Abstract
Proline-, glutamic acid-, and leucine-rich protein-1)PELP1/MNAR [modulator of nongenomic activity of estrogen receptor (ER)], a novel coregulatory protein, modulates genomic as well as nongenomic activity of ERs. We characterized the expression and localization of PELP1 in both benign and cancerous endometrium. Our results suggest that PELP1 is expressed in all stages of endometrium; however, this protein exhibits distinct localization depending on the phase. PELP1 is expressed in both the stroma and epithelial cells. Using the Ishikawa endometrial cancer model cell line and ER subtype-specific ligands, we found that PELP1 functionally interacts with both ERα and ERβ and enhances their transcriptional responses. However, in endometrial cancer cells, endogenous PELP1 is also required for optimal ligand-mediated transcription and proliferation responses. PELP1 promoted a tamoxifen-mediated agonistic action in endometrial, but not in breast cancer cells. PELP1 expression and localization are widely deregulated in endometrial cancers. In addition, PELP1 and ERβ were localized predominantly in the cytoplasm of high-grade endometrial tumors. Our results suggest that PELP1 plays an essential role in the proliferation of cancerous endometrial cells.
Abbreviations: DCC, Dextran-coated, charcoal-treated fetal calf serum; DPN, diarylpropionitrile; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen response element; luc, luciferase; MNAR, modulator of nongenomic activity of estrogen receptor; PELP1, proline-, glutamic acid-, and leucine-rich protein-1; PPT, propyl-pyrazole-triol; siRNA, short interference RNA; SRC, steroid receptor coactivator
ALTHOUGH ENDOMETRIAL CANCER is the most common malignancy of the female genital tract, the molecular aspects of this malignancy remain poorly understood. Some understanding can probably come from an analysis of benign endometrium and comparison with cancerous tissue. During the menstrual cycle, the endometrium undergoes sequential stages of proliferation, differentiation, and degeneration in response to steroid hormones (1). One such hormone is 17β-estradiol (E2), which plays an important role in the normal development of the uterus (2). It is also involved in the pathogenesis of endometrial cancer (3). The biological effects of estrogen are mediated by its binding to the estrogen receptor (ER), which has two subtypes, ERα and ERβ (4, 5).
The ER subtypes share a similar modular structure but differ in function and expression and have isoform-specific effects on gene expression (6). Upon binding to E2, ligand-activated ERs bind to ER-responsive elements and modulate respective gene expression (genomic signaling) (7, 8). It is increasingly recognized that the biological actions of ERs result from both genomic and nongenomic signaling. For example, ERs participate in transcription-independent functions (nongenomic action) through activation of cytosolic signaling pathways, including the Src, MAPK, protein kinase C, and protein kinase B (AKT) pathways (4, 9–11).
The transcription functions of ERs are influenced by several coactivators (12–14). We recently cloned a novel ER-regulatory protein named proline-, glutamic acid-, and leucine-rich protein-1 (PELP1) (15), also referred to as modulator of nongenomic activity of ER (MNAR) (16–18). PELP1 is abundantly expressed in a number of tissues, including the mammary gland and the endometrium (15). PELP1 plays a permissive role in E2-mediated cell cycle progression through its regulatory interactions with the retinoblastoma pathway (18). Although PELP1 is predominantly localized in the nucleus (15, 19), recent studies suggest that, under certain conditions, PELP1 could be localized in the cytoplasm of breast cancer cells (20), which may suggest that it contributes toward the nongenomic action of ERs in cancer cells.
The differential action of estrogen in the breast and endometrium depends on the tissue-specific expression of the ER isoform, coregulators, and availability of novel integrators (21, 22). Emerging data and the identification of novel ER cofactors with diverse functions are beginning to shed light on the complex events of E2-ER signaling. Until now, however, few studies have looked at the expression of several coactivators such as steroid receptor coactivator 1 (SRC1), CEBP binding protein (CBP), and amplified in breast cancer-1 (AIB1) and corepressors such as nuclear receptor corepressor (NCOR) in the benign and cancerous endometrium (23–25). Because of the increasing awareness of the potential contribution of nongenomic and extranuclear functions of ER, it is important to examine the status and localization of novel steroid receptor coactivators such as PELP1 to fully understand the mechanisms of E2 signaling in the endometrium. The aim of present study was to analyze the expression and the localization of novel ER coactivator in the benign and cancerous endometrium. In addition, we have also examined the relevance of PELP1 in endometrial cancer cell proliferation and its potential role in tamoxifen-mediated partial agonist action in the endometrium.
Materials and Methods
Human samples
Residual endometrial tissue was derived from hysterectomy surgical specimens submitted to the Department of Pathology, M. D. Anderson Cancer Center. The use of human residual tissues used in this study was approved by M. D. Anderson Cancer Center institutional human research committee (protocol no. Lab01–718). Formalin-fixed, paraffin-embedded sections (3-μm thickness) derived from six Proliferative phase endometrium, six secretory phase endometrium, and six inactive postmenopausal endometrium samples were examined. In addition, a tissue array containing 60 endometrial cancer samples [endometrial adenocarcinoma, International Federation of Gynecology and Obstetrics (FIGO) grades 1, 2, and 3] was used for immunohistochemistry experiments. Frozen endometrial tissue (benign and malignant) was also derived from hysterectomy surgical specimens. All tissue classifications were verified by light microscopic examination of hematoxylin and eosin-stained slides by a gynecologic pathologist (R.R.B.). The benign endometrial samples used for this study were derived from hysterectomies for cervical squamous cell carcinoma, uterine leiomyomas, and dysfunctional uterine bleeding secondary to endometriosis. In addition, we were careful to check the clinical records for such patients, and we did not use benign endometrial samples from patients with a prior history of hormone use, radiation treatment, or chemotherapy.
