Abstract
The phasing of neuronal activity in a rhythmic motor network is determined by a neuron’s intrinsic firing properties and synaptic inputs; these could vary in their relative importance under different modulatory conditions. In the lobster pyloric network, the firing of eight follower PY neurons is shaped by their intrinsic rebound after pacemaker inhibition and by synaptic input from the LP neuron, which inhibits all PY neurons and is electrically coupled to a subset of them. Under control conditions, LP inhibition is weak and has little influence on PY firing. We examined modulation that could theoretically enhance the LP’s synaptic contribution to PY firing. We measured the effects of dopamine (DA) on LP→PY synapses, driving the LP neuron with trains of realistic waveforms constructed from prerecorded control and DA LP oscillations, which differed in shape and duration. Under control conditions, chemical inhibition underwent severe depression and disappeared; in the mixed synapses, electrical coupling dominated. Switching between control and DA LP waveforms (with or without DA present) caused only subtle changes in synaptic transmission. DA markedly enhanced synaptic inhibition, reduced synaptic depression and weakened electrical coupling, reversing the sign of the mixed synapses. Despite this, removal of the LP from the intact network still had only weak effects on PY firing. DA also enhances PY intrinsic rebound properties, which still control the onset of PY firing. Thus, in a rhythmic network, the functional importance of synaptic modulation can only be understood in the context of parallel modulation of intrinsic properties.
Keywords: lobster, pyloric network, central pattern generator, mixed synapse
Introduction
The phasing of rhythmic activity of neurons and muscles shapes rhythmic motor behaviors, for example, distinguishing walking from skipping or galloping. This timing of neuronal activity during a rhythmic motor cycle is generally considered to be determined by the interactions of neurons in the central pattern generator (CPG) network that organizes the behavior (Getting 1989; Harris-Warrick et al. 1992; Orlovsky et al. 1999), as well as the pattern of sensory feedback (Pearson 2004; Pearson and Ramirez 1997). In many CPGs, the timing of onset and offset of neuronal firing in the networks is determined by a dynamic interaction between the intrinsic firing properties of the neurons (such as post-inhibitory rebound, bistability and bursting) and the pattern of synaptic interactions within the network (Cymbalyuk et al. 2002; Getting 1989; Harris-Warrick et al. 1992; Hooper 1997; Katz 1999; Ramirez et al. 2004; Stein et al. 1997). The phasing of neurons in the cycle is not fixed, but can vary, generating different motor patterns, due to sensory input or the actions of neuromodulators such as amines and peptides. Plasticity in phasing can arise from the recruitment of new neurons that provide new synaptic inhibition or excitation (Jing et al. 2003; Jing and Weiss 2001; Selverston et al. 1997), modulation of existing synapses (Johnson et al. 1995; Marder and Thirumalai 2002; Sillar et al. 1998), and modulation of the intrinsic properties of the CPG neurons (Harris-Warrick et al. 1995a,b, 1998; Kloppenburg et al. 1999; Marder and Thirumalai 2002; Matsushima et al. 1993). While the involvement of these parameters is widely accepted, their relative contributions in determining changes in phasing are not well understood in any system.
In this paper, we look at a specific example of modulation of phasing, how dopamine (DA) modifies the phasing of the 8 Pyloric (PY) neurons in the pyloric network of the crustacean stomatogastric ganglion (STG). The pyloric network contains 14 neurons, and generates a triphasic rhythmic motor pattern driven by a 3-neuron pacemaker kernel (Ayali and Harris-Warrick 1999; Johnson and Hooper 1992). Under control conditions, the pacemaker Anterior Burster (AB)/Pyloric Dilator (PD) neuron group fires first and inhibits all other neurons; then the Lateral Pyloric (LP) neuron fires, followed by the PY neurons. The firing phase of the PY neurons is thought to be established by the combined actions of intrinsic post-inhibitory rebound (PIR) after pacemaker inhibition and by synaptic input from the LP neuron (Hartline and Gassie 1979; Selverston et al. 1998). The LP chemically inhibits the PY neurons via graded transmission, and is additionally electrically coupled to a subset of them (Fig. 1A; Johnson et al. 1994). Under control conditions, the chemical inhibition is weak and undergoes significant synaptic depression during normal LP oscillations, leaving the electrotonic coupling between LP and PY as the dominant synaptic interaction (Mamiya et al. 2003). Removal of the LP neuron has only slight effects on the phasing of PY firing in the intact network (Mamiya et al. 2003; Weaver and Hooper 2003a).
Figure 1.
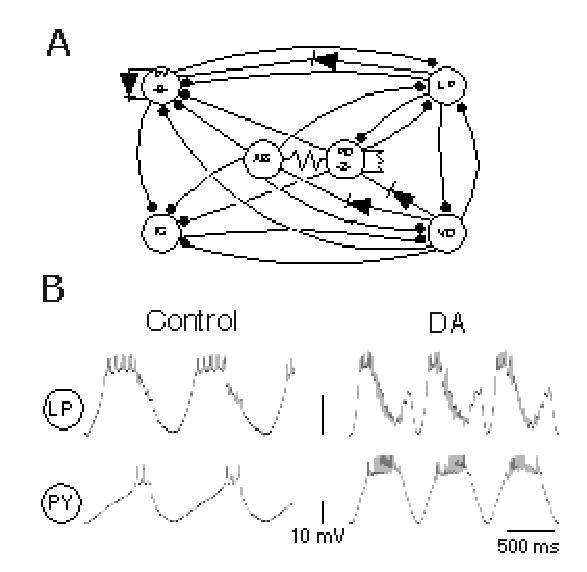
Schematic diagram of the pyloric network of the spiny lobster Panulirus interruptus (A) and typical LP and PY neuron activity under control and dopamine (DA, 10−4M) conditions (B). In the pyloric network, the synaptic connections are either electrical (nonrectifying: resistor symbols; and rectifying: diode symbols) or chemical inhibitory (filled circles). The LP→PY synapses examined in this study are marked in bold lines.
Dopamine causes a significant PY phase advance in the pyloric rhythm. DA enhances PY post-inhibitory rebound after pacemaker inhibition primarily by reducing IA (Harris-Warrick et al. 1995a). At the same time, it enhances LP→PY chemical inhibition and weakens LP→PY electrical coupling, reversing the sign of the LP→PY interaction from depolarizing to hyperpolarizing (Johnson et al. 1993b, 1994). In theory, these changes should cause the LP→PY synapse to oppose and constrain the advance in PY onset phase caused by PY PIR. However, two factors might lessen its impact. First, the LP neuron appears to oscillate with a significantly narrower waveform in DA compared to control (Flamm and Harris-Warrick 1986a), potentially reducing synaptic transmission. Second, the degree of synaptic depression of the DA-modified LP→PY synapse is unknown, as previous studies were done with single square pulse depolarizations of the LP neuron (Johnson et al. 1994). Here, we address these two factors and examine the relative role of the LP→PY synapse in determining the onset phase of PY firing in the DA-modified pyloric rhythm. Using pre-recorded realistic waveforms to drive synaptic transmission, we show that the LP waveform shape plays a surprisingly small role in determining the strength of the LP→PY synapse. Even though the synapse undergoes synaptic depression, it still becomes strongly inhibitory in the presence of DA. Despite this sign reversal, the LP→PY synapse continues to play only a minor role in setting PY onset phase, which is dominated by DA’s enhancement of the PY neurons’ intrinsic PIR properties.
Materials and Methods
California spiny lobsters (Panulirus interruptus) were supplied by Don Tomlinson Commercial Fishing (San Diego, CA) and maintained in marine aquaria at 16° C. Lobsters were cooled in ice until immobile. The stomatogastric nervous system (STNS) was removed as previously described (Selverston et al. 1976), and pinned in a Sylgard–coated petri dish in chilled Panulirus saline of the following composition (mM): 479 NaCl, 12.8 KCl, 13.7 CaCl2, 3.9 Na2SO4, 10.0 MgSO4, 2 glucose, 11.1 Tris base, pH 7.35 (Mulloney and Selverston 1974). The STG was desheathed, enclosed in a 1ml pool walled with Vaseline and superfused at 5 ml/min with oxygenated Panulirus saline (18–19°C). In all experiments, DA was prepared in the appropriate control solution to a final concentration of 10−4M just before application (DA conditions). All measurements were taken after 5 min perfusion with DA. Results were discarded if the effects of DA did not show reversal after a 30 min wash. All chemicals were purchased from Sigma Chemical Co., St. Louis, MO.
Electrophysiological recording and cell identification
Pyloric neuron activity was monitored using extracellular pin electrodes and standard intracellular recording techniques. We identified pyloric neuron somata during ongoing rhythmic activity by: 1) matching extracellularly recorded action potentials from the appropriate motor roots with intracellularly recorded action potentials, 2) by the characteristic shape and amplitude of membrane potential oscillations and action potentials, and 3) by the neuron’s synaptic connectivity (Johnson and Harris-Warrick 1997; Johnson et al. 1994). We examined the LP → PY synaptic interaction using two electrode voltage-clamp of the pre-synaptic LP neuron and two electrode current clamp to maintain the post-synaptic PY membrane potential at the desired level (3 M KCl filled electrodes, 10 to 15 MΩ resistance) using Axoclamp-2A and 2B amplifiers (Axon Instruments) as previously described (Johnson et al. 1994; Mamiya et al. 2003).
