Abstract
Previous studies demonstrated that antibodies to live vaccinia virus infection are needed for optimal protection against orthopoxvirus infection. The present report is the first to compare the protective abilities of individual and combinations of specific polyclonal and monoclonal antibodies that target proteins of the intracellular (IMV) and extracellular (EV) forms of vaccinia virus. The antibodies were directed to one IMV membrane protein, L1, and to two outer EV membrane proteins, A33 and B5. In vitro studies showed that the antibodies to L1 neutralized IMV and that the antibodies to A33 and B5 prevented the spread of EV in liquid medium. Prophylactic administration of individual antibodies to BALB/c mice partially protected them against disease following intranasal challenge with lethal doses of vaccinia virus. Combinations of antibodies, particularly anti-L1 and -A33 or -L1 and -B5, provided enhanced protection when administered 1 day before or 2 days after challenge. Furthermore, the protection was superior to that achieved with pooled immune gamma globulin from human volunteers inoculated with live vaccinia virus. In addition, single injections of anti-L1 plus anti-A33 antibodies greatly delayed the deaths of severe combined immunodeficiency mice challenged with vaccinia virus. These studies suggest that antibodies to two or three viral membrane proteins optimally derived from the outer membranes of IMV and EV, may be beneficial for prophylaxis or therapy of orthopoxvirus infections.
Following the eradication of smallpox in the 1970s, vaccination almost entirely ceased, leaving large segments of the population unprotected or poorly protected should there be a reoccurrence of the disease. Such concerns have led to the production and stockpiling of a modern version of the currently licensed vaccine as well as renewed efforts to develop or evaluate safer ones (18). The latter include attenuated live vaccinia virus (VACV) (6, 12, 22, 32, 44, 50), DNA vaccines (19, 20), and protein vaccines (15, 16). There may also be a role for passive antibody to treat adverse effects of a live VACV vaccine or to provide immediate protection against smallpox in an emergency situation (8, 51). VACV immune gamma globulin (VIG), prepared from pooled sera of vaccinated human donors, has been used to treat complications of vaccination and smallpox (14, 21). Although not conclusive, some studies suggested that the incidence of smallpox in close contacts of patients who received VIG was about one-quarter that in close contacts who did not receive VIG (21). The production of large quantities of VIG, however, would require continued vaccination of volunteers. Moreover, the use of human blood products has inherent dangers. For these and other reasons, an alternative to VIG that consists of antibodies to specific viral antigens would have advantages.
The choice of antigens is complicated by the existence of several related infectious forms of VACV (33, 43). Intracellular mature virions (IMV) have a complex core structure surrounded by a lipid membrane containing nonglycosylated viral proteins and are released by cell lysis. Some IMV are enveloped by additional membranes containing viral glycoproteins and are transported to the periphery of the cell where exocytosis occurs. Most extracellular virions adhere to the outside of the cell and are known as cell associated enveloped virions. Those particles that are released from the cell are called extracellular enveloped virions. Because the membranes of the two extracellular forms are similar if not identical, we will refer to them collectively as extracellular virions (EV). It is thought that because of their stability, IMV are primarily responsible for virus spread from host to host, whereas the two extracellular forms mediate cell-to-cell and longer-range spread within a host. The proteins within the outer membrane of EV are entirely different from those present in the membrane of IMV, although the latter is present beneath the relatively fragile EV membrane and must be exposed for fusion to occur with the cell membrane (39, 40). Studies with a rabbit orthopoxvirus model indicated that killed IMV vaccines were less protective than live vaccines, and passive transfer experiments confirmed that the difference was due to the absence of antibodies to EV membrane proteins (4, 7). Similar results with antibodies to killed and live VACV were recently reported using a mouse pneumonia model (27).
Active immunization studies have identified a number of VACV immunogens that induce protective immunity. Mice immunized with individual proteins including the A27 and H3 proteins of IMV (25, 29) and the A33 and B5 proteins of EV (16) are partially protected against VACV challenge. Using a DNA vaccine approach, Hooper et al. (19, 20) showed that combinations of genes encoding A27, L1, A33, and B5 are more protective than single genes. Similarly, combinations of protein vaccines comprised of L1, A33, and B5 are more effective than individual proteins (15). Partly because of the lack of reagents, there have been few published studies on passive immunization with antibodies to specific VACV proteins (16, 36) and none that evaluated combinations of such antibodies. The purpose of the present study was to determine whether individual or combinations of antibodies to specific IMV and EV proteins would enhance protection in a mouse pneumonia model. We inoculated normal or severe combined immunodeficiency disease (SCID) mice with individual or combinations of rabbit polyclonal antibodies (PAbs) or rodent monoclonal antibodies (MAbs) to one IMV membrane protein, L1, and to two EV membrane proteins, A33 and B5. The mice were challenged with a potentially lethal dose of the Western Reserve (WR) strain of VACV through the respiratory route. We report that antibodies to individual IMV or EV proteins protected against a lethal infection but that combinations provided more complete protection against disease. These data suggest that human or humanized MAbs could be considered a replacement for VIG.
(Portions of this work were done to partially fulfill the Ph.D. thesis requirements of C.F. at the University of Maryland.)
MATERIALS AND METHODS
Viruses and cells.
HeLa S3 cells (ATCC CCL-2.2) were maintained in suspension in modified Eagle medium for spinner cells (Quality Biologicals) supplemented with 5% heat-inactivated horse serum. BS-C-1 cell monolayers were maintained at 37°C and 5% CO2 in Earle's modified Eagle medium (Quality Biologicals) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 10 U/ml of penicillin, and 10 μg/ml of streptomycin. Stocks of VACV WR (ATCC VR-1354), IHD-J (from S. Dales, Rockefeller University), and VV-NP-siinfekl-EGFP (3, 34) were prepared in HeLa S3 cells and purified by sucrose gradient centrifugation (13). Titers of viral stocks were measured by plaque assay on BS-C-1 monolayers.