Cell culture and reagents
The human endometrial Ishikawa cell line, a model of well-differentiated endometrial adenocarcinoma, was kindly provided by Dr. Bruce A. Lessey (University of North Carolina at Chapel Hill, NC) (26). The endometrial cancer cell line RL 95–2 and breast cancer cell lines MCF-7 and MDA-MB-231 were obtained from the American Type Culture Collection (Manassas, VA). All the cells were maintained in DMEM/nutrient mixture F-12 (1:1) supplemented with 10% fetal calf serum. Dextran-coated, charcoal-treated fetal calf serum (DCC serum) and antibodies against actin, vinculin, and E2 were purchased from Sigma Chemical Company (St. Louis, MO). Antibodies against ERα (MS-750-S1) were purchased from NeoMarkers (Fremont, CA) and Upstate Biotechnology, Inc. (Lake Placid, NY). The chicken antihuman ERβ 503 antibody and antihuman ERβ antibody have been previously described (27). ERα-specific ligand propyl-pyrazole-triol (PPT) and the ERβ-specific ligand diarylpropionitrile (DPN) were purchased from TOCRIS (Ellisville, MO). ERβ-specific monoclonal antibody purchased from Oncogene Research products (San Diego, CA) was used for ERβ immunoprecipitation.
Immunohistochemistry and interpretation of immunoreactivity
For immunohistochemical detection of ERα, ERβ, and PELP1, formalin-fixed, paraffin-embedded endometrial sections were deparaffinized with xylene and rehydrated using graded ethanol. Sections were incubated in 0.3% hydrogen peroxide and methanol for 30 min to inactivate the endogenous peroxidase. The sections were then boiled for 10 min in 0.01 m citrate buffer and cooled for 30 min at room temperature to expose antigenic epitopes. The sections were blocked with 2% normal goat serum in 1% BSA and PBS for 30 min and then incubated overnight at room temperature with primary antibodies against ERα, 1:50 dilution (NeoMarkers); chicken ERβ 503, 1:100 dilution (27); and PELP1, 1:500 dilution (15). Primary antibodies were diluted in diluent buffer (2% normal goat serum, 1% BSA, and PBS). For ERα and PELP1, the sections were washed three times with 0.05% Tween in PBS for 10 min; incubated with biotinylated secondary antibody, 1:100 dilution (Vector Laboratories, Inc. Burlingame, CA) for 1 h; and then washed three times with 0.05% Tween in PBS for 10 min; incubated with a streptavidin horse-radish peroxidase reagent (Dako Corporation, Carpinteria, CA) for 15 min; and washed three times with 0.05% Tween in PBS for 10 min. For ERβ, the sections were washed three times with 0.05% Tween in PBS for 10 min; incubated with a rabbit antichicken secondary antibody, 1:100 dilution (Sigma Chemical Company, St. Louis, MO) for 1 h; and then washed three times with 0.05% Tween in PBS for 10 min and three times with 0.05% Tween in PBS for 10 min. The sections were then developed with diaminobenzidine-H2O2 and counterstained with Mayer’s hematoxylin. For negative control, slides were incubated with nonspecific mouse IgG for ERα; chicken IgG for ERβ. For PELP1 slides, peptide-absorbed PELP1 antibody was used as a negative control. The specific staining of each antibody was assessed semiquantitatively using a four-point scale: 0 = no staining, 1 = mild staining, 2 = moderate staining, and 3 = intense staining. Staining pattern (cytoplasmic vs. nuclear), as well as cell compartment stained (glandular epithelial cells vs. endometrial stromal cells), was also examined.
Cell growth and reporter assays
For the cell growth assay, cells were grown in phenol red-free medium supplemented with 5% DCC serum for 48 h, and growth was stimulated with supplemental estrogen. The growth rate of the cells was measured by counting them in a Beckman Coulter Counter (Kendal, FL) (18). For reporter-gene transient transfections, estrogen response element (ERE)-luciferase (luc) reporter constructs were cotransfected with or without PELP1/MNAR, ERα, and ERβ or a control vector (0.5 μg each) using FuGENE 6 (Roche Diagnostics Corporation, Indianapolis, IN) according to the manufacturer’s instructions as previously described (15). Ishikawa cells cultured in DCC serum were cotransfected with 3XERE-luc reporter gene with or without the PELP1 expression vector. After 24 h, cells were treated with E2 (10−9 m), and ERE-luc reporter activation was measured after 16 h. The activity of the β-galactosidase reporter was used to correct the transfection efficiencies.
Western and immunoprecipitation analysis
Total protein lysates from cancer cells and endometrial benign and tumor samples were prepared using radioimmunoprecipitation buffer as described (15). For Western analysis, 100 μg total cellular extract was analyzed using antibodies specific to PELP1, ERα, and ERβ. Actin was used as a loading control. For immunoprecipitation, a total of 2 mg protein/each sample was incubated with 1 μg of the respective antibody.
RNA interference
For PELP1 knockdown, siGenome SMARTpool [short interference RNA (siRNA)] duplexes were purchased from Dharmacon (Lafayette, CO). The catalogue number and targeted sequences are as follows: D-004463–01-ggaagaagaaggugaguuauu (PELP1 siRNA no. 1); d-004463–02-caagguguaugcgauauuauu (PELP1 siRNA no. 2); d-004463–03-ccacagagccugacuccuauu (PELP1 siRNA no. 3); D-004463–04-ggaaugaaggcuuguaugauu (PELP1 siRNA no. 4). The catalog number for the PELP1 SMART pool siRNA is D-001210–01-20. The catalog number for siControl nontargeting siRNA is D001210–01-20. The GenBank accession number used to design siRNA is NM_014389. siRNA transfections were performed using oligofectamine according to the manufacturer’s protocol (Qiagen, Valencia, CA). In the initial screen, we have checked all the four siRNA individually as well as the SMART pool of four siRNAs. Of all the four PELP1 siRNAs tested, siRNA no. 3 and PELP1 pool showed substantial and consistent reduction in the PELP1 levels, and therefore these two siRNAs were used in the study.
Results
Expression profile of PELP1, ERα, and ERβ
To understand the significance of PELP1 and ERs, we first surveyed the spatial distribution of PELP1, ERα, and ERβ immunostaining in the proliferative and secretory phases of the menstrual cycle and in the postmenopausal phase of the human endometrium. The representative pattern is shown in Fig. 1. Staining intensity and the cellular localization of these proteins in the glandular and stromal compartments in the proliferative and secretory phases and the postmenopausal phases are summarized in Table 1. In the proliferative and secretory phases, PELP1 was expressed in both the glandular and stromal compartments and was localized in both the nucleus and cytoplasm of these cells. Similarly, there were no marked differences in staining between the proliferative and secretory phases (Fig. 1A, top, and Table 1). In contrast, in the postmenopausal sections, PELP1 staining was observed only in the glandular compartment and confined in the cytoplasm of the cells (Fig. 1B, top, and Table 1).
Fig. 1.
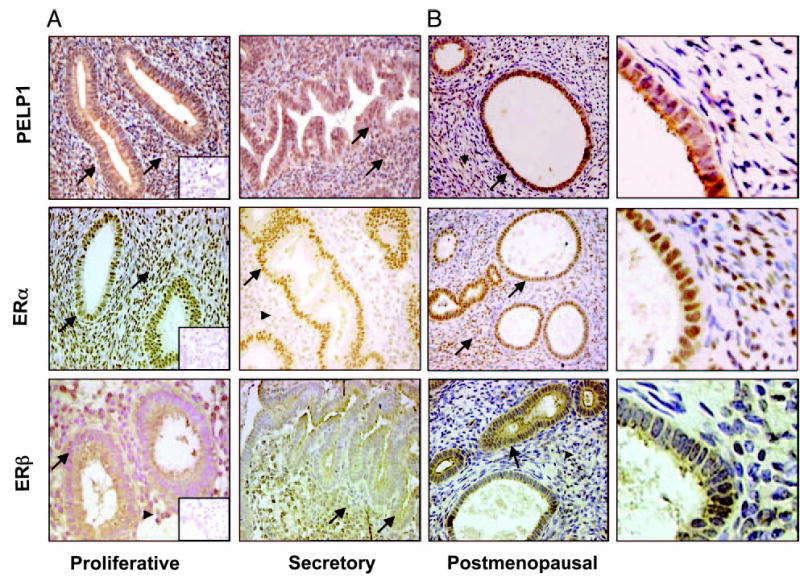
Immunohistochemical localization of ERα , ERβ, and PELP1 in various stages of benign endometrium. A, PELP1 immunostaining was high in glandular and stromal cells (arrow) during the proliferative and secretory phases of benign endometrium. High ERα staining was observed in the glandular cells of the proliferative and secretory phases. In stromal cells, ERα staining was high in the proliferative phase (arrow) and decreased in the secretory phase (arrowhead). Prominent staining of ERβ in the glandular cells was observed in both the proliferative and secretory phases (arrow). In stromal cells, ERβ expression was less prominent in the proliferative phase (arrowhead) and increased in the secretory phase (arrow). Insets (magnification, ×200) show negative controls. B, In postmenopausal endometrium, PELP1 staining was observed only in the glandular compartment (arrow), strong ERα staining in both the glandular and stromal cells (arrow), and strong ERα staining in the glandular cells (arrow) and weak staining in stromal cells (arrowhead) (magnification, ×200). Higher magnification of PELP1, ERα, and ERβ expression in postmenopausal sections is also shown (far right).
TABLE 1.
Summary of immunoreactive staining of ERα, ERβ, and PELP1 in the human endometrium
| Proliferative
|
Secretory
|
Postmenopausal
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epithelium
|
Stroma
|
Epithelium
|
Stroma
|
Epithelium
|
Stroma
|
|||||||
| Staining | Staining intensity | N/C | Staining intensity | N/C | Staining intensity | N/C | Staining intensity | N/C | Staining intensity | N/C | Staining intensity | N/C |
| ERα | 3+ | N | 3+ | N | 2+ | N | 1+ | N | 3+ | N | 3+ | N |
| ERβ | 2+ | C | 1+ | C | 2+ | C | 2+ | C | 2+ | C | 1+ | C |
| PELP1 | 2–3+ | N/C | 2–3+ | N/C | 2+ | N/C | 2+ | N/C | 2+ | C | 1+ | C |
1–3 represents the intensity of staining: 1 = weak, 2 = moderate, 3 = strong. N, Nuclear; C, cytoplasm.
Strong nuclear staining of ERα was observed in the glandular cells of both proliferative and secretory phases. In the stromal cells, however, nuclear staining was prominent in the proliferative phase and decreased drastically in the secretory phase (Fig. 1A, middle, and Table 1). In the post-menopausal sections, the nuclei stained strong for ERα in both the glandular and stromal cells (Fig. 1B, middle, and Table 1). The glandular cells showed prominent cytoplasmic staining for ERβ in both the proliferative and secretory phases. In the stromal cells, staining was less prominent in the proliferative phase and increased in the secretory phase (Fig. 1A, lower, and Table 1). However, in the postmenopausal stage, the glandular cells showed strong cytoplasmic staining, and the cells of the stroma showed very weak staining (Fig. 1B, lower). Because both PELP1 and ERβ were confined to the cytoplasm of the glandular cells in tissue from postmenopausal women, a time when women are more prone to endometrial cancer, these findings raised the possibility of a relationship between these molecules and tumorigenesis.
PELP1 interacts with ERs and acts as a coactivator of both ERα and ERβ in endometrial cancer cells
We then analyzed the expression of PELP1 in widely used endometrial cell lines (Ishikawa and RL 95–2) and in control MCF-7 (ERα positive) and MDA-MB-231 (ERβ positive) breast cancer cells. PELP1 was widely expressed in endometrial cancer cells (Fig. 2A). To examine whether PELP1 positively contributes to E2-mediated signaling in endometrial cells, we performed ER transactivation assays using ER-positive Ishikawa cells as a model system. Coexpression of PELP1 increased ERE-luc activity in ligand-stimulated cells by 9-fold, compared with 6-fold observed in vector-transfected cells, suggesting that PELP1 also acts as a coactivator of ER in endometrial cells (Fig. 2B).