LP waveform construction and LP → PY synaptic transmission
We constructed artificial, realistic LP waveforms from pre-recorded LP activity in control and DA conditions to use as pre-synaptic voltage clamp commands. LP recordings were low pass filtered at 30 Hz to preserve the slope of LP rebound from pacemaker inhibition and to filter out spike transients. An averaged, normalized LP waveform, sampled at 1000 points with the first and last points corresponding to the beginning and ending midpoint voltage values of a single oscillation, was constructed from the average of 10 consecutive oscillation cycles in a preparation. The original average period and amplitude of these cycles were preserved as separate values. The waveforms from 6 different preparations were then averaged and adjusted for the appropriate averaged period. Since LP waveform amplitudes were not significantly different in control and 10−4 DA conditions (see Results), both control and DA waveforms were scaled to 30 mV amplitude. This waveform amplitude drove the LP neuron from a holding value of −55 mV, near the resting potential of silent LP neurons (Johnson et al. 1992), to a peak of approximately −25 mV, a value that evokes the largest chemical synaptic response in PY neurons (Johnson et al. 1994).
Pyloric cells release transmitter as a continuous function of pre-synaptic voltage, by a process called graded synaptic transmission (Hartline and Graubard 1992). These graded synaptic interactions shape the pyloric pattern in the lobster (Hartline et al. 1988). To record PY graded inhibitory post-synaptic potentials (IPSPs), we added 10−7 M TTX to the saline to block spiking activity. In these experiments, the AB neuron was killed by intracellular iontophoresis of 5,6-carboxyflourescein and illumination with bright blue light (Miller and Selverston 1979). In control-TTX conditions, the PY neuron was held at −55 mV with current injection in current clamp, while 10 linked control or DA waveforms were injected as voltage clamp commands into LP. This was repeated in the presence of 10−4 M DA; in separate runs, the PY was either held at − 55 mV or allowed to depolarize to its DA-induced value. We measured the PY peak response to the first LP oscillation, and the mean steady state response to repeated LP oscillations, as calculated from the average amplitudes of the last 5 PY IPSPs. A synaptic depression index (DI) was calculated as the steady state peak response divided by the initial peak response. In addition, we examined the voltage dependence of the electrotonic synapse between LP and PY in control-TTX conditions after adding 5 X 10−6 M picrotoxin (PTX) to block the LP glutamatergic chemical transmission (Bidaut 1980; Eisner and Marder 1982). In one experiment we also added 20 mM TEA to block voltage-gated K+ currents to ensure that shunting alone did not reduce electrical coupling during PY depolarization.
Firing properties of PY neurons during rhythmic activity
To examine the functional importance of DA modulation of LP→PY synaptic dynamics on PY onset phasing during rhythmic pyloric activity, we measured the delay between the onset of LP and PY spiking in control and DA conditions; these experiments were done with intact descending modulatory inputs activating the pyloric network. In addition, we hyperpolarized the LP neuron to temporarily remove it from network activity, and measured the timing of PY firing onset relative to the AB pacemaker. In these experiments we also characterized the following PY parameters in control and 10−4 M DA conditions: number of spikes/burst, burst duration, duty cycle, and PY onset phase relative to AB onset. For each PY neuron, we averaged burst and firing measurements from 5 to 10 oscillation cycles in control and DA conditions.
Data acquisition and analysis
Electrophysiological recordings were digitized at 4KHz using a PCI-6070-E board (National Instruments), and stored on a PC using custom-made recording software written in Lab Windows/CVI (National Instruments). The same software was also used to inject artificial control and DA waveforms as voltage clamp commands into the LP neuron. All data were analyzed using another custom-made software program also written in Lab Windows/CVI (software available upon request). For statistical comparisons we used Statview, SAS and SPSS software to run paired and unpaired t-tests, two-way repeated measures analysis of variance (ANOVA), and Levene’s test for equality of variances, as appropriate. ANOVA tests were followed by post-hoc t tests to determine specific statistical differences between individual data groups. Statistical differences between mean values were accepted with p < 0.05 (2-tailed probability) for F or t values. Mean measured values and percentages are reported ± SD.
Results
Construction of realistic waveforms to drive the LP neuron
The LP neuron displays quite different waveform shapes during control and DA-modulated pyloric rhythms (Fig. 1B). The control LP waveform is monophasic: it rebounds from AB/PD pacemaker inhibition to fire a burst of spikes, which is terminated by synaptic inhibition from the PY and VD neurons (Fig. 1A) before the next round of pacemaker inhibition (Fig. 1B top left trace). During modulation by 10−4 M DA, the LP waveform is biphasic (Fig. 1B top right trace; Flamm and Harris-Warrick 1986a). Its rebound from pacemaker inhibition is accelerated by DA (Flamm and Harris-Warrick 1986a ; Harris-Warrick et al. 1995b), but its firing is quickly terminated by DA-enhanced PY inhibition (Fig.1b, PY right traces; Johnson et al. 1995). The second, brief depolarizing phase in DA is caused by its release from tonic PY inhibition as the AB/PD pacemaker group inhibits the PY cells (Johnson and Harris–Warrick 1997). This second depolarizing phase is terminated by pacemaker inhibition, which is also enhanced by DA (Johnson et al. 1995).
We generated realistic waveforms for the LP neuron in control and 10−4 M DA conditions. As synaptic transmission between pyloric neurons is primarily graded, we filtered (30 Hz) and averaged recordings from 6 LP neurons in control and in DA; these averaged waveforms (Fig. 2A, top traces) reflected the shapes of the LP slow wave oscillations in Figure 1B. The control and DA waveforms did not differ significantly in amplitude (12.2 ± 1.84 mV and 13.7 ± 1.79 mV, respectively; n = 6, paired t test; p = 0.27) or period (645 ± 40.62 ms and 692 ± 57.9 ms, respectively; n = 6, paired t test; p = 0.53). The trend to a longer mean period of the DA waveform reflects the tendency of DA to slow down the rhythm slightly (Ayali and Harris-Warrick 1998). However, the half-durations of the depolarizing phase of the control and the first depolarizing component of the DA waveforms were significantly different (389.6 ± 57.45 ms vs. 211.3 ± 17.54 ms; n = 6, paired t test; p = 0.03). These two waveforms were applied periodically in trains of 10 as pre-synaptic voltage commands in the LP neuron to examine DA modulation of LP→PY graded synaptic dynamics. We drove the LP neuron with both the control and the DA waveforms under both control conditions and in the presence of 10−4M DA; this allowed us to discriminate between the direct effects of DA on the LP→PY synapse and its indirect effects due to changes in the LP waveform.
Figure 2.
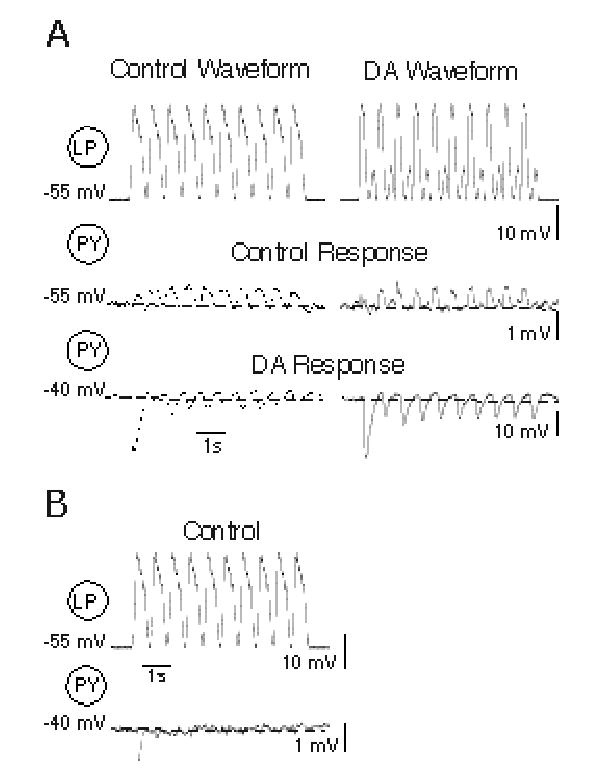
Dopamine modulation of synaptic transmission at LP→PY mixed synapses. A: Pre-synaptic Control and dopamine (DA) LP waveforms (top traces) and PY responses to these waveforms in Control (middle traces) and DA (10−4M) conditions (bottom traces). Note 10-fold difference in voltage scales in control and DA traces, and DA-evoked depolarization. B: PY response to the control LP waveform under control conditions with the PY neuron artificially depolarized to the membrane potential (−40 mV) seen in the presence of DA. Dashed line in PY traces indicates resting potential. Note different PY voltage scales in A and B.
