Abstract
This study investigated the efficacy of a 10-session, HIV-risk-reduction intervention with 221 women and 187 men receiving outpatient psychiatric care for a mental illness. Patients were randomly assigned to the HIV intervention, a structurally equivalent substance use reduction (SUR) intervention, or standard care; they were assessed pre- and postintervention and at 3- and 6-month follow-ups. Patients receiving the HIV-risk-reduction intervention reported less unprotected sex, fewer casual sex partners, fewer new sexually transmitted infections, more safer sex communications, improved HIV knowledge, more positive condom attitudes, stronger condom use intentions, and improved behavioral skills relative to patients in the SUR and control conditions. Patients receiving the SUR intervention reported fewer total and casual sex partners compared with control patients. Exploratory analyses suggested that female patients and patients diagnosed with a major depressive disorder were more likely to benefit from the HIV-risk-reduction intervention.
Adults living with a mental illness such as schizophrenia, major depression, bipolar disorder, and schizoaffective disorder are disproportionately vulnerable to infection with HIV. For example, Rosenberg et al. (2001) assessed 931 psychiatric patients undergoing inpatient or outpatient treatment in four states and reported that 3.1% were infected with HIV. More than a dozen seroprevalence studies completed with psychiatric patients reveal infection rates that range from 3% to 23%, rates that are 8 to 70 times higher than the rate (0.3% to 0.4%) estimated for the general population (M. P. Carey, Weinhardt, & Carey, 1995; Cournos & McKinnon, 1997).
Mental illness may increase risk for infection with HIV for many reasons (M. P. Carey, Carey, & Kalichman, 1997). Psychiatric illnesses and medication side effects may lead to life circumstances that result in patients being less well-informed about HIV disease (Kalichman, Kelly, Johnson, & Bulto, 1994); in addition, living with a mental illness involves many life challenges, decreasing the salience of many health threats, including HIV. Indeed, research has often found that psychiatric patients are poorly motivated to adopt risk-reduction strategies (Blanchard, Mueser, & Bellack, 1998; M. P. Carey, Carey, Weinhardt, & Gordon, 1997). Psychiatric patients often lack the interpersonal and social skills needed to negotiate for safer sexual relationships (Mueser et al., 1996), and the social networks of economically disadvantaged psychiatric patients tend not to be supportive of self-protection (Kelly et al., 1997). Patients with persistent impairment are typically unable to work and may experience periods of homelessness (Drake et al., 1991). These disease characteristics, and their social sequelae, tend to emerge in early adulthood, the same time when sexual activity also peaks. The confluence of these events may increase patients’ vulnerability to sexual coercion (Wenzel, Koegel, & Gelberg, 2000) and sexual risk behavior (Ramrakha, Caspi, Dickson, Moffitt, & Paul, 2000). Considerable evidence suggests that, although psychiatric patients engage in fewer occasions of sexual intercourse compared with other subgroups, they often engage in risky sexual practices, such as having multiple or high-risk partners, sex trading, and having unprotected penetrative sex (M. P. Carey, Carey, et al., 1999; M. P. Carey, Carey, Maisto, Gordon, & Vanable, 2001; Kalichman et al., 1994; Kelly et al., 1995).
Implementing HIV-risk-reduction interventions with adults who have a mental illness can be uniquely challenging (Kelly, 1997). The psychological and social impairments that characterize these illnesses require considerable therapeutic effort and resources, and broadening the scope of psychiatric care creates additional burden for patients and providers. Deinstitutionalization, transitory housing, and limited access to transportation may create additional barriers to patient recruitment, participation, and retention. Despite the challenges of implementing risk-reduction interventions, several investigations have examined the use of multiple-session, HIV-risk-reduction interventions for psychiatric outpatients (Kalichman, Sikkema, Kelly, & Bulto, 1995; Kelly et al., 1997; Otto-Salaj, Kelly, Stevenson, Hoffmann, & Kalichman, 2001; Susser, Valencia, et al., 1998; Weinhardt, Carey, & Carey, 1997; Weinhardt, Carey, Carey, & Verdecias, 1998). Collectively, these studies provide preliminary evidence of the feasibility and the efficacy of HIV-risk-reduction interventions tailored to the unique needs of the mentally ill. Many of these pioneering efforts, however, have relied on relatively small samples, weak control groups, suboptimal measures (i.e., arbitrary composites of risk behavior or proportions of condom use rather than counts of actual risk behavior), brief follow-up intervals, or poorly matched data analytic strategies (e.g., failure to use intent-to-treat analyses or use of analytic approaches whose fundamental assumptions are violated). Overall, these studies provide encouraging but inconclusive evidence of the efficacy of HIV-risk-reduction interventions for adults with a psychiatric disorder.
To build on prior research, we developed an HIV-risk-reduction intervention that was guided by the information–motivation–behavioral skills (IMB) model (Fisher & Fisher, 1992). In presenting their model, Fisher and Fisher (1992) argued that a person is more likely to adopt HIV-risk-reduction strategies (e.g., condom use) if he or she is well-informed about HIV transmission and prevention, motivated to reduce his or her risk, and capable of demonstrating the necessary self-management and interpersonal skills needed to avoid risk behavior. Fisher and Fisher also recognized the need for formative research to identify population-specific determinants of risk taking and risk reduction. Consistent with these tenets, the HIV intervention developed for the current study was guided by qualitative (Gordon, Carey, Carey, Maisto, & Weinhardt, 1999) and quantitative (M. P. Carey, Carey, et al., 1997) formative work with the target population as well as by a comprehensive review of the literature (M. P. Carey, Carey, & Kalichman, 1997). We also conducted pilot intervention work (Weinhardt et al., 1997; Weinhardt, Carey, et al., 1998) and, on the basis of these formative efforts, developed a manual to guide the implementation of this intervention.
In addition, we sought to develop a strong test of intervention efficacy by considering alternative interventions that, although not targeted directly at HIV-risk behavior, could be expected to reduce such risk behavior indirectly. In this regard, evidence suggests that persons living with a mental illness are at increased risk of substance use (Blanchard, Brown, Horan, & Sherwood, 2000; Regier et al., 1990), and correlational research indicates that substance use is often associated with increased HIV-related risk taking (M. P. Carey, Chandra, Carey, & Neal, 2003; Chandra et al., 2003).
Prior experimental research from our laboratory has also provided support for the notion that acute intoxication may impair the theoretical antecedents of HIV-risk behavior suggested by the IMB model. In our first study, we found that intoxicated men endorsed less favorable condom attitudes and reported lower self-efficacy to initiate condom use, relative to sober controls (Gordon & Carey, 1996). In a subsequent study, we found that men who consumed alcohol demonstrated lower skill to negotiate for condom use relative to sober controls. In addition, more negative condom attitudes were expressed by participants with stronger sex-related alcohol expectancies, especially when these expectancies were triggered by subjective intoxication (Gordon, Carey, & Carey, 1997). Our most recent study showed that men who consumed alcohol had poorer negotiation skills and greater intentions to engage in risky sex in a role-play compared with participants who did not drink (Maisto et al., in press). Overall, these three studies demonstrate that alcohol use affects two hypothesized determinants of risky sexual behavior, namely, HIV-related motivation and behavioral skills.
Substance use may affect motivation and skills through direct pharmacologic degradation of cognitive and/or motivational processes and behavioral skills or through the influence of alcohol-related sexual expectancies. In this regard, alcohol expectancy theory suggests that the initiation and continuation of alcohol use is motivated in large part by a person’s beliefs about the likely outcomes of drinking (Goldman, Del Boca, & Darkes, 1999). Therefore, beliefs that alcohol enhances sexual experiences are likely to lead to alcohol seeking in contexts where a sexual encounter is anticipated. In contrast, reducing alcohol and other substance use and challenging beliefs that alcohol facilitates sexual opportunities and/or performance can be expected to reduce sexual risk behavior.
Therefore, we developed a substance use reduction (SUR) intervention as a structurally equivalent comparison condition for the current research. This intervention also benefited from considerable formative research and pilot testing with psychiatric patients who use alcohol and other substances (K. B. Carey, Carey, Maisto, & Purnine, 2002; K. B. Carey, Maisto, Carey, Gordon, & Correia, 1999; K. B. Carey, Purnine, Maisto, & Carey, 2001; K. B. Carey, Purnine, Maisto, Carey, & Barnes, 1999; K. B. Carey, Purnine, Maisto, Carey, & Simons, 2000). The SUR condition also provided a time- and attention-matched control condition separate from the purported substance use–HIV association.
The primary purpose of this study was to investigate the efficacy of HIV and SUR interventions with respect to HIV-related risk behavior and its theoretical antecedents in a large sample of male and female psychiatric outpatients. We selected an assessment interval of 6 months to afford ample opportunity to observe behavior change. Given the different content and hypothesized mechanisms of change for the two interventions, we predicted a tiered effect (i.e., HIV > SUR > control) for the sexual behavior outcomes. Specifically, we predicted that patients who received the HIV intervention would reduce their risk more than would those who received the SUR intervention, who, in turn, would reduce their risk more than patients in the control condition. For the skills and communication outcomes, however, we expected the HIV and the SUR interventions to lead to equivalent improvements because both groups involved interpersonal-skills training (i.e., HIV = SUR > control). For the remaining HIV-specific antecedent variables suggested by the IMB model (i.e., knowledge, attitudes, intentions, and decisional balance), we predicted that patients in the HIV condition would improve more than patients in both the SUR and control conditions (i.e., HIV > SUR = control). Exploratory analyses addressed whether any observed intervention effects were moderated by patients’ gender, as has been found in the one previous study that examined the moderating role of patient gender (Otto-Salaj et al., 2001). Additional exploratory analyses addressed the potential for psychiatric diagnosis to moderate intervention effects. Given the exploratory nature of these analyses, we made no a priori predictions regarding the effects of diagnosis.
Method
Design
This randomized controlled trial had three conditions (HIV-risk reduction [HIV], SUR, standard care control [CTL]) and four assessment occasions (pre- and postintervention and 3- and 6-month follow-ups). The schedule of assessments was selected to provide an interval of time that afforded ample opportunity for sexual risk and protective behavior to occur; we assessed sexual behavior at 3-month intervals because this interval has become the standard for reliable reporting of sexual behavior events (M. P. Carey, Carey, Maisto, Gordon, & Weinhardt, 2001; Jaccard, McDonald, Wan, Dittus, & Quinlan, 2002) and because it allowed us to maintain contact with participants to optimize retention (Ostrow & Kalichman, 2000). The primary outcome variables were self-reported sexual risk behaviors (unprotected vaginal sex), self-reported history of a diagnosed sexually transmitted infection (STI), and communication regarding safer sex; secondary outcome variables included the hypothesized antecedents of behavior change (i.e., information, motivation, and behavioral skills; Fisher & Fisher, 1992). Data collection occurred during 1997–2000.
Participants
The sample was drawn from 2,906 outpatients attending a psychiatric clinic at two not-for-profit hospitals in Syracuse, New York. All patients were being seen for an intake appointment or receiving ongoing treatment (see Figure 1). Eligibility criteria were (a) age 18 years or older; (b) sexually active in the past year; (c) alcohol or drug use in the past year (alcohol or drug use was an eligibility criterion, so that the patients assigned to the SUR intervention would find it interesting and relevant); (d) diagnosis of a major mood or thought disorder using the Structured Clinical Interview for the DSM–IV (SCID; First, Spitzer, Gibbon, & Williams, 1995); and (e) ability to participate meaningfully as determined by patients’ mental status, assessed with the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975) and minimal literacy, assessed by the Rapid Estimate of Adult Literacy in Medicine (REALM; Davis et al., 1993). Four hundred eight patients completed the preintervention assessment, met all inclusion criteria, and consented to participate.
Figure 1.