Fig. 2.
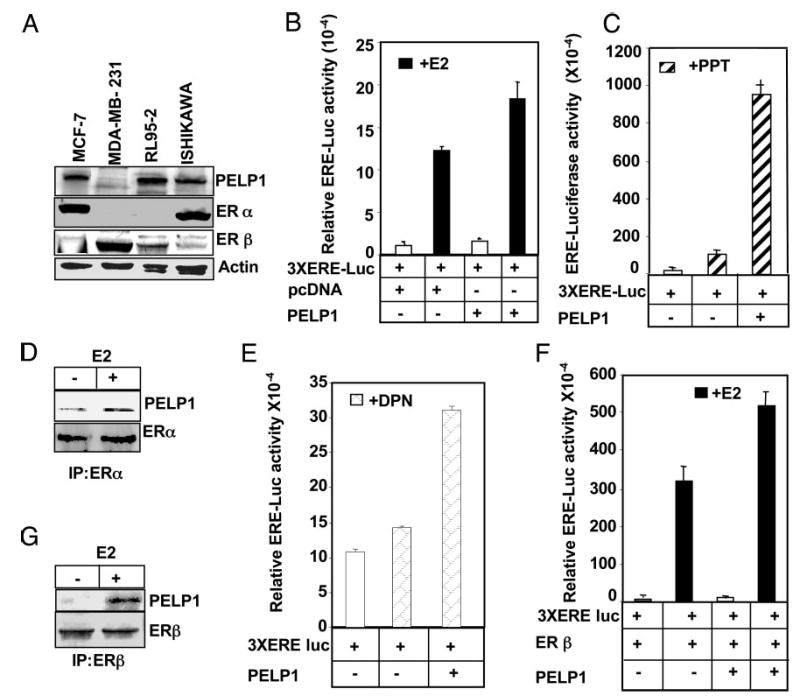
Functional interaction of PELP1 with ERα and ERβ in endometrial cells. A, Western blot, analyzing the expression of PELP1 in endometrial cancer cell lines. B, PELP1 enhances the E2-mediated reporter gene activation in Ishikawa cells. C and E, PELP1 potentiates ERα-and ERβ-specific ligand PPT- and DPN-mediated ERE reporter gene transcription. D and G, Equal amounts of cell lysates from Ishikawa cells cultured in 10% serum were subjected to immunoprecipitation (IP) with ERα and ERβ antibodies, followed by Western blotting with PELP1 antibody. F, PELP1 potentiates ERβ-mediated reporter gene activation. Ishikawa cells were cotransfected with 3XERE-luc reporter gene, the ERβ expression vector, with or without the PELP1 expression vector; cells were treated with E2 (10−9 m), and ERE-luc reporter activation was measured after 16 h.
Because the endometrium expresses both subtypes of ERs (28), we next investigated whether PELP1 modulates the transactivation functions of both ER subtypes using ERα- or ERβ-specific ligands. When PELP1-transfected cells were treated with PPT (29), the ERα-specific ligand, 3XERE-luc reporter activity was increased nine times more than the vector-transfected control (Fig. 2C), suggesting that PELP1 coactivates ERα-dependent transcription and cooperates with the endogenous ERα and its specific ligand PPT. In consonance with these results, treatment of Ishikawa cells with E2 resulted in an enhanced association of PELP1 with ERα in vivo (Fig. 2D). As shown in Fig. 2E, treatment of Ishikawa cells with an ERβ-specific ligand DPN (29) also stimulated ERE-luc activity, though only three times more than seen in control cells, suggesting that PELP1 can also cooperate with the transcriptional activity of ERβ. To validate these results, we showed that PELP1 also promotes ERβ transcriptional activity in Ishikawa cells when cotransfected with PELP1 (Fig. 2F). These findings are relevant to the biology of endometrial cancer cells because endogenous PELP1 effectively interacts with ERβ in Ishikawa cells in a ligand-dependent manner (Fig. 2G). Collectively these results suggest that PELP1 acts as a coactivator of both ER subtypes; however, PELP1 exhibited more magnitude of coactivation with ERα compared with ERβ in Ishikawa cells.
Status of the PELP1 protein controls the magnitude of ER response
To examine the potential role of PELP1 in ERE-mediated transcription in endometrial cells, we selectively knocked down the endogenous PELP1 expression using the siRNA methodology. The efficacy of PELP1-specific siRNA in down-regulating endogenous PELP1 was confirmed by Western blotting (Fig. 3A), which showed a 70–80% reduction in the level of endogenous PELP1 compared with cells treated with control siRNA. We also found that down-regulation of PELP1 caused a significant decrease in 3XERE-luc reporter gene activity after ligand treatment, compared with cells treated with control siRNA (Fig. 3B). We next examined the effect of PELP1 down-regulation on the growth of E2-stimulated Ishikawa cells. Estrogen stimulated growth of control siRNA-treated Ishikawa cells, whereas down-regulation of PELP1 by siRNA was accompanied by some reduction in the basal proliferation as well as the ability of estrogen to stimulate the growth of Ishikawa cells (Fig. 3C). These results suggest that PELP1 signaling plays an important role in generating optimal E2 responses in endometrial cells.
Fig. 3.