Dopamine reverses the sign of mixed LP→PY synapses driven by realistic LP waveforms
In the pyloric network, a subset of PY neurons is both chemically inhibited by and electrically coupled to the LP neuron. The remaining PY neurons only receive chemical inhibition from the LP neuron. We first looked at DA’s effects on the mixed chemical-electrical LP→PY synapses with TTX added to the saline to block spontaneous activity and spike-evoked transmitter release (Fig. 2). In response to both control and DA pre-synaptic LP waveforms, the steady-state PY responses in 5 LP→PY synapses from different preparations under control conditions was weakly depolarizing. However, in 10−4M DA, the synaptic response to both LP waveforms reversed in sign at 4 of these synapses to become strongly hyperpolarizing. In the example of Figure 2, which is the same cell pair shown in Figure 1B, the first LP waveform in the series elicited weak, biphasic PY responses, consisting of an electrotonic depolarization that outweighed the weak chemical inhibition (Fig. 2A, middle traces). This response occurred with both the control and the DA LP waveforms. By the second or third waveform in the series, only depolarizing electrotonic responses were obvious, due to marked synaptic depression of the chemical component (Fig. 2A, middle traces, note PY responses above dashed line marking the resting potential; see also Mamiya et al. 2003). Application of DA depolarized this PY by 15 mV. Both control and DA LP waveforms elicited large, hyperpolarizing graded chemical synaptic potentials, which depressed to a steady state hyperpolarized value by the forth or fifth repeated LP waveform (Fig. 2A, bottom traces, note PY IPSPs below dashed line marking the resting potential, and the ten-fold reduced voltage scale). In between IPSPs in DA, the PY neuron depolarized above the initial resting potential, reflecting the marked enhancement of post-inhibitory rebound that DA evokes in these neurons (Harris-Warrick et al. 1995a).
In theory, the DA-induced depolarization of the post-synaptic PY neuron could by itself explain the enhanced chemical IPSP in Figure 2A by increasing the driving force on the inhibitory synapse. At the 4 mixed synapses where synaptic sign was reversed, DA depolarized the PY neurons an average of 14 ± 1.5 mV. However, when the PY neuron was depolarized to the same extent under control conditions without DA, only a small, initial hyperpolarization was seen, and this initial response depressed into the noise level with repeated LP oscillations (Fig. 2B, same synapse as shown in 2A, note the dashed line marking the resting potential, and the expanded voltage scale). We could not analyze PY IPSPs at −55 mV in the presence of DA because these PY neurons generated slow rhythmic membrane potential oscillations when hyperpolarized by current injection.
Figure 3 shows the mean peak PY responses to the first LP waveform and the steady state PY responses at the end of the LP train at these 4 mixed synapses, using both control and DA waveforms under both control and DA conditions. DA had significant main effects on the initial and steady state PY responses, using either the control or the DA LP waveforms (repeated measures, two-way ANOVA, p = 0.005 and 0.003, respectively). DA caused significant increases in both peak initial and steady state PY responses using either the control (post hoc t tests; p = 0.02, and 0.007, respectively) or DA waveforms (post hoc t tests; p = 0.03, and 0.02, respectively). The initial peak PY response in DA was also significantly larger than the initial response under control conditions when the PY neuron was depolarized to the same membrane potential as seen during DA application (n = 4; paired t test, p = 0.03). Surprisingly, the different LP waveform shapes (control and DA) did not have any significant main effect on the peak amplitudes of either the initial or steady state PY responses in either control or DA conditions (repeated measures, two-way ANOVA, p = 0.77 and 0.33, respectively). Notably, in DA, the second depolarizing component of the DA waveform had no detectable effect on the PY neuron. These experiments suggest that the rather dramatic change in LP waveform evoked by DA has only subtle effects on its synaptic output (see below). A minority of PY neurons across different preparations do not respond to DA (Johnson, Schneider and Harris-Warrick, unpublished observations), and at a fifth mixed LP→PY synapse, DA did not depolarize the PY neuron. The chemical synapse onto this PY was undetectable under control conditions, and became apparent only during the first LP oscillation in DA. Like the other mixed synapses we studied in control conditions, the chemical component of this synapse depressed with repeated LP oscillations to leave a predominantly electrotonic, steady state component (not shown). Thus, at all of these synapses, DA enhanced chemical inhibition, and in the majority of the mixed synapses (4 of 5 from different preparations), this reversed the synaptic sign from depolarizing to hyperpolarizing. This predominant hyperpolarization was maintained during repeated LP oscillations despite relatively strong chemical synaptic depression.
Figure 3.
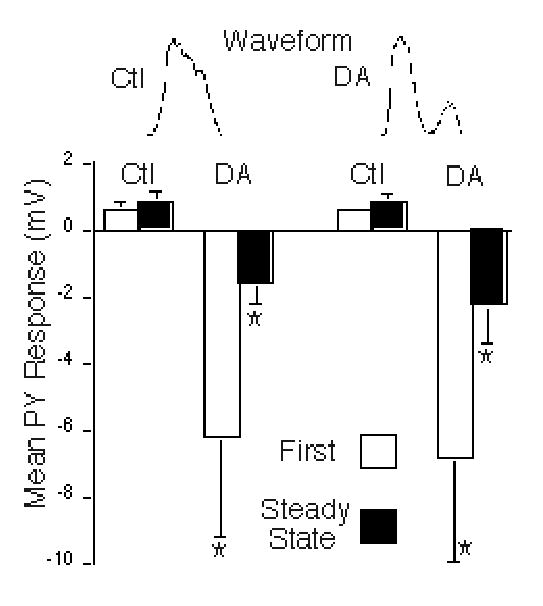
Summary of DA effects on LP→PY mixed synapses. Mean PY peak synaptic responses to LP control (Ctl, left bars) and dopamine (DA, right bars) waveforms are shown in control (Ctl) and dopamine (DA) conditions. The PY responses to the first waveform in each train are shown by the open bars, while the steady state PY responses, averaged from the last 5 waveforms in the train, are shown by the closed bars. PY responses were depolarizing in control conditions and hyperpolarizing in DA conditions. Asterisks: DA had significant main effects on the initial and steady state PY responses, using either the control or the DA LP waveforms (repeated measures, two-way ANOVA, p = 0.005 and 0.003, respectively). DA caused significant differences in peak initial and steady state PY responses to the control (post hoc t tests; p = 0.02, and 0.007, respectively) and DA waveforms (post hoc t tests; p = 0.03, and 0.02, respectively).
Dopamine is known to weaken the electrical coupling between LP and PY neurons when measured at constant pre- and post-synaptic membrane potentials (Johnson et al. 1993a). This is a rectifying electrical junction where depolarization of the LP is transferred to the PY neuron as a function of the difference between their membrane potentials. Thus, DA-induced PY depolarization, by lessening the differential voltage between the LP and PY neurons, could further contribute to weakening the electrotonic component of the mixed synapse. This is demonstrated in Figure 4, which shows the isolated electrotonic component of the LP→PY synapse after PTX was added to block the chemical component, and 20 mM TEA was added to partially block voltage-gated K+ conductances and enhance control of the electrotonic component. Under control conditions (with no DA), LP depolarization drove a relatively large electrotonic potential (1.9 mV) in the PY neuron (Fig. 4, left traces). Depolarization of the PY neuron by 15 mV (to mimic the effect of DA) reduced the electrotonic potential amplitude to 0.62 mV (Fig. 4, middle PY trace). This diminished PY electrotonic response could be partially restored when the LP waveform amplitude was in turn increased by 10 mV (Fig. 4a, right PY trace). ). In 4 experiments, the mean PY electrotonic response at −55 mV to a 30 mV LP depolarization (1.3 ± 0.63 mV) was significantly greater than the PY response at −40 mV (0.43 ± 0.16 mV; paired t-test; p = 0.048). This shows that LP→PY rectifying electrical coupling depends on the voltage difference between the two neurons.
Figure 4.
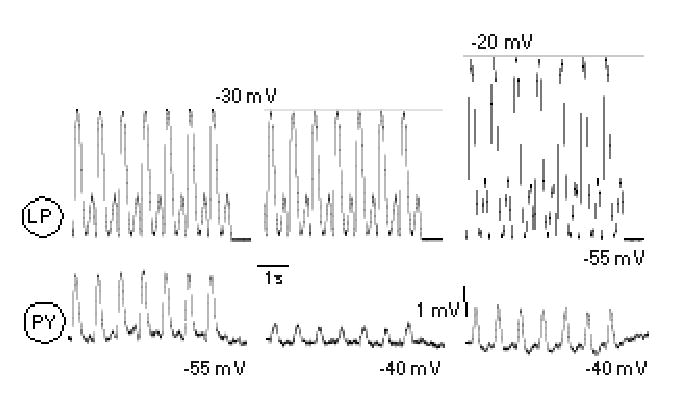
Effects of LP and PY voltage differences on LP→PY electrotonic coupling. To isolate the electrotonic coupling between these neurons, chemical inhibition was eliminated with 5 x 10−6 M pictrotoxin, and 20 mM TEA was added to reduce the shunting effect of IK(V). An LP depolarization of 25 mV from −55 to −30 mV, using the DA waveform, produced a PY electrotonic response at −55 mV (left traces) which was reduced when the PY neuron was depolarized to −40 mV with current injection (middle traces). Subsequently increasing the LP depolarization to −20 mV while the PY was depolarized to −40 mV restored about half of the PY electrotonic response (right traces).
Dopamine activates silent purely chemical LP→ PY synapses during realistic network activity
A subset of the PY neurons are not electrically connected to the LP and show only chemical inhibition upon LP stimulation (Johnson et al. 1994). At these purely chemical LP→PY synapses, the PY neuron displayed a hyperpolarizing response to the first LP waveform in control conditions, but this depressed to little or no postsynaptic response with repeated LP oscillations using either the control or the DA LP waveforms (Fig. 5A, middle traces; note dashed line marking the resting potential). DA enhanced synaptic inhibition in response to the first control and DA waveforms (Fig. 5A, bottom traces). Although these synapses depressed strongly, there remained a small hyperpolarizing response at steady state during the LP train using both pulse types (Fig. 5A, bottom traces; note PY responses below dashed line marking the resting potential). In these neurons, DA evoked a depolarization in the PY neurons that was weaker than in the PYs receiving mixed synapses (4 ± 2.6 mV; n= 4); we could eliminate this slight depolarization with current injection, but the IPSP was still enhanced (Fig. 5B, same synapse as in Fig. 5A). Figure 6 shows the mean peak PY responses to the first LP waveform and the steady state PY responses at the end of the LP train at these chemical synapses, using both control and DA waveforms under both control and DA conditions. In 3 out of 4 experiments, under control conditions synaptic depression was strong enough to completely eliminate the PY steady state response. Again, DA had significant main effects on the initial and steady state PY responses, using either the control or the DA LP waveforms (repeated measures, two-way ANOVA, p = 0.007 and 0.01, respectively). DA significantly increased the peak initial and steady state PY responses to the control (post hoc t tests; p = 0.03, and 0.04, respectively) and DA waveforms (post hoc t tests; p = 0.01, and 0.03, respectively). When the PYs were hyperpolarized back to the control resting potential in DA conditions, the initial PY IPSP was still significantly larger in response to the control LP waveform (n = 4; paired t test, p = 0.046). Again, switching between control and DA LP waveform shapes had no significant main effect on the initial PY responses or the steady state PY responses under control conditions or during DA application (repeated measures, two way ANOVA, p = 0.52 and 0.62, respectively; Fig. 6). Thus, during realistic network activity, DA enhanced the purely chemical LP→PY synapses strongly enough to maintain chemical inhibition that depressed to silence in most cases under control conditions.
Figure 5.
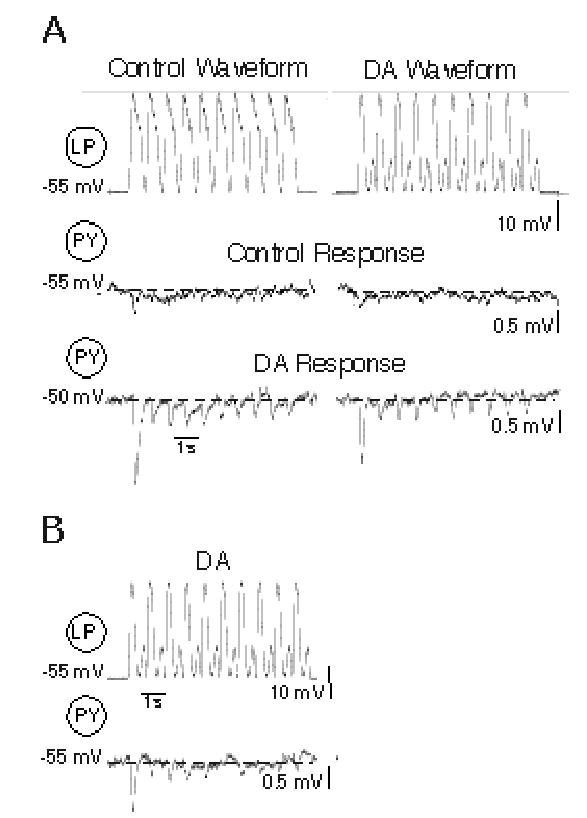
Dopamine modulation of synaptic transmission at LP→PY purely chemical synapses. A: Pre-synaptic Control and dopamine (DA) LP waveforms (top traces) and PY responses to these LP waveforms under Control (middle traces) and DA (10−4M) conditions (bottom traces). Note PY depolarization from −55 mV to −50 mV in DA. B: PY response to the DA LP waveform in the presence of DA, while the PY was hyperpolarized back to the control membrane potential (−55 mV). Dashed lines in PY traces indicate resting potentials; values shown to the left of each trace.
Figure 6.
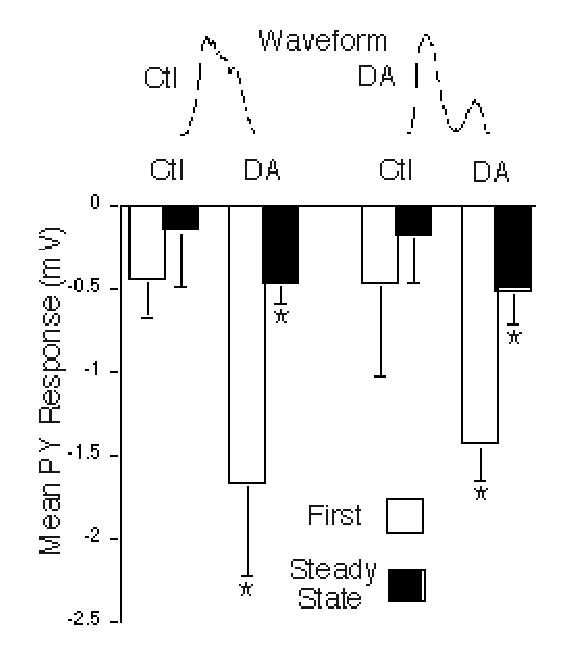
Summary of DA effects on LP→PY purely chemical synapses. Mean PY peak synaptic responses are shown to LP control (Ctl, left bars) and dopamine (DA, right bars) waveforms in control and DA conditions. The PY responses to the first waveform in each train are shown by the open bars, while the steady state PY responses, averaged from the last 5 waveforms in the train, are shown by the closed bars. Asterisks: DA had significant main effects on the initial and steady state PY responses, using either the control or the DA LP waveforms (repeated measures, two-way ANOVA, p = 0.007 and 0.01, respectively). DA significantly increased the peak initial and steady state PY responses to the control (post hoc t tests; p = 0.03, and 0.04, respectively) and DA waveforms (post hoc t tests; p = 0.01, and 0.03, respectively).
Control and DA pre-synaptic LP waveforms cause subtle differences in the shape and depression of DA-enhanced PY inhibitory responses
We were surprised that changing the pre-synaptic LP waveform from the control to the DA shape did not cause significant differences in the amplitudes of the initial and steady state PY IPSPs (Figs. 3 and 6). We looked for more subtle effects of waveform shape and found two small, but statistically significant, differences in the duration and depression of PY inhibitory responses to the two types of waveforms during DA application. We combined the IPSP measurements that were enhanced by DA from both mixed and purely chemical LP→PY synapses (n = 8) because these IPSPs were large enough to measure accurately, and because DA-enhanced steady state IPSPs did not depress completely, allowing calculation of the depression index (DI). In the presence of DA, the mean half-duration of the first PY IPSP in response to the control LP waveform was significantly longer (225 ± 71.6 ms) than the response to the DA waveform (199 ± 63.3 ms; paired t test; p = .05), correlating with the different durations of the two LP waveforms (Fig. 7A, bottom traces).
Figure 7.
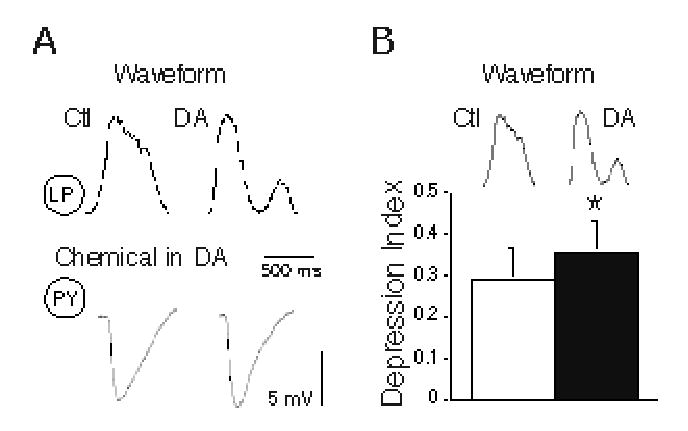
Effects of LP waveform shape on PY IPSP duration and synaptic depression. A: Control (Ctl) and dopamine (DA) LP waveforms (top traces) elicited different duration IPSPs in PY neurons in DA conditions (bottom traces). B: Depression indices for PY IPSPs in DA conditions are significantly smaller (asterisks) to control (Ctl; open bar) than to DA waveforms (DA, closed bar; paired t test, p = 0.0001).
