Abstract
Proline-, glutamic acid-, and leucine-rich protein-1 (PELP1) is a novel estrogen receptor coactivator that plays an important role in the genomic and nongenomic actions of estrogen receptor by interacting with histones and src-mitogen-activated protein kinase pathway, respectively. A great deal of information has emerged in recent years about the possible role of PELP1 in estrogen receptor signaling. However, the participation and significance of PELP1 in other cellular signaling pathways remains unknown. Using a yeast two-hybrid screen, we identified PELP1 as a novel interacting protein of signal transducers and activators of transcription 3 (STAT3) and found evidence of physiologic interaction between PELP1 and STAT3. We also found that these interactions played a mechanistic role in the positive regulation of STAT3 transcription from synthetic promoters and endogenous target genes such as cyclin D1, c-myc, and c-fos. Overexpression of PELP1 enhanced phosphorylation of STAT3 at Ser727 in a src-mitogen-activated protein kinase–sensitive manner and, conversely, down-regulation of PELP1 compromised growth factor–mediated induction of STAT3 target genes. We also discovered that PELP1 interacts with STAT3 in the nuclear compartment and down-regulation of PELP1 interfered with the recruitment of STAT3 to its target gene promoters. In summary, our results highlight a novel role for PELP1 in growth factor signaling and indicate that PELP1-mediated genomic and nongenomic functions play a role in the growth factor–mediated STAT3 transactivation functions. Such regulatory interactions of PELP1 may have important functional implications in the cross-talk of estrogen receptor and growth factor signaling.
Introduction
Proline-, glutamic acid-, and leucine-rich protein-1 (PELP1)/modulator of nongenomic activity of estrogen receptor is a novel coactivator of estrogen receptor widely expressed in a number of tissues (1). PELP1 participates in estrogen receptor genomic signaