Abstract
The V protein of simian virus 5 (SV5) facilitates the ubiquitination and subsequent proteasome-mediated degradation of STAT1. Here we show, by visualizing direct protein-protein interactions and by using the yeast two-hybrid system, that while the SV5 V protein fails to bind to STAT1 directly, it binds directly and independently to both DDB1 and STAT2, two cellular proteins known to be essential for SV5-mediated degradation of STAT1. We also demonstrate that STAT1 and STAT2 interact independently of SV5 V and show that SV5 V protein acts as an adaptor molecule linking DDB1 to STAT2/STAT1 heterodimers, which in the presence of additional accessory cellular proteins, including Cullin 4a, can ubiquitinate STAT1. Additionally, we show that the avidity of STAT2 for V is relatively weak but is significantly enhanced by the presence of both STAT1 and DDB1, i.e., the complex of STAT1, STAT2, DDB1, and SV5 V is more stable than a complex of STAT2 and V. From these studies we propose a dynamic model in which SV5 V acts as a bridge, bringing together a DDB1/Cullin 4a-containing ubiquitin ligase complex and STAT1/STAT2 heterodimers, which leads to the degradation of STAT1. The loss of STAT1 results in a decrease in affinity of binding of STAT2 for V such that STAT2 either dissociates from V or is displaced from V by STAT1/STAT2 complexes, thereby ensuring the cycling of the DDB1 and SV5 V containing E3 complex for continued rounds of STAT1 ubiquitination and degradation.
Simian virus type 5 (SV5) is classified within the genus Rubulavirus of the subfamily Paramyxovirinae of the family Paramyxoviridae (18). It is now well established that most members of the Paramyxovirinae subfamily at least partially circumvent the interferon (IFN) response by blocking IFN signaling and reducing the production of IFN by infected cells (for reviews see (1, 10, 13, 22, 32). In human cells, SV5 blocks both IFN-α/β and IFN-γ signaling by targeting STAT1 (a transcription factor which is essential for IFN signaling) for proteasome-mediated degradation (3, 7, 23, 25, 36, 37). The molecular mechanisms by which SV5 targets STAT1 for degradation have been the subject of several recent investigations, and of the virus proteins, only the V protein is required to mediate this process (3, 7, 26).
The SV5 V protein is the 222-amino-acid product of a faithful mRNA copy of the second open reading frame (the V/P gene) of the virus genome. V has been shown to be a multifunctional protein that, apart from its involvement in STAT1 degradation, also interacts with an IFN-inducible DExD/H box helicase mda-5 to limit the production of IFN (1), binds single-stranded RNA (20) and may act as a chaperone keeping the nucleoprotein of the virus soluble (28). STAT1 degradation, mediated by SV5 V protein, is independent of IFN signaling or phosphorylation of STAT proteins (3, 24). However, there is an absolute requirement for STAT2 in STAT1 degradation and consequently, SV5 infection fails to induce the degradation of STAT1 in STAT2-deficient (U6A) cells (26) or in 2fTGH cells that express PIV2 V protein (a virus related to SV5 that preferentially degrades STAT2) (3). Additionally, SV5 cannot induce the degradation of STAT1 in murine cells (5). However, murine STAT1 is degraded if human STAT2 is expressed in murine cells, indicating that STAT2 is a host range determinant (24). A requirement for human STAT2 was also observed in a rabbit reticulocyte lysate-based in vitro assay that demonstrated that STAT1 was both ubiquitinated and degraded in the presence of purified SV5 V (27). However, while it has been shown both in vitro and in vivo that SV5 V protein can associate with STAT2, it was not clear whether this was a direct or indirect interaction. Additionally, the specific role of SV5 V in the degradation process has not been determined (13, 27).
Recent studies have established that the proteasomal degradation of STAT1 is mediated by ubiquitination and have identified some additional cellular proteins required in the degradation process. SV5 V protein interacts strongly with the 127-kDa subunit (DDB1) of the UV-damaged DNA-binding protein (21), and a clear role for DDB1 in the targeted degradation of STAT1 by the V protein of SV5 has now been firmly established (2, 19, 33). Direct interaction between SV5 V protein and DDB1 have been demonstrated in the yeast two-hybrid system that has also shown the first 20 amino acids of the V protein are not required for DDB1 binding (2).
In normal uninfected cells DDB1 has been shown to interact with several proteins, including Cullin 4a (Cul4a) (30). Cullins are highly conserved molecules that play an essential role in cell cycle control, development, and genomic stability (14). Cullins function by assembling multiprotein complexes as ubiquitin E3 ligases. In human cells there is a family of six closely related cullins and three more distantly related proteins. All members of this group exhibit a highly conserved carboxy-terminal domain that binds to a small RING finger protein, Rbx1 (or Roc1). Rbx1 activates an E2 ubiquitin-conjugating enzyme to catalyze polyubiquitination (14). In addition, cullins interact with substrate recognition proteins to complete an E3 ligase complex that specifically ubiquitinate the substrate protein and hence targets it for proteasome-mediated degradation (for reviews on ubiquitination and proteasome-mediated degradation, see references 12, 15, and 34).
It has been recently shown that DDB1 and Cul4a form part of E3 ligase complexes which regulate c-Jun activity (35), target CDT1for ubiquitination in Caenorhabditis elegans embryos (14), and stimulate ubiquitination and degradation of the HOXA9 homeodomain protein (38). Evidence for a direct role of Cul4a in the targeted degradation of STAT1 by SV5 comes from the observations that Cul4a is coimmunoprecipitated with V and DDB1, and that treatment of cells with short interfering RNA to Cul4a slightly affects the degradation of STAT1 (27, 33). It thus appears that the V protein of SV5 and other rubulaviruses can form novel ubiquitin ligase complexes that include STAT1, STAT2, DDB1 and probably Cul4a that lead to the ubiquitination and degradation of either STAT1 or STAT2 and this complex has been termed the VDC complex (33). However, the details of complex action and assembly have not been worked out.
We have previously reported the development of an in vitro assay, based on a reticulocyte lysate coupled transcription/translation system and have demonstrated the ubiquitination and degradation of STAT1 in the presence of STAT2 and SV5 V (27). However, the large number of cellular proteins in the reticulocyte lysate background limited the information regarding specific molecular interactions between cellular and viral proteins necessary for the degradation of STAT1. Here we examine in detail the molecular interactions of STAT1, STAT2, DDB1, and SV5 V, from which we derive a dynamic model in which the relative affinities of the interacting proteins alter during the degradation process, thereby ensuring efficient degradation of STAT1 by recycling the component parts that make up the VDC complex. Additionally, we demonstrate the position of SV5 V protein in the VDC complex as an adaptor between STAT2 and DDB1.
MATERIALS AND METHODS
Cell culture, infection and preparation of cell extracts.
Spodoptera frugiperda (Sf9) cells were maintained as suspension culture in TC-100 medium (Invitrogen) supplemented with 7.5% fetal calf serum at 28°C. Monolayers of approximately 108 Sf9 cells in 300-cm2 flasks (Helena Biosciences Ltd.) were infected with recombinant baculoviruses at a multiplicity of infection of 3 for 1 h at 28°C. After infection, cells were incubated at 28°C for 72 h in fresh medium. The medium was removed and cells scraped into 10 ml phosphate-buffered saline, centrifuged at 500 x g 5 min, and the cell pellet was resuspended in 5 ml of a lysis buffer (buffer A) containing 50 mM HEPES, pH 7.6, 10 mM KCl, 1.5 mM MgCl2, 1% Nonidet P40 (Sigma), 5 mM dithiothreitol, and 10% glycerol supplemented with protease inhibitors (Complete mini EDTA free [Roche]). Samples were centrifuged at 35,000 x g for 30 min at 4°C in a Beckman SW50.1 rotor and supernatants were frozen at −70°C.
293T cells were grown in suspension culture in Joklik's modified minimal essential medium (ICN Biomedical Ltd.) supplemented with 10% newborn calf serum at 37°C to a density of approximately 106 cells/ml. Cell extracts from approximately 109 cells were prepared essentially as described above.
Recombinant baculoviruses.
Recombinant baculoviruses containing human STAT2 and human STAT1 genes were the kind gift of I. Julkunen (Laboratory of Infectious Disease Immunology, Department of Microbiology, National Public Health Institute, Helsinki, Finland) (9).
Recombinant baculovirus containing a gene for human DDB1 was made using the BAC-to-BAC Baculovirus Expression System (Invitrogen) following the manufacturer's protocols. Briefly, DDB1 was amplified by PCR from a preexisting clone using the following primers; Fwd: 5′AAATTTCCCGGGCGCCATGTCGTACAACTACGTGGTAACGG and Rev: 5′TTTTATGCGGCCGCTGCAGGTCG, and the resulting, amplified product was digested with restriction enzymes EheI and NotI (New England Biolabs), ligated into pFastBac HTc digested with the same restriction enzymes, and transformed into Escherichia coli XL-1Blue. Plasmid DNA, from isolated clones, was sequenced and, correct clones were transformed into E. coli DH10Bac. Clones were isolated, the DNA extracted and transfected into Sf9 cells. Baculovirus isolated from this transfection was amplified over several rounds of infection and monitored for DDB1 expression.
GST-SV5 V cloning and purification.
Cloning, expression, and purification of glutathione S-transferase (GST)-SV5 V protein have been described previously (27).
Gel electrophoresis and immunoblotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was carried out using 4 to 12% Novex NuPAGE gradient gels using morpholinepropanesulfonic acid (MOPS) buffer (Invitrogen). Transfer to nitrocellulose by Western blotting was carried out using the Novex blotting module and buffer at a constant voltage of 10 V for 16 h.
STAT1 was detected using a polyclonal anti-STAT1 antibody raised against the amino-terminal 194 amino acids of the protein (Transduction Laboratories; catalog number G16930). DDB1 was detected using a rabbit polyclonal anti DDB1 antibody (Zymed cat no 34 to 2300). Cul4a was detected by a goat polyclonal antibody directed to the carboxy-terminus of the protein (Santa Cruz catalog number sc-8557). SV5 V protein was detected using a monoclonal antibody (PK) that has been described previously (29)
The protein-antibody interactions were visualized by enhanced chemiluminescence (ECL) using, where appropriate, horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G, or anti-goat protein A (Amersham International Ltd.., United Kingdom).
STAT2 capture of expressed proteins.
We incubated 50 μl protein G beads (Sigma) with 40 μg of a rabbit polyclonal anti-carboxy-terminal STAT2 antibody (Santa Cruz catalog number sc-476) for 1 h at 4°C followed by three washes of 1 ml with 50 mM Tris HCl, pH 7.5, 200 mM NaCl, 0.1% Tween 20 (buffer B); 1 ml of an Sf9 cell extract prepared from cells infected with baculovirus containing the human STAT2 gene was added to the antibody-coated beads and incubated for 1 h at 4°C before being washed as above, and 100 μg SV5 V protein (in 1 ml of buffer B) or buffer B only (control) was incubated with these beads for 1 h before washing. This was followed by further incubation of the beads with 0.5 ml of soluble cell extracts from Sf9 cells that had, or had not been, infected with baculoviruses expressing STAT1, DDB1, or mixtures of extracts containing STAT1 and DDB1. STAT2 beads without SV5 V were also incubated with 0.5 ml partially purified SV5 V-DDB1 complex (see below). The volume of each sample was equalized to 1 ml with uninfected Sf9 cell extract. After incubation for 2 h the beads were washed with buffer B as above. Where appropriate, further incubation of samples with either 1 ml 293T cell extract or Sf9 uninfected cell extract, with the addition of 10 μM of the proteasome inhibitor MG132, followed by washing, as above, completed the sequential capture of proteins for analysis. Samples were then analyzed by SDS-PAGE and immunoblotting.