The depression index (DI, calculated as the average steady state peak IPSP divided by the initial peak IPSP) was slightly, though significantly, smaller for the control LP waveform than the DA waveform during DA application (Fig. 7B; paired t test; p = 0.001). This indicated slightly greater synaptic depression in DA with the longer control waveform than with the shorter DA waveform. Despite variability in the initial and steady state amplitudes of the PY IPSPs in DA (Figs. 3 and 6). the ratio of steady state to initial amplitude was consistently lower with the control waveform (see bottom traces in Figs. 2A and 5A); the effect is, however, subtle. These results show that fairly large differences in LP waveform shape cause only small changes in PY inhibitory response. The changes that we did detect are probably due to differences in the two LP waveform durations (see Discussion).
LP→PY firing delay in control and DA conditions
Under control conditions, the LP→PY synapse appears to have little effect on the onset of PY firing in the rhythmic pyloric motor pattern, in that LP hyperpolarization does not strongly advance the onset of PY spiking (Weaver and Hooper 2003a; Mamiya et al. 2003). We hypothesized that application of DA, by weakening electrical coupling and very significantly strengthening the functional LP→PY inhibition, may increase the LP control of PY firing onset. We first examined the onset time delay between LP and PY (first LP spike to first PY spike) in experiments where the cycling network activity of both neuron types was recorded simultaneously in the absence and presence of DA. Apparently consistent with our hypothesis, the mean LP-PY delay across our population of 21 cell pairs from 14 preparations was 42% greater in DA than in control conditions (Fig. 8A). This effect was not, however, statistically significant (paired t test; p = 0.10). During DA application the pyloric cycle period also became slightly longer, as described above (Ayali and Harris-Warrick 1999), so when the LP-PY delay was converted into a fraction of the period to take this into account, the phasing between LP and PY was unchanged by DA (Fig. 8B; paired t test; p = 0.734).
Figure 8.
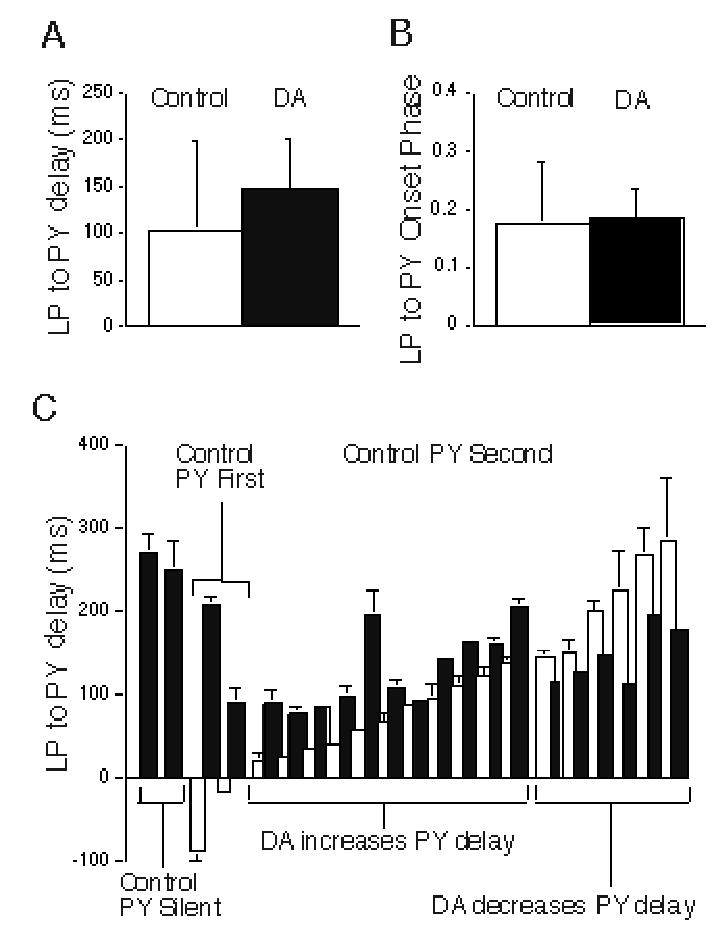
Dopamine (DA) effects on time delay between LP and PY onset, and LP to PY onset phase difference during normal pyloric network activity. A: Mean effect of DA on LP to PY time delay. B: Mean effect of DA on LP to PY onset phase difference. Neither difference is statistically significant (LP to PY time delay, paired t test, p = 0.10; LP to PY onset phase, paired t test, p = 0.73). C: Time delay between LP and PY onset for each of 21 PY neurons under control (white bars) and DA (black bars) conditions. PY neurons are grouped by their firing in control conditions: silent, firing before LP, and firing after LP. The last group is subdivided by whether DA causes a phase advance or a phase delay.
The lack of a significant DA effects on LP to PY time delay was caused by the large variability in firing delay of individual PY neurons. Figure 8C shows the range of PY firing delays in control and DA conditions. This figure plots the LP to PY firing delay for each of 21 PY neurons under control and DA conditions; it divides these PY neurons by their firing onset relative to LP and by the effects of DA on their firing times. Most (17 out of 21) PY neurons began firing after the LP neuron under control conditions; 11 (from 9 preparations) of these increased, while 6 (from 5 preparations) decreased their firing delay in DA (Fig. 8C; see also example in Fig. 1B). Interestingly, the PY neurons that increased their firing delay in DA all had shorter control delays than those that decreased their firing delay in DA. Thus, the major effect of DA was to make the PY firing onset relative to LP more uniform by significantly decreasing the delay variability in this set of PY cells (control delay= 120.7 ± 82.86 ms; DA delay= 132.8 ± 41.07 ms; Levene’s test for equality of variances; p = 0.021). When these PY firing delays were converted to onset phase, DA also made the LP→PY onset phase more uniform. Although we did not systematically examine multiple PY neurons from the same preparation, in two preparations we recorded 2 PYs that had short and long delays; these were lengthened and shortened, respectively, by DA. Thus, these cells fired together to a much greater extent in DA. Two PYs were silent under control conditions and fired only after a long delay during application of DA. The remaining two PY neurons fired before the LP neuron in control and switched to fire after the LP neuron in DA (Fig. 8C). Although we did not test the LP→PY synaptic connection for every cell pair, the PY firing delay did not seem to correlate with the type of synaptic connection with the LP (purely chemical vs. mixed chemical/electrical). Thus, for most PY neurons, DA appeared to make the onset of their firing more uniform relative to the LP neuron.
LP influence on the AB→PY firing delay and PY burst properties
To further examine the LP’s influence on PY firing onset, we examined the effect of removing its synaptic input by hyperpolarizing the LP neuron and measuring the change in the delay of PY firing relative to the AB pacemaker during network activity in the presence and absence of DA. If the LP neuron exerted more control over PY firing during DA application due to its stronger synaptic inhibition, its temporary removal from the network should advance PY firing relative to the pacemaker AB to a greater extent in DA than under control conditions. Figure 9A shows an experiment to test this hypothesis in a cycling preparation; the LP neuron was hyperpolarized under control conditions and during application of DA. Here, block of LP activity weakly accelerated the cycle frequency in both control (8% decrease in period; see also Hooper and Marder 1987; Selverston and Miller 1980; Weaver and Hooper 2003b) and DA conditions (10% decrease in period), due to the removal of LP inhibitory feedback to the pacemaker kernel (Fig. 9A, Fig. 1A). When the LP neuron was released from hyperpolarization, it fired a strong post-inhibitory rebound burst which inhibited AB firing in control (Fig. 9A, left traces), and delayed AB firing in DA (Fig. 9a, right traces). This showed that LP chemical synapses were active, and their steady state depression was removed by the hyperpolarization.
Figure 9.