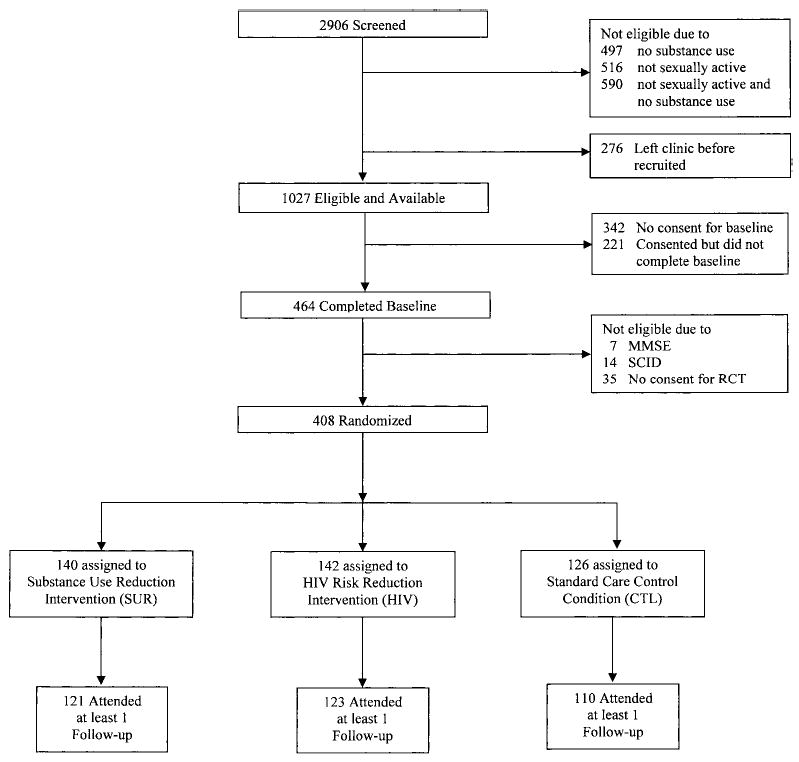
Participant flow. SCID = Structured Clinical Interview for the DSM–IV; MMSE = Mini-Mental State Exam; RCT = randomized controlled trial.
The sample included 221 female and 187 male psychiatric outpatients (see Table 1). The mean age of patients was 36.5 years (SD = 9.5); self-described race/ethnicity was 67% European American, 21% African American, and 12% other. Twenty-eight percent were currently married, and 60% had at least one child. Two thirds of the participants had at least a high school education, but most (83%) were unemployed; patients reported an average annual income of $6,759. Psychiatric diagnoses consisted of 49% depressive disorder, 19% bipolar disorder, 18% schizophrenia, and 15% schizoaffective disorder. Average global assessment of functioning score, as determined by the SCID, was 46.7 (SD = 12.5), and patients reported a lifetime average of 6.1 psychiatric hospitalizations. A lifetime history of an STI was reported by 38% of the sample. Eight participants (2%) reported that they were infected with HIV at baseline.
Table 1.
Patient Demographic, Social, and Psychiatric Characteristics by Condition
| HIV (n = 142)
|
SUR (n = 140)
|
CTL (n = 126)
|
All (N = 408)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | M | SD | M | SD | M | SD | M | SD |
| Yearly income ($) | 7,295 | 8,091 | 6,322 | 5,818 | 6,638 | 5,170 | 6,759 | 6,528 |
| Age (years) | 35.7 | 9.3 | 37.2 | 9.6 | 36.8 | 9.7 | 36.5 | 9.5 |
| Age at first treatment (years) | 20.2 | 8.6 | 21.3 | 10.6 | 21.9 | 9.0 | 21.1 | 9.4 |
| No. of hospitalizations | 7.5 | 19.4 | 5.2 | 13.1 | 5.6 | 12.3 | 6.1 | 15.4 |
| Mental status (MMSE) | 28.0 | 2.1 | 28.1 | 1.9 | 27.6 | 2.5 | 27.9 | 2.2 |
| Literacy (REALM) | 56.0 | 16.2 | 58.9 | 12.8 | 58.0 | 15.6 | 57.6 | 14.9 |
| Psychiatric impairment (GAF) | 46.4 | 12.0 | 46.2 | 12.8 | 47.6 | 12.7 | 46.7 | 12.5 |
| % | % | % | % | |||||
| Gender (% female) | 56 | 54 | 53 | 54 | ||||
| Ethnicity | ||||||||
| European American | 64 | 70 | 67 | 67 | ||||
| African American | 26 | 20 | 16 | 21 | ||||
| Other | 10 | 10 | 17 | 12 | ||||
| Marital status (% married) | 28 | 24 | 30 | 28 | ||||
| Living with sex partner | 30 | 25 | 25 | 27 | ||||
| Parents (% with children) | 64 | 61 | 56 | 60 | ||||
| Education | ||||||||
| < high school | 36 | 29 | 34 | 33 | ||||
| High school graduate | 34 | 34 | 33 | 34 | ||||
| Some college | 22 | 26 | 24 | 24 | ||||
| College graduate | 8 | 11 | 9 | 9 | ||||
| Employed | 17 | 16 | 20 | 17 | ||||
| Diagnosis | ||||||||
| Bipolar disorder | 18 | 18 | 19 | 19 | ||||
| Depressive disorder | 43 | 51 | 52 | 49 | ||||
| Schizophrenia | 21 | 18 | 15 | 18 | ||||
| Schizoaffective disorder | 18 | 12 | 14 | 15 | ||||
Note. HIV = HIV-risk reduction condition; SUR = substance use reduction condition; CTL = standard care control condition; MMSE = Mini-Mental State Exam (Folstein et al., 1975); REALM = Rapid Estimate of Adult Literacy in Medicine (Davis et al., 1993); GAF = Global Assessment of Functioning (First et al., 1995).
Primary Outcome Measures
Sexual behavior was assessed with a comprehensive interview that included the timeline followback (TLFB; M. P. Carey, Carey, Maisto, Gordon, & Weinhardt, 2001), a validated assessment strategy that uses detailed calendars, landmark events, personally meaningful dates, and other memory prompts to facilitate accurate recall (Weinhardt, Carey, et al., 1998). The assessment interval was 3 months, and the primary outcome was the frequency count of unprotected vaginal intercourse. (Anal sex was also assessed, but this risk behavior was not sufficiently common to use as an outcome measure.) Additional behavioral outcomes included counts of the (a) total number of sex partners, (b) number of casual sex partners, (c) number of safer sex communications before intercourse, and (d) self-report of newly diagnosed STIs. The latter outcome was collected only at baseline and the 6-month follow-up because of the expected low frequency of STIs. The TLFB has been used in previous studies with psychiatric patients (Weinhardt, Carey, et al., 1998).
Secondary Outcome Measures
Consistent with the IMB model that guided the risk reduction intervention, we assessed HIV-related information, motivation, and behavioral skills, constructs that are hypothesized to influence sexual risk behavior. All measures had been used and validated in prior research.
Information
Knowledge about the transmission and prevention of HIV was assessed with the HIV Knowledge Questionnaire (HIV-KQ; M. P. Carey, Morrison-Beedy, & Johnson, 1997). Patients were asked to indicate whether each of 45 statements was true or false or to indicate that they did not know. Factor analyses with diverse samples indicate that the HIV-KQ contains a single, internally consistent factor (M. P. Carey, Morrison-Beedy, & Johnson, 1997). Possible scores on the HIV-KQ range from 0% to 100%, with higher scores reflecting greater HIV-related knowledge. The HIV-KQ has been used in previous studies with psychiatric patients (Weinhardt, Carey, et al., 1998). Coefficient alpha for the current sample from the baseline assessment was .87.
Motivation
Three self-report measures, developed and validated in prior research, were used to assess HIV-related motivation. Attitudes toward condoms were measured with 10 items adapted from a longer, validated measure (Sacco, Levine, Reed, & Thompson, 1991). Patients were asked to use 6-point Likert scales (ranging from 1 = strongly disagree to 6 = strongly agree) to indicate their agreement with each of 10 statements (e.g., “Condoms ruin the mood”). Possible scores ranged from 10 to 60, with higher scores reflecting more favorable attitudes toward condoms. Similar attitude items have been used in previous studies with psychiatric patients (M. P. Carey, Carey, Weinhardt, & Gordon, 1997; Otto-Salaj et al., 2001). Coefficient alpha from the baseline assessment in the current sample was .74.
The pros and cons of condom use were assessed with the Decisional Balance Scale (Galavotti et al., 1995). Patients used 5-point Likert scales (ranging from 1 = not at all important to 5 = extremely important) to indicate how important each statement (e.g., pro: “I would be safe from disease”; con: “My partner would think that I do not trust him/her”) was when deciding whether to use condoms. Possible scores ranged from 5 to 25, with higher scores indicating more pros or cons, respectively. Decisional Balance Scale items have been used in previous studies with psychiatric patients (K. B. Carey, Carey, et al., 2002; K. B. Carey, Maisto, Carey, & Purnine, 2001; K. B. Carey, Purnine, Maisto, & Carey, 2002). Coefficient alpha from the baseline assessment in the current sample was .76 for the pros and .70 for the cons.
Behavioral intentions for safer sex were assessed using a six-item measure adapted from prior research (M. P. Carey, Maisto, et al., 1997). Patients were presented with a scenario describing a possible sexual encounter and asked to rate how likely it was that they would engage in six risky or protective behaviors (e.g., “I will tell the person I don’t want to have sex without a condom”). Patients responded to each behavior using a 6-point scale (ranging from 0 = definitely will not do to 5 = definitely will do). Possible scores ranged from 0 to 30, with higher scores reflecting stronger risk reduction intentions. Similar intention items have been used in previous studies with psychiatric patients (Kalichman et al., 1995; Otto-Salaj et al., 2001). Coefficient alpha from the baseline assessment in the current sample was .89.
Behavioral skills
Assertion skills for safer sex were observed using standardized behavioral simulations (Gordon et al., 1997; Weinhardt, Carey, et al., 1998). Patients listened to four audiotaped high-risk scenarios and responded to three prompts for unsafe sex from hypothetical partners. The simulations were video recorded and later rated by trained assistants who were masked to the patient’s assigned condition. The assistants rated each response on a 0–2 scale on four dimensions: (a) the presence of a clear refusal of risky sexual behavior; (b) a clear reason for preferring a safer sexual behavior; (c) suggestion of a safer alternative; and (d) the degree of assertiveness in the request. To assess reliability in the current sample, a second assistant selected and rated 50 samples at random. The exact agreement rate of these ratings was 88% (κ = .77). Scores from the four dimensions were summed to yield a composite index of behavioral skills, which ranged from 0 to 96, with higher scores reflecting greater skill. This role-play measure has been used in previous studies with psychiatric patients (Weinhardt, Carey, et al., 1998). Coefficient alphas from all assessments in the current sample exceeded .77.
Procedures
All procedures were approved by the Institutional Review Boards at Syracuse University and the two participating hospitals. The intervention trial was preceded by a period of formative research, including focus groups, survey development, and pilot intervention testing.
Screening, preintervention assessment, and random assignment
During standard clinical care, outpatients were screened by a research assistant regarding their sexual activity, alcohol use, and drug use in the past year. Patients who reported both sexual activity and substance use were invited by the research assistant to participate in a more detailed assessment. They were informed about the purpose and procedures of the study and that information they provided to the research team would not be released to anyone (including clinical staff) unless patients specifically requested this in writing. We explained that having a Federal Certificate of Confidentiality protected our data from subpoena. Those who agreed and provided informed written consent met with a member of the assessment staff (separate from the intervention staff); they completed a SCID, the MMSE, and the REALM as well as the preintervention battery of self-report questionnaires, the TLFB, and the role-play assessment. Completion of these assessments required two to three sessions, and patients were reimbursed $30 for their time and expenses. At the end of this comprehensive assessment phase, patients who met all inclusion criteria were then invited to participate in the intervention phase of the project. Interested patients were asked to sign a second informed consent and were then randomly assigned to one of three conditions: (a) CTL, (b) SUR, or (c) HIV.
Interventions
Patients in the CTL condition received standard outpatient psychiatric care including medication, psychotherapy, and case management services. The standard of care at the hospitals where the trial was conducted mandates that individual therapists provide HIV and substance use education and intervention tailored to the needs and circumstances of individual patients. There was no prohibition regarding the discussion of HIV or substance use as a condition of participating in this clinical trial.