Hybridomas and MAbs.
Anti-B5 MAb, anti-A33 MAb (immunoglobulin G3 [IgG3]), and anti-L1 MAb (IgG2a) were produced from rat hybridoma 19C2 (38), mouse hybridoma 1G10 (provided by Alan Schmaljohn), and mouse hybridoma 7D11 (provided by Alan Schmaljohn), respectively. Anti-Kb-ova MAb (35) was a gift of Jack Bennink. The 19C2 and 1G10 hybridomas were maintained in Dulbecco's modified Eagle medium (Quality Biologicals) supplemented with 5% fetal bovine serum, l-glutamine, and antibiotics as described above. Hybridoma 7D11 was maintained in RPMI 1640 (Quality Biologicals) supplemented in the same manner. IgGs were purified from the medium using the Montage antibody purification-Prosep A kit (Millipore, Billerica, MA), dialyzed against phosphate-buffered saline (PBS), and concentrated by centrifugation with an Amicon Ultra centrifugal filter device (Millipore). Aliquots of purified 2D5 and 7D11 MAbs were stored at −20°C; 1G10 MAb was stored at 4°C because its IgG3 isotype resulted in precipitation upon freezing and thawing. Antibodies used for one experiment, in which mice were passively immunized at times before or after challenge, were purified on protein A Sepharose from ascites fluid prepared in ICR SCID mice. Protein concentration was determined from absorbance at 280 nm. Antibody purity was confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and Coomassie blue staining.
Polyclonal antibodies.
Soluble L1, A33, or B5 protein was affinity purified from the culture supernatant of recombinant baculovirus-infected cells as described in reference 24. Rabbits were injected at subcutaneous and intramuscular sites with 100 μg of purified recombinant protein in PBS mixed with an equal volume of Freund's complete adjuvant. Rabbits were boosted a total of four times at 2-week intervals with 50 μg of protein mixed with an equal volume of incomplete Freund's adjuvant via the same routes. Herpes simplex virus (strain Savage) gD-2 was purified from infected cells, and rabbit polyclonal IgG was prepared as described previously (9).
VIG.
VIG intravenous (VIGIV; Dynport Vaccine Company LLC) lot 1 was provided by Dorothy Scott (Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, MD). The product contained 50 mg per ml of immunoglobulin, primarily IgG.
Enzyme-linked immunosorbent assays (ELISAs).
Recombinant A33, B5, and L1 proteins or a lysate of VACV-infected cells were used as antigens in 96-well plates as previously described (15). Titers were determined on serial twofold dilution of purified IgG (1 mg per ml) or serum from the tail vein of passively immunized mice using anti-mouse IgG (γ-chain) peroxidase-conjugated antibody (Roche Diagnostics GmbH, Mannheim, Germany) and a ready-to-use solution of 3,3′,5,5′-tetramethlybenzidine (BM Blue, POD substrate; Roche). A370 and A492 values were measured spectrophotometrically and endpoint titers were calculated as the dilution, which gave an absorbance 2 standard deviations above that measured in wells not incubated with primary antibody or serum.
Neutralization and comet reduction assays.
The concentration of IgG required for 50% inhibition of virus replication (IC50) was determined by flow cytometry after infecting HeLa S3 cells with purified VV-WR-NP-siinfekl-EGFP IMV that had been incubated with antibody (11). The comet reduction assay was carried out by infecting confluent BS-C-1 cells in six-well plates with approximately 50 PFU of the IHD-J strain of VACV per well for 2 h at 37°C. The inoculum was removed, and infected monolayers were covered with Earle's modified Eagle medium with 2% fetal bovine serum, l-glutamine, and antibiotics and dilutions of purified antibodies. The plates were incubated for 36 h and then stained with crystal violet.
Passive immunization and virus challenge.
Female 7- or 14-week-old BALB/c mice from Taconic (Germantown, NY) and 14-week-old female BALB/c SCID mice (strain C3Smn.CB17- Prkdc scid/J) from the Jackson Laboratory (Bar Harbor, ME) were used. The 50% lethal dose (LD50) of VACV WR was determined to be 1.5 × 105 PFU for 7-week-old mice and 3 × 105 PFU for 14-week-old mice by the intranasal (i.n.) route. Antibodies were diluted in PBS for passive intraperitoneal (i.p.) injection. Unimmunized mice were injected with the same volume of phosphate-buffered saline. Before or after passive immunization, mice were challenged intranasally (i.n.) with 20-μl suspensions of VACV (WR) as previously described (15). Mice were weighed daily for 2 to 12 weeks and sacrificed if their weight fell below 70% of the initial value. The National Institute of Allergy and Infectious Diseases (NIAID) Animal Care and Use Committee approved animal protocols.
Statistical analysis.
The significance of the effect of different immunizations on change in weight following virus challenge was examined by analysis of variance (ANOVA) using the StatView statistical software package (SAS Institute Inc., Cary, NC). The Fisher protected least significant difference test was used post hoc if some effects of immunization were determined to be significant, and this test allowed us to determine which immunizations were significantly better than no immunization. Significance levels were set at a P value of 0.05.
RESULTS
PAbs and MAbs used for passive protection studies.
PAbs were generated by repeatedly immunizing rabbits with individual VACV recombinant proteins that were engineered to be naturally folded in the endoplasmic reticulum of insect cells, secreted into the medium, and purified by metal affinity chromatography (1, 15). The proteins consisted of L1, a component of the IMV membrane, and A33 and B5, components of the outer EV membrane. IgG was purified, and the reactivity of each was determined by ELISA using recombinant proteins and VACV-infected cell lysates as antigens (Table 1). Each specific antiserum also reacted with an infected cell lysate, though the titers were lower, presumably due to limiting amounts of the target antigen. We also analyzed the amounts of antibody to L1, A33, and B5 in human VIG; as expected, the ELISA titers were much lower than those obtained with the specific antibodies (Table 1).
TABLE 1.