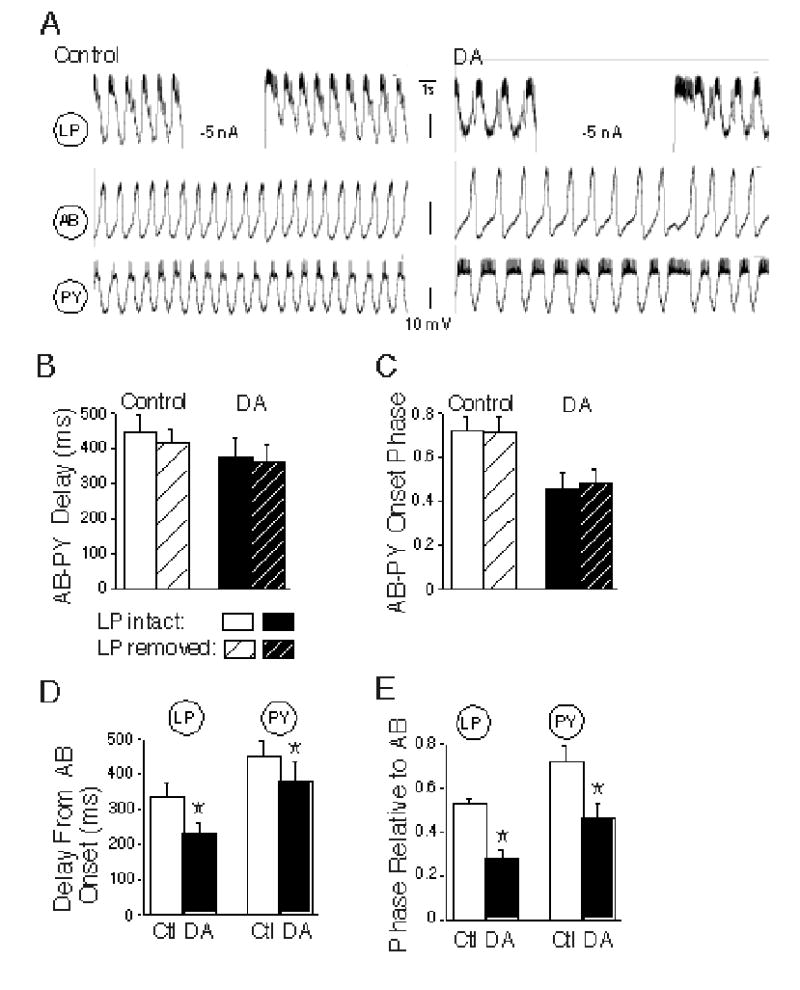
Effect of removing the LP→PY synapse on PY onset time and phase, and comparison of LP and PY firing phases in control and dopamine conditions. A: Example showing the protocol for testing the LP neuron’s contribution to pyloric network activity. The LP neuron was connected to the PY by a mixed synapse. B-C: Comparisons of the effects of LP hyperpolarization on time delay between AB and PY firing onset (B), and AB to PY firing onset phase (C) under control and dopamine (DA) conditions. None of these differences are significant (repeated measures two way ANOVAs, p > 0.27). D-E: Comparison of LP and PY time delay to onset (D), and phase delay to onset under control (white bars) and dopamine (black bars) conditions. DA caused a significant time and phase advance of both LP and PY relative to AB (Asterisks: DA significantly reduces AB to LP and PY delay and AB to LP and PY onset phase; post hoc t tests, p <0.002 for all comparisons).
LP hyperpolarization had only a small and insignificant effect to decrease the AB to PY delay under control conditions (PY delay decrease: 30.5 ± 15.9 ms, n = 6, from 4 preparations; Fig. 9B, control). Surprisingly, this effect of removing LP did not change significantly when LP→PY synaptic inhibition was enhanced by DA (17.5 ± 18.1 ms; Fig. 9B, DA; no main effect of LP hyperpolarization on AB to PY delays; repeated measures, 2 way ANOVA; p = 0.27). Due to the acceleration of the pyloric rhythm, there was no main effect of LP hyperpolarization on the phase of PY onset relative to AB during LP hyperpolarization in either control or DA conditions (Fig. 9C; 2 way ANOVA; p = 0.39). These results suggest that, contrary to our earlier hypothesis, the addition of DA did not increase LP control of PY firing onset.
These small changes in AB to PY delay during LP hyperpolarization cannot account for the tendency for the LP to PY time delay to increase during DA application (Fig. 8A); if this increase were due to stronger LP inhibition of the PY neurons, it would also delay PY onset relative to the AB neuron. We further compared the AB to LP and AB to PY time delays during control and DA network activity. These experiments showed that in fact, DA decreases the AB to LP firing delay more (32 ± 4.1%) than the AB to PY firing delay (16 ± 6.5%; post hoc t test, p = 0.002); that is, the increases in LP to PY delay (Fig. 8C) are caused by the LP advancing more, relative to the AB, than the PY, rather than the LP delaying the PY (Fig. 9D). The slightly longer cycle period during DA application neutralized the slightly longer time delay between LP and PY onset; indeed, in these experiments, the DA-evoked phase advances for LP (0.25 ± 0.02) and PY (0.26 ± 0.09) relative to AB were similar (post hoc t test; p= 0.48; Fig. 9E), consistent with the constancy of LP to PY phase under control and DA conditions (Fig. 8B). These results did not depend upon the LP→PY synaptic connection; the LP neuron made mixed synapses with half of these PY neurons and purely chemical synapses with the rest.
LP hyperpolarization also had no significant main effects on other aspects of PY activity in the presence or absence of DA, including the burst duration (Fig. 10A), the number of APs per burst (Fig. 10B), or the duty cycle (Fig. 10C; repeated measures ANOVAs; p > 0.42 for all comparisons). Note, however, that DA did have a significant main effect to enhance excitability in these PY neurons, as indicated by the increases in burst duration, number of action potentials per burst and duty cycle (Figs. 10A, B and C; repeated measures ANOVAs; p < 0.003 for all comparisons). We have previously documented DA’s significant enhancement of post-inhibitory rebound in the PY neurons (Harris-Warrick et al. 1995a).
Figure 10.
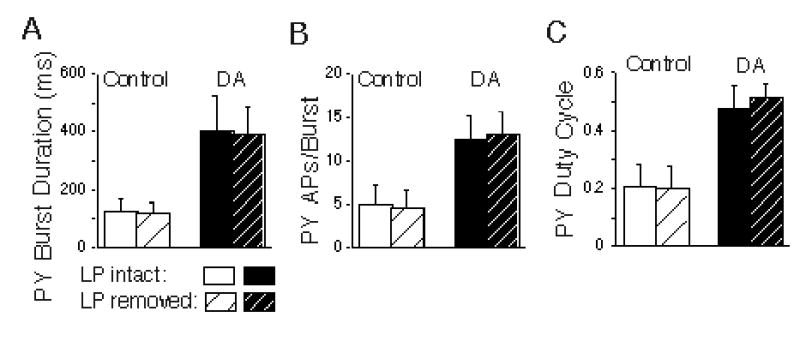
DA enhanced PY excitability, and this was unaffected by removal of LP. Comparisons of the effects of LP hyperpolarization (to remove the LP→PY synaptic interaction) on PY burst duration (A), the number of action potentials per PY bursts (B), and the PY duty cycle (C), under control and dopamine (DA) conditions. DA significantly excited the PY neurons by all three measures (repeated measures ANOVAs; p < 0.003 for all comparison), but removal of LP had no effect (repeated measures ANOVAs; p > 0.42 for all comparison).
In essence, the timing of LP and PY firing onset is primarily determined by their rates of intrinsic post-inhibitory rebound from the pacemaker AB/PD inhibition. During DA application, both the LP and most of the PY neurons are highly excited (Figs. 1B, 9), but the LP shows a more rapid rebound after pacemaker inhibition than the PY neurons. Thus the LP neuron fires earlier and increases the time delay (though not the phase delay) between the LP and PY neurons. Despite DA’s enhancement of LP→PY inhibition (and even its sign reversal in isolated conditions), this synapse is still not strong enough to significantly delay PY firing onset in an intact, rhythmic network. Thus, intrinsic PY firing properties and their enhancement by DA determine PY firing under both control and DA conditions.
Discussion
Mechanisms of DA modulation of LP→PY synaptic transmission
Using single square pulses, we previously showed that DA strengthens LP→PY chemical inhibition (Johnson et al. 1994) and weakens electrical coupling between the neurons (Johnson et al. 1993a). However, these graded chemical synapses show marked depression during normal membrane potential oscillations (Mamiya et al. 2003; Mamiya and Nadim 2004; Manor et al. 1997), and it was not clear whether DA could still enhance a depressed synapse. Here we have shown that under partially depressed conditions using realistic LP waveforms, DA can still dramatically strengthen the inhibitory component and weaken the electrotonic component of the mixed LP→PY synapse, reversing the sign of most LP→PY synapses from depolarizing to strongly hyperpolarizing. DA increases chemical inhibition by both pre-synaptic enhancement of ICa (Johnson et al. 2003) to enhance transmitter release from LP terminals (Johnson and Harris-Warrick 1997), and by post-synaptic enhancement of the PY neurons’ response to glutamate, the LP neuron’s transmitter (Johnson and Harris-Warrick 1997), accompanied by depolarization and increased input resistance (Harris-Warrick et al. 1995a; Johnson et al. 1993a). These post-synaptic actions are partially caused by a decrease in IA (Harris-Warrick et al. 1995a), and perhaps to a small extent by an increase in Ih (J. Peck and R. Harris-Warrick, unpublished observations). In addition, electrical coupling at mixed LP→PY synapses is weakened directly by DA (Johnson et al. 1993a), and as we show here, also indirectly as a result of postsynaptic depolarization which decreases the voltage difference between the two neurons at this rectifying synapse.
Dopamine might also reduce the steady state level of synaptic depression at the LP→PY synapse. In our experiments, for both mixed and purely chemical synapses, the chemical inhibition was almost completely depressed at steady state under control conditions. In control conditions similar to ours, Mamiya and Nadim (2005) estimate that the chemical component of LP→PY mixed synapses depresses by approximately 90%. In contrast, the chemical inhibition was only depressed by 65% at steady state in the presence of DA. Dopamine has been shown to prevent the development of synaptic depression in other systems (Baimoukhametova et al. 2004).