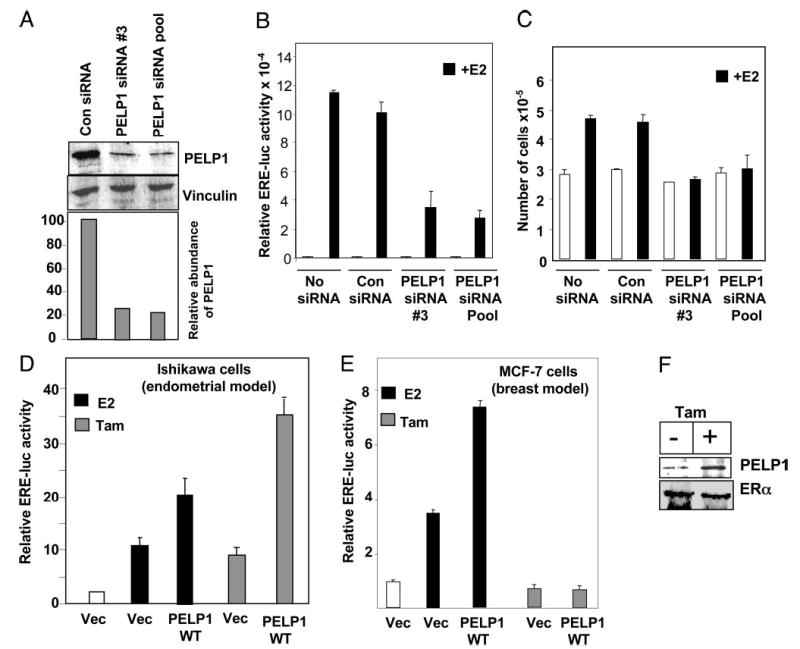
PELP1 down-regulation by siRNA reduces E2-mediated responses in Ishikawa cells. A, Ishikawa cells were treated with nonspecific or PELP1-specific siRNAs. Total cell lysates were analyzed by Western blotting using the PELP1 antibody. B, Ishikawa cells were transfected with ERE reporter gene along with nonspecific siRNA or PELP1-specific siRNAs. After 48 h, cells were treated with or without E2 (10−9 m) for 16 h, and the reporter gene activity was measured. C, Ishikawa cells were transfected with nonspecific or PELP1-specific siRNAs, treated with or without E2 (10−9 m), and cell number was counted after 72 h. D and E, PELP1 promotes tamoxifen (Tam)-mediated agonist action in the endometrium. Ishikawa cells (D) and MCF-7 cells (E) were transfected with 3XERE reporter gene along with or without PELP1 expression vector (Vec). After 48 h, cells were treated with either E2 (10−9 m) or Tam (10−8 m), and the reporter gene activity was measured after 24 h. F, Ishikawa cells were treated with or without Tam, lysates were immunoprecipitated with ERα antibody, and the presence of PELP1 in the immunoprecipitates was analyzed by Western blotting.
Potential role of PELP1 in tamoxifen agonism in endometrial cells
Tamoxifen behaves as an agonist in endometrial tissue. It is speculated that the tissue-specific expression of novel regulators or the specific modification of coregulators contributes to the tamoxifen’s agonism (4). Because PELP1 is a novel coactivator, we examined whether PELP1 cooperates with tamoxifen in endometrial cancer cells. We found that the expression of PELP1 substantially increased the tamoxifen-mediated agonist response in the endometrial cell line (Fig. 3D), whereas it had no such effect in MCF-7 cells, in which tamoxifen behaved as an antagonist of the estrogen response (Fig. 3E). Consistent with a potential interaction of PELP1 with tamoxifen-responsive pathways, the interaction of PELP1 with ER increased after treatment with tamoxifen (Fig. 3F). These results suggest that PELP1 has a role in tamoxifen-mediated agonist actions in endometrial cells.
Expression of PELP1 and ERs in endometrial tumors
To understand the physiological significance of PELP1 in endometrial cancer, we next examined the expression of PELP1 in sections of cancerous endometrium. For this, we first determined the levels of PELP1 in five endometrial tumor lysates. The expression of PELP1 was higher in endometrial tumor samples than in the postmenopausal specimens, which showed very weak expression (Fig. 4A). Interestingly, four of five endometrial tumors exhibited weak ERα expression compared with ERβ, which showed increased expression in all tumors. To gain further insight into the subcellular localization of PELP1 in tumors, we immunostained PELP1 in a subset of 60 endometrial tumors. PELP1 was expressed in all the tumors, but the subcellular localization of PELP1was variable. Specifically, in 31.6% (19 of 60) of the tumor samples, cytoplasmic staining was prominent (Table 2), and an intense nuclear staining was observed in only 6.6% (4 of 60) of tumors (Table 2), whereas both nuclear and cytoplasmic PELP1 staining was observed in 61.6% (37 of 60) of the endometrial tumor specimens. The percentages of tumors showing cytoplasmic, nuclear, or both nuclear and cytoplasmic staining and their staining intensities are summarized in Table 2.
Fig. 4.
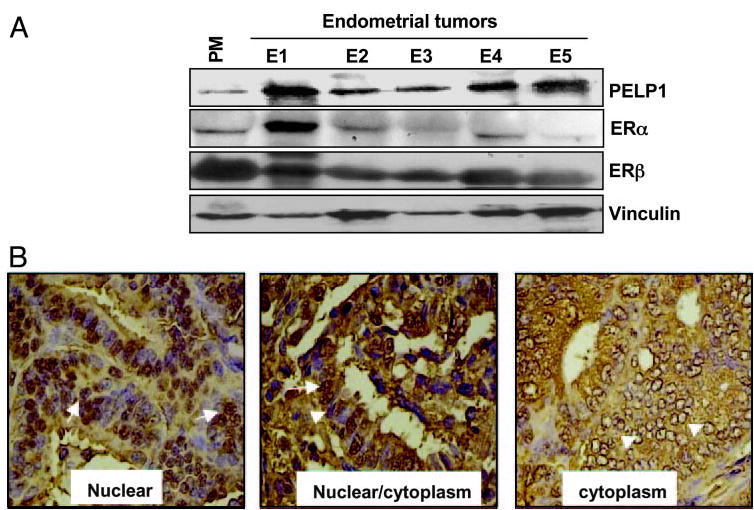
PELP1 expression in human endometrial carcinomas. A, Total lysates from endometrial tumors were analyzed by Western blot analysis using PELP1 antibody. Postmenopausal (PM) samples were used as a control. B, Immunohistochemical localization of PELP1 in human endometrial carcinoma. Nuclear staining is shown by arrows and cytoplasmic staining by arrowheads. Magnification, ×200.