GST-SV5 V capture of Sf9 expressed proteins.
Capture of proteins expressed in baculovirus infected Sf9 cells by GST-SV5 V protein was carried out essentially as previously described (27). Briefly glutathione beads were first saturated with E. coli expressed GST-SV5 V protein or GST alone. After washing three times with buffer B, 10 μl beads were incubated with 1 ml Sf9 cell extracts for 1 h at 4°C followed by three further washes in buffer B and analysis by SDS-PAGE. To isolate SV5 V-DDB1 complexes; after incubation with the Sf9 cell extract containing expressed DDB1 the GST-SV5 V-DDB1 complex was eluted using 10 mM glutathione in buffer B. The eluate was then incubated overnight at room temperature with recombinant TEV protease (Invitrogen) using a 50:1 ratio of protein to enzyme and with accompanying dialysis in buffer B. GST was removed from the dialysate by a glutathione column and the flow through (containing SV5 V-DDB1) used for capture assays without further purification.
STAT1 ubiquitination assays.
Protein G beads containing proteins captured as described above were tested for specific ubiquitination activity of STAT1; 5 μl of beads were mixed with 3.5 μl of a ubiquitination buffer containing the following components: 240 mM Tris HCl, pH 7.5, 30 mM MgCl2, 12 mM dithiothreitol, 3 mM ATP, 60 mM creatine phosphate, 20 units/ml creatine phosphokinase, 1.2 mg/ml ubiquitin (Sigma), 5 μg/ml ubiquitin ligase E2 UBCH5a prepared as described previously (4), and 50 ng ubiquitin ligase E1. The total reaction mix was adjusted to 20 μl and the samples were incubated for 1.5 h at 37°C with mixing followed by SDS-PAGE and immunoblotting for the detection of STAT1.
Yeast two-hybrid assay.
Plasmids directing the expression in yeast of GAL4 DNA binding domain fusion proteins were constructed by inserting a full-length open reading frame of the appropriate gene into pGBKT7 (Clontech; pGBKT7.SV5 V) or pHON1 (a plasmid with features similar to pGBKT7, including an identical alcohol dehydrogenase [ADH] promoter) (S. Goodbourn, unpublished) (pHON1.DDB1 and pHON1.STAT1). Plasmids directing the expression in yeast of GAL4 activation domain fusion proteins were constructed by inserting a full-length open reading frame of the appropriate gene into pGADT7 (Clontech; pGADT7.STAT2) or pHON3 (a plasmid with similar features to pGADT7, including an identical ADH promoter) (S. Goodbourn, unpublished) (pHON3.DDB1 and pHON3.STAT1).
Combinations of GAL4 DNA binding domain and activation domain fusion plasmids were introduced into Saccharomyces cerevisiae strain CG1945 (Clontech) or PJ69-4α (16) using lithium acetate-polyethylene glycol-mediated transformation (11), and positive transformants were selected by growth at 30°C on synthetic dropout medium (SD) lacking leucine and tryptophan. Double transformants were then streaked sequentially onto SD plates lacking leucine and tryptophan, and lacking leucine, tryptophan, and histidine and containing 2 mM 3-aminotriazole (for CG1945). For PJ69-4α selections, adenine was also omitted. Growth was monitored at 30°C.
The choices of strain used to give the results shown here were determined experimentally. Thus, CG1945 was chosen when V was used as the bait (Fig. 1b) because it gave the lowest background of several strains examined. PJ69-4α was chosen to examine interactions when DDB1 or STAT1 was used as the bait (Fig. 1c) because double transformants grew faster than those generated in CG1945, although equivalent interactions were seen in the latter strain (data not shown).
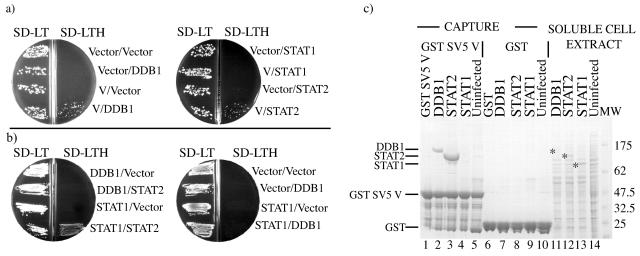
FIG. 1.
SV5 V binds directly to DDB1, STAT2 but not STAT1. (a). Saccharomyces cerevisiae CG1945 was transformed with a plasmid directing the expression of a GAL4 DNA binding domain fusion with SV5 V (pGBKT7.SV5 V) or the parental nonfusion vector pGBKT7, and a plasmid directing the expression of either a GAL4 activation domain fusion with DDB1 (pHON3.DDB1), STAT1 (pHON3.STAT1), or STAT2 (pGADT7.STAT2), or the parental vector pHON3 or pGADT7. The double transformants were selected and replated sequentially on SD without leucine and tryptophan or leucine, tryptophan, and histidine as described in the Materials and Methods. All combinations of plasmids should confer growth on medium lacking leucine and tryptophan (SD-LT), whereas only combinations of proteins that can interact confer growth on medium lacking leucine, tryptophan, and histidine and containing 2 mM 3-aminotriazole (SD-LTH). (b). S. cerevisiae PJ69-4α was transformed with a plasmid directing the expression of a GAL4 DNA binding domain fusion with DDB1 (pHON1.DDB1) or STAT1 (pHON1.STAT1) or the parental nonfusion vector pHON1, and a plasmid directing the expression of either a GAL4 activation domain fusion with STAT2 (pGADT7.STAT2) or DDB1 (pHON3.DDB1) or the parental vectors pHON3 or pGADT7. The double transformants were selected and replated sequentially on SD-LT and SD-LTH. All combinations of plasmids should confer growth on medium lacking leucine and tryptophan (SD-LT), whereas only combinations of proteins that can interact confer growth on medium lacking leucine, tryptophan adenine and histidine and containing 2 mM 3-aminotriazole (SD-LTH). (c). We incubated 500 μl of soluble cell extracts of Sf9 cells that had, or had not, been infected with recombinant baculoviruses which express DDB1, STAT2 or STAT1 with 10 μl of glutathione beads saturated with either GST-SV5 V (lanes 2 to 5) or GST (lanes 7 to 10). Bound proteins were visualized by Coomassie blue staining of the proteins following their separation through a 4 to 12% SDS-PAGE gel. Lanes 1 and 6 are controls of GST-SV5 V and GST, respectively incubated with buffer only (the bands seen below GST-SV5 V have been identified by Western blotting as truncations of GST-SV5 V[data not shown]) Lanes 11 to 14 represent 5 μl of the soluble cell extracts (i.e., 100 times this amount was mixed with the GST beads). Asterisks on lanes 11 to 14 indicate the positions of DDB1 STAT2 and STAT1, respectively, in the Sf9 cell extracts.