Endpoint reciprocal titers of PAbs and MAbs
| Antibodya | Antigen
|
|||
|---|---|---|---|---|
| A33 | B5 | L1 | Lysate | |
| A33 PAb | 330,000 | —b | — | 25,600 |
| B5 PAb | — | 300,000 | — | 12,800 |
| L1 PAb | — | — | 61,000 | 1,600 |
| VIG | 1,600 | 6,400 | 800 | 6,400 |
| A33 MAb | 800,000 | — | — | — |
| B5 MAb | — | 800,000 | — | — |
| L1 MAb | — | — | 1,000,000 | — |
IgG (1 mg/ml).
—, not determined.
IMV neutralizing activity was measured by a recently described flow cytometric assay using a VACV-expressing green fluorescent protein (GFP) (11). The neutralizing activity of anti-L1 PAb IgG (IC50 of 2.3 μg) was only threefold higher than that of VIG (IC50 of 6.7 μg), though the former had 76-fold greater binding to L1 protein than the latter. These data suggest that L1 is not the major target of neutralizing antibody in VIG, a supposition that agrees with other studies (2). As expected, IgG from rabbits immunized with the EV proteins A33 and B5 had no significant IMV neutralizing activity (data not shown). Two assays are available for measuring the effects of antibody on EV: a neutralization assay and the comet reduction test (4, 26). The former, however, apparently measures only anti-B5 antibodies in VIG (5) and does not detect antibodies to A33 (and perhaps other proteins) that are protective in vivo (16). The comet reduction test measures the formation of satellite plaques formed by released EV, which is inhibited by antibodies to A33 as well as B5 (1, 15), and was therefore preferred for this study. The rabbit anti-A33 and anti-B5 PAbs reduced comet formation at 20 μg per ml (Fig. 1). More than 10 times that amount of VIG was required for comet reduction (not shown), in agreement with Bell et al. (5). Anti-L1 PAb did not inhibit comet formation at 20 μg per ml (Fig. 1) or higher (not shown) concentrations. Thus, the PAbs exhibited the expected in vitro biological activities.
FIG. 1.
Comet reduction by anti-EV antibodies. BS-C-1 monolayers were inoculated with VACV IHD-J and 2 h later were incubated with no antibody (None, top two wells) or 20 μg per ml of MAbs or PAbs to A33, B5, or L1 in liquid medium. After 36 h, the cells were stained with crystal violet to visualize comets. The left and right no-antibody controls correspond to the PAb and MAb experiments, respectively, which were carried out on different days.
We also used MAbs to L1, A33, and B5. MAb 7D11 is a conformation-specific IMV-neutralizing antibody that reacts with the L1 protein (42, 49). The nonneutralizing MAb 1G10 reacts with native A33 protein (19) and MAb 19C2 with the B5 protein (38). The reciprocal endpoint ELISA titers of the MAbs were higher than those of the PAbs on a weight basis, as expected since the latter undoubtedly contained antibodies with other specificities (Table 1). Although the L1 binding of 7D11 MAb was 16-fold higher than L1 PAb, its relative neutralizing activity was much greater (IC50 of 3.1 ng for MAb 7D11 versus 2.3 μg for L1 PAb). The abilities of the A33 and B5 MAbs to reduce comet formation were also documented (Fig. 1).
Individual and combinations of PAbs protect mice against respiratory challenge.
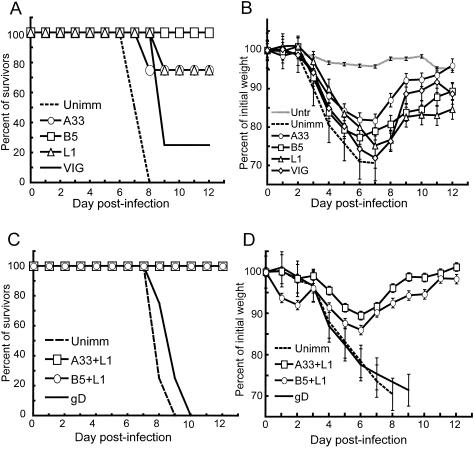
A mouse respiratory infection model employing the WR strain of VACV (46, 48) was used to measure the protective ability of passively administered antibodies. In this model, weight loss correlates with virus replication in the lungs (27) and therefore the former was used as a noninvasive measure of disease that allowed us to follow the onset and recovery phases using a minimal number of animals. According to our animal protocol, individual mice were sacrificed if their weights dropped to 70% of the starting values. Others have shown that 98% of mice that lose 30% of their weight will die naturally (37). IgG was administered i.p., followed 1 day later by a lethal dose of VACV WR by the i.n. route. In most experiments, we used BALB/c mice that were approximately 14 weeks old to correspond with our previous active vaccination study (15) and challenged them with 106 PFU (3 LD50) of VACV. In an initial experiment, all mice that were given 1 mg of anti-L1, -B5, or -A33 IgG died or were sacrificed because of weight loss following challenge. Therefore, subsequent experiments were carried out with 5 mg of IgG per mouse. At this dose, the serum antibody titers present 24 h after administration were in the range previously obtained by active immunization (Table 2) (15). In this experiment, the four unimmunized mice died within 8 days (Fig. 2A). Of four mice that received anti-L1, -A33, or -B5 IgG, at least three in each group survived (Fig. 2A) but lost weight (Fig. 2B). The difference in weight loss between mice receiving anti-L1 PAb and unimmunized mice was statistically significant on days 3 and 4 (P = 0.0114 and P = 0.0235, respectively) whereas statistical significance was achieved on days 5 and 6 (P = 0.0477 and P = 0.0320, respectively) for mice receiving anti-A33 PAb. Significance could not be determined at later times because of deaths of control mice. Although mice receiving anti-B5 PAb lost less weight than controls, the difference did not reach statistical significance. As a positive control, we passively immunized the mice i.p. with VIG at approximately 2.5× the recommended weight-based human dose. Protection with VIG, however, was poorer than with the rabbit PAbs; of the four mice that received VIG, three died or were sacrificed (Fig. 2A) and the weight gain after 7 days represents the one surviving mouse (Fig. 2B).