Patients in the HIV and SUR conditions also received standard care; in addition, they were invited to attend a prevention program designed to reduce either risky sexual behavior or substance use. The HIV and SUR intervention groups were structurally equivalent. Both met twice weekly for 5 weeks for a total of 10 sessions. The interventionist team included two male and four female doctoral-level facilitators and two male and one female master’s-level facilitators. Each intervention group was led by a female and a male cofacilitator pair; a mixed-gender team was used to model healthy male–female interpersonal interactions and to permit both matched-gender and mixed-gender role-play exercises. In every team, the primary facilitator had completed doctoral-level clinical training and the cofacilitator had completed at least master’s-level clinical training. The facilitators received extensive training in both interventions prior to leading groups, followed detailed intervention manuals, and received weekly supervision from a licensed clinical psychologist. To avoid confounding condition with facilitator, all facilitators led both interventions. Refreshments were served during each group, and participants received $10 at each session to offset travel expenses.
The two interventions also shared conceptual underpinnings. First, both included a strong informational component to facilitate the acquisition of domain-specific knowledge. Information was presented using explicit and simple terms and was reviewed frequently (so that patients who missed sessions could catch up, to compensate for learning impairments that sometimes accompany persistent psychiatric disorders, and to allow for a review of key prevention concepts). Second, both interventions sought to facilitate the acquisition of behavioral skills through the use of interactive role-play exercises (Bellack, Mueser, Gingerich, & Agresta, 1997; Kelly, 1982; Liberman, DeRisi, & Mueser, 1989). To strengthen skill acquisition and provide opportunities for rehearsal, both interventions included homework assignments.
Third, both interventions reflected new developments in motivational interventions. Each was guided by the “harm reduction” conceptualization to risk reduction (Marlatt, 1996) and used motivational techniques to enhance participants’ readiness to change (Miller & Rollnick, 1991). Five therapeutic guidelines derived from past research were followed: (a) express empathy using nonjudgmental listening, acceptance of the patient, and recognition that ambivalence about behavior change is normal, (b) develop a discrepancy between the patient’s current behavior and his or her long-term goals, (c) avoid argumentation and encourage the patient to articulate reasons for change, (d) tolerate resistance to change and do not force new perspectives, and (e) support self-efficacy for change. Both interventions reflected these motivational principles and incorporated the active ingredients of successful brief interventions, summarized by the acronym FRAMES (Miller & Rollnick, 1991); thus, facilitators provided feedback on personal risk, emphasized personal responsibility for change, provided clear advice to change, provided a menu of change options, demonstrated empathy, and encouraged participants’ self-efficacy and optimism for change.
The essential difference between the two interventions involved the targeted risk behaviors. Thus, the HIV intervention focused on sexual behavior and was explicitly guided by the IMB model. This model holds that sexual risk (and protective) behavior is a function of information about HIV transmission and prevention, motivation to reduce personal risk, and behavioral skills for performing the specific acts involved in risk reduction. Empirical support for the fundamental assumptions of the IMB model comes from multivariate correlational research in a variety of populations (Fisher & Fisher, 2000).
A session-by-session outline for the HIV intervention is provided in Appendix A. (The intervention manual can be obtained at http:// www.chb.syr.edu/PDF_Resources/.) The first four sessions emphasized informational and motivational components. Using an interactive style, facilitators provided current information about sexual behavior, HIV, and other STIs; debunked misunderstandings about disease transmission; and discussed the value of HIV testing and counseling and related topics. Motivational exercises were designed to increase risk awareness and sensitization, to encourage patients to identify the pros and cons of various risk reduction strategies, to discuss social norms regarding risky and safer sex, to alter cognitions related to behavior change, and to encourage mutual social support for risk reduction. Six sessions were devoted to the development of behavioral skills for safer sex, self-management strategies, and sexual assertiveness training (Kelly, 1995). Consistent with the behavioral skills component of the IMB model, this involved preparing patients to obtain, store, and use condoms correctly; to develop coping strategies and self-efficacy for dealing with high-risk situations; and to develop effective strategies for negotiating condom use with sexual partners.
The SUR intervention sought to enhance participants’ knowledge, motivation, and behavioral skills regarding harmful substance use. The SUR intervention used a social–cognitive theoretical framework to promote responsible use of alcohol, nicotine, and caffeine (Maisto, Carey, & Bradizza, 1999). Although we were interested in exploring the benefits of alcohol use reduction on sexual risk behavior, we provided a broader intervention that addressed an array of substances so that the intervention was acceptable to patients and not stigmatizing as an alcohol-only intervention might have been.
Exercises included an introduction to the concept of risk “triggers,” development of skills and strategies for coping with high-risk situations, and development of effective refusal skills for avoiding pressure to consume substances (Drake et al., 2001; Monti, Abrams, Kadden, & Cooney, 1989). The SUR intervention controlled for nonspecific factors, such as group interaction and additional contact time with facilitator-therapists, that might explain effects resulting from the HIV intervention (Cook & Campbell, 1979). In addition, because research suggests that reduction or elimination of substance use might also reduce risky sexual behavior (Avins et al., 1997), this alternative intervention provided an ambitious comparison condition for the HIV-risk reduction intervention (Ostrow & Kalichman, 2000).
Past research indicates that reduction or elimination of alcohol, caffeine, and nicotine use can improve the adaptive functioning of patients with a psychiatric illness (Drake, Mercer-McFadden, Mueser, McHugo, & Bond, 1998). As depicted in Appendix B, Sessions 1, 2, and 3 focused on enhancing knowledge, motivation, and self-management skills related to reducing caffeine use; Sessions 4 and 5 focused on preventing, reducing, or eliminating tobacco use; Sessions 6 through 9 focused on reducing or eliminating harmful alcohol consumption (for patients who were not currently drinking, group processes focused on relapse prevention). Although the groups did not focus explicitly on recreational drug use (because only a subset of participants would view such an intervention as being relevant), participants who wished to discuss struggles with illicit drug use (e.g., marijuana) were allowed to do so, permitting the facilitators the opportunity to illustrate the general principles related to any substance use. Session 10 included a review of earlier group sessions along with solidification of longer term substance use behavior change goals.
Follow-up assessments
Three postintervention assessments were completed. The first took place 1 to 2 weeks following the final intervention session; the postintervention assessment occurred approximately 3 months after the baseline assessment because of a lag between the baseline assessment and the beginning of intervention groups. Two additional assessments occurred approximately 3 and 6 months after the postinter-vention assessment. All assessments covered a 3-month period and were conducted by an assessor who was not associated with the intervention or the patients’ standard care to reduce reporting bias associated with demand characteristics. Assessors were masked to intervention condition and the research hypotheses. Participants were scheduled for the first follow-up during the last week of groups (participants in the CTL condition received a reminder letter and were scheduled by phone). A reminder letter for subsequent follow-up assessments was sent to all participants, and appointments were scheduled by phone (when possible) or by mail for those participants who lacked access to a telephone. Participants were paid $25 to complete each of the follow-up assessments.
Data analytic plan
Data were double entered and compared to ensure accuracy. All outcome variables were standardized and checked for outliers (i.e., cases with a z score > 3). Outliers were replaced with the unstandardized score corresponding to z = 3. Across all variables and assessment occasions, there were 37 outliers (i.e., 0.2%). Also, to avoid possible bias from deleting patients who did not attend all of the follow-ups (i.e., listwise deletion), we imputed missing scores using the estimation maximization procedure of SPSS 10.5 (SPSS, 2000). Data were missing from 8% of patients at postintervention, 11% at the 3-month follow-up, and 12% at 6-month follow-up.
Three preliminary analyses were completed. First, we compared the three conditions on the baseline demographic, social, psychiatric, and outcome variables to determine whether random assignment produced equivalent groups. Second, we compared patients who attended one or more follow-up sessions to those patients who did not attend any follow-ups to determine whether differential attrition occurred as a function of either intervention condition or patient characteristics. Third, we compared the HIV and SUR conditions on group attendance to determine if differential intervention participation occurred.
The primary analyses involved planned contrasts between (a) HIV versus CTL, (b) HIV versus SUR, and (c) SUR versus CTL; in cases indicated by a priori hypotheses, one-tailed tests were used. The analyses used an intent-to-treat approach (Kleinman, Ibrahim, & Laird, 1998); that is, all patients who provided any outcome data were included in the analyses regardless of whether they attended the intervention sessions.
Outcome analyses with dichotomous variables used logistic regression, and those with continuous variables used the generalized estimation equation (GEE) approach (Zeger & Liang, 1986; Zeger, Liang, & Albert, 1988). The GEE approach has been increasingly used to analyze data from HIV prevention trials as a means of assessing change over time with extremely skewed sexual behavior data (e.g., Kamb et al., 1998; National Institute of Mental Health [NIMH] Multisite HIV Prevention Trial Group, 1998; Otto-Salaj et al., 2001; Voluntary HIV-1 Counselling and Testing Efficacy Study Group, 2000).1 GEE analyses were performed using STATA (Stata-Corp, 2003). For each dependent variable, the appropriate distribution family was determined. The behavioral outcomes were characterized by a high number of zero scores and extreme skewness, indicating either Poisson or negative binomial regression. Variables fitting the Poisson model were analyzed by GEE with Poisson regression. Variables that were only slightly overdispersed were analyzed using Poisson regression with robust estimates. A negative binomial distribution was specified for overdispersed variables that deviated clearly from the Poisson model. Normally distributed outcome variables were also analyzed by GEE, specifying a Gaussian distribution. The analyses were performed specifying an exchangeable correlation matrix for the repeated measures.
GEE analyses tested for hypothesized differences between the regression coefficients for the Condition × Time interactions. For the pairwise comparisons, condition was dummy coded with “1” indicating the condition predicted to elicit the stronger intervention effect (e.g., HIV = 1 vs. CTL = 0). For the computation of the interaction terms only, the time factor was dummy coded with 0, indicating preintervention assessment, and 1, indicating the follow-up assessments. The regression equations estimating the regression coefficients for the hypothesized Condition × Time interactions had the form y = a + B1 × Condition + B2 × Time + B3 × Condition × Time + e. Thus, each analysis provided regression coefficients for the main effects of condition and time as well as for the Condition × Time interaction.
Exploratory analyses were conducted to examine whether any observed effects were moderated by gender or psychiatric diagnosis. Potential moderator effects were tested by performing the GEE analyses separately for men and women. Nonoverlapping confidence intervals for the Condition × Time interactions ( p < .05) were interpreted as indicating significant moderator effects of gender on the observed intervention effects. Psychiatric diagnosis was dichotomized into patients diagnosed with major depression (n = 164) and those with other psychiatric diagnosis (n = 187). Moderator effects were tested by performing the GEE analyses separately for the two genders (and diagnostic groups), an approach that avoids problems associated with multicollinearity of the predictors. Nonoverlapping confidence intervals for the Condition × Time interactions ( p < .05) were interpreted as indicating significant moderator effects of gender (or diagnostic group) on the observed intervention effects.
Results
Preliminary Analyses
Recruitment success
Figure 1 provides patient flow data and results regarding recruitment success. A total of 2,906 patients were screened; of these, 1,603 did not meet initial eligibility criteria, and 276 left the treatment facility before they could be invited to participate. Thus, 1,027 patients were eligible on the basis of initial criteria (e.g., sexual activity status) and were available to be invited; from this pool, 342 patients declined our invitation to participate in the baseline assessment, and 221 consented but did not complete the three-session baseline assessment, leaving a total of 464 patients. Of these, 14 did not meet diagnostic eligibility criteria, 7 were not eligible on the basis of their MMSE scores, and 35 declined the invitation to participate in the intervention trial; thus, 408 of the 1,006 eligible and available patients (41%) were randomized to the trial.