RESULTS
STAT2 and DDB1 bind to SV5 V independently.
Two approaches were undertaken to dissect the molecular interactions between the SV5 V protein and cellular partners involved in the STAT1 ubiquitination process; yeast two-hybrid assays and a biochemical analysis using extracts from baculovirus-infected Sf9 cells. In the first of these approaches, the yeast two-hybrid system showed that SV5 V interacts independently with both DDB1 and STAT2 but not STAT1 (Fig. 1a). In addition, these experiments also showed that while there was no direct interaction between DDB1 and either STAT1 or STAT2 there was a clear interaction between STAT1 and STAT2 in this system (Fig. 1b). To confirm these results STAT1, STAT2 and DDB1 were individually expressed from recombinant baculoviruses in Sf9 cells. Following expression of the proteins, soluble cell extracts were prepared (Fig. 1c, lanes 11 to 13) and mixed with glutathione agarose beads that had been saturated with bacterially expressed GST-SV5 V or GST alone. Figure 1c clearly shows that GST-SV5 V was able to bind to either DDB1 (lane 2) or STAT2 (lane 3) but not to STAT1 (lane 4). By comparison, neither STAT2 nor DDB1 bound to GST-only-saturated beads.
SV5 V acts as a bridge linking STAT2 to DDB1.
Since these results demonstrated direct and independent interactions between SV5 V protein and DDB1 and SV5 V protein and STAT2, we next determined whether SV5 V bound to one protein could capture the other to form a tertiary complex. In the approach taken, protein G beads were first coated with an antibody to the carboxy terminus of STAT2, which was then saturated with baculovirus-expressed STAT2. These STAT2-coated beads were in turn used to capture either SV5 V or preformed and purified DDB1-SV5 V complexes (as detailed in Materials and Methods) such that all the available SV5 V attached to the beads was bound through its interaction with STAT2. The STAT2, STAT2/V, STAT2/V/DDB1 beads were then further incubated with Sf9 cell extracts that did or did not contain STAT1, DDB1 or a mixture of both proteins. The composition of the protein complexes attached to the beads was visualized by Western blot analysis and Coomassie blue staining of the proteins following their separation on SDS-PAGE (Fig. 2).
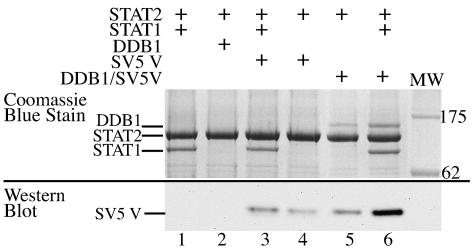
FIG. 2.
Assembly of DDB/SV5 V/STAT2/STAT1 complexes. Protein G beads were saturated with anti-STAT2 antibody and 40-μl aliquots of the resulting suspension of beads (50% vol/vol) were incubated with 500 μl of soluble Sf9 cell extract infected with a recombinant baculovirus that expressed STAT2 (Fig. 1, lane 12). These STAT2-saturated beads were then incubated with various mixtures of soluble extracts of; purified bacterially expressed SV5 V, soluble extracts of SF9 cells infected with recombinant baculoviruses that express either DDB1 or STAT1 (Fig. 1, lanes 11 and 13, respectively), or preformed and purified DDB1/SV5 V complexes. Samples equivalent to 5 μl of the original bead suspension were subjected to 4 to 12% SDS-PAGE; the SV5 V protein was detected by Western blot analysis, and other bound proteins were detected by Coomassie blue staining of the gel (note that the V protein was detected by Western blot analysis because it comigrated with antibody light chain on SDS-PAGE). The + symbol above each lane indicates which proteins or cell extracts were incubated with the immobilized anti-STAT2-saturated beads (Note that all comparable lanes had exactly the same amount of expressed protein cell extracts in the same volume added to the beads, the volume being corrected, where appropriate, with uninfected Sf9 cell extract.)
The results confirmed the yeast two-hybrid results, which showed that in the absence of SV5 V protein, STAT2 can interact with and specifically capture STAT1 (Fig. 2, lane 1). Furthermore, the binding of STAT2 to STAT1 is apparently unaffected by the presence of SV5 V bound to STAT2 (lane 3). Figure 2 also shows that while DDB1 does not bind directly to STAT2 (Fig. 2, lane 2) it is captured by STAT2 if it is in a preformed SV5 V/DDB1 complex, presumably through the interaction of SV5 V with STAT2 (lane 5). Significantly, preformed DDB1/SV5 V complexes were more efficiently captured by the STAT2/STAT1 beads (Fig. 2, lane 6) than by the STAT2 only beads (lane 5). Densitometry comparison of Fig. 2, lanes 5 and 6, indicated a fourfold increase in bound DDB1 in lane 6 compared to lane 5 despite the same amount of DDB1 being available for capture in both samples. It is also of note that the relative level of SV5 V was also highest when all the component parts of the complex (STAT1, STAT2, and DDB1) were present (compare lane 6 with lanes 3, 4, and 5in Fig. 2), suggesting that the affinity of binding of SV5 V to STAT2 may be higher in the presence of both STAT1 and DDB1, thereby also explaining the increase in the relative amount of DDB1 present in lane 6. These results clearly show that SV5 acts as an adaptor molecule bringing DDB1 and STAT2 together and imply that the relative affinity of DDB1/V for STAT2 is increased by the presence of STAT1 bound to STAT2
Affinity of binding of SV5 V to STAT2 is increased by the presence of STAT1 and DDB1.