TABLE 2.
Comparison of endpoint ELISA titers in sera of mice following passive or active immunizations
| Antigen
|
|||
|---|---|---|---|
| A33 | B5 | L1 | |
| A33 passivea | 304,437 | —c | — |
| A33 activeb | 565,685 | — | — |
| B5 passive | — | 304,437 | — |
| B5 active | — | 100,000 | — |
| L1 passive | — | — | 59,460 |
| L1 active | — | — | 70,711 |
Titers 24 h after injection of 5 mg PAb.
Titers after three immunizations with protein (15).
—, not determined.
FIG. 2.
Protection of mice by PAbs. (A and B) Groups of four 14-week-old female BALB/c mice received 5 mg of polyclonal rabbit antibodies against A33, B5, or L1 or 5 mg of VIG by the i.p. route. Unimmunized (Unimm) mice were injected with phosphate-buffered saline and untreated (Untr) mice with nothing. After 24 h, all mice except the untreated group were challenged i.n. with 106 PFU of VACV WR and weighed daily for 12 days. Mice were sacrificed when their weight fell below 70% of initial weight. Percentages of survivors are shown in panel A. Percentages of initial mean weights of all live mice ± standard errors of the means are shown in panel B. (C and D) Mice were immunized with either 2.5 mg of anti-A33 plus 2.5 mg of anti-L1 or 2.5 mg of anti-B5 plus 2.5 mg of anti-L1. Control mice were unimmunized or immunized with 5 mg of rabbit anti-herpes simplex virus gD. Challenges were the same as described for panels A and B. Percentages of initial mean weights of all live mice ± standard errors of the means are shown in panel D.
We also administered combinations of PAbs such that each mouse received a total of 5 mg of IgG. The serum titers determined 24 h after inoculation were proportionate to the amount of PAb inoculated (not shown). In addition to unimmunized mice, another control group received rabbit IgG to the unrelated herpes simplex virus gD protein. Following challenge with 106 PFU of VACV, mice in each of the passively immunized groups survived, whereas none of the mice in the control groups lived (Fig. 2C). The mice receiving 2.5 mg each of L1 and A33 PAbs suffered the least weight loss (Fig. 2D), and the difference from the unimmunized controls was significant by day 4 (P = 0.0281 and P = 0.0150 relative to the no antibody and gD controls, respectively). These data indicated that passive administration of high titer rabbit PAbs was more protective than human VIG in the mouse respiratory model and that the best protection was achieved with combinations of antibodies to IMV and EEV proteins e.g., anti-L1 plus anti-A33 or anti-B5.
Individual and combinations of MAbs protected mice against respiratory challenge.
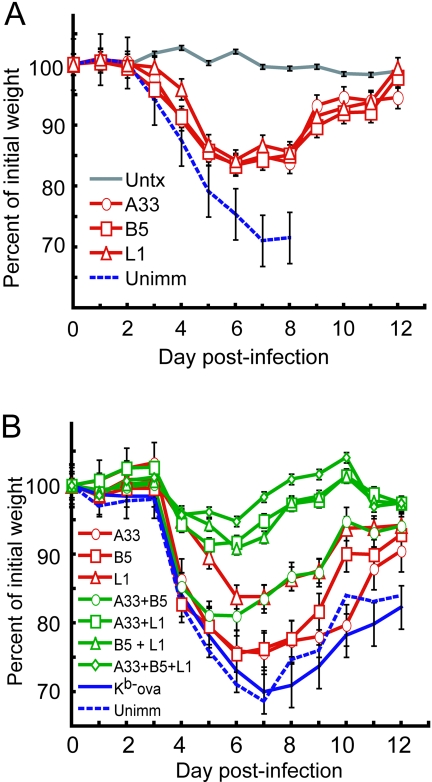
In the next series of experiments, we used the same respiratory challenge model to determine the efficacy of MAbs. First, we checked individual MAbs. Mice receiving 200 μg of the anti-L1, -A33, or -B5 MAb survived, whereas all control mice died. In each case, there was approximately a 15% weight loss before the passively immunized mice recovered (Fig. 3A). Even when the amount of MAb was reduced to 100 μg, all mice receiving anti-L1 or -A33 and three of four receiving anti-B5 survived. Although there was overall greater weight loss with the lower amounts of MAb (Fig. 3B), the difference between mice receiving 100 μg of anti-L1 MAb and unimmunized mice was statistically significant on days 4 and 6 (P = 0.0356 and P = 0.0290, respectively) and statistical significance was achieved on day 6 for mice receiving anti-A33 or anti-B5 MAb (P = 0.0400 and P = 0.0444, respectively).
FIG. 3.
Protection of mice by MAbs. (A) Groups of four 14-week-old female BALB/c mice received 200 μg of A33, B5, or L1 MAb i.p. and were challenged i.n. as described in the legend to Fig. 2. All immunized mice survived, and percentages of initial mean weights ± standard errors of the means are plotted. (B) Groups of four 14-week-old female BALB/c mice were immunized i.p. with 100 μg of each antibody alone or in all possible combinations and challenged as described above. All immunized mice survived except for one mouse that received anti-B5 alone and all control mice that received mouse anti-Kb-ova MAb. Percentages of initial mean weights ± standard errors of the means are shown.
The best protection was achieved with combinations of MAbs.
Three of four mice that received no MAb or MAb to an irrelevant protein (Kb-ova) died, and the weight gains for these control groups are for the single surviving animals (Fig. 3B). No deaths occurred in mice given 100 μg of anti-L1 plus 100 μg of anti-A33 or anti-B5 or 100 μg of each of the three MAbs (Fig. 3B). The difference in weight loss for mice receiving a combination of anti-L1 and anti-A33, anti-L1, and anti-B5 or all three MAbs relative to controls was highly significant (P = <0.001 on days 4, 5, and 6 for each combination). Mice receiving a combination of the two anti-EV MAbs to A33 and B5 were less well protected against weight loss, though statistical significance relative to controls was reached on day 6 (P = 0.0012). We concluded that the best protection was achieved by passive administration of combinations of MAbs to IMV and EEV proteins, e.g., anti-L1 plus anti-A33 or anti-B5 or all three.