Equivalence of groups at baseline
To determine whether random assignment produced equivalent groups, we compared the three conditions (N = 408) on the baseline demographic, social, psychiatric, and outcome variables listed in Tables 1 and 2 using ANOVA, t test, and chi-square tests. The only significant difference at baseline among conditions was that participants who were assigned to the HIV condition had more positive condom attitudes compared with participants assigned to the SUR condition (Ms = 41.5 vs. 38.8; F[2, 350] = 3.1, p < .05). Given this difference, subsequent analyses comparing the HIV and the SUR conditions included baseline condom attitudes as a covariate.
Table 2.
Means and Standard Deviations for Outcome Variables by Condition
| Preintervention
|
Postintervention
|
3-month follow-up
|
6-month follow-up
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable (possible range) | Condition | M | SD | M | SD | M | SD | M | SD |
| Frequency of unprotected vaginal sex (count) | HIV | 14.0 | 31.2 | 8.7 | 14.5 | 9.5 | 23.1 | 7.2 | 14.5 |
| SUR | 10.8 | 22.1 | 9.4 | 22.9 | 8.1 | 20.3 | 8.8 | 20.2 | |
| CTL | 11.6 | 25.9 | 12.1 | 25.6 | 10.0 | 21.1 | 8.0 | 17.9 | |
| No. of partners (count) | HIV | 1.25 | 1.11 | 1.04 | 0.84 | 0.93 | 0.80 | 0.97 | 0.78 |
| SUR | 1.41 | 1.67 | 1.12 | 1.12 | 1.02 | 1.12 | 0.95 | 0.99 | |
| CTL | 1.24 | 1.12 | 1.19 | 1.29 | 1.12 | 1.16 | 1.07 | 1.38 | |
| No. of casual partners (count) | HIV | 0.54 | 1.05 | 0.28 | 0.60 | 0.21 | 0.57 | 0.30 | 0.58 |
| SUR | 0.63 | 1.31 | 0.38 | 0.79 | 0.33 | 0.93 | 0.30 | 0.67 | |
| CTL | 0.49 | 1.05 | 0.52 | 1.21 | 0.47 | 0.97 | 0.48 | 1.19 | |
| Communications about safer sex (count) | HIV | 1.17 | 2.92 | 1.34 | 2.62 | 1.28 | 2.61 | 0.98 | 2.06 |
| SUR | 1.12 | 2.81 | 0.94 | 2.79 | 0.53 | 1.71 | 0.69 | 2.08 | |
| CTL | 1.02 | 2.17 | 1.20 | 3.17 | 0.79 | 1.99 | 0.91 | 2.47 | |
| Knowledge (0%–100%) | HIV | 69.6 | 16.6 | 80.1 | 13.1 | 77.7 | 14.1 | 77.3 | 15.1 |
| SUR | 73.2 | 13.7 | 73.9 | 14.1 | 74.5 | 14.4 | 74.7 | 14.6 | |
| CTL | 72.7 | 15.0 | 73.0 | 15.2 | 73.7 | 15.2 | 73.7 | 15.8 | |
| Condom attitudes (10–60) | HIV | 41.5 | 8.4 | 43.0 | 8.5 | 43.1 | 8.3 | 43.7 | 8.7 |
| SUR | 38.8 | 9.4 | 40.3 | 9.4 | 40.5 | 9.3 | 40.1 | 10.0 | |
| CTL | 40.5 | 8.3 | 40.4 | 8.3 | 40.1 | 8.8 | 40.4 | 8.8 | |
| Decisional balance (pros of condom use; 5–25) | HIV | 20.5 | 3.9 | 20.7 | 3.8 | 20.8 | 3.7 | 20.6 | 3.7 |
| SUR | 19.9 | 4.3 | 20.2 | 4.0 | 20.2 | 3.9 | 19.8 | 4.0 | |
| CTL | 20.4 | 4.2 | 21.0 | 3.7 | 20.6 | 4.2 | 20.5 | 4.1 | |
| Decisional balance (cons of condom use; 5–25) | HIV | 13.4 | 4.6 | 13.0 | 4.4 | 12.8 | 4.5 | 13.0 | 4.3 |
| SUR | 13.7 | 5.0 | 13.2 | 4.7 | 12.7 | 4.4 | 12.4 | 4.2 | |
| CTL | 13.1 | 4.2 | 12.3 | 4.9 | 12.7 | 4.4 | 12.2 | 4.3 | |
| Condom use intentions (0–30) | HIV | 20.8 | 7.7 | 23.1 | 6.3 | 22.6 | 6.6 | 21.7 | 7.5 |
| SUR | 19.2 | 8.5 | 21.0 | 7.8 | 20.5 | 8.0 | 20.0 | 7.7 | |
| CTL | 21.2 | 7.5 | 21.6 | 7.1 | 21.7 | 7.7 | 21.4 | 7.6 | |
| Behavioral skills (0–96) | HIV | 43.8 | 13.3 | 46.9 | 13.3 | 46.3 | 12.3 | 44.4 | 12.7 |
| SUR | 44.7 | 13.4 | 48.5 | 12.5 | 46.3 | 13.1 | 45.7 | 12.5 | |
| CTL | 45.6 | 14.3 | 46.9 | 11.5 | 45.6 | 13.3 | 45.6 | 12.9 | |
| % | % | ||||||||
| Sexually transmitted infections (0%–100%) | HIV | 10 | 2 | ||||||
| SUR | 8 | 8 | |||||||
| CTL | 7 | 5 | |||||||
Note. HIV = HIV-risk reduction condition (n = 123); SUR = substance use reduction condition (n = 121); CTL = standard care control condition (n = 110).
Attendance at the intervention sessions
Patients in the HIV and SUR conditions were considered enrolled in the assigned treatment if they attended at least one session, and they were considered treated if they completed five or more sessions. Of the 142 patients assigned to the HIV condition, 103 (73%) enrolled in the intervention, and 95 (67%) completed it; of the 140 patients assigned to the SUR condition, 106 (76%) enrolled in the intervention, and 94 (67%) completed it. Patients assigned to the HIV and SUR interventions attended an average of 5.7 and 6.0, respectively, of the intervention sessions, F(1, 281) = 0.25, p = .87.
Attendance at the follow-up assessments
Of the 408 patients who completed the baseline assessment, 354 (87%) attended at least one of the follow-up assessments (Figure 1); 54 patients completed the baseline assessment but did not attend any subsequent assessment sessions. Chi-square tests indicated that the conditions did not differ significantly in the percentage of patients retained at the postintervention ( p = .71), 3-month ( p = .30), or 6-month follow-up assessments ( p = .70). Comparison of those who attended one of the follow-up assessments (n = 354) with those who were lost to follow-up (n = 54) revealed that these two groups did not differ on any of the demographic, social, or psychiatric variables listed in Table 1. Comparison on the outcome variables measured at baseline and listed in Table 2 indicated that those patients who were lost to follow-up (n = 54) reported more pros of condom use (Ms = 21.7 vs. 20.2), t(406) = 2.52, p = .012, and demonstrated better HIV-related knowledge (Ms = 76.2 vs. 71.8), t(406) = 2.04, p < .04, than did patients who attended one or more postintervention assessments.
Primary Analyses
Table 2 provides the raw means and standard deviations for the outcome variables by condition and for each of the four assessment occasions. At baseline, patients reported an average of one sexual partner and having unprotected vaginal sex approximately once per week over the 3-month period prior to baseline. Eight percent of the sample reported a sexually transmitted infection in the previous year.
Effects of the HIV intervention
Table 3 shows the results of the HIV–CTL planned contrast, and Table 4 shows the results of the HIV–SUR contrast. Results for the behavioral outcomes are presented first, followed by the results for the hypothesized antecedents of HIV-related risk behavior.
Table 3.
Pairwise Comparisons Between HIV and CTL Conditions Testing Condition × Time Interactions
| Variable | Comparison | Est. | SE | z | p |
|---|---|---|---|---|---|
| Frequency of unprotected vaginal sex | HIV vs. CTL | 0.129 | 0.127 | 1.02 | .308 |
| Time | −0.118 | 0.029 | −4.09 | .001 | |
| HIV vs. CTL × Time | −0.269 | 0.102 | −2.64 | .004 | |
| No. of partners | HIV vs. CTL | 0.011 | 0.111 | 0.10 | .918 |
| Time | −0.047 | 0.025 | −1.90 | .057 | |
| HIV vs. CTL × Time | −0.153 | 0.085 | −1.79 | .037 | |
| No. of casual partners | HIV vs. CTL | 0.093 | 0.190 | 0.49 | .622 |
| Time | −0.005 | 0.046 | −0.10 | .920 | |
| HIV vs. CTL × Time | −0.717 | 0.166 | −4.33 | .001 | |
| Communicating about safer sex | HIV vs. CTL | 0.028 | 0.166 | 0.17 | .864 |
| Time | −0.101 | 0.041 | −2.49 | .013 | |
| HIV vs. CTL × Time | 0.228 | 0.141 | 1.62 | .053 | |
| Knowledge | HIV vs. CTL | −3.981 | 1.929 | −2.06 | .039 |
| Time | −0.174 | 0.209 | −0.83 | .405 | |
| HIV vs. CTL × Time | 9.175 | 0.744 | 12.34 | .001 | |
| Condom attitudes | HIV vs. CTL | 1.226 | 1.071 | 1.14 | .252 |
| Time | 0.048 | 0.167 | 0.29 | .772 | |
| HIV vs. CTL × Time | 1.661 | 0.594 | 2.80 | .003 | |
| Condom use intentions | HIV vs. CTL | −0.917 | 0.910 | −1.01 | .314 |
| Time | −0.180 | 0.147 | −1.22 | .221 | |
| HIV vs. CTL × Time | 2.052 | 0.522 | 3.93 | .001 | |
| Decisional balance (pros of condom use) | HIV vs. CTL | −0.191 | 0.486 | −0.39 | .694 |
| Time | −0.017 | 0.086 | −0.19 | .846 | |
| HIV vs. CTL × Time | 0.274 | 0.305 | 0.90 | .184 | |
| Decisional balance (cons of condom use) | HIV vs. CTL | 0.628 | 0.554 | 1.13 | .257 |
| Time | −0.163 | 0.100 | −1.63 | .103 | |
| HIV vs. CTL × Time | −0.164 | 0.354 | −0.46 | .322 | |
| Behavioral skills | HIV vs. CTL | −2.781 | 1.624 | −1.71 | .087 |
| Time | −0.456 | 0.283 | −1.61 | .108 | |
| HIV vs. CTL × Time | 2.994 | 1.005 | 2.98 | .002 |
Note. HIV = HIV-risk reduction condition (n = 123); CTL = standard care control condition (n = 110). Positive regression coefficients indicate increase, negative coefficients indicate decrease over time. The p values for Condition × Time interaction effects reflect one-tailed values for beta coefficients that show the hypothesized change. Est. = estimate.
Table 4.