To determine whether the binding of STAT1 and DDB1 increased the affinity of binding of SV5 V to STAT2, the dissociation of SV5 V from STAT2 in the presence and absence of DDB1 and STAT1 was investigated. STAT2/SV5 V-coated beads (Fig. 3, lane 1), generated as described above, were incubated for 180 min with mixtures of soluble extracts of baculovirus-infected Sf9 cells that did or did not contain STAT1 and/or DDB1 (Fig. 1c, lanes 11, 13, and 14). Following incubation, the supernatants were collected and the presence of SV5 V was detected by Western blot analysis. The composition of the proteins remaining attached to the beads was determined by Coomassie blue staining of the SDS-PAGE gel and by Western blotting (Fig. 3).
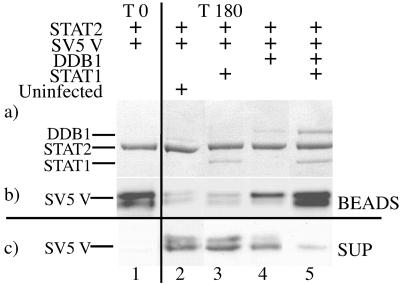
FIG. 3.
Interaction of SV5 V with STAT2 is stabilized by the presence of both DDB1 and STAT1. Complexes of SV5 V and STAT2 were assembled on protein G beads as described for Fig. 2. These complexes were subsequently incubated for 180 min with 500 μl of soluble antigen extracts of Sf9 cells that had or had not been infected with recombinant baculoviruses that expressed DDB1 or STAT1 (as shown in Fig. 1, lanes 11, 13, and 14). After incubation the beads were centrifuged and supernatant fluids were collected. Samples from washed beads and the supernatants were analyzed by SDS-PAGE followed by Coomassie blue staining of the gel (a) or by Western blotting with anti-Pk SV5 V antibody (b). The presence of SV5 V in the supernatant fluids was also detected by Western blot analysis (c). It is important to note that lane 1 represents the composition of the STAT2/SV5 V beads prior to incubation with the indicated cell extracts. Also note all comparable lanes had exactly the same amount of expressed protein cell extracts in the same volume added to the beads, the volume being corrected, where appropriate, with uninfected Sf9 cell extract.
When the beads were incubated with uninfected cell extract, most of the SV5 V dissociated into the supernatant and little remained bound to the beads (Fig. 3, compare lanes 1 and 2), indicating that the STAT2/SV5 V complex is not very stable. (Note: a similar dissociation of SV5 V from STAT2 as seen in lane 2 was obtained when buffer B was substituted for uninfected cell extracts during the incubation period, ruling out the possibility that dissociation was influenced by components of the Sf9 cell extract; data not shown.) Addition of STAT1 (Fig. 3, lane 3) did not affect the stability of the STAT2 SV5 V complex. In striking contrast, significantly less SV5 V dissociated from the beads into the supernatant when the STAT2/SV5 V beads were incubated in the presence of both STAT1 and DDB1 (Fig. 3, lane 5). Also in agreement with Fig. 2, the amount of DDB1 attached to the STAT2/SV5 V beads was highest in the presence of STAT1 (Fig. 3, compare lanes 4 and 5). It should also be noted that less SV5 V was detected in the supernatant and more SV5 V remained bound to the beads in the presence of DDB1 than when the beads were incubated with either extracts of uninfected cells or cells that contained STAT1 (Fig. 3, compare lanes 2, 3, and 4), suggesting that the binding of DDB1 to V also increases its affinity for STAT2 but not as much as the presence of both DDB1 and STAT1 does. (note in the preparation of V used for this experiment a doublet band can be seen, with the faster-migrating fragment having a lower affinity for STAT2 than the upper full-length protein).
Cul4a binds to DDB1/SV5 V/STAT2/STAT1 complexes to generate a functional E3 ligase complex.
Members of the cullin family of proteins bind to cellular proteins to form SCF-like ubiquitin complexes (14, 35, 38). Evidence has also previously been presented that Cul4a may play a role in the degradation of STAT1, mediated by SV5 V protein (26). Furthermore, in our previous in vitro studies, GST-SV5 V captured Cul4a from rabbit reticulocyte lysates. However, since these lysates also contained rabbit DDB1, it was assumed that this was an indirect interaction between SV5 V and Cul4a mediated through DDB1 (27).
In order to determine whether DDB1/SV5 V/STAT2/STAT1 complexes formed on protein G beads could also bind Cul4a, complexes (see Fig. 2, lane 6) were incubated with cell extracts prepared from 293T cells and bound proteins were visualized by either Coomassie blue staining of the SDS-PAGE gel or subjected to Western blot analysis using an antibodies specific for either STAT1, DDB1, or Cul4a (Fig. 4). The results clearly showed that Cul4a was captured from the 293T cell extracts by the DDB1/SV5 V/STAT2/STAT1 complexes However, it was not possible in this experiment to determine whether or not any other cellular proteins (e.g., Rbx1) were recruited onto the immobilized complex.
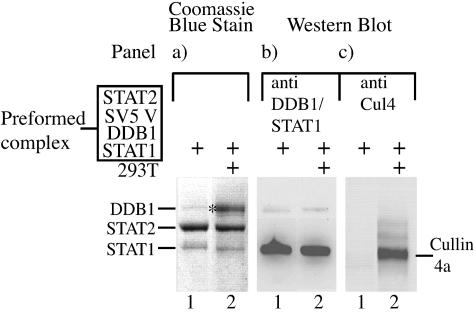
FIG. 4.