Effect of time of administration of MAbs relative to challenge.
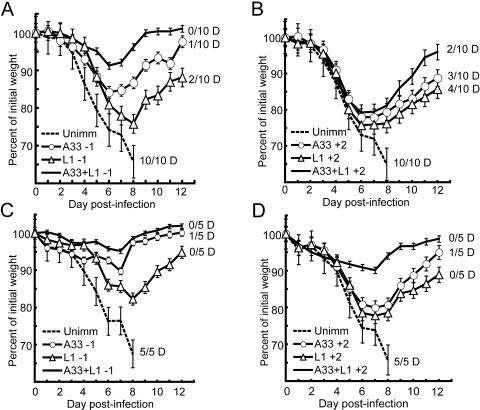
Further experiments were carried out to determine whether anti-L1 and anti-A33 MAbs could protect when administered therapeutically after challenge. Seven-week-old mice were used for these experiments instead of 14-week-old mice. Because of the greater susceptibility of young mice to VACV WR, the challenge dose of 106 PFU represented 6 LD50 instead of 3 LD50. The data collected for Fig. 4 came from two separate experiments with a total of 10 mice for each immunization and control group. As expected, all unimmunized mice died after challenge. All mice receiving 100 μg of anti-L1 MAb plus anti-A33 MAb prior to challenge survived, though two of the 10 mice receiving only anti-L1 and one mouse receiving only anti-A33 died. Mice receiving the two MAbs had less weight loss than those receiving only one (Fig. 4A). Prior immunization with anti-L1 or anti-L1 plus anti-A33 antibodies protected mice significantly better from weight loss relative to controls (P ≤ 0.0022 on days 3, 4, 5, and 6 for anti-L1 or anti-L1 plus anti-A33 antibodies compared to control). Similarly, mice preimmunized with anti-A33 were protected from significant weight loss when compared to controls (P ≤ 0.0003 on days 4, 5, and 6 for anti-A33 compared to control). When the MAbs were given 2 days after challenge, two to four mice died in each group and weight loss was more severe, though again, the best results were obtained with the combination of MAbs (Fig. 4B).
FIG. 4.
Post- and preimmunization challenge with VACV WR. (A and B) In two separate experiments, groups of five 7-week-old female BALB/c mice were immunized with 100 μg of anti-A33 or -L1 MAbs or a combination of both 1 day prior to challenge (-1) or 2 days after challenge (+2) i.n. with 106 PFU of VACV WR. The mice were weighed daily, and the number (N/10) that died or was sacrificed is indicated. (C and D) Groups of five mice were challenged as described above with 5 × 105 PFU of VACV WR. The number (N/5) that died or were sacrificed is indicated. Percentages of initial mean weights ± standard errors of the means are shown.
Because of severity of the 106 PFU challenge for 7-week-old mice, we repeated the experiment with half the challenge dose (5 × 105 PFU, 3 LD50), which was the equivalent LD50 dose used for the 14-week-old mice in earlier parts of this study. Again, none of the control mice survived. However, there was now higher survival and lower weight loss in the prechallenge passive immunization groups (P ≤ 0.0340 on days 3 to 6 for the combination of antibodies compared to control and P ≤ 0.0064 on days 4 to 6 for anti-A33 or anti-L1 compared to control) as shown in Fig. 4C. With this lower challenge dose, there was also higher survival and lower weight loss in the postchallenge passive immunization groups (P ≤ 0.0042 on days 5 to 7 for the combination of antibodies versus control and P = 0.0266 on day 7 for anti-A33 versus control) as shown in Fig. 4D. In particular, there was relatively little weight loss for groups receiving anti-A33 and anti-L1 MAbs.
Passive protection of SCID mice.
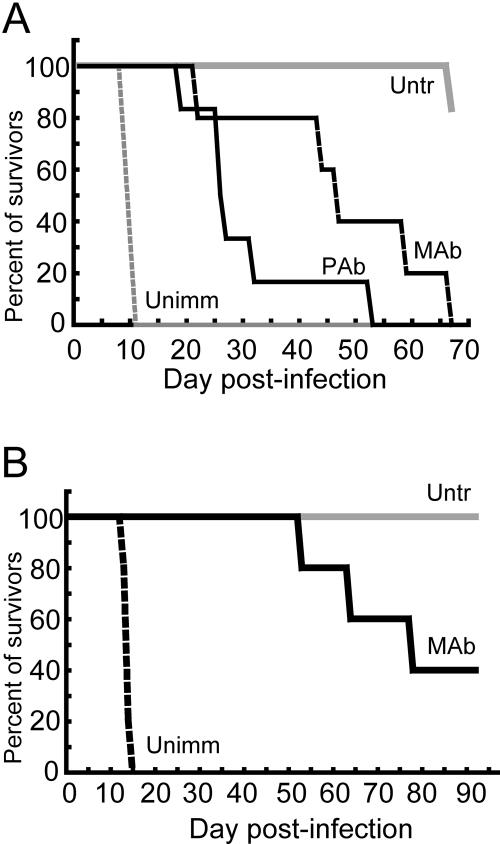
The above experiments demonstrated that passive antibody protected BALB/c mice against lethal infection with VACV. BALB/c mice, however, mount an immune response to the challenge virus, which could contribute to a favorable outcome. To diminish the latter contribution to protection, we carried out additional experiments with BALB/c SCID mice, which are deficient in T and B cells. The mice were inoculated with 100 μg of each of the three MAbs or 1.7 mg of each of the three PAbs and challenged i.n. with 106 PFU of VACV WR. The i.n. LD50 for SCID mice is 2.5 × 102 (S. Lustig, unpublished data), and therefore our challenge dose represented 4,000 LD50. The control mice all died or were sacrificed by day 11, whereas the 50% survival times were 26 and 46.5 days for animals receiving PAbs or MAbs, respectively (Fig. 5A). As mice started to lose weight rapidly, they exhibited signs of pneumonia, including labored breathing and a hunched posture. We repeated the experiment with a 104 PFU challenge representing 40 LD50 to see if survival would be extended. The control mice died within 2 weeks, whereas two of the five passively immunized mice were still alive after 3 months (Fig. 5B), when the experiment was terminated.