TABLE 2.
Summary of immunostaining intensity and localization of PELP1 in endometrial tumors
| n | Localization | Immunohistochemistry score |
|---|---|---|
| 4 of 60 | Nuclear | Weak/focal (1+) |
| 37 of 60 | Nuclear/cytoplasm | Moderate (2+) |
| 19 of 60 | Cytoplasm | Strong (3+) |
To determine the potential relationship of PELP1 and ER isoforms as a function of tumor grades in endometrial tumors, we also performed immunohistochemical analyses of PELP1, ERα, and ERβ in a series of carefully selected endometrial tumors from patients with grades 1 and 3 disease (n = 9). The expression of nuclear ERα was reduced as one went from grade 1 to 3 (Fig. 5). Interestingly, however, the expression of both PELP1 and ERβ was maintained in grade 3 tumors, and PELP1 and ERβ showed a distinct cytoplasmic localization in grade 1 and 3 tumors.
Fig. 5.
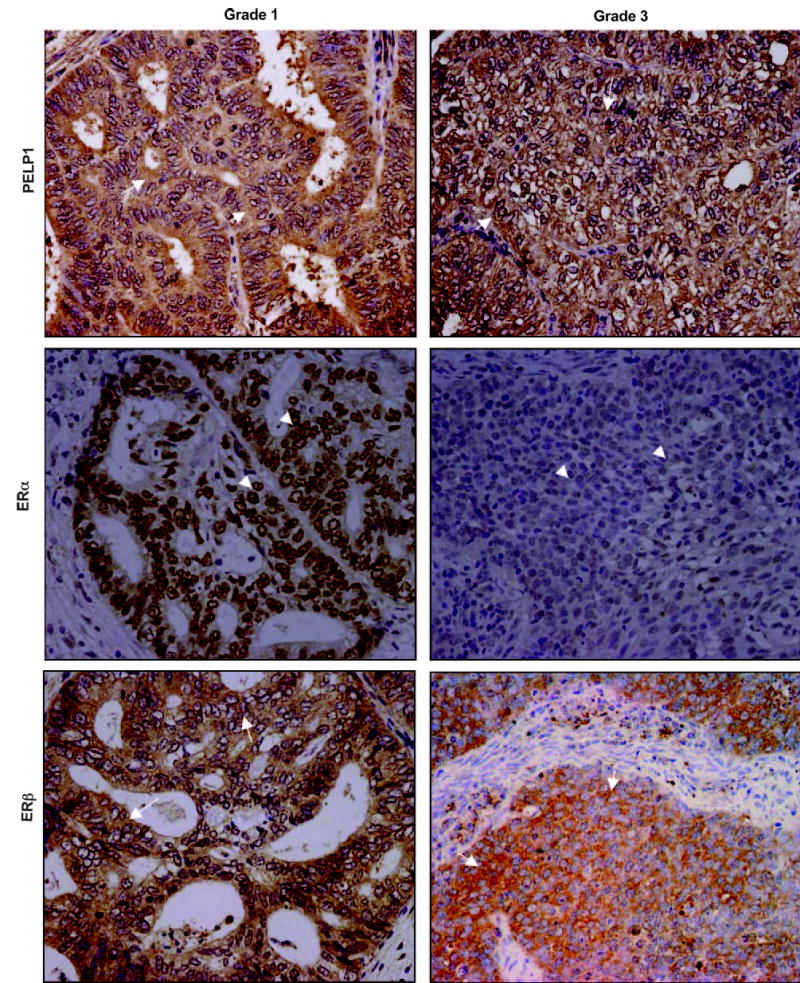
Immunohistochemical expression of ERα, ERβ, and PELP1 in grades 1 and 3 endometrial tumors. ERα expression (arrowheads) was high in grade 1 tumor and reduced in grade 3 tumor. Increased expression of both PELP1 and ERβ (arrows) was observed in grade 1 and 3 tumors. Magnification, ×200.
Discussion
We have shown that the newly discovered ER coactivator PELP1 is differently expressed and localized in the cells of different stages of the benign endometrium and in endometrial adenocarcinoma. Using a variety of independent biochemical and cellular assays, we found that PELP1 is a transcriptional coactivator for both ERα and ERβ and that PELP1 interacts with both receptors in a ligand-dependent manner in endometrial cancer cells. Expression of PELP1 in different stages of endometrial development and its functional interactions with both ER subtypes further suggest that it plays an important role in ER signaling in the endometrium.
Estradiol regulates cell proliferation in a wide variety of tissues. Several studies demonstrated that coactivators are critical for efficient proliferation induced by E2 (3, 5, 13). Earlier studies also suggested that ER coactivator PELP1 plays an important role in E2-mediated G1-S progression in breast cancer cells (18). PELP1 interacts with cell cycle switch protein pRb and plays a permissive role in E2-mediated cell cycle progression, presumably via its regulatory interactions with pRb pathway (18). In the present study, using siRNAs that specifically target PELP1, we have demonstrated that PELP1 also plays an essential role in E2-mediated cell proliferation of endometrial cells, further supporting a role of PELP1 in E2-mediated cell cycle progression.