Pairwise Comparisons Between HIV and SUR Conditions Testing Condition × Time Interactions
| Variable | Comparison | Est. | SE | z | p |
|---|---|---|---|---|---|
| Frequency of unprotected vaginal sex | HIV vs. SUR | 0.246 | 0.126 | 1.96 | .025 |
| Time | −0.093 | 0.028 | −3.34 | .001 | |
| HIV vs. SUR × Time | −0.325 | 0.100 | −3.24 | .001 | |
| No. of partners | HIV vs. SUR | −0.065 | 0.109 | −0.60 | .550 |
| Time | −0.107 | 0.024 | −4.39 | .001 | |
| HIV vs. SUR × Time | −0.036 | 0.085 | −0.42 | .339 | |
| No. of casual partners | HIV vs. SUR | −0.028 | 0.180 | −0.15 | .878 |
| Time | −0.192 | 0.049 | −3.91 | .001 | |
| HIV vs. SUR × Time | −0.363 | 0.167 | −2.17 | .015 | |
| Communicating about safer sex | HIV vs. SUR | 0.046 | 0.167 | 0.27 | .785 |
| Time | −0.188 | 0.040 | −4.73 | .001 | |
| HIV vs. SUR × Time | 0.416 | 0.137 | 3.05 | .001 | |
| Knowledge | HIV vs. SUR | −5.327 | 1.804 | −2.95 | .003 |
| Time | −0.046 | 0.217 | −0.21 | .833 | |
| HIV vs. SUR × Time | 8.918 | 0.789 | 11.31 | .001 | |
| Condom attitudes | HIV vs. SUR | 0.088 | 0.707 | 0.12 | .901 |
| Time | 0.375 | 0.168 | 2.24 | .025 | |
| HIV vs. SUR × Time | 1.006 | 0.611 | 1.65 | .045 | |
| Condom use intentions | HIV vs. SUR | 0.065 | 0.914 | 0.07 | .944 |
| Time | −0.070 | 0.151 | −0.49 | .624 | |
| HIV vs. SUR × Time | 1.841 | 0.549 | 3.35 | .001 | |
| Decisional balance (pros of condom use) | HIV vs. SUR | 0.068 | 0.466 | 0.15 | .885 |
| Time | −0.039 | 0.080 | −0.49 | .623 | |
| HIV vs. SUR × Time | 0.319 | 0.290 | 1.10 | .136 | |
| Decisional balance (cons of condom use) | HIV vs. SUR | 0.375 | 0.531 | 0.71 | .480 |
| Time | −0.303 | 0.097 | −3.11 | .002 | |
| HIV vs. SUR × Time | 0.116 | 0.354 | 0.33 | .742 | |
| Behavioral skills | HIV vs. SUR | −3.360 | 1.581 | −2.13 | .034 |
| Time | −0.305 | 0.284 | −1.07 | .283 | |
| HIV vs. SUR × Time | 2.693 | 1.031 | 2.61 | .005 |
Note. HIV = HIV-risk reduction condition (n = 123); SUR = substance use reduction condition (n = 121). Positive regression coefficients indicate increase, negative coefficients indicate decrease over time. The p values for Condition × Time interaction effects reflect one-tailed values for beta coefficients that show the hypothesized change. Est. = estimate.
Compared with patients in both the CTL and SUR conditions, participants in the HIV intervention showed a significantly stronger decrease over time in the overall frequency of unprotected vaginal sex (zs = –2.64 and −3.24, ps < .004 and .001, respectively)2 and in the number of casual partners (zs = −4.33 and –2.17, ps < .0001 and .015, respectively), and they increased the number of communications about safer sex (zs = 1.62 and 3.05, ps < .053 and .001, respectively). Patients in the HIV condition also reduced the total number of partners (z = −1.79, p < .037) compared with patients in the CTL group; there was no difference between the HIV and SUR groups on this variable.
For four of the six hypothesized antecedents of HIV-risk behavior, significant HIV × Time interactions were found (see Tables 3 and 4). Compared with the patients in both the CTL and SUR groups, patients in the HIV intervention showed a significantly stronger increase over time in HIV-related knowledge (zs = 12.34 and 11.31, respectively, ps < .0001), positive condom attitudes (zs = 2.80 and 1.65, ps < .003 and .045, respectively), condom use intentions (zs = 3.93 and 3.35, ps = .0001 and .001, respectively), and behavioral skills as indicated by the role-play ratings (zs = 2.98 and 2.61, ps < .002 and .005, respectively). No significant effects were found for the two subscales of the decisional balance measure.
Two hierarchical logistic regressions performed for the STI data (Table 2) revealed that the report of an STI at baseline predicted the report of an STI at the 6-month follow-up. These analyses also revealed that the HIV group factor, entered in Step 2, added to the prediction of STIs in both analyses, with Δχ2(1, N = 208) = 2.86, p < .046, in the comparison of HIV versus CTL, and Δχ2(1, N = 215) = 4.97, p < .013, for the comparison of HIV versus SUR. These two analyses indicate that participation in the HIV-risk-reduction group significantly reduced the likelihood of a self-reported STI at the final follow-up.
Effects of the SUR intervention
The results of the SUR–CTL planned contrast are depicted in Table 5. Compared with patients in the CTL condition, participants in the SUR intervention reduced the total number of partners (z = −2.48, p < .007) and the number of casual partners (z = −3.64, p < .0001); patients in the SUR intervention also increased positive condom attitudes (z = 2.83, p < .003), strengthened their condom use intentions (z = 2.91, p < .002), and demonstrated improved behavioral skills as indicated by the role-play ratings (z = 3.05, p < .001). There were no differences between the SUR and CTL groups on the number of incident STIs, the overall frequency of unprotected sex over time, the number of communications about safer sex, HIV knowledge, or the two subscales of the decisional balance measure.
Table 5.
Pairwise Comparisons Between SUR and CTL Conditions Testing Condition × Time Interactions
| Variable | Comparison | Est. | SE | z | p |
|---|---|---|---|---|---|
| Frequency of unprotected vaginal sex | SUR vs. CTL | −0.101 | 0.129 | −0.78 | .436 |
| Time | −0.098 | 0.026 | −3.70 | .001 | |
| SUR vs. CTL × Time | −0.016 | 0.094 | −0.17 | .864 | |
| No. of partners | SUR vs. CTL | 0.113 | 0.109 | 1.03 | .301 |
| Time | −0.062 | 0.023 | −2.69 | .007 | |
| SUR vs. CTL × Time | −0.193 | 0.078 | −2.48 | .007 | |
| No. of casual partners | SUR vs. CTL | 0.192 | 0.194 | 0.99 | .322 |
| Time | −0.037 | 0.043 | −0.86 | .388 | |
| SUR vs. CTL × Time | −0.547 | 0.150 | −3.64 | .001 | |
| Communicating about safer sex | SUR vs. CTL | −0.006 | 0.169 | −0.04 | .970 |
| Time | −0.102 | 0.041 | −2.47 | .013 | |
| SUR vs. CTL × Time | −0.249 | 0.143 | −1.74 | .081 | |
| Knowledge | SUR vs. CTL | 0.486 | 1.895 | 0.26 | .798 |
| Time | 0.369 | 0.210 | 1.76 | .078 | |
| SUR vs. CTL × Time | 0.412 | 0.747 | 0.55 | .291 | |
| Condom attitudes | SUR vs. CTL | −1.740 | 1.151 | −1.51 | .131 |
| Time | −0.096 | 0.170 | −0.56 | .573 | |
| SUR vs. CTL × Time | 1.714 | 0.606 | 2.83 | .003 | |
| Condom use intentions | SUR vs. CTL | −2.450 | 0.984 | −2.49 | .013 |
| Time | −0.109 | 0.146 | −0.74 | .458 | |
| SUR vs. CTL × Time | 1.521 | 0.522 | 2.91 | .002 | |
| Decisional balance (pros of condom use) | SUR vs. CTL | −0.807 | 0.507 | −1.59 | .111 |
| Time | −0.073 | 0.089 | −0.83 | .407 | |
| SUR vs. CTL × Time | 0.289 | 0.316 | 0.91 | .181 | |
| Decisional balance (cons of condom use) | SUR vs. CTL | 0.677 | 0.567 | 1.19 | .232 |
| Time | −0.272 | 0.097 | −2.80 | .005 | |
| SUR vs. CTL × Time | −0.317 | 0.347 | −0.91 | .181 | |
| Behavioral skills | SUR vs. CTL | −1.946 | 1.630 | −1.19 | .232 |
| Time | −0.505 | 0.289 | −1.75 | .080 | |
| SUR vs. CTL × Time | 3.138 | 1.030 | 3.05 | .001 |
Note. SUR = substance use reduction condition (n = 121); CTL = standard care control condition (n = 110). Positive regression coefficients indicate increase, negative coefficients indicate decrease over time. The p values for Condition × Time interaction effects reflect one-tailed values for beta coefficients that show the hypothesized change. Est. = estimate.
Exploratory Analyses
Moderator analyses using GEE revealed that the impact of the HIV intervention varied as a function of gender for two outcome variables. Specifically, women were more responsive than men to the HIV intervention with regard to the total frequency of unprotected vaginal sex; this moderator effect was observed both in the comparison to the CTL condition (B = −.477, confidence interval [CI] = −0.73, −0.231, z = −3.80, p < .001, for the women; and B = .091, CI = −0.229, 0.412, z = 0.56, p = .576, for the men) and the SUR condition (B = −.538, CI = −0.785, −0.291, z = −4.27, p < .001, for the women; and B = .050, CI = −0.258, 0.359, z = 0.32, p = .749, for the men). In contrast, although both men and women in the HIV condition improved their HIV-related knowledge, men improved their HIV knowledge more than women but only in the HIV–SUR contrast (B = 11.57, CI = 8.964, 14.175, z = 8.70, p < .001, for the men; and B = 6.816, CI = 5.004, 8.628, z = 7.37, p < .001, for the women). These gender differences were significant at p < .05.
Moderator analyses using GEE revealed that the impact of the HIV intervention also varied as a function of patients’ diagnoses for two outcome variables. Thus, in the HIV–SUR contrast, persons diagnosed with major depression (a) reduced the frequency of unprotected vaginal sex (B = −0.640, CI = −0.948, –0.333, z = −4.08, p < .001, for the patients diagnosed with major depression; and B = –0.051, CI = −0.294 to 0.193, z = −0.41, p = .684, for the other patients) and (b) reported more safer sex communications in response to the HIV intervention than did patients diagnosed with the other disorders (B = 1.101, CI = 0.567, 1.636, z = 4.04, p < .001, for the patients diagnosed with major depression; and B = –0.079, CI = −0.377, 0.218, z = −0.52, p = .601, for the other patients). These group differences were significant at p < .05.
Discussion
The current study investigated the feasibility and efficacy of an HIV-risk-reduction intervention developed specifically for a vulnerable but understudied population, namely, adults receiving outpatient treatment for a persistent mental illness. We sought to test predictions that (a) the HIV-risk-reduction intervention would reduce sexual behavior risk more than the SUR intervention, which, in turn, would reduce risk more than the CTL condition; (b) the HIV and the SUR interventions would lead to equivalent improvements for the skills and communication outcomes because both conditions used interpersonal-skills training; and (c) the HIV condition would lead to improved HIV-specific knowledge and motivation relative to both the SUR and control conditions. We also explored whether any observed intervention effects were moderated by patients’ gender and psychiatric diagnosis.
Recruitment data indicated that 41% of the eligible and available patients completed the baseline assessment and consented to the clinical trial. Although authors of prior studies with psychiatric patients typically have not provided data regarding recruitment, recent large studies with other clinical populations have reported such data. For example, the NIMH Multisite Study (1998) succeeded in recruiting 33% of eligible patients to a seven-session intervention, and Project Respect recruited 44% of eligible patients (Kamb et al., 1998) to a two- or four-session intervention. Thus, the 41% recruitment rate obtained in the current study—for a 10-session intervention, with a lower functioning sample of psychiatric outpatients—compares favorably to these studies.
Attendance patterns indicated that three quarters of the patients invited to attend risk reduction sessions did so; and two thirds of those invited completed the intervention (defined as attending at least 50% of the sessions). In a preliminary report regarding this trial, we compared patients who completed the baseline portion of this study with those who did not (Vanable, Carey, Carey, & Maisto, 2002). As reported there, we found that study completion was associated with older age, a more severe psychiatric diagnosis, and a recent STI diagnosis. We interpreted these findings as suggestive that patients who could most benefit from risk-reduction interventions were more likely to participate. Thus, findings from the current trial corroborate findings obtained in earlier trials with fewer sessions (Kalichman et al., 1995; Kelly et al., 1997; Otto-Salaj et al., 2001; Weinhardt et al., 1997). Taken together, the recruitment and retention data demonstrate the feasibility of implementing HIV-risk-reduction interventions with psychiatric outpatients. Nevertheless, efforts to enhance patient participation and optimize retention in psychiatric and prevention research represent a promising direction for future research.
Outcome analyses demonstrate that the small-group HIV intervention helped psychiatric outpatients to increase their HIV-related knowledge and interpersonal skills, develop attitudes more favorable to condom use, and express stronger intentions to avoid unsafe sexual practices. More important, patients who received the HIV intervention reported that they communicated more with their sexual partners, reduced the number of casual sexual partners, were less likely to engage in unprotected vaginal intercourse, and experienced fewer sexually transmitted infections. The pattern of findings clearly documents that a theoretically based, behavioral intervention can help psychiatric patients to reduce their risk of HIV infection.
