltransferase RrmJ. J. Bacteriol. 186:6634-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond, D. C., and J. A. Lesnaw. 1987. The fates of undermethylated mRNA cap structures of vesicular stomatitis virus (New Jersey) during in vitro transcription. Virology 159:229-236. [DOI] [PubMed] [Google Scholar]
- 30.Hercyk, N., S. M. Horikami, and S. A. Moyer. 1988. The vesicular stomatitis virus L protein possesses the mRNA methyltransferase activities. Virology 163:222-225. [DOI] [PubMed] [Google Scholar]
- 31.Hodel, A. E., P. D. Gershon, X. Shi, and F. A. Quiocho. 1996. The 1.85 A structure of vaccinia protein VP39: a bifunctional enzyme that participates in the modification of both mRNA ends. Cell 85:247-256. [DOI] [PubMed] [Google Scholar]
- 32.Horikami, S. M., and S. A. Moyer. 1995. Alternative amino acids at a single site in the Sendai virus L protein produce multiple defects in RNA synthesis in vitro. Virology 211:577-582. [DOI] [PubMed] [Google Scholar]
- 33.Horikami, S. M., and S. A. Moyer. 1982. Host range mutants of vesicular stomatitis virus defective in in vitro RNA methylation. Proc. Natl. Acad. Sci. USA 79:7694-7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iverson, L. E., and J. K. Rose. 1981. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell 23:477-484. [DOI] [PubMed] [Google Scholar]
- 35.Keene, J. D., and R. A. Lazzarini. 1976. A comparison of the extents of methylation of vesicular stomatitis virus messenger RNA. Virology 69:364-367. [DOI] [PubMed] [Google Scholar]
- 36.Lehrach, H., D. Diamond, J. M. Wozney, and H. Boedtker. 1977. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry 16:4743-4751. [DOI] [PubMed] [Google Scholar]
- 37.Lodish, H. F., and M. Porter. 1980. Translational control of protein synthesis after infection by vesicular stomatitis virus. J. Virol. 36:719-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodish, H. F., and M. Porter. 1981. Vesicular stomatitis virus mRNA and inhibition of translation of cellular mRNA-is there a P function in vesicular stomatitis virus? J. Virol. 38:504-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moyer, S. A. 1981. Alteration of the 5′ terminal caps of the mRNAs of vesicular stomatitis virus by cycloleucine in vivo. Virology 112:157-168. [DOI] [PubMed] [Google Scholar]
- 40.Moyer, S. A., G. Abraham, R. Adler, and A. K. Banerjee. 1975. Methylated and blocked 5′ termini in vesicular stomatitis virus in vivo mRNAs. Cell 5:59-67. [DOI] [PubMed] [Google Scholar]
- 41.Moyer, S. A., and A. K. Banerjee. 1976. In vivo methylation of vesicular stomatitis virus and its host-cell messenger RNA species. Virology 70:339-351. [DOI] [PubMed] [Google Scholar]
- 42.Obijeski, J. F., and R. W. Simpson. 1974. Conditional lethal mutants of vesicular stomatitis virus. II. Synthesis of virus-specific polypeptides in nonpermissive cells infected with “RNA-” host-restricted mutants. Virology 57:369-377. [DOI] [PubMed] [Google Scholar]
- 43.Ogino, T., M. Kobayashi, M. Iwama, and K. Mizumoto. 2005. Sendai virus RNA-dependent RNA polymerase L protein catalyzes cap methylation of virus-specific mRNA. J. Biol. Chem. 280:4429-4435. [DOI] [PubMed] [Google Scholar]
- 44.Pattnaik, A. K., and G. W. Wertz. 1990. Replication and amplification of defective interfering particle RNAs of vesicular stomatitis virus in cells expressing viral proteins from vectors containing cloned cDNAs. J. Virol. 64:2948-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poch, O., B. M. Blumberg, L. Bougueleret, and N. Tordo. 1990. Sequence comparison of five polymerases (L proteins) of unsegmented negative-strand RNA viruses: theoretical assignment of functional domains. J. Gen. Virol. 71(Pt. 5):1153-1162. [DOI] [PubMed] [Google Scholar]
- 46.Qanungo, K. R., D. Shaji, M. Mathur, and A. K. Banerjee. 2004. Two RNA polymerase complexes from vesicular stomatitis virus-infected cells that carry out transcription and replication of genome RNA. Proc. Natl. Acad. Sci. USA 101:5952-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinisch, K. M., M. L. Nibert, and S. C. Harrison. 2000. Structure of the reovirus core at 3.6 Å resolution. Nature 404:960-967. [DOI] [PubMed] [Google Scholar]