Recently, using biochemical methods and confocal microscopy, we have provided evidence that in breast cancer cells, a substantial amount of PELP1 localizes in the nuclear compartment (19). Ligand stimulation promotes PELP1 localization to the ER target gene promoters and colocalizes with active chromatin. PELP1 also interacts with histones, and such functions have a role in ER transactivation functions (19). In this study, we found that PELP1 localizes to the cytoplasm in the postmenopausal endometrium but localizes to the nucleus during the proliferative and secretory phases of the premenopausal endometrium. Further, we have also observed that in postmenopausal endometrium, ERβ is also localized in the cytoplasm. In addition, endogenous PELP1 interacts with ERβ and plays a mechanistic role in the enhanced ligand sensitivity of ERβ in endometrial cancer cells. Collectively, the results from this study suggest that PELP1-mediated genomic and nongenomic signaling plays an important role in proliferative and secretory phases of endometrium, whereas PELP1-mediated nongenomic functions may play a role in postmenopausal endometrium.
ER coregulatory proteins are suspected to play a role in the generally observed tissue-specific effects of tamoxifen (4). Although tamoxifen has been used to treat breast cancer, tamoxifen use has also been associated with endometrial thickening, dysfunctional uterine bleeding, endometrial polyps, endometrial hyperplasia, and uterine sarcoma (30). Our findings suggest that the coregulator PELP1 may play a role in the tamoxifen-mediated partial agonist action seen in the endometrium, because overexpression of PELP1 promoted tamoxifen-mediated partial agonist action in the endometrial cells but not in the breast cells. It is increasingly accepted that deregulation of nongenomic pathways, such as MAPK activation, plays a role in tamoxifen-mediated agonist actions in endometrial cells (31, 32). Because PELP1 stimulates the Src-MAPK pathway (16), it is possible that the noted partial agonist activity of PELP1 in endometrial cells is also influenced by the nongenomic action of PELP1.
In this study, we observed that ER coactivator PELP1 was widely expressed in endometrial cancer cell lines and also in endometrial adenocarcinoma, which constitutes more than 80% of the cases of endometrial cancer. Interestingly, in tumors, PELP1 was localized predominantly to the cytoplasm; and in several cases, ERβ was also localized to the cytoplasm. Because PELP1 acts as a coactivator of ERβ, presence of ERβ and PELP1 in endometrial tumors raises a possibility that ERβ-PELP1 signaling may play a role in endometrial tumorigenesis. Because the level of PELP1 is modulated by a ligand-dependent pathway (33), the increased expression seen in endometrial tumors could reflect up-regulation of the E2-ER signaling pathway. It is also possible that the up-regulation of PELP1 helps to hypersensitize ER signaling, thus causing ER signaling to function as an autocrine loop in the ER-positive tumor cells. Future studies are warranted to test the possibility that PELP1 expression is a marker of endometrial tumor progression.
Several studies have shown ERβ to be localized to membranes (34), cytoplasm (35), and mitochondria (36), in addition to its nuclear localization, supporting a potential involvement of ERβ in nongenomic signaling. Our findings that ERβ is present in the cytoplasm of postmenopausal and grade 3 endometrial tumors suggest nongenomic functions for ERβ in the development of endometrial cancer. Interestingly, in platelets, which preferentially express ERβ isoform, it was shown that ERβ activates Src kinase via nongenomic actions, suggesting a possibility that similar activation of nongenomic signaling may occur in the endometrium (37). Because PELP1/MNAR interacts with ERβ and activates Src kinase in an E2-dependent manner, the coexpression and localization of both ERβ and PELP1 in a subset of endometrial tumors indicate a role for PELP1 and ERβ in the pathobiology of endometrial tumors via activation of nongenomic pathways.
In summary, we have characterized the expression and localization of PELP1, a novel ER coregulatory protein, in benign and cancerous endometrium. Our results suggest that PELP1 plays an important role in the transcriptional responses of both ER isoforms in the endometrial cancer cells. Furthermore, the ability of PELP1 to enhance tamoxifen agonist action, the overexpression of PELP1 in endometrial tumors, and its distinct localization in various stages of endometrium suggest that PELP1 plays a role in the biology of benign and cancerous endometrium.
Acknowledgments
This work was supported, in part, by the National Institutes of Health Grants CA095681 (to R.K.V.) and CA90970 and CA109379 (to R.K.) and by the M. D. Anderson Cancer Center Multidisciplinary Research Program-Uterine Cancer.
References
- 1.Irwin JC, Utian WH, Eckert RL. Sex steroids and growth factors differentially regulate the growth and differentiation of cultured human endometrial stromal cells. Endocrinology. 1991;129:2385–2392. doi: 10.1210/endo-129-5-2385. [DOI] [PubMed] [Google Scholar]
- 2.Punyadeera C, Verbost P, Groothuis P. Oestrogen and progestin responses in human endometrium. J Steroid Biochem Mol Biol. 2003;84:393–410. doi: 10.1016/s0960-0760(03)00061-x. [DOI] [PubMed] [Google Scholar]
- 3.Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9:1980–1989. [PubMed] [Google Scholar]
- 4.Shao W, Brown M. Advances in estrogen receptor biology: prospects for improvements in targeted breast cancer therapy. Breast Cancer Res. 2004;6:39–52. doi: 10.1186/bcr742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafsson JA. What pharmacologists can learn from recent advances in estrogen signalling. Trends Pharmacol Sci. 2003;24:479–485. doi: 10.1016/S0165-6147(03)00229-3. [DOI] [PubMed] [Google Scholar]