The favorable outcomes obtained by the HIV intervention are even more encouraging because (a) baseline levels of risk behavior in this sample were not high (relative to other population groups at elevated risk for HIV), which afforded less opportunity to observe risk reduction, and (b) because many of these outcomes were obtained in comparison to a structurally equivalent substance-use-reduction intervention. The latter provided a rigorous methodological control for two reasons. First, the SUR condition controlled for the time and attention received by HIV-group members (Ostrow & Kalichman, 2000). Thus, it is unlikely that the gains observed in the HIV condition can be attributed simply to the nonspecific effects of additional contact with a therapist or facilitator. Second, the SUR condition was also expected to reduce HIV-related risk behavior, albeit indirectly. Thus, the present study corroborates findings obtained from earlier investigations with psychiatric patients that have used less rigorous comparison conditions and smaller samples (e.g., Kalichman et al., 1995; Susser, Valencia, et al., 1998; Weinhardt, Carey, et al., 1998).
Exploratory analyses revealed that gender and psychiatric diagnosis moderated some of the observed intervention effects. Women who received the HIV intervention showed greater reductions in unprotected sex relative to men, whereas men demonstrated greater improvements in HIV-related knowledge. Interestingly, these findings replicate findings from the only other HIV-risk-reduction study that has explored gender differences (Otto-Salaj et al., 2001). The other extant intervention studies with psychiatric patients have examined only men (e.g., Susser, Valencia, et al., 1998) or women (e.g., Weinhardt, Carey, et al., 1998), or they did not have a large enough sample to examine differential intervention response as a function of gender. The risk reduction demonstrated by women, who are more likely to become infected with HIV through heterosexual intercourse (Padian, Shiboski, Glass, & Vittinghoff, 1997), may reflect women’s tendency to perceive HIV as more of a health threat than do men (Schiemen, 1998). Alternatively, the interpersonal and self-management-skills components of the intervention may have been seen as more relevant to women who often need to negotiate safer sex with male partners (Amaro, 1995). Given the enhanced vulnerability to sexual coercion experienced by women receiving psychiatric care (Coverdale & Turbott, 2000; Weinhardt, Bickham, & Carey, 1999), continued development of sexual health promotion interventions is warranted.
Patients diagnosed with a major depressive disorder were more likely to benefit from the HIV intervention than were patients diagnosed with schizophrenia-spectrum or bipolar disorder. This finding may reflect greater cognitive and social impairment among patients with a more severe psychotic disorder, and it corroborates previous findings that indicate that psychiatric rehabilitation is often more successful with less impaired persons (Wykes & Dunn, 1992). One implication of this finding is that the intervention as currently designed may be most appropriate for people with major depression who also have severe functional disabilities but not for those with psychotic illnesses. Patients with more severe schizophrenia-spectrum disorders may profit more from alternative interventions or different delivery strategies (e.g., individual interventions). However, the exploratory nature of the analyses suggests the need for replication and permits only cautious interpretations. Future research should investigate whether tailoring interventions specifically to patients’ levels of functioning can enhance intervention efficacy.
The results also supported our prediction that a substance-use-reduction intervention would help to indirectly reduce sexual risk behavior. As expected, patients who received the SUR intervention were more likely than control participants to demonstrate enhanced interpersonal skills and to reduce the number of sexual partners. They were also more likely than control patients to express more favorable attitudes toward condoms and to endorse stronger intentions to use condoms. They did not, however, report fewer instances of unprotected vaginal sex. These findings resulted from a prevention-oriented intervention, but they are consistent with earlier reports that effective alcohol (Avins et al., 1997) and drug treatment (Metzger, Navaline, & Woody, 1998) may have the secondary benefit of reducing HIV-related sexual risk behavior. Taken together, these prevention and treatment findings suggest that interventions designed primarily to reduce alcohol and drug use (or misuse) may also reduce sexual risk behavior. It is premature to assume a causal relationship between substance use reduction and HIV risk reduction, but such findings are intriguing and warrant further investigation.
The results of this study need to be considered in light of its strengths and limitations. Strengths of the study included the use of a randomized controlled trial, with the largest and most diagnostically diverse sample of psychiatric outpatients studied to date. Although a strength of this study, the diagnostic diversity of our sample does make comparison to earlier studies complex; earlier intervention studies (e.g., Kalichman et al., 1995; Otto-Salaj et al., 2001; Susser, Valencia, et al., 1998; Weinhardt, Carey, et al., 1998) used smaller, more diagnostically homogeneous samples. Another strength of this study was that both the HIV and the structurally equivalent comparison condition were guided by prior theory and formative research, and were implemented by trained facilitators who followed a detailed manual. Both interventions were compared with a standard care control condition using validated and established measures, and data analyses tested a priori hypotheses using procedures appropriate for nonnormally distributed count data (Schroder, Carey, & Vanable, 2003).
Several limitations need to be acknowledged. First, most of the primary outcomes were measured by self-report, an assessment modality that can be vulnerable to bias. However, we took several steps to reduce potential biases and to optimize data quality. To minimize the cognitive burden on participants, the timeline followback method uses calendars, landmark events, and other cues (M. P. Carey, Carey, Maisto, Gordon, & Weinhardt, 2001). To minimize demand characteristics, all assessments were completed by assessors not associated with the intervention and masked to the study’s hypotheses (Weinhardt, Forsyth, Carey, Jaworski, & Durant, 1998). To optimize patient candor, we explained the public health importance of the research project and highlighted the opportunity that patients had to contribute to our understanding of HIV-risk reduction; we also explained that we obtained a Federal Certificate of Confidentiality, which protected our data from subpoena; and we assured patients that their research records were separate from clinical records and that the information that they shared with our research team could not be shared without their written approval.
The limitations of self-report are perhaps most apparent with respect to the self-report of STIs. Although self-report of STIs is necessary (because biological testing will not detect bacterial infections that have already been treated), it is not sufficient because some STIs (e.g., Chlamydia trachomatis) are frequently asymptomatic; therefore, future research will be strengthened by the collection of biological samples to supplement self-report (Fishbein & Pequegnat, 2000). Although such testing will be challenging because it requires coordination with the public health system for reporting infections and partner notification, the scientific, clinical, and public health benefits of supplemental testing warrant such efforts.
A second limitation of the current study was our use of a relatively brief follow-up interval. When designing this study, we chose to follow patients for 6 months to provide a sufficient opportunity to observe behavior change but also a brief enough interval to retain research participants. In this intent-to-treat trial, however, we were able to retain nearly 88% of patients enrolled in the intervention at the 6-month follow-up. Thus, future studies can extend the follow-up interval to investigate the stability of intervention gains over a longer interval. Previous research with a small sample of men that used longer follow-ups has been encouraging regarding the maintenance of risk reduction (Susser, Valenica, et al., 1998), but this warrants future study.
Third, recruitment and retention of voluntary research participants always raises the possibility of a self-selection or participation bias, especially when the study protocol requires attendance at multiple assessment and intervention sessions over long periods. In this trial, 41% of the patients who were eligible consented to participate, a recruitment rate that compares favorably to other large-scale intervention trials (e.g., Kamb et al., 1998; NIMH Multisite HIV Prevention Trial Group, 1998). Previous analyses from this trial identified few clinically significant differences between patients who consented to participate and those who declined (Vanable et al., 2002). Additional analyses reported herein identified few differences between patients who attended intervention and follow-up sessions and those who were lost to follow-up. In sum, no obvious self-selection biases emerged in this study; nevertheless, caution is always advised when generalizing results from a single sample to the larger population from which it is drawn.
Overall, the pattern of findings provides evidence that a theoretically based HIV preventive intervention that provides information, motivational enhancement, and behavioral skills training can help psychiatric outpatients to reduce their risk for HIV. The findings also suggest that a substance use reduction intervention provides some benefits for HIV-risk reduction. Given that psychiatric patients are disproportionately vulnerable to HIV, continued refinement, implementation, and dissemination of HIV-risk-reduction interventions is strongly encouraged.
Appendix A
Overview of HIV Risk-Reduction Intervention
Enhancing knowledge about HIV transmission and prevention (Sessions 1–3)
Introduction: Intervention goals and ground-rules for participation.
Concerns and questions about HIV: a group discussion to enhance interest and motivation to participate in the intervention.
HIV transmission basics: In-depth overview of HIV transmission modes, common myths about HIV transmission, methods for self-protection against HIV, and facts about HIV testing.
“Risk continuum” exercise: Awareness enhancement of how different sexual activities vary in their degree of risk for HIV and highlight the fact that there are many healthy, pleasurable activities to choose from.
Assessing partner risks: exercise to enhance awareness that HIV serostatus cannot be determined based on physical attributes. Participants view pictures of men and women of varying backgrounds and discuss the likelihood that each person is HIV-positive, followed by a discussion of common misperceptions regarding perceptions of “safe” vs. “risky” partners.
“Crowded bedroom” exercise: Reinforcement of the importance of considering the sexual history of sexual partners when assessing the risks for contracting HIV.
Enhancing motivation for HIV behavior change (Sessions 3–4)
HIV-risk sensitization: After reviewing high and lower risk sexual activities using a stop-light exercise (i.e., behaviors that are in the red, yellow, or green zone), group members discuss which if any of their own behaviors fall in the “risky” zone.
Healthy sexual choices exercise: Members generate a list of steps that could be taken to reduce their risks of contracting HIV.
Decisional balance exercise: Members discuss the pros and cons of different risk reduction strategies including condom use and HIV testing.
Realistic change options: Group members identify and discuss behavior changes strategies that they plan to adopt for protection against HIV.
Strengthening HIV behavioral skills and self-management training (Sessions 5–10)
Condom acquisition discussion: Discussion of barriers and strategies for obtaining condoms and increasing the availability of condoms in contexts where sex may occur.
Condom application skills: Group members are instructed in proper application of male and female condoms; group members practice applying and removing a condom using models. Session also includes an overview of condom use “do’s and don’ts,” including the importance of water- rather than oil-based lubricants, proper condom storage, and the importance of using latex condoms.
Risk “triggers” exercise: Group members identify personally relevant situations, mood states, and thought processes that may lead to unsafe sex; participants discuss strategies for coping with risk triggers.
Reaffirming specific behavior change goals to reduce HIV risk: Personalized discussion of risk reduction goals, and specific steps required to reach and maintain those goals.
Assertive communication overview: Overview of critical components of an assertive statement.
Assertiveness Training I: Facilitators provide demonstration of assertive interactions using role-plays. Group members are then paired with a facilitator to practice a simple (nonsexual) role-play in which they are asked to practice making an assertive statement. Throughout role-play exercises, corrective feedback and reinforcement are provided by facilitators and group members.
Assertiveness Training II: Role-play exercises continue, with participants rehearsing assertiveness skills in hypothetical sexual situations in which they are asked to discuss the need for safer sex.
Assertiveness Training III: Role-play exercises conclude with challenging scenarios in which a hypothetical sexual partner initially refuses a request for condom use or safer sex activity.
Final intervention review and reaffirmation of goals: Core content areas of Sessions 1–9 are reviewed, and each group member presents their long-term behavior change goals.
Appendix B
Overview of Substance Use Reduction (SUR) Intervention
Enhancing knowledge, motivation, and skills to reduce caffeine consumption (Sessions 1–3)
Introduction: SUR intervention and ground-rules for participation.
Discussion of concerns about caffeine use: Discussion designed to enhance motivation to reduce caffeine use.
Facts about caffeine: In-depth discussion of the positive and negative consequences of caffeine use.
Self-monitoring of caffeine use: Participants monitor the number of caffeinated beverages consumed per day.
Risk sensitization: A stoplight exercise in which participants contrast their caffeine use to national averages and label their use as falling in the green, yellow, or red zone depending on their level of use and risk for negative consequences.
Strategies for change: Participants generate and discuss a menu of caffeine use reduction strategies.