- 48.Rhodes, D. P., and A. K. Banerjee. 1975. 5′-terminal sequence of vesicular stomatitis virus mRNAs synthesized in vitro. J. Virol. 17:33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhodes, D. P., S. A. Moyer, and A. K. Banerjee. 1974. In vitro synthesis of methylated messenger RNA by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell 3:327-333. [DOI] [PubMed] [Google Scholar]
- 50.Rose, J. K. 1975. Heterogeneous 5′-terminal structures occur on vesicular stomatitis virus mRNAs. J. Biol. Chem. 250:8098-8104. [PubMed] [Google Scholar]
- 51.Rose, J. K., H. F. Lodish, and M. L. Brock. 1977. Giant heterogeneous polyadenylic acid on vesicular stomatitis virus mRNA synthesized in vitro in the presence of S-adenosylhomocysteine. J. Virol. 21:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In D. Knipe and P. M. Howley (ed.), Fields virology, vol. 1. Lippincott Williams & Wilkins Co., New York, N.Y. [Google Scholar]
- 53.Schluckebier, G., M. O'Gara, W. Saenger, and X. Cheng. 1995. Universal catalytic domain structure of AdoMet-dependent methyltransferases. J. Mol. Biol. 247:16-20. [DOI] [PubMed] [Google Scholar]
- 54.Schnierle, B. S., P. D. Gershon, and B. Moss. 1992. Cap-specific mRNA (nucleoside-O2′-)-methyltransferase and poly(A) polymerase stimulatory activities of vaccinia virus are mediated by a single protein. Proc. Natl. Acad. Sci. USA 89:2897-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schnitzlein, W. M., M. K. O'Banion, M. K. Poirot, and M. E. Reichmann. 1983. Effect of intracellular vesicular stomatitis virus mRNA concentration on the inhibition of host cell protein synthesis. J. Virol. 45:206-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schubert, M., G. G. Harmison, C. D. Richardson, and E. Meier. 1985. Expression of a cDNA encoding a functional 241-kilodalton vesicular stomatitis virus RNA polymerase. Proc. Natl. Acad. Sci. USA 82:7984-7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shinshi, H., M. Miwa, and T. Sugimura. 1976. Enzyme cleaving the 5′-terminal methylated blocked structure of messenger RNA. FEBS Lett. 65:254-257. [DOI] [PubMed] [Google Scholar]
- 58.Sleat, D. E., and A. K. Banerjee. 1993. Transcriptional activity and mutational analysis of recombinant vesicular stomatitis virus RNA polymerase. J. Virol. 67:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smallwood, S., C. D. Easson, J. A. Feller, S. M. Horikami, and S. A. Moyer. 1999. Mutations in conserved domain II of the large (L) subunit of the Sendai virus RNA polymerase abolish RNA synthesis. Virology 262:375-383. [DOI] [PubMed] [Google Scholar]
- 60.Smallwood, S., T. Hovel, W. J. Neubert, and S. A. Moyer. 2002. Different substitutions at conserved amino acids in domains II and III in the Sendai L RNA polymerase protein inactivate viral RNA synthesis. Virology 304:135-145. [DOI] [PubMed] [Google Scholar]
- 61.Stanners, C. P., A. M. Francoeur, and T. Lam. 1977. Analysis of VSV mutant with attenuated cytopathogenicity: mutation in viral function, P, for inhibition of protein synthesis. Cell 11:273-281. [DOI] [PubMed] [Google Scholar]
- 62.Testa, D., and A. K. Banerjee. 1977. Two methyltransferase activities in the purified virions of vesicular stomatitis virus. J. Virol. 24:786-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Villarreal, L. P., M. Breindl, and J. J. Holland. 1976. Determination of molar ratios of vesicular stomatitis virus induced RNA species in BHK21 cells. Biochemistry 15:1663-1667. [DOI] [PubMed] [Google Scholar]
- 64.Wertz, G. W., and J. S. Youngner. 1972. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J. Virol. 9:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whelan, S. P., L. A. Ball, J. N. Barr, and G. T. Wertz. 1995. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA 92:8388-8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whelan, S. P., J. N. Barr, and G. W. Wertz. 2004. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:61-119. [DOI] [PubMed] [Google Scholar]
- 67.Whelan, S. P., and G. W. Wertz. 2002. Transcription and replication initiate at separate sites on the vesicular stomatitis virus genome. Proc. Natl. Acad. Sci. USA 99:9178-9183. [DOI] [PMC free article] [PubMed] [Google Scholar]