- 6.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 7.Warner M, Nilsson S, Gustafsson JA. The estrogen receptor family. Curr Opin Obstet Gynecol. 1999;11:249–254. doi: 10.1097/00001703-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Speirs V, Carder PJ, Lane S, Dodwell D, Lansdown MR, Hanby AM. Oestrogen receptor β: what it means for patients with breast cancer. Lancet Oncol. 2004;5:174–181. doi: 10.1016/S1470-2045(04)01413-5. [DOI] [PubMed] [Google Scholar]
- 9.Simoncini T, Fornari L, Mannella P, Varone G, Caruso A, Liao JK, Genazzani AR. Novel non-transcriptional mechanisms for estrogen receptor signaling in the cardiovascular system. Interaction of estrogen receptor α with phosphotidylinositol 2-OH kinase. Steroids. 2002;67:935–939. doi: 10.1016/s0039-128x(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 11.Levin ER. Cellular functions of the plasma membrane estrogen receptor. Trends Endocrinol Metab. 1999;10:374–377. doi: 10.1016/s1043-2760(99)00192-7. [DOI] [PubMed] [Google Scholar]
- 12.Glass CK, Rose DW, Rosenfeld MG. Nuclear receptor coactivators. Curr Opin Cell Biol. 1997;9:222–232. doi: 10.1016/s0955-0674(97)80066-x. [DOI] [PubMed] [Google Scholar]
- 13.Barnes CJ, Vadlamudi RK, Kumar R. Novel estrogen receptor coregulators and signaling molecules in human diseases. Cell Mol Life Sci. 2004;61:281–291. doi: 10.1007/s00018-003-3222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards DP. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia. 2000;5:307–324. doi: 10.1023/a:1009503029176. [DOI] [PubMed] [Google Scholar]
- 15.Vadlamudi RK, Wang RA, Mazumdar A, Kim Y, Shin J, Sahin A, Kumar R. Molecular cloning and characterization of PELP1, a novel human co-regulator of estrogen receptor α. J Biol Chem. 2001;276:38272–38279. doi: 10.1074/jbc.M103783200. [DOI] [PubMed] [Google Scholar]
- 16.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol. 2004;18:1096–1108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- 18.Balasenthil S, Vadlamudi RK. Functional interactions between the estrogen receptor coactivator PELP1/MNAR and retinoblastoma protein. J Biol Chem. 2003;278:22119–22127. doi: 10.1074/jbc.M212822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair S, Mishra S, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. PELP1/MNAR modulate ER genomic functions via Histone H1 interactions. Cancer Res. 2004;64:6416–6423. doi: 10.1158/0008-5472.CAN-04-1786. [DOI] [PubMed] [Google Scholar]
- 20.Balasenthil S, Zhibo Y, Gustafsson JA, Kumar R, Vadlamudi RK. Potential role of PELP1 in tamoxifen resistance. Breast Cancer Res Treat. 2003;85:S14. [Google Scholar]
- 21.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 22.Clarke R, Leonessa F, Welch JN, Skarr TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001;53:25–71. [PubMed] [Google Scholar]
- 23.Uchikawa J, Shiozawa T, Shih HC, Miyamoto T, Feng YZ, Kashima H, Oka K, Konishi I. Expression of steroid receptor coactivators and corepressors in human endometrial hyperplasia and carcinoma with relevance to steroid receptors and Ki-67 expression. Cancer. 2003;98:2207–2213. doi: 10.1002/cncr.11760. [DOI] [PubMed] [Google Scholar]
- 24.Shiozawa T, Shih HC, Miyamoto T, Feng YZ, Uchikawa J, Itoh K, Konishi I. Cyclic changes in the expression of steroid receptor coactivators and corepressors in the normal human endometrium. J Clin Endocrinol Metab. 2003;88:871–878. doi: 10.1210/jc.2002-020946. [DOI] [PubMed] [Google Scholar]
- 25.Gregory CW, Wilson EM, Apparao KB, Lininger RA, Meyer WR, Kowalik A, Fritz MA, Lessey BA. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab. 2002;87:2960–2966. doi: 10.1210/jcem.87.6.8572. [DOI] [PubMed] [Google Scholar]
- 26.Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod. 2002;66:297–304. doi: 10.1095/biolreprod66.2.297. [DOI] [PubMed] [Google Scholar]
- 27.Cheng G, Weihua Z, Warner M, Gustafsson JA. Estrogen receptors ERα and ERβ in proliferation in the rodent mammary gland. Proc Natl Acad Sci USA. 2004;101:3739–3746. doi: 10.1073/pnas.0307864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M. Presence of estrogen receptor β in the human endometrium through the cycle: expression in glandular, stromal, and vascular cells. J Clin Endocrinol Metab. 2001;86:1379–1386. doi: 10.1210/jcem.86.3.7322. [DOI] [PubMed] [Google Scholar]
- 29.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor α- and α-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 30.Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres’ ALERT Group. Assessment of liver and endometrial cancer risk following tamoxifen. Lancet. 2000;356:881–887. doi: 10.1016/s0140-6736(00)02677-5. [DOI] [PubMed] [Google Scholar]
- 31.Marsaud V, Gougelet A, Maillard S, Renoir JM. Various phosphorylation pathways, depending on agonist and antagonist binding to endogenous estrogen receptor α(ERα), differentially affect ERα extractability, proteasome-mediated stability, and transcriptional activity in human breast cancer cells. Mol Endocrinol. 2003;17:2013–2027. doi: 10.1210/me.2002-0269. [DOI] [PubMed] [Google Scholar]
- 32.Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331S–336S. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 33.Mishra SK, Balasenthil S, Nguyen D, Vadlamudi RK. Cloning and functional characterization of PELP1 promoter. Gene. 2004;330:115–122. doi: 10.1016/j.gene.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERβ has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- 35.Skliris GP, Parkes AT, Limer JL, Burdall SE, Carder PJ, Speirs V. Evaluation of seven oestrogen receptor β antibodies for immunohistochemistry, Western blotting, and flow cytometry in human breast tissue. J Pathol. 2002;197:155–162. doi: 10.1002/path.1077. [DOI] [PubMed] [Google Scholar]
- 36.Yang SH, Liu R, Perez EJ, Wen Y, Stevens Jr SM, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor β. Proc Natl Acad Sci USA. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moro L, Reineri S, Piranda D, Pietrapiana D, Lova P, Bertoni A, Graziani A, Defilippi P, Canobbio I, Torti M, Sinigaglia F 2004 Nongenomic effects of E2 in human platelets: potentiation of thrombin-induced aggregation through estrogen receptor β and src kinase. Blood, in press, DOI10.1182 [DOI] [PubMed]