SV5 V, DDB1, STAT2 and STAT1 complexes recruit Cul4a from 293T cell extracts. We incubated 40 μl of DDB1/SV5 V/STAT2/STAT1 complexes captured on protein G beads (as described and shown in Fig. 2, lane 6) with 500 μl of either buffer (lanes 1) or soluble antigen extracts of 293T cells (lanes 2) as described in Materials and Methods. The proteins captured on the beads were separated by electrophoresis through a 4 to 12% PAGE and either visualized by staining the gel with Coomassie blue (panel a) or subjected to Western blot analysis using a pool of antibodies to STAT1 and DDB1 (panel b) or an anti-Cul4 antibody (panel c). Note that the Coomassie blue-stained band marked * in panel A was analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF-mass spectrometry and found to contain DDB1 and bovine immunoglobulin G (heavy and light chains, which presumably was captured from the 293T cell extract).
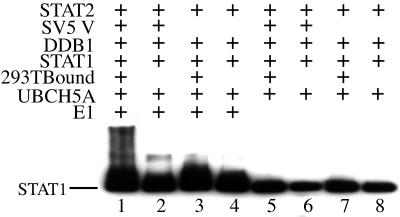
We next determined whether complexes containing DDB1/SV5 V/STAT2/STAT1 and Cul4a could ubiquitinate STAT1. Thus, a series of ubiquitination assays using a variety of complex assemblies were carried out in the presence of E1 and UBCH5a (which is the most potent E2 enzyme that we have yet identified using in vitro-based assays; data not shown). Figure 5 shows that STAT1 was polyubiquitinated in complexes of DDB1/SV5 V/STAT2/STAT1 when additional proteins, including Cul4a, were captured from a 293T cell extract as described in the legend to Fig. 4 (lane 1) but not if 293T cell extract (lane 2) was omitted from the complex assembly protocol. (Note that no ubiquitination of SV5 V could be detected in these experiments; data not shown.) Ubiquitination of STAT1 also did not occur if SV5 V was omitted from the complex. Similarly, no ubiquitination of STAT1 took place in the absence of either ubiquitin ligase E1 (Fig. 5, lanes 5 to 8) or Ubch5a (data not shown) regardless of the complex that had been formed.
FIG. 5.
SV5 V, DDB1, STAT2, STAT1, Cul4a containing complexes can induce ubiquitination after of STAT1. Complexes containing various combinations of DDB1, SV5 V, STAT2, STAT1 and proteins captured from 293T cells (including Cul4a) were assembled on beads as previously described; 5 μl of washed beads were then used for ubiquitination assays in the presence and absence of E1 and UBCH5a. The presence of polyubiquitinated STAT1 in the samples was detected by Western blot analysis using anti-STAT1 antibody followed by horseradish peroxidase-labeled protein A. Labeled proteins were then visualized on x-ray film following exposure to ECL reagents. (Note that lanes 2 and 6 and lanes 1 and 5 are the same complexes as shown in Fig. 4, lanes 1 and 2, respectively.)
DISCUSSION
In our investigations on the molecular basis for the degradation of STAT1 by SV5 we have sought to establish in vitro systems to demonstrate the role of both virus and cellular proteins in the degradation process. We have previously demonstrated that STAT1 could be both ubiquitinated and degraded in in vitro assays based on the synthesis of appropriate proteins in reticulocyte lysate coupled transcription/translation systems (27). However, while such systems proved useful in establishing suitable experimental conditions for in vitro ubiquitination, the reticulocyte lysate cellular background limited the information that could be gained regarding the specific interaction(s) of the proteins involved in the degradation process.
Here, using protein capture assays in conjunction with the yeast two-hybrid system, we have established that although STAT1 does not bind directly to either DDB1 or SV5 V, it does bind directly to STAT2 in the absence of IFN-induced phosphorylation. Association of STAT1 with STAT2 in the absence of IFN-induced phosphorylation has been reported previously (31), but what is striking in the experiments presented here is the apparent in vitro stability of such STAT1/STAT2 complexes. Although SV5 V does not bind STAT1 directly, we show that SV5 V binds directly to STAT2, and can thereby indirectly capture STAT1 through the association of STAT1 with STAT2. Furthermore, since SV5 V also binds directly to DDB1, it can act as a molecular bridge between DDB1 and complexes of STAT1/STAT2. Significantly, the affinity of binding of SV5 V to STAT2 is relatively weak but is enhanced by the formation of the DDB1/SV5 V/STAT2/STAT1 complex. In vitro ubiquitin assays in the presence of E1 and UBCH5a showed that while DDB1/SV5 V/STAT2/STAT1 complexes were unable to polyubiquitinate STAT1, following the capture of Cul4a (presumably through its known interaction with DDB1 (6, 14, 38) from extracts of 293T cells a functional ubiquitination ligase complex was formed that led to the ubiquitination of STAT1. Whether capture of cellular proteins in addition to Cul4a from 293T cell extracts, e.g., Rbx1, is required for ubiquitination of STAT1 is currently under investigation.
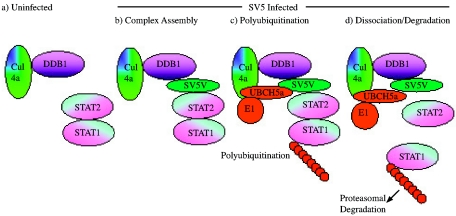
These results have enabled us to propose a dynamic model for of the degradation of STAT1. In essence the model proposes that two normally non interacting systems, the DDB1/Cul4a ubiquitination system and the STAT1/STAT2 signaling system (Fig. 6a) are brought together via SV5 V protein (Fig. 6b). As a result of this interaction, STAT1 becomes polyubiquitinated via a ubiquitin E3 complex containing DDB1, Cul4a, E1, and UBCH5a (Fig. 6c). Polyubiquitinated STAT1 then dissociates at some point from STAT2 before it is degraded by the proteasome (Fig. 6d). The loss of STAT1 destabilizes the interaction of STAT2 with the SV5 V/DDB1/Cul4a complex such that STAT2 either dissociates from the complex or is displaced by preformed STAT1/2 complexes. Since SV5 V clearly has a high affinity for DDB1, as it can displace other proteins, including DDB2 and HBX, from DDB1 (19), SV5 V probably remains bound to the DDB1/Cul4a complex, which would thus be primed to accept new STAT2/STAT1 complexes.