Behavioral skills to reduce caffeine use: Participants engage in role-play exercises involving rehearsal of refusal and assertiveness skills required to avoid or reduce caffeine consumption.
Behavior change goals: Group members generate concrete goals and strategies for reducing caffeine use.
Enhancing knowledge, motivation, and skills to reduce smoking (Sessions 4–5)
Nicotine sources and consequences of use: Brief discussion of the major sources of nicotine, as well as the major short- and long-term consequences of regular nicotine use.
Motives for nicotine use: Participants generate a list of the major reasons why people smoke (now or in the past). For homework, group members are asked to observe their own smoking behavior and identify the most important motives for smoking using a checklist. Nonsmokers are asked to complete the homework on the basis of what they know of the experience of other smokers.
Risk-sensitization exercise: Exercise begins with a discussion of the pros and cons of smoking. Facilitators then present data on smoking in the United States to illustrate that many people have quit in recent years.
Pros and cons of nicotine reduction: Discussion of benefits and costs of quitting and/or reducing smoking.
Self-management skills for quitting: Facilitators introduce the concept of risk “triggers.” Group members generate a list of self-relevant triggers for smoking. Facilitators then lead a discussion of skills and strategies for coping with smoking triggers, including the use of avoidance and substitution strategies.
Setting the stage for change: Session concludes with a review of the major benefits of quitting or reducing smoking. Group members identify change strategies and set goals for reducing or eliminating smoking.
Enhancing knowledge, motivation, and skills to reduce alcohol use (Sessions 6–10)
Review of basic information on alcohol: Discussion of the short- and long-term effects of alcohol consumption, emphasizing both positive and negative consequences.
Risk sensitization: After self-monitoring their alcohol use, group members discuss how their current alcohol consumption compares with national averages. A traffic-light exercise is then used to illustrate that drinking-related harm occurs along a continuum from low to higher risk drinking, and group members assess whether their current drinking falls in the green, yellow, or red zone.
Pros and cons of alcohol reduction: A decisional-balance exercise is carried out involving consideration of costs and benefits of drinking as it relates to relationships, psychiatric functioning, and other relevant dimensions.
Strategies for change: Discussion of strategies for reducing or eliminating alcohol use and emphasizing avoidance of high-risk settings and behavioral strategies for reducing the number of drinks consumed.
Assertiveness training to reduce drinking: Facilitators provide an overview of critical components of an assertive statement and provide demonstration of drink-refusal skills. Group members then rehearse drink-refusal skills through a series of role-play exercises that become progressively more challenging.
Self-management skills to reduce alcohol-related harm: Facilitators reintroduce the concept of risk triggers, and group members generate a list of personally relevant triggers for alcohol use. Members then discuss strategies for reducing alcohol use, emphasizing avoidance of high-risk drinking situations.
Goal setting for alcohol use: Group members generate individualized goals for reducing their alcohol use, and potential barriers are discussed as a group.
Final intervention review and reaffirmation of goals: Core content areas for SUR are reviewed, and each group member presents their long-term behavior change goals related to caffeine, nicotine, and alcohol.
Footnotes
This work was supported by Grants R01-MH54929 and K02-MH01582 from the National Institute of Mental Health. We gratefully acknowledge the patients for their participation and the therapists and administrators at the Richard H. Hutchings Psychiatric Center (Syracuse, NY) and St. Joseph’s Hospital (Syracuse, NY) for their enthusiasm and support. We also express our gratitude to the Health Improvement Project team, including Kristin Barnes, Jennifer Becker, Susan Bland, Brian Borsari, Laura Braaten, Kristen Brewer, Susan Collins, Christopher Correia, Tracy Cromp, Lauren Durant, Julie Fuller, Jack Gleason, Julie Ann Hartley, Beth Jaworski, Jaejin Kim, Vardit Konsens, Jeanette Mattson, Dianne Morrison-Beedy, Dan Neal, Teal Pedlow, MaryBeth Pray, Paul Preczewski, Daniel Purnine, Jeffrey Simons, Lance Weinhardt, Adrienne Williams, Emily Wright, and Denise Zona, for its excellent contributions to this work.
GEE provides a useful tool for group comparisons with nonnormal outcomes and multiple postintervention assessments (Schroder et al., 2003). GEE assumes that a known transformation of the marginal distribution of the outcome (e.g., log10 [X = 1]) is a linear function of the covariates or predictors. In contrast to the common fixed-effects models (e.g., analysis of variance; ANOVA), GEE estimates “population-averaged” models, using an extension of the quasi-likelihood approach. Quasi-likelihood makes few assumptions about the distribution of the dependent variable and, for that reason, is applicable to a wide variety of nonnormally distributed outcome variables (Zeger & Liang, 1986). The only requirement involves the specification of the mean-covariance structure. GEE uses an iterative procedure for the development of an estimator whose error has a mean of zero and is asymptotically multivariate Gauss-ian. However, this requires that missing observations be missing at random (Liang & Zeger, 1986).
In GEE, the data are modeled by specifying the appropriate distribution family for the dependent variable (e.g., Poisson, negative binomial). If the data are not Gaussian, this approach is likely to yield considerably more test power compared to repeated measures ANOVA with normalized variables. However, it is important to ensure that the specified distribution family provides a good fit for the dependent variable. A Poisson model is quite restrictive in its assumptions and may not be appropriate for most count measures. Falsely specifying a Poisson distribution may produce misleading results and indicate significant effects that may not apply to the true distribution of the outcome. In general, the significance achieved with the specification of a particular distribution family or correlation structure is not a valid indicator of the appropriateness of the model. The applicability of a distribution needs to be tested using diagnostics that are available in some standard statistical software (e.g., STATA).
Even when working with normally distributed outcome variables, GEE might be preferred over classical ANOVA models because GEE treats individual change as a random variable. The advantages of a mixed design with a random individualized-change variable and a fixed treatment-group-effect factor may be seen in a potentially increased test power. Further, GEE analyses are flexible in that they allow specifying the within-group correlation structure for the panels.
Because anal sex was not frequently reported, inferential statistics would not be appropriate, and the results would not have been sufficiently powered to permit a strong test. Therefore, to test whether the inclusion of anal sex would have affected study outcomes, we calculated a separate composite index, the vaginal episode equivalent (VEE; Susser, Desvarieux, & Wittkowski, 1998). Parallel analyses using the VEE as an outcome yielded an equivalent pattern of results across all analyses.
References
- Amaro H. Love, sex, and power. American Psychologist. 1995;50:437–447. doi: 10.1037//0003-066x.50.6.437. [DOI] [PubMed] [Google Scholar]
- Avins AL, Lindan CP, Woods WJ, Hudes ES, Boscarino JA, Kay J, et al. Changes in HIV-related behaviors among heterosexual alcoholics following addiction treatment. Drug and Alcohol Dependence. 1997;44:47–55. doi: 10.1016/s0376-8716(96)01321-x. [DOI] [PubMed] [Google Scholar]
- Bellack, A. S., Mueser, K. T., Gingerich, S., & Agresta, J. (1997). Social skills training for schizophrenia: A step-by-step guide. New York: Guilford Press.
- Blanchard JJ, Brown SA, Horan WP, Sherwood AR. Substance use disorders in schizophrenia: review, integration, and a proposed model. Clinical Psychology Review. 2000;20:207–234. doi: 10.1016/s0272-7358(99)00033-1. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophrenia Bulletin. 1998;24:413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- Carey KB, Carey MP, Maisto SA, Purnine DM. The feasibility of enhancing psychiatric outpatients’ readiness to change their substance use. Psychiatric Services. 2002;53:602–608. doi: 10.1176/appi.ps.53.5.602. [DOI] [PubMed] [Google Scholar]
- Carey KB, Maisto SA, Carey MP, Gordon CM, Correia CJ. Use of legal drugs by psychiatric outpatients: Benefits, costs, and change. Cognitive and Behavioral Practice. 1999;6:15–22. doi: 10.1016/S1077-7229(99)80037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Maisto SA, Carey MP, Purnine DM. Measuring readiness-to-change substance misuse among psychiatric outpatients: I. Reliability and validity of self-report measures. Journal of Studies on Alcohol. 2001;62:79–88. doi: 10.15288/jsa.2001.62.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Purnine DM, Maisto SA, Carey MP. Enhancing readiness-to-change substance abuse in persons with schizophrenia. A four-session motivation-based intervention. Behavior Modification. 2001;25:331–384. doi: 10.1177/0145445501253001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Purnine DM, Maisto SA, Carey MP. Correlates of stages of change for substance abuse among psychiatric outpatients. Psychology of Addictive Behaviors. 2002;16:283–289. [PubMed] [Google Scholar]
- Carey KB, Purnine DM, Maisto SA, Carey MP, Barnes KL. Decisional balance regarding substance use among persons with schizophrenia. Community Mental Health Journal. 1999;35:289–299. doi: 10.1023/a:1018705722246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Purnine DM, Maisto SA, Carey MP, Simons JS. Treating substance abuse in the context of severe and persistent mental illness: Clinicians’ perspectives. Journal of Substance Abuse Treatment. 2000;19:189–198. doi: 10.1016/s0740-5472(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Carey MP, Carey KB, Kalichman SC. Risk for human immunodeficiency virus (HIV) infection among adults with a severe mental disorder. Clinical Psychology Review. 1997;17:271–291. doi: 10.1016/s0272-7358(97)00019-6. [DOI] [PubMed] [Google Scholar]
- Carey MP, Carey KB, Maisto SA, Gleason JR, Gordon CM, Brewer KK. HIV-risk behavior among outpatients at a state psychiatric hospital: Prevalence and risk modeling. Behavior Therapy. 1999;30:389–406. doi: 10.1016/S0005-7894(99)80017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Carey KB, Maisto SA, Gordon CM, Vanable PA. Prevalence and correlates of sexual activity and HIV-related risk behavior among psychiatric outpatients. Journal of Consulting and Clinical Psychology. 2001;69:500–505. doi: 10.1037//0022-006x.69.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Carey KB, Maisto SA, Gordon CM, Weinhardt LS. Assessing sexual risk behaviour with the timeline follow-back (TLFB) approach: Continued development and psychometric evaluation with psychiatric outpatients. International Journal of STD and AIDS. 2001;12:365–375. doi: 10.1258/0956462011923309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Carey KB, Weinhardt LS, Gordon CM. Behavioral risk for HIV infection among adults with a severe and persistent mental illness: Patterns and psychological antecedents. Community Mental Health Journal. 1997;33:133–142. doi: 10.1023/a:1022423417304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Chandra PS, Carey KB, Neal D. Predictors of HIV risk behavior among men seeking treatment for substance abuse in India. Archives of Sexual Behavior. 2003;32:339–349. doi: 10.1023/a:1024043032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Maisto SA, Kalichman SC, Forsyth AD, Wright EM, Johnson BT. Enhancing motivation to reduce the risk of HIV infection for economically disadvantaged urban women. Journal of Consulting and Clinical Psychology. 1997;65:531–541. doi: 10.1037//0022-006x.65.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Morrison-Beedy D, Johnson BT. The HIV-Knowledge Questionnaire: Development and evaluation of a reliable, valid, and practical self-administered questionnaire. AIDS and Behavior. 1997;1:61–74. [Google Scholar]
- Carey MP, Weinhardt LS, Carey KB. Prevalence of infection with HIV among the seriously mentally ill: Review of research and implications for practice. Professional Psychology: Research and Practice. 1995;26:262–268. [Google Scholar]
- Chandra PS, Carey MP, Carey KB, Rao P, Jairam KR, Thomas T. HIV-related risk behavior among psychiatric inpatients: Results from a hospital-wide screening study in southern India. International Journal of AIDS and STDs. 2003;14:532–538. doi: 10.1258/095646203767869147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, T. D., & Campbell, D. T. (1979). Quasi-experimentation: Design and analysis issues for field settings. Chicago: Rand McNally.