FIG. 5.
Passive protection of SCID mice against VACV WR challenge. (A) Groups of five 8-week-old female BALB/c SCID mice were inoculated i.p. with a mixture of 1.67 mg each of anti-A33, -B5, and -L1 PAbs or of 100 μg each of anti-A33, -B5, and -L1 MAbs. After 1 day, the mice were challenged i.n. with 106 PFU of VACV WR and weighed daily. (B) Groups of five 8-week-old female BALB/c SCID mice received MAbs as described for panel A and challenged 1 day later with 104 PFU of VACV WR and weighed daily. Abbreviations: Untr, untreated; Unimm, unimmunized.
DISCUSSION
Previous studies indicated that antibodies to live virus provide optimal protection against orthopoxvirus infection (4, 7, 27). The inability of inactivated virus to completely protect was attributed to the absence of EV-specific antibodies. The present study is the first to compare the abilities of combinations of specific PAbs and MAbs to protect against orthopoxvirus infections. We prepared antibodies to the L1 IMV membrane protein and to the B5 and A33 EV membrane proteins because active immunization with them had provided protection against lethal infection with VACV WR (15). The antibodies were prepared against the extracellular domains of the VACV membrane proteins, which were engineered for the secretion from baculovirus-infected insect cells (1, 2). In the case of L1, this allowed the conformationally important intramolecular disulfide bonds to form in the endoplasmic reticulum, whereas they normally form in the cytoplasm by the poxvirus redox system (41, 45). In addition, MAbs to L1, A33, and B5 were available for this study. We found that the PAb and MAb to L1 neutralized IMV and that the corresponding antibodies to A33 and B5 inhibited the spread of EV in liquid medium.
The BALB/c mouse pneumonia model with VACV WR challenge was used for several reasons: (i) weight loss and death are correlated with replication in the lungs, allowing the onset and progress of disease to be monitored by a noninvasive method that greatly reduces the number of animals needed for significance (27); (ii) the model has been used for active immunization studies with live VACV (6, 50) as well as with individual VACV proteins (15) and for passive immunization studies with antisera prepared against live and inactivated VACV (27); (iii) use of the i.n. route for challenge and the i.p. route for passive antibody allowed us to avoid using the latter route for both as had been done in some previous studies; and (iv) the i.n. route is believed to be the major avenue for transmission of variola virus. In addition, previous studies showed that CD8 T cells are not required for protection in this model (6, 50).
The 106 PFU challenge dose of VACV WR used for most experiments in this study was considerably higher than that used for passive protection with VACV immune IgG in a recent report (27). Nevertheless, protection against death and partial protection against weight loss were achieved with prophylactic administration of individual PAbs or MAbs. However, the most complete protection was obtained with combinations of antibodies, particularly anti-L1 plus anti-A33 or anti-B5. Anti-L1 antibody targets the IMV, which constitutes the challenge virus and is probably the most abundant replicated form. Anti-A33 and -B5 antibodies target the EV, which are required for efficient cell-to-cell spread. The action of anti-L1 might involve in vivo neutralization of the inoculum IMV, of IMV released by lysis of infected cells or lysis of the outer membrane of the EV by immune mechanisms such as complement-mediated lysis (30) or during virus entry, which is mediated by fusion of the IMV membrane with the cell membrane (39, 40). Antibodies to EV membrane proteins would have no effect on the inoculum virus but might bind and neutralize EV, agglutinate progeny virus on the cell surface (28), or participate in complement-mediated lysis of the EV membrane (30) or infected cells. We noticed repeatedly that mice passively immunized with anti-L1 or actively immunized with L1 protein (15) had delayed initial weight loss compared to animals immunized with the EV proteins, although the effect was small and not statistically significant. If anti-L1 antibody mainly worked by neutralizing the virus inoculum as the latter result might suggest, we reasoned that anti-L1 would be relatively less important when given 2 days after challenge compared to 1 day prior to challenge. However, anti-L1 provided partial protection and enhanced the protection provided by anti-A33 even when given postchallenge. Thus, anti-L1 as well as anti-A33 and anti-B5 probably reduce the replication and spread of progeny virus. An alternative strategy of using a MAb to the orthopoxvirus-encoded epidermal growth factor in conjunction with anti-L1 MAb has been advocated (23). However, in the mouse intranasal challenge model, the incremental effect of the growth factor antibody was minor compared to that seen here with anti-A33 or anti-B5 MAb.
Using SCID mice, we showed that passive antibody could provide considerable protection in the absence of an adaptive immune response. At the highest challenge dose used (4,000 LD50), survival was greatly prolonged but all mice eventually succumbed to VACV infection. With a lower challenge dose (40 LD50), there were some survivors at 3 months when the experiment was terminated. Presumably, as the titer of antibody decreases, replication of residual virus can occur. In this regard, we experimentally determined the half-lives of the MAbs against L1 and A33 as 10.2 and 4.8 days, respectively, in BALB/c mice, which are close to that determined previously for murine IgG2a and IgG3 (47). The half-life of rabbit IgG in mice has been reported to be approximately 5 to 6 days (17). We are unsure whether these numbers hold precisely for SCID mice; however, they do suggest that the antibody levels were very low when they finally succumbed at 1 to 3 months after challenge and that survival might have been further prolonged by additional immunizations.