FIG. 6.
Model for the role of SV5 V in the ubiquitination of STAT1. (a). In uninfected cells DDB1 and Cul4a complexes do not associate with STAT1 and STAT2, which can associate together in the absence of IFN stimulated phosphorylation. (b). After infection with SV5, the V protein acts as a linker bringing DDB1/Cul4a complexes into a close and stable association with STAT1/STAT2 complexes. (c). An E3 ligase complex is formed by the recruitment of other cellular proteins (including E1 and UBCH5a) and STAT1 becomes polyubiquitinated. (d). STAT1 is degraded by the proteasome. The DDB1/SV5 V/STAT2 complex is destabilized. While SV5 V remains bound to DDB1/Cul4a complex, STAT2 either captures STAT1 to form a new degradation complex or dissociates from the complex (perhaps through competition with STAT2/STAT1 complexes) and can thus bind any free STAT1 before the resulting STAT1/STAT2 complex is recaptured by the DDB1/SV5 V-containing E3 ligase. Note that the model does not attempt to predict the stoichiometry of the components of the complex, only their relative positions.
The novelty of this model is that the adaptor molecule (SV5 V) does not interact directly with the target molecule (STAT1) but with its partner (STAT2) to bring the target molecule for ubiquitination into contact with the DDB1/Cul4a ubiquitin ligase complex. The results and model presented here show how SV5 V protein might interact with other components of the STAT1 ubiquitination complex. However, the current study does not address the question of whether, in its role as an adaptor, the V protein is either polymeric or has more than the adaptor function in the E3 ligase complex described. Nevertheless, the formation of a stable complex between DDB1, STAT2, SV5 V, and STAT1 which can subsequently be activated for STAT1 ubiquitination suggests that the adaptor role of SV5 V may be the key initial step in STAT1 degradation. Furthermore, this model would help to explain why small amounts of SV5 V can rapidly induce the degradation of STAT1 in cells, as occurs upon infection of cells with UV-inactivated virus (8).
SV5 V protein has now been shown to bind to at least three cellular proteins, DDB1, STAT2, and mda-5, and at least one viral protein, the nucleoprotein (28). It may therefore be that the natively unfolded nature of paramyxovirus V proteins (17) facilitates the formation of different structural conformations depending on the binding partner(s). The results presented here suggest that perhaps only the DDB1/SV5 V/STAT1/STAT2 complex will be stable enough to give meaningful data on the structure of SV5 V in the functional E3 ubiquitin ligase complexes that leads to the degradation of STAT1.
Acknowledgments
We acknowledge the following for their help: C Boutell, Institute of Virology, University of Glasgow, for the gift of plasmids containing UBCH5a; and C. Botting and R. Antrobus, Huan Ting Liu, and E. Jaffray, School of Biology, University of St. Andrews, for mass spectrometer analysis, assistance with baculovirus cloning, and expression and for the gift of E1 ligase, respectively.
This work was supported by the Wellcome Trust and BBSRC
REFERENCES
- 1.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrejeva, J., E. Poole, D. F. Young, S. Goodbourn, and R. E. Randall. 2002. The p127 subunit (DDB1) of the UV-DNA damage repair binding protein is essential for the targeted degradation of STAT1 by the V protein of the paramyxovirus simian virus 5. J. Virol. 76:11379-11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatziandreou, N., D. Young, J. Andrejeva, S. Goodbourn, and R. E. Randall. 2002. Differences in interferon sensitivity and biological properties of two related isolates of simian virus 5: a model for virus persistence. Virology 293:234-242. [DOI] [PubMed] [Google Scholar]
- 6.Chen, X., Y. Zhang, L. Douglas, and P. Zhou. 2001. UV-damaged DNA-binding proteins are targets of CUL-4A-mediated ubiquitination and degradation. J. Biol. Chem. 276:48175-48182. [DOI] [PubMed] [Google Scholar]
- 7.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J. Virol. 73:3125-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagerlund, R., K. Melen, L. Kinnunen, and I. Julkunen. 2002. Arginine/lysine-rich nuclear localization signals mediate interactions between dimeric STATs and importin alpha 5. J. Biol. Chem. 277:30072-30078. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Sastre, A. 2004. Identification and characterisation of viral antagonists of type 1 interferon in negative-strand RNA viruses, p. 249-280. In Y. Kawaoka (ed.), Biology of negative strand RNA viruses. Springer-Verlag, New York, NY. [DOI] [PubMed]
- 11.Gietz, R. D., R. H. Schiestl, A. R. Willems, and R. A. Woods. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11:355-360. [DOI] [PubMed] [Google Scholar]
- 12.Glickman, M. H., and A. Ciechanover. 2002. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82:373-428. [DOI] [PubMed] [Google Scholar]
- 13.Horvath, C. M. 2004. Silencing STAT's: lessons from paramyxovirus interferon evasion. Cytokine Growth Factor Rev. 15:117-127. [DOI] [PubMed] [Google Scholar]
- 14.Hu, J., C. M. McCall, T. Ohta, and Y. Xiong. 2004. Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat. Cell Biol. 6:1003-1009. [DOI] [PubMed] [Google Scholar]
- 15.Jackson, P. K., A. G. Eldridge, E. Freed, L. Furstenthal, J. Y. Hsu, B. K. Kaiser, and J. D. Reimann. 2000. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 10:429-439. [DOI] [PubMed] [Google Scholar]