- Cournos F, McKinnon K. HIV seroprevalence among people with severe mental illness in the United States: A critical review. Clinical Psychology Review. 1997;17:259–269. doi: 10.1016/s0272-7358(97)00018-4. [DOI] [PubMed] [Google Scholar]
- Coverdale JH, Turbott SH. Sexual and physical abuse of chronically ill psychiatric outpatients compared with a matched sample of medical outpatients. Journal of Nervous and Mental Disease. 2000;188:440–445. doi: 10.1097/00005053-200007000-00008. [DOI] [PubMed] [Google Scholar]
- Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, et al. Rapid estimate of adult literacy in medicine: A shortened screening instrument. Family Medicine. 1993;25:391–395. [PubMed] [Google Scholar]
- Drake RE, Essock SM, Shaner A, Carey KB, Minkoff K, Kola L, et al. Implementing dual diagnosis services for clients with severe mental illness. Psychiatric Services. 2001;52:469–476. doi: 10.1176/appi.ps.52.4.469. [DOI] [PubMed] [Google Scholar]
- Drake RE, Mercer-McFadden C, Mueser KT, McHugo GJ, Bond GR. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophrenia Bulletin. 1998;24:589–608. doi: 10.1093/oxfordjournals.schbul.a033351. [DOI] [PubMed] [Google Scholar]
- Drake RE, Wallach MA, Teague GB, Freeman DH, Paskus TS, Clark TA. Housing instability and homelessness among rural schizophrenic patients. American Journal of Psychiatry. 1991;148:330–336. doi: 10.1176/ajp.148.3.330. [DOI] [PubMed] [Google Scholar]
- First, M. G., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (1995). Structured Clinical Interview for the DSM–IV—Patient Version (SCID-I/P, Version 2.0) New York: New York State Psychiatric Institute.
- Fishbein M, Pequegnat W. Evaluating AIDS prevention interventions using behavioral and biological outcome measures. Sexually Transmitted Diseases. 2000;27:101–110. doi: 10.1097/00007435-200002000-00008. [DOI] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychological Bulletin. 1992;111:455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- Fisher, J. D., & Fisher, W. A. (2000). Theoretical approaches to individual-level change in HIV risk behavior. In J. L. Peterson & R. J. DiClemente (Eds.), Handbook of HIV prevention (pp. 3–55). New York: Kluwer Academic/Plenum.
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician”. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galavotti C, Cabral RJ, Lansky A, Grimley DM, Riley GE, Prochaska JO. Validation of measures of condom and other contraceptive use among women at high risk for HIV infection and unintended pregnancy. Health Psychology. 1995;14:570–578. doi: 10.1037//0278-6133.14.6.570. [DOI] [PubMed] [Google Scholar]
- Goldman, M. S., Del Boca, F. K., & Darkes, J. (1999). Alcohol expectancy theory: The application of cognitive neuroscience. In K. E. Leonard & B. T. Blane (Eds.), Psychological theories of drinking and alcoholism (2nd ed., pp. 203–246). New York: Guilford Press.
- Gordon CM, Carey MP. Alcohol’s effects on requisites for sexual risk-reduction in men: An initial experimental investigation. Health Psychology. 1996;15:56–60. doi: 10.1037//0278-6133.15.1.56. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Carey MP, Carey KB. Effects of a drinking event on behavioral skills and condom attitudes in men: Implications for HIV risk from a controlled experiment. Health Psychology. 1997;16:490–495. doi: 10.1037//0278-6133.16.5.490. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Carey MP, Carey KB, Maisto SA, Weinhardt LS. Understanding HIV-related risk among persons with a severe and persistent mental illness: Insights from qualitative inquiry. Journal of Nervous and Mental Disease. 1999;187:208–216. doi: 10.1097/00005053-199904000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard J, McDonald R, Wan CK, Dittus PJ, Quinlan S. The accuracy of self reports of condom use and sexual behavior. Journal of Applied Social Psychology. 2002;32:1863–1905. [Google Scholar]
- Kalichman SC, Kelly JA, Johnson JR, Bulto M. Factors associated with risk for HIV infection among chronic mentally ill adults. American Journal of Psychiatry. 1994;151:221–227. doi: 10.1176/ajp.151.2.221. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Sikkema KJ, Kelly JA, Bulto M. Use of a brief behavioral skills intervention to prevent HIV infection among chronic mentally ill adults. Psychiatric Services. 1995;46:275–280. doi: 10.1176/ps.46.3.275. [DOI] [PubMed] [Google Scholar]
- Kamb ML, Fishbein M, Douglas JM, Jr, Rhodes F, Rogers J, Bolan G, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: A randomized controlled trial. Project RESPECT Study Group. JAMA. 1998;280:1161–1167. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- Kelly, J. A. (1982). Social skills training: A practical guide for interventions New York: Springer.
- Kelly, J. A. (1995). Changing HIV risk behavior: Practical strategies. New York: Guilford Press.
- Kelly JA. HIV risk reduction interventions for persons with severe mental illness. Clinical Psychology Review. 1997;17:293–310. doi: 10.1016/s0272-7358(97)00020-2. [DOI] [PubMed] [Google Scholar]
- Kelly JA, McAuliffe TL, Sikkema KJ, Murphy DA, Somlai AM, Mulry G, et al. Reduction in risk behavior among adults with severe mental illness who learned to advocate for HIV prevention. Psychiatric Services. 1997;48:1283–1288. doi: 10.1176/ps.48.10.1283. [DOI] [PubMed] [Google Scholar]
- Kelly JA, Murphy DA, Sikkema KJ, Mulry GW, Fernandez MI, Miller JG, et al. Predictors of high and low levels of HIV risk behavior among adults with chronic mental illness. Psychiatric Services. 1995;46:813–818. doi: 10.1176/ps.46.8.813. [DOI] [PubMed] [Google Scholar]
- Kleinman KP, Ibrahim JG, Laird NM. A Bayesian framework for intent-to-treat analysis with missing data. Biometrics. 1998;54:265–278. [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Liberman, R. P., DeRisi, W. J., & Mueser, K. T. (1989). Social skills training for psychiatric patients. Boston: Allyn & Bacon.
- Maisto, S. A., Carey, K. B., & Bradizza, C. M. (1999). Social learning theory. In H. Blane & K. Leonard (Eds.), Psychological theories of drinking and alcoholism (2nd ed., pp. 106–163). New York: Guilford Press.
- Maisto, S. A., Carey, M. P., Carey, K. B., Gordon, C. M., Schum, J. L., & Lynch, K. G. (in press). The relationship between alcohol and participant characteristics to attitudes and behavioral skills relevant to safer sex among heterosexual young adult men. Archives of Sexual Behavior. [DOI] [PMC free article] [PubMed]
- Marlatt GA. Harm reduction: Come as you are. Addictive Behaviors. 1996;21:779–788. doi: 10.1016/0306-4603(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Navaline H, Woody GE. Drug abuse treatment as AIDS prevention. Public Health Reports. 1998;113(Suppl 1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Miller, W. R., & Rollnick, S. (Eds.). (1991). Motivational interviewing: Preparing people to change addictive behaviors New York: Guilford Press.
- Monti, P. M., Abrams, D. A., Kadden, R. M., & Cooney, N. L. (1989). Treating alcohol dependence: A coping skills training guide New York: Guilford Press.
- Mueser KT, Doonan R, Penn DL, Blanchard JJ, Bellack AS, Nishith P, et al. Emotion recognition and social competence in chronic schizophrenia. Journal of Abnormal Psychology. 1996;105:271–275. doi: 10.1037//0021-843x.105.2.271. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group. The NIMH Multisite HIV Prevention Trial: Reducing HIV sexual risk behavior. Science. 1998 June 19;280:1889–1894. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- Ostrow, D. G., & Kalichman, S. C. (2000). Methodological issues in HIV behavioral interventions. In J. L. Peterson & R. J. DiClemente (Eds.), Handbook of HIV prevention (pp. 67–80). New York: Kluwer Academic/ Plenum.
- Otto-Salaj LL, Kelly JA, Stevenson LY, Hoffmann R, Kalichman SC. Outcomes of a randomized small-group HIV prevention intervention trial for people with serious mental illness. Community Mental Health Journal. 2001;37:123–144. doi: 10.1023/a:1002709715201. [DOI] [PubMed] [Google Scholar]
- Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in Northern California: Results from a ten-year study. American Journal of Epidemiology. 1997;146:350–357. doi: 10.1093/oxfordjournals.aje.a009276. [DOI] [PubMed] [Google Scholar]
- Ramrakha S, Caspi A, Dickson N, Moffitt TE, Paul C. Psychiatric disorders and risky sexual behaviour in young adulthood: Cross-sectional study in birth cohort. British Medical Journal. 2000;321:263–266. doi: 10.1136/bmj.321.7256.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Rosenberg SD, Goodman LA, Osher FC, Swartz MS, Essock SM, Butterfield MI, et al. Prevalence of HIV, Hepatitis B, and Hepatitis C in people with severe mental illness. American Journal of Public Health. 2001;91:31–37. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco WP, Levine B, Reed DL, Thompson K. Attitudes about condom use as an AIDS-relevant behavior: Their factor structure and relation to condom use. Psychological Assessment. 1991;3:265–272. [Google Scholar]
- Schiemen S. Gender and AIDS-related psychological processes: A study of perceived susceptibility, social distance, and homophobia. AIDS Education and Prevention. 1998;10:264–277. [PubMed] [Google Scholar]
- Schroder KEE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: I. Item content, scaling, and data analytical options. Annals of Behavioral Medicine. 2003;26:76–103. doi: 10.1207/s15324796abm2602_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. (2000). SPSS base 10.5 for Windows user’s guide. Chicago: Author.
- StataCorp. (2003). Stata statistical software: Release 8.0 [software]. College Station, TX: Author.
- Susser E, Desvarieux M, Wittkowski KM. Reporting sexual risk behavior for HIV: A practical risk index and a method for improving risk indices. American Journal of Public Health. 1998;88:671–674. doi: 10.2105/ajph.88.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Valencia E, Berkman A, Sohler N, Conover S, Torres J, et al. Human immunodeficiency virus sexual risk reduction in homeless men with mental illness. Archives of General Psychiatry. 1998;55:266–272. doi: 10.1001/archpsyc.55.3.266. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Carey MP, Carey KB, Maisto SA. Predictors of participation and attrition in a health promotion study involving psychiatric outpatients. Journal of Consulting and Clinical Psychology. 2002;70:362–368. doi: 10.1037//0022-006x.70.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voluntary HIV-1 Counselling and Testing Efficacy Study Group. Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: A randomised trial. Lancet. 2000;356:103–112. [PubMed] [Google Scholar]
- Weinhardt LS, Bickham NL, Carey MP. Sexual coercion among women living with a severe and persistent mental illness: Review of the literature and recommendations for mental health providers. Aggression and Violent Behavior. 1999;4:307–317. [Google Scholar]
- Weinhardt LS, Carey MP, Carey KB. HIV risk reduction for the seriously mentally ill: Pilot investigation and call for research. Journal of Behavior Therapy and Experimental Psychiatry. 1997;28:87–96. doi: 10.1016/s0005-7916(97)00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhardt LS, Carey MP, Carey KB, Verdecias RN. Increasing assertiveness skills to reduce HIV risk among women living with a severe and persistent mental illness. Journal of Consulting and Clinical Psychology. 1998;66:680–684. doi: 10.1037//0022-006x.66.4.680. [DOI] [PubMed] [Google Scholar]
- Weinhardt LS, Forsyth AD, Carey MP, Jaworski BC, Durant LE. Reliability and validity of self-report measures of HIV-related sexual behavior: Progress since 1990 and recommendations for research and practice. Archives of Sexual Behavior. 1998;27:155–180. doi: 10.1023/a:1018682530519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel SL, Koegel P, Gelberg L. Antecedents of physical and sexual victimization among homeless women: A comparison to homeless men. American Journal of Community Psychology. 2000;28:367–390. doi: 10.1023/A:1005157405618. [DOI] [PubMed] [Google Scholar]
- Wykes T, Dunn G. Cognitive deficit and the prediction of rehabilitation success in a chronic psychiatric group. Psychological Medicine. 1992;22:389–398. doi: 10.1017/s0033291700030336. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]