The contributions of anti-L1, anti-A33, and anti-B5 antibodies to protection against orthopoxvirus challenge points out the difficulty in evaluating VIG or screening MAbs using a single in vitro test. The IMV neutralization assay is sensitive, reproducible and simple to perform, especially as it has been adapted for use with reporter genes (10, 11, 31). However, it does not measure antibodies to EV proteins. The EV neutralization test is difficult to perform because the EV are fragile and cannot be stored frozen; moreover, broken EV must be neutralized with an anti-IMV antibody or the results will be inaccurate; and most importantly the assay does not work at all with anti-A33 antibody and has only been shown to work with anti-B5 antibody. Anti-A33 can neutralize extracellular enveloped virus in the presence of complement, which lyses the outer membrane and allows anti-L1 to neutralize the released IMV (30). The latter assay, however, would be cumbersome to perform routinely. For the present study we used the comet inhibition test in conjunction with protein-specific ELISAs to measure anti-A33 and anti-B5 antibodies. It is our feeling, however, that in vivo protection studies are necessary to evaluate the potency of individual and mixtures of antibodies.
Human VIG was used as a positive control for the passive immunization experiments. However, it was evident that the protection achieved with VIG was less than that obtained with the PAb or MAb cocktails. This result correlated with the lower IMV neutralizing and comet inhibition activities of the VIG compared to the specific antibody combinations. It seems likely that human MAbs to selected viral membrane proteins, e.g., L1, A33, and B5, may be superior to, as well as safer than, pooled sera from vaccinated human volunteers, the source of VIG. Potential uses of human MAbs would include treatment of adverse reactions to vaccination and prophylactic administration to individuals exposed or suspected of being exposed to variola virus. In the mouse model, both the challenge virus titer and time of administration of antibody affected the outcome. For this reason, one would expect the best clinical results to be obtained by administering antibody prophylactically or soon after infection.
Acknowledgments
We thank Alan Schmaljohn for hybridomas, Norman Cooper for cells and virus stocks, and Pat Earl for advice on purification of MAbs.
This research was supported in part by the Intramural Research Program of the NIAID, NIH, and by Public Health Service grants AI53044 and NIAID Mid-Atlantic Regional Center of Excellence grant U54 AI057168 and by a grant from the Pennsylvania Department of Health.
The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
REFERENCES
- 1.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 79:6260-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. K. Pannell, J. Lebowitz, C. Fogg, C. White, B. Moss, G. H. Cohen, and R. J. Eisenberg. 3August2005, posting date. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology. [Online.] doi: 10.1016/j.virol.2005.07.006. [DOI] [PubMed]
- 3.Anton, L. C., U. Schubert, I. Bacik, M. F. Princiotta, P. A. Wearsch, J. Gibbs, P. M. Day, C. Realini, M. C. Rechsteiner, J. R. Bennink, and J. W. Yewdell. 1999. Intracellular localization of proteasomal degradation of a viral antigen. J. Cell Biol. 146:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appleyard, G., A. J. Hapel, and E. A. Boulter. 1971. An antigenic difference between intracellular and extracellular rabbitpox virus. J. Gen. Virol. 13:9-17. [DOI] [PubMed] [Google Scholar]
- 5.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulter, E. A., H. T. Zwartouw, D. H. J. Titmuss, and H. B. Maber. 1971. The nature of the immune state produced by inactivated vaccinia virus in rabbits. Am. J. Epidemiol. 94:612-620. [DOI] [PubMed] [Google Scholar]
- 8.Casadevall, A. 2002. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg. Infect. Dis. 8:833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, G. H., M. Katze, C. Hydrean-Stern, and R. J. Eisenberg. 1978. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J. Virol. 27:172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosma, A., S. Buhler, R. Nagaraj, C. Staib, A. L. Hammarin, B. Wahren, F. D. Goebel, V. Erfle, and G. Sutter. 2004. Neutralization assay using a modified vaccinia virus Ankara vector expressing the green fluorescent protein is a high-throughput method to monitor the humoral immune response against vaccinia virus. Clin. Diagn. Lab. Immunol. 11:406-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earl, P. L., J. L. Americo, and B. Moss. 2003. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J. Virol. 77:10684-10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earl, P. L., J. L. Americo, L. S. Wyatt, L. A. Eller, J. C. Whitbeck, G. H. Cohen, R. J. Eisenberg, C. J. Hartmann, D. L. Jackson, D. A. Kulesh, M. J. Martinez, D. M. Miller, E. M. Mucker, J. D. Shamblin, S. H. Zwiers, J. W. Huggins, P. B. Jahrling, and B. Moss. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182-185. [DOI] [PubMed] [Google Scholar]
- 13.Earl, P. L., B. Moss, L. S. Wyatt, and M. W. Carroll. 1998. Generation of recombinant vaccinia viruses, p. 16.17.1-16.17.19. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates & Wiley Interscience, New York, N.Y. [Google Scholar]
- 14.Ferry, B. J. 1976. The efficacy of vaccinial immune globulin. Vox Sang. 31:68-76. [DOI] [PubMed] [Google Scholar]
- 15.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galmiche, M. C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254:71-80. [DOI] [PubMed] [Google Scholar]
- 17.Gitlin, J. D., J. I. Gitlin, and D. Gitlin. 1976. Selective transfer of plasma proteins across mammary gland in lactating mouse. Am. J. Physiol. 230:1594-1602. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, D. A. 1999. The looming threat of bioterrorism. Science 283:1279-1282. [DOI] [PubMed] [Google Scholar]
- 19.Hooper, J. W., D. M. Custer, C. S. Schmaljohn, and A. L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266:329-339. [DOI] [PubMed] [Google Scholar]