Effect of control and DA LP waveform shape on LP→PY dynamics
The amplitude and time courses of graded IPSPs have been shown to depend on the shape of the pre-synaptic waveform (Mamiya and Nadim 2004; Manor et al 1997; Olsen and Calabrese 1996; Simmons 2002). Our ability to drive the LP cell with either control or DA waveforms in the absence or presence of DA allows us to distinguish direct DA modulation of the pre-synaptic release process itself from indirect modulation caused by DA-induced changes in the LP pre-synaptic waveform. Despite the very marked differences in LP waveform shape under control and DA conditions (Fig. 1A, 2A), we saw no significant effect of LP waveforms on the amplitudes of the initial or steady state PY IPSPs, when tested under the same conditions (control or during application of DA). Thus, DA enhancement of the peak LP→PY inhibition arises from direct actions of DA at the synapse and not from indirect changes in pre-synaptic waveform shape. The different waveform shapes did cause subtle changes in the duration of the IPSP and on the depression index, but these effects are probably too small to have significant functional consequences for the network, especially since the enhanced LP→PY inhibition did not contribute much to the onset time of PY firing (see below).
Intrinsic rebound properties outweigh LP synaptic inhibition in determining the onset of PY firing
The control of patterned firing in motor networks is normally thought to be achieved through a balance between synaptic interactions and the intrinsic firing properties of the network neurons. Under control conditions of ongoing rhythmic network activity, we found, as others had before (Mamiya et al. 2003; Weaver and Hopper 2003a) that removal of the LP neuron had only a small effect to advance the onset of PY firing. It appears that the PY neurons are primarily responding to the very strong periodic inhibition from the pacemaker kernel (AB-PD), and their intrinsic PIR from this pacemaker inhibition plays the dominant role in setting their onset time under control conditions. However, we expected that after DA strongly enhanced LP→PY inhibition, this synapse would assume a more important role in delaying PY onset. This was not observed: removal of LP had equally small effects on PY onset times in the presence and absence of DA (Fig. 9A,B) and had no effect on PY phasing relative to the pacemaker kernel (Fig. 9C). DA strongly enhances the PY neurons’ intrinsic post-inhibitory rebound after pacemaker inhibition (Harris-Warrick et al. 1995a), and this still outweighs the effects of the LP→PY synapse in setting PY onset times. While the overall effect of DA was to phase advance the PY neurons relative to the pacemaker kernel (AB/PD; Fig. 9C), the LP neuron was time-advanced even more than the PY, relative to the pacemaker AB neuron (Fig. 9D); this then led to the small average increase in timing delay of PY relative to LP.
A more important effect of DA is to make the delay between LP and PY onset more uniform (Fig. 8B). PY neurons that had short delays relative to LP had them prolonged by DA, while PY neurons with long delays had them shortened, so the variance of the LP to PY delay was significantly smaller in DA. Thus, the PY neurons fire more synchronously as a group in DA than under control conditions. Since the PY neurons poly-innervate non-spiking muscles, this more synchronized and strengthened PY activity should evoke stronger PY contractions in DA than under control conditions. It is likely that this enhanced regularity of PY firing onset arises primarily from intrinsic changes in their rebound from AB/PD inhibition, with LP→PY inhibition playing a minor role.
These reset experiments were performed with an actively cycling pyloric preparation, driven by mixed modulatory inputs from higher ganglia. It is thus possible that DA failed to enhance the effect of hyperpolarizating the LP on PY onset because these synapses are already fully modulated by the inputs from higher centers. DA is a natural neuromodulator in crustaceans (Nusbaum and Beenhakker 2002; Tierney et al. 2003), and DA and other modulators may already be affecting the LP→PY synapses during pyloric activity, occluding the effects of additional DA. However, we think this unlikely. Under the same conditions, bath-applied DA has a number of other effects similar to those seen in the absence of other modulators, including exciting PY firing (Fig. 10; Flamm and Harris-Warrick 1986b; Harris-Warrick et al. 1995a), and strengthening LP→PD inhibition (Ayali et al. 1998; Johnson et al. 1995), which leads to a larger acceleration of cycle period than under control conditions (Johnson, Schneider and Harris-Warrick, unpublished observations). In addition, in preliminary experiments, we have monitored the effect of LP hyperpolarization on AB-PY timing in an isolated pyloric network, with no neuromodulators present except DA. Even in this simplified preparation, LP removal does not appear to significantly advance the onset of PY firing relative to the pacemaker group.
We still do not understand why DA’s enhancement of the LP→PY synapse fails to increase the efficacy of this synapse in determining the PY onset time. The competing increase in PY excitability appears to counterbalance the strengthening of this synaptic inhibition, yet when the inhibition is removed (by hyperpolarizing the LP) we do not see a dramatic advance in PY onset relative to control conditions. Further work will be needed to test how this arises from complex network interactions that are not yet fully understood.
Separating the complementary contributions of intrinsic and synaptic mechanisms that shape the patterns of neuronal output in a functioning neural network is a difficult challenge in most systems. This is especially true for complex vertebrate networks, though progress has been made in studies of contrast adaptation in the cat visual cortex (Nowak et al. 2005). New models of the mechanisms underlying memory consolidation are also beginning to consider both synaptic and intrinsic mechanisms (Xu et al. 2005; Zhang and Linden 2003). As we have shown here, modulatory actions that dramatically alter network synaptic interactions may in fact not be quantitatively very important for follower cell activity, when compared to intrinsic changes that are happening simultaneously. This emphasizes our finding in the pyloric network that the functional importance of synaptic modulation can only be understood in the context of parallel modulation of intrinsic properties in a rhythmic network.
Acknowledgments
We thank Drs. John Guckenheimer and Matthias Gruhn, and Ms. Marie-Luise Göritz for helpful discussions, Dr. Jack Peck, Karen Grace-Martin and Jennifer Schaub for statistical help, and Niranjan Mulay for data acquisition and analysis software development. This work was supported by National Institutes of Neurological Disorders and Stroke Grant NS-17323 (R. M. H-W), NIH MH-60605 (FN) and the Cornell Hughes Scholars Research Program (LS).
References
- Ayali A, Harris-Warrick RM. Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network. J Neurosci. 1999;19:6712–6722. doi: 10.1523/JNEUROSCI.19-15-06712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayali A, Johnson BR, Harris-Warrick RM. Dopamine modulates graded and spike-evoked synaptic inhibition independently at single synapses in pyloric network of lobster. J Neurophysiol. 1998;79:2063–2069. doi: 10.1152/jn.1998.79.4.2063. [DOI] [PubMed] [Google Scholar]
- Bidaut M. Pharmacological dissection of the pyloric network of the lobster stomatogastric ganglion using picrotoxin. J Neurophysiol. 1980;44:1089–1101. doi: 10.1152/jn.1980.44.6.1089. [DOI] [PubMed] [Google Scholar]
- Baimoukhametova DV, Hewitt SA, Sank CA, Baine JS. Dopamine modulates use-dependent plasticity of inhibitory synapses. J Neurosci. 2004;24:5162–5171. doi: 10.1523/JNEUROSCI.4979-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cymballyuk GS, Gaudry Q, Masino MA, Calabrese RL. Bursting in leech heart interneurons: cell-autonomous and network-based mechanisms. J Neurosci. 2002;22:10580–10592. doi: 10.1523/JNEUROSCI.22-24-10580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JS, Marder E. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. III. Synaptic connections of electrically coupled pyloric neurons. J Neurophysiol. 1982;48:1392–1415. doi: 10.1152/jn.1982.48.6.1392. [DOI] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM. Aminergic modulation in lobster stomatogastric ganglion. I. Effects on motor pattern and activity of neurons within the pyloric circuit. J Neurophysiol. 1986a;55:847–865. doi: 10.1152/jn.1986.55.5.847. [DOI] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM. Aminergic modulation in lobster stomatogastric ganglion. II. Target neurons of dopamine, octopamine and serotonin within the pyloric circuit. . J Neurophysiol. 1986b;55:866–8 881. doi: 10.1152/jn.1986.55.5.866. [DOI] [PubMed] [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–205. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Barazangi N, Guckenheimer J, Gueron S. Dopamine modulation of transient potassium current evokes phase shifts in a central pattern generator network. J Neurosci. 1995a;15:342–358. doi: 10.1523/JNEUROSCI.15-01-00342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Levini RM, Gueron S, Guckenheimer J. Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron. J Neurophysiol. 1995b;74:1404–1420. doi: 10.1152/jn.1995.74.4.1404. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR, Peck JH, Kloppenburg P, Ayali A, and Skarbinski J. Distributed effects of dopamine modulation in the crustacean pyloric network. In: Neuronal Mechanisms for Generating Locomotor Activity, edited by Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, and Kudo N. New York: NY Acad Sci, vol 860, 1998, p.155–167. [DOI] [PubMed]
- Harris-Warrick RM, Marder E, Selverston AI, and Moulins M. Dynamic Biological Networks. The Stomatogastric Nervous System Cambridge, MA: The MIT Press, 1992.
- Hartline DK, Gassie DV. Pattern generation in the lobster (Panilurus) stomatogastric ganglion. 1. Pyloric neuron kinetics and synaptic interactions. Biol Cybern. 1979;33:209–222. doi: 10.1007/BF00337410. [DOI] [PubMed] [Google Scholar]
- Hartline DK and Graubard K. Cellular and synaptic properties in the crustacean stomatogastric nervous system. In: Dynamic Biological Networks: The Stomatogastric Nervous System, edited by Harris-Warrick RM, Marder E, Selverston AI, and Moulins M. Cambridge, MA: MIT Press, 1992, p. 31–85.