- 16.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlin, D., S. Longhi, V. Receveur, and B. Canard. 2002. The N-terminal domain of the phosphoprotein of morbilliviruses belongs to the natively unfolded class of proteins. Virology 296:251-262. [DOI] [PubMed] [Google Scholar]
- 18.Lamb, R. A., and D. Kolakofsky. 2001. Paramyxviridae: the viruses and their replication, p. 1305-1340. In B. N. Fields, D. M. Knipe, P. M. Howley, and D. E. Griffen (ed.), Field's virology, 4th ed., vol. 1. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 19.Leupin, O., S. Bontron, and M. Strubin. 2003. Hepatitis B virus X protein and simian virus 5 V protein exhibit similar UV-DDB1 binding properties to mediate distinct activities. J. Virol. 77:6274-6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, G. Y., R. G. Paterson, and R. A. Lamb. 1997. The RNA binding region of the paramyxovirus SV5 V and P proteins. Virology 238:460-469. [DOI] [PubMed] [Google Scholar]
- 21.Lin, G. Y., R. G. Paterson, C. D. Richardson, and R. A. Lamb. 1998. The V protein of the paramyxovirus SV5 interacts with damage-specific DNA binding protein. Virology 249:189-200. [DOI] [PubMed] [Google Scholar]
- 22.Nagai, Y., and A. Kato. 2004. Accessory genes of the Paramyxoviridae, a large family of nonsegmented negative-strand RNA viruses, as a focus of active investigation by reverse genetics, p. 197-248. In Y. Kawaoka (ed.), Biology of negative strand RNA viruses. Springer-Verlag, New York, NY. [DOI] [PubMed]
- 23.Nishio, M., M. Tsurudome, M. Ito, M. Kawano, H. Komada, and Y. Ito. 2001. High resistance of human parainfluenza type 2 virus protein-expressing cells to the antiviral and anti-cell proliferative activities of alpha/beta interferons: cysteine-rich V-specific domain is required for high resistance to the interferons. J. Virol. 75:9165-9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parisien, J. P., J. F. Lau, and C. M. Horvath. 2002. STAT2 acts as a host range determinant for species-specific paramyxovirus interferon antagonism and simian virus 5 replication. J. Virol. 76:6435-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parisien, J. P., J. F. Lau, J. J. Rodriguez, B. M. Sullivan, A. Moscona, G. D. Parks, R. A. Lamb, and C. M. Horvath. 2001. The V protein of human parainfluenza virus 2 antagonizes type I interferon responses by destabilizing signal transducer and activator of transcription 2. Virology 283:230-239. [DOI] [PubMed] [Google Scholar]
- 26.Parisien, J. P., J. F. Lau, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2002. Selective STAT protein degradation induced by paramyxoviruses requires both STAT1 and STAT2 but is independent of alpha/beta interferon signal transduction. J. Virol. 76:4190-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Precious, B., D. F. Young, L. Andrejeva, S. Goodbourn, and R. E. Randall. 2005. In vitro and in vivo specificity of ubiquitination and degradation of STAT1 and STAT2 by the V proteins of the paramyxoviruses simian virus 5 and human parainfluenza virus type 2. J. Gen. Virol. 86:151-158. [DOI] [PubMed] [Google Scholar]
- 28.Randall, R. E., and A. Bermingham. 1996. NP:P and NP:V interactions of the paramyxovirus simian virus 5 examined using a novel protein:protein capture assay. Virology 224:121-129. [DOI] [PubMed] [Google Scholar]
- 29.Randall, R. E., D. F. Young, K. K. Goswami, and W. C. Russell. 1987. Isolation and characterization of monoclonal antibodies to simian virus 5 and their use in revealing antigenic differences between human, canine and simian isolates. J. Gen. Virol. 68:2769-2780. [DOI] [PubMed] [Google Scholar]
- 30.Shiyanov, P., A. Nag, and P. Raychaudhuri. 1999. Cullin 4A associates with the UV-damaged DNA-binding protein DDB. J. Biol. Chem. 274:35309-35312. [DOI] [PubMed] [Google Scholar]
- 31.Stancato, L. F., M. David, C. Carter-Su, A. C. Larner, and W. B. Pratt. 1996. Preassociation of STAT1 with STAT2 and STAT3 in separate signalling complexes prior to cytokine stimulation. J. Biol. Chem. 271:4134-4137. [DOI] [PubMed] [Google Scholar]
- 32.Stock, N., S. Goodbourn, and R. E. Randall. 2004. The anti-interferon mechanisms of paramyxoviruse. Kluwer Plenum, New York, NY.
- 33.Ulane, C. M., and C. M. Horvath. 2002. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology 304:160-166. [DOI] [PubMed] [Google Scholar]
- 34.Weissman, A. M. 2001. Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell. Biol. 2:169-178. [DOI] [PubMed] [Google Scholar]
- 35.Wertz, I. E., K. M. O'Rourke, Z. Zhang, D. Dornan, D. Arnott, R. J. Deshaies, and V. M. Dixit. 2004. Human De-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science 303:1371-1374. [DOI] [PubMed] [Google Scholar]
- 36.Yokosawa, N., S. Yokota, T. Kubota, and N. Fujii. 2002. C-terminal region of STAT-1α is not necessary for its ubiquitination and degradation caused by mumps virus V protein. J. Virol. 76:12683-12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young, D. F., L. Didcock, S. Goodbourn, and R. E. Randall. 2000. Paramyxoviridae use distinct virus-specific mechanisms to circumvent the interferon response. Virology 269:383-390. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, Y., G. Morrone, J. Zhang, X. Chen, X. Lu, L. Ma, M. Moore, and P. Zhou. 2003. CUL-4A stimulates ubiquitylation and degradation of the HOXA9 homeodomain protein. EMBO J. 22:6057-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]