- 20.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempe, C. H., C. Bowles, G. Meiklejohn, T. O. Berge, L. St Vincent, B. V. Babu, S. Govindarajan, N. R. Ratnakannan, A. W. Downie, and V. R. Murthy. 1961. The use of vaccinia hyperimmune gamma-globulin in the prophylaxis of smallpox. Bull. W. H. O. 25:41-48. [PMC free article] [PubMed] [Google Scholar]
- 22.Kidokoro, M., M. Tashiro, and H. Shida. 2005. Genetically stable and fully effective smallpox vaccine strain constructed from highly attenuated vaccinia LC16m8. Proc. Natl. Acad. Sci. USA 102:4152-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, M., H. L. Yang, S. K. Kim, P. A. Reche, R. S. Tirabassi, R. E. Hussey, Y. Chishti, J. G. Rheinwald, T. J. Morehead, T. Zech, I. K. Damon, R. M. Welsh, and E. L. Reinherz. 2004. Biochemical and functional analysis of smallpox growth factor (SPGF) and anti-SPGF monoclonal antibodies. J. Biol. Chem. 279:25838-25848. [DOI] [PubMed] [Google Scholar]
- 24.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai, C. F., S. C. Gong, and M. Esteban. 1991. The purified 14-kilodalton envelope protein of vaccinia virus produced in Escherichia coli induces virus immunity in animals. J. Virol. 65:5631-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law, M., R. Hollinshead, and G. L. Smith. 2002. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 83:209-222. [DOI] [PubMed] [Google Scholar]
- 27.Law, M., M. M. Putz, and G. L. Smith. 2005. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J. Gen. Virol. 86:991-1000. [DOI] [PubMed] [Google Scholar]
- 28.Law, M., and G. L. Smith. 2001. Antibody neutralization of the extracellular enveloped form of vaccinia virus. Virology 280:132-142. [DOI] [PubMed] [Google Scholar]
- 29.Lin, C. L., C. S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lustig, S., C. Fogg, J. C. Whitbeck, and B. Moss. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 328:30-35. [DOI] [PubMed] [Google Scholar]
- 31.Manischewitz, J., L. R. King, N. A. Bleckwenn, J. Shiloach, R. Taffs, M. Merchlinsky, N. Eller, M. G. Mikolajczyk, D. J. Clanton, T. Monath, R. A. Weltzin, D. E. Scott, and H. Golding. 2003. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J. Infect. Dis. 188:440-448. [DOI] [PubMed] [Google Scholar]
- 32.McCurdy, L. H., J. A. Rutigliano, T. R. Johnson, M. Chen, and B. S. Graham. 2004. Modified vaccinia virus Ankara immunization protects against lethal challenge with recombinant vaccinia virus expressing murine interleukin-4. J. Virol. 78:12471-12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 34.Norbury, C. C., D. Malide, J. S. Gibbs, J. R. Bennink, and J. W. Yewdell. 2002. Visualizing priming of virus-specific CD8(+) T cells by infected dendritic cells in vivo. Nat. Immunol. 3:265-271. [DOI] [PubMed] [Google Scholar]
- 35.Porgador, A., J. W. Yewdell, Y. Deng, J. R. Bennink, and R. N. Germain. 1997. Localization, quantitation, and in situ detection of specific peptide- MHC class I complexes using a monoclonal antibody. Immunity 6:715-726. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez, J. C., E. Tapia, and M. Esteban. 2002. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J. Gen. Virol. 83:1059-1067. [DOI] [PubMed] [Google Scholar]
- 37.Rees, D. G. C., A. J. Gates, M. Green, L. Eastaugh, R. A. Lukaszewski, K. F. Griffin, A. M. Krieg, and R. W. Titball. 2005. CpG-DNA protects against a lethal orthopoxvirus infection in a murine model. Antivir. Res. 65:87-95. [DOI] [PubMed] [Google Scholar]
- 38.Schmelz, M., B. Sodeik, M. Ericsson, E. J. Wolffe, H. Shida, G. Hiller, and G. Griffiths. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J. Virol. 68:130-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senkevich, T. G., and B. Moss. 2005. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J. Virol. 79:4744-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senkevich, T. G., B. M. Ward, and B. Moss. 2004. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J. Virol. 78:2357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senkevich, T. G., C. L. White, E. V. Koonin, and B. Moss. 2002. Complete pathway for protein disulfide bond formation encoded by poxviruses. Proc. Natl. Acad. Sci. USA 99:6667-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senkevich, T. G., C. L. White, E. V. Koonin, and B. Moss. 2000. A viral member of the ERV1/ALR protein family participates in a cytoplasmic pathway of disulfide bond formation. Proc. Natl. Acad. Sci. USA 97:12068-12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 44.Stittelaar, K. J., G. van Amerongen, I. Kondova, T. Kuiken, R. F. van Lavieren, F. H. Pistoor, H. G. Niesters, G. van Doornum, B. A. van der Zeijst, L. Mateo, P. J. Chaplin, and A. D. Osterhaus. 2005. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 79:7845-7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su, H. P., S. C. Garman, T. J. Allison, C. Fogg, B. Moss, and D. N. Garboczi. 2005. The 1.51-A structure of the poxvirus L1 protein, a target of potent neutralizing antibodies. Proc. Natl. Acad. Sci. USA 102:4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner, G. S. 1967. Respiratory infection of mice with vaccinia virus. J. Gen. Virol. 1:399-402. [DOI] [PubMed] [Google Scholar]
- 47.Vieira, P., and K. Rajewsky. 1988. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 18:313-316. [DOI] [PubMed] [Google Scholar]
- 48.Williamson, J. D., R. W. Reith, L. J. Jeffrey, J. R. Arrand, and M. Mackett. 1990. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J. Gen. Virol. 71:2761-2767. [DOI] [PubMed] [Google Scholar]
- 49.Wolffe, E. J., S. Vijaya, and B. Moss. 1995. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 211:53-63. [DOI] [PubMed] [Google Scholar]
- 50.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 101:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeitlin, L., R. A. Cone, T. R. Moench, and K. J. Whaley. 2000. Preventing infectious disease with passive immunization. Microbes Infect. 2:701-708. [DOI] [PubMed] [Google Scholar]