- Hartline DK, Russell DF, Raper JA, Graubard K. Special cellular and synaptic mechanisms in motor pattern generation. Comp Biochem Physiol. 1988;91C:115–131. doi: 10.1016/0742-8413(88)90177-6. [DOI] [PubMed] [Google Scholar]
- Hooper SL. Phase maintenance in the pyloric pattern of the lobster (Panulirus interruptus) stomatogastric ganglion. J Comput Neurosci. 1997;4:191–205. doi: 10.1023/a:1008822218061. [DOI] [PubMed] [Google Scholar]
- Hooper SL, Marder E. Modulation of the lobster pyloric rhythm by the peptide proctolin. J Neurosci. 1987;7:2097–2112. doi: 10.1523/JNEUROSCI.07-07-02097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Wu JS, Park JH, Weiss KR. Concerted GABAergic actions of Aplysia feeding interneurons in motor program specification. J Neurosci. 2003;23:5283–5294. doi: 10.1523/JNEUROSCI.23-12-05283.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Neural mechanisms of motor program switching in Apylsia. J Neurosci. 2001;21:7349–7362. doi: 10.1523/JNEUROSCI.21-18-07349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Harris-Warrick RM. Amine modulation of glutamate responses from pyloric motor neurons in lobster stomatogastric ganglion. J Neurophysiol. 1997;78:3210–3221. doi: 10.1152/jn.1997.78.6.3210. [DOI] [PubMed] [Google Scholar]
- Johnson BR and Hooper SL. Overview of the stomatogastric nervous system. In: Dynamic Biological Networks: The Stomatogastric Nervous System, edited by Harris-Warrick RM, Marder E, Selverston AI, and Moulins M. Cambridge, MA: MIT Press, 1992, p. 1–30.
- Johnson BR, Kloppenburg P, Harris-Warrick RM. Dopamine modulation of calcium currents in pyloic neurons of the lobster stomatogastric ganglion. J Neurophysiol. 2003;90:631–643. doi: 10.1152/jn.00037.2003. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris-Warrick RM. Elevated temperature alters the ionic dependence of amine-induced pacemaker activity in a conditional burster neuron. J Comp Physiol A. 1992;170:201–209. doi: 10.1007/BF00196902. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris-Warrick RM. Amine modulation of electrical coupling in the pyloric network of the lobster stomatogastric ganglion. J Comp Physiol A. 1993a;172:715–732. doi: 10.1007/BF00195397. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris-Warrick RM. Dopamine induces sign reversal at mixed chemical-electrical synapses. Brain Res. 1993b;625:159–164. doi: 10.1016/0006-8993(93)90149-h. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris-Warrick RM. Differential modulation of chemical and electrical components of mixed synapses in the lobster stomatogastric ganglion. J Comp Physiol A. 1994;175:233–249. doi: 10.1007/BF00215119. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris-Warrick RM. Distributed amine modulation of graded chemical transmission in the pyloric network of the lobster stomatogastric ganglion. J Neurophysiol. 1995;74:437–452. doi: 10.1152/jn.1995.74.1.437. [DOI] [PubMed] [Google Scholar]
- Katz PS. Beyond Neurotransmission Oxford: Oxford University Press, 1999.
- Kloppenburg P, Levini RM, Harris-Warrick RM. Dopamine modulates two potassium currents and inhibits the intrinsic firing properties of an identified motor neuron in a central pattern generator network. J Neurophysiol. 1999;81:29–38. doi: 10.1152/jn.1999.81.1.29. [DOI] [PubMed] [Google Scholar]
- Mamiya A, Manor Y, Nadim F. Short-term dynamics of a mixed chemical and electrical synapse in a rhythmic network. J Neurosci. 2003;23:9557–9564. doi: 10.1523/JNEUROSCI.23-29-09557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya A, Nadim F. Dynamic interaction of oscillatory neurons coupled with reciprocally inhibitory synapses acts to stabilize the rhythm period. J Neurosci. 2004;24:5140–5150. doi: 10.1523/JNEUROSCI.0482-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya A and Nadim F. target-specific short-term dynamics are important for function of synapses in an oscillatory neural network. J Neurophysiol, in press. [DOI] [PubMed]
- Manor Y, Nadim F, Abbott LF, Marder E. Temporal dynamics of graded synaptic transmission in the lobster stomatogastic ganglion. J Neurosci. 1997;17:5610–5621. doi: 10.1523/JNEUROSCI.17-14-05610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Thirumalai V. Cellular, synaptic and network effects of neuromodulation. Neural Networks. 2002;15:479–493. doi: 10.1016/s0893-6080(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Tegner J, Hill RH, Grillner S. GABAB receptor activation causes a depression of low- and high- voltage-activated Ca2+ currents, postinhibitory rebound, and post-spike afterhyperpolarization in lamprey neurons. J Neurophysiol. 1993;70:2606–2619. doi: 10.1152/jn.1993.70.6.2606. [DOI] [PubMed] [Google Scholar]
- Miller JP, Selverston AI. Rapid killing of single neurons by irradiation of intracellularly injected dye. Science. 1979;206:702–704. doi: 10.1126/science.386514. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Selverston AI. Organization of the stomatogastric ganglion of the spiny lobster. I. Neurons driving the lateral teeth. J Comp Physiol. 1974;91:1–32. [Google Scholar]
- Nowak LG, Sanchez-Vives MV, McCormick DA. Role of synaptic and intrinsic membrane properties in short-term receptive field dynamics in cat area 17. J Neurosci. 2005;25:1866–1880. doi: 10.1523/JNEUROSCI.3897-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen OH, Calabrese RL. Activation of intrinsic and synaptic currents in leech heart interneurons by realistic waveforms. J Neurosci. 1996;16:4958–4970. doi: 10.1523/JNEUROSCI.16-16-04958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, and Grillner S. Neuronal Control of Locomotion Oxford: Oxford University Press, 1999.
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res. 2004;143:123–129. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Pearson KG and Ramirez J-M. Sensory modulation of pattern-generating circuits. In: Neurons, Networks, and Motor Behavior, edited by Stein PSG, Grillner S, Selverston AI, and Stuart DG. Cambridge, MA: The MIT Press, 1997, p. 225–235.
- Ramirez J-R, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Selverston A, Elson R, Rabinovich M, Huerta R, and Abarbanel H. Basic principles for generating motor output in the stomatogastric ganglion. In: Neuronal Mechanisms for Generating Locomotor Activity, edited by Kiehn O, Harris-Warrick RM, Jordan LM, Hultburn H, and Kudo N. New York: NY Acad. Sci. vol. 860, 1998, p. 35–50. [DOI] [PubMed]
- Selverston AI, Miller JP. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. I. Pyloric System. J Neurophysiol. 1980;44:1102–1121. doi: 10.1152/jn.1980.44.6.1102. [DOI] [PubMed] [Google Scholar]
- Selverston AI, Panchin YV, Arshavsky YI, and Orlovsky GN. Shared features of invertebrate central pattern generators. In: Neurons, Networks, and Motor Behavior, edited by Stein PSG, Grillner S, Selverston AI, and Stuart DG. Cambridge, MA: The MIT Press, 1997, p. 105–117.
- Selverston AI, Russell DF, Miller JP, King DG. The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol. 1976;7:215–290. doi: 10.1016/0301-0082(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Reith CA, and McDearmid JR. Development and aminergic neuromodulation of a spinal locomotor network controlling swimming in Xenopus larvae. In: Neuronal Mechanisms for Generating Locomotor Activity, edited by Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, and Kudo N. New York: NY Acad Sci, vol 860, 1998, p. 318-332. [DOI] [PubMed]
- Simmons PJ. Presynaptic depolarization rate controls transmission at an invertebrate synapse. Neuron. 2002;35:749–758. doi: 10.1016/s0896-6273(02)00791-2. [DOI] [PubMed] [Google Scholar]
- Stein PSG, Grillner S, Selverston AI, and Stuart DG. Neurons, Networks, and Motor Behavior Cambridge, MA: The MIT Press, 1997.
- Tierney AJ, Kim T, Abrams R. Dopamine in crayfish and other crustaceans: distribution in the central nervous system and physiological functions. Microsc Res Tech. 2003;60:325–335. doi: 10.1002/jemt.10271. [DOI] [PubMed] [Google Scholar]
- Weaver A, Hooper SL. Relating network synaptic connectivity and network activity in the lobster (Panulirus interruptus) pyloric network. J Neurophysiol. 2003a;90:2378–2386. doi: 10.1152/jn.00705.2002. [DOI] [PubMed] [Google Scholar]
- Weaver A, Hooper SL. Follower neurons in lobster (Panulirus interruptus) pyloric network regulate pacemaker period in complementary ways. J Neurophysiol. 2003b;89:1327–1338. doi: 10.1152/jn.00704.2002. [DOI] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:1750–1760. doi: 10.1523/JNEUROSCI.4217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nature Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]


