Abstract
The increased transmission and geographic spread of dengue fever (DF) and its more severe presentation, dengue hemorrhagic fever (DHF), make it the most important mosquito-borne viral disease of humans (50 to 100 million infections/year) (World Health Organization, Fact sheet 117, 2002). There are no vaccines or treatment for DF or DHF because there are no animal or other models of human disease; even higher primates do not show symptoms after infection (W. F. Scherer, P. K. Russell, L. Rosen, J. Casals, and R. W. Dickerman, Am. J. Trop. Med. Hyg. 27:590-599, 1978). We demonstrate that nonobese diabetic/severely compromised immunodeficient (NOD/SCID) mice xenografted with human CD34+ cells develop clinical signs of DF as in humans (fever, rash, and thrombocytopenia), when infected in a manner mimicking mosquito transmission (dose and mode). These results suggest this is a valuable model with which to study pathogenesis and test antidengue products.
Dengue virus infections in humans can be subclinical or can cause illnesses ranging from a mild, flu-like syndrome with rash and some hemorrhagic manifestations (dengue fever [DF]) to a severe and sometimes fatal disease, with coagulopathy, capillary leakage, and hypovolemic shock (dengue hemorrhagic fever [DHF]). The development of dengue vaccines and antivirals is complicated by the viruses ' diversity: they would have to protect against four different serotypes and numerous genotypes within each serotype. We have shown that dengue viruses differ in their rates of infection and replication in primary human cells, and these rates vary from one donor to another (3). In addition, secondary infection by a heterologous virus enhances disease severity, and multivalent vaccine preparations could prove dangerous to vaccinees if they induce subneutralizing levels of antibodies (5). Thus, it is hypothesized that viral and host (genetic and prior immune status) factors contribute to dengue pathogenesis, but there has been no in vivo system in which to measure their contributions (9).
Little is understood about the events that lead to DF or DHF after an infected mosquito (mainly Aedes aegypti) bites and injects virus into the human epidermis. The main targets for viral infection and replication seem to be dendritic cells (DCs) and macrophages, mainly Langerhans and monocyte-derived DCs (7, 13). A full repertoire of DCs develops in nonobese diabetic/severely compromised immunodeficient (NOD/SCID) mice transplanted with human cord blood hematopoietic progenitor (CD34+) cells (4, 8). The NOD/SCID strain of mice lacks T and B cells and has defects in natural killer cell function and antigen-presenting cell development and function and genetically lacks C5, resulting in a deficiency in hemolytic complement; it therefore provides an excellent environment for reconstitution with human hematopoetic cells and tissues.
All animal procedures were reviewed and approved by our Institutional Animal Care and Use Committees.
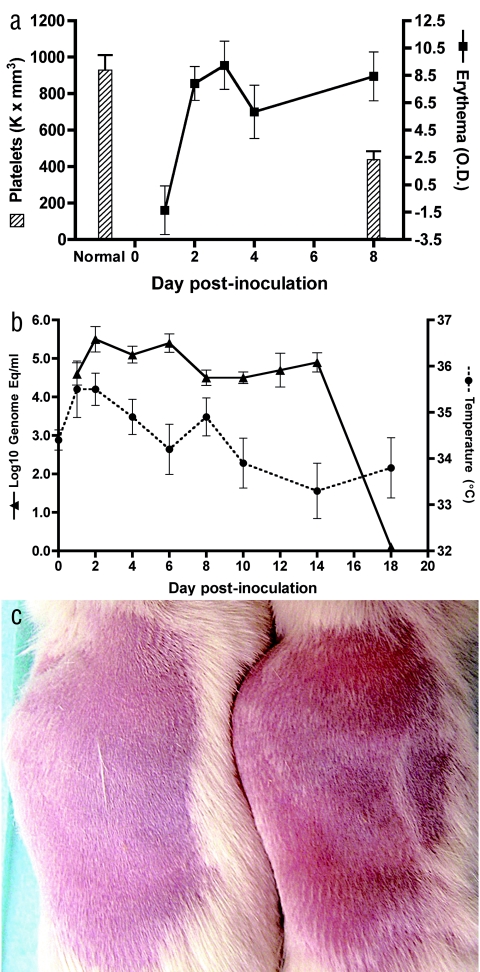
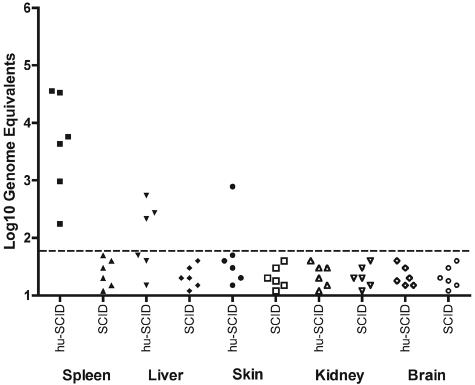
CD34+ cells were isolated from human cord blood with immunomagnetic beads and transplanted into preconditioned mice (325 rads) as we have previously described (4, 8). Mice were analyzed for reconstitution with human cells (CD45+) and for the presence of human DCs (defined as CD11c+ or CD123+ lineage negative, HLA-DR+) in peripheral blood by flow cytometry using human-specific antibodies, as we have previously described (4, 8). We obtained high levels of reconstitution and DC development in multiple organs in these mice: human CD45+ cells in whole blood before inoculation (8+ weeks posttransplant) ranged from 0.9 to 43% (n = 18; median, 14%). A total of 18 reconstituted NOD/SCID mice were inoculated subcutaneously with approximately 4.7 log10 PFU (7.7 log10 genome equivalents) of dengue serotype 2 strain K0049 (Southeast Asia genotype; C6/36 cell passage 3); this virus was selected because it grows to high levels in human DCs in vitro (3). Six reconstituted and 15 nonreconstituted NOD/SCID mice (same genetic background but not receiving human CD34+ cells) served as negative controls (inoculated with saline, C6/36 cell culture supernatant, or virus). The most dramatic signs of dengue infection in humanized mice were erythema and thrombocytopenia (Fig. 1a), as occurs in humans. We measured erythema using a DermaSpectrometer (Cortex Technology, Denmark) while mice were anesthesized; when compared to nonreconstituted, infected mice, erythema mean values were significantly different on days 1 through 8 (all pairwise t tests, P < 0.05; reconstituted mouse range, −1.36 to 8.44; nonreconstituted mouse range, −5.35 to −1.51). A marked decrease in platelets was measurable on day 8 postinoculation and was statistically highly significant (Mann-Whitney test, P < 0.001) when comparing six infected, reconstituted mice (range, 320,000 to 594,000/mm3) to three nonreconstituted, infected and six reconstituted, noninfected mice (normal mouse range, 600,000 to 1,200,000/mm3; range for our negative controls, 610,000 to 1,251,000/mm3). Rash was visible on days 2 to 4 in the majority (eight of nine) of infected, reconstituted mice and continued through day 14 in some mice (Fig. 1c). Viremias were measured in sera (25- to 50-μl retro-orbital bleed on alternate days) by quantitative, real-time reverse transcription-PCR (RT-PCR) (Fig. 1b) (11); virus levels peaked on days 2 to 6 postinoculation (range, 4.2 to 5.4 log10 genome equivalents/ml). A rise in body temperature followed the viremia, and temperature returned to normal levels on day 10. For reconstituted mice, changes in temperature were statistically significant by analysis of variance (P < 0.02); for nonreconstituted mice, changes in temperature were not significant by analysis of variance (P ≫ 0.05). Nonreconstituted, infected NOD/SCID mice showed consistently lower viremias (undetectable by day 10; range, 3.6 to 4.1 log10 genome equivalents/ml) and, as noted above, showed no rash, rise in temperature, or decrease in platelets and therefore served as negative controls for statistical analyses throughout. Body weight decreased dramatically in some reconstituted, infected animals (20% loss in four mice); there were no gastrointestinal abnormalities on necropsy, and weight loss seemed to be due to lethargy or lack of eating at the time of viremia or fever. Several organs were tested for viral positive- and negative-strand RNA template (surrogate for replicating virus) by quantitative, real-time RT-PCR (3) at different times postinfection in reconstituted and nonreconstituted mice. Only the reconstituted, infected mice had dengue virus RNA in various tissues on day 8: spleen (six of six mice), liver (three of six mice), and skin (one of six mice) had positive-strand RNA, while negative-strand RNA was detectable in spleen (two of six mice) and skin (one of six mice) (Fig. 2). These data are consistent with viral replication in this model.
FIG. 1.
Signs of dengue virus infection in humanized NOD/SCID mice. (a) Thrombocytopenia and erythema in infected mice by day 8 (means and standard errors of the means); platelet levels in nonreconstituted, infected and noninfected (“normal”; n = 9) versus reconstituted, infected (n = 6); and erythema development in the latter group. O.D., optical density. (b) Viremia and fever on days 1 to 18 (mean and standard error) in mice reconstituted with human CD34+ cells. (c) Comparison of rashes on the backs of shaved mice. The nonreconstituted, infected mouse is on the left, and the reconstituted, infected mouse is on the right (both day 7 postinfection).
FIG. 2.
Comparison of dengue virus RNA in reconstituted, infected NOD/SCID mice (hu-SCID) and nonreconstituted, infected NOD/SCID mice (SCID) on day 8. Virus positive-strand RNA was estimated in each organ (samples in triplicate), using quantitative, real-time RT-PCR, as described in reference 3. The dotted line reflects the limit of detection: approximately 60 genome equivalents per organ sample.
Others have used transplanted SCID or inbred AG129 or A/J mice to show variable thrombocytopenia, paralysis, or death after injection of massive amounts (8 log10 PFU, intravenous) of cell-culture-passaged dengue virus or smaller amounts (4 to 6 log10 PFU, intraperitoneal) of mouse-adapted dengue virus; however, these mice did not show signs of dengue disease as in humans (1, 2, 6, 10). The model described here is therefore highly relevant to human infection and can now be used to test antiviral preparations and vaccine attenuation. It may eventually also prove useful for understanding the immunopathogenesis (antibody-dependent enhancement of infection and cross-reactive T-cell activation) of DHF, after transplantation of other factors or tissues, to obtain a functional adaptive immune system.
Acknowledgments
We thank R. Wolf and staff at the University of Texas Health Science Center at San Antonio for animal facilities and care, A. Padgett-Thomas and M. del P. Martin for transplantation of mice, and M. Sharp and A. Hopstetter for statistics and graphics. Cord blood samples were obtained through the Department of Obstetrics and Gynecology's Tissue Procurement Facility of the University of Texas Southwestern Medical Center at Dallas (NIH grant HD-011149).
Funding was provided by The Ellison Medical Foundation and NIH grants AI50123 (to R.R.-H.) and CA82055 (to J.V.G.).
REFERENCES
- 1.An, J., J. Kimura-Kuroda, Y. Hirabayashi, and K. Yasui. 1999. Development of a novel mouse model for dengue virus infection. Virology 263:70-77. [DOI] [PubMed] [Google Scholar]
- 2.Blaney, J. E., Jr., C. T. Hanson, K. A. Hanley, B. R. Murphy, and S. S. Whitehead. 4. October 2004, posting date. Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect. Dis. 4:39. [Online.] doi: 10.1186/1471-2334-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cologna, R., P. M. Armstrong, and R. Rico-Hesse. 2005. Selection for virulent dengue viruses occurs in humans and mosquitoes. J. Virol. 79:853-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cravens, P. D., M. W. Melkus, A. Padgett-Thomas, M. Islas-Ohlmayer, M. del P. Martin, and J. V. Garcia. 2005. Development and activation of human dendritic cells in vivo in a xenograft model of human hematopoiesis. Stem Cells 23:264-278. [DOI] [PubMed] [Google Scholar]
- 5.Halstead, S. B., and J. Deen. 2002. The future of dengue vaccines. Lancet 360:1243-1245. [DOI] [PubMed] [Google Scholar]
- 6.Johnson, A. J., and J. T. Roehrig. 1999. New mouse model for dengue virus vaccine testing. J. Virol. 73:783-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwan, W. H., A. M. Helt, C. Maranon, J. B. Barbaroux, A. Hosmalin, E. Harris, W. H. Fridman, and C. G. Mueller. 2005. Dendritic cell precursors are permissive to dengue virus and human immunodeficiency virus infection. J. Virol. 79:7291-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palucka, A. K., J. Gatlin, J. P. Blanck, M. W. Melkus, S. Clayton, H. Ueno, E. T. Kraus, P. Cravens, L. Bennett, A. Padgett-Thomas, F. Marches, M. Islas-Ohlmayer, J. V. Garcia, and J. Banchereau. 2003. Human dendritic cell subsets in NOD/SCID mice engrafted with CD34+ hematopoietic progenitors. Blood 102:3302-3310. [DOI] [PubMed] [Google Scholar]
- 9.Scherer, W. F., P. K. Russell, L. Rosen, J. Casals, and R. W. Dickerman. 1978. Experimental infection of chimpanzees with dengue viruses. Am. J. Trop. Med. Hyg. 27:590-599. [DOI] [PubMed] [Google Scholar]
- 10.Shresta, S., J. L. Kyle, P. Robert Beatty, and E. Harris. 2004. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J. mice. Virology 319:262-273. [DOI] [PubMed] [Google Scholar]
- 11.Wang, W.-K., T.-L. Sung, Y.-C. Tsai, C.-L. Kao, S.-M. Chang, and C.-C. King. 2002. Detection of dengue virus replication in peripheral blood mononuclear cells from dengue virus type 2-infected patients by a reverse transcription-real-time PCR assay. J. Clin. Microbiol. 40:4472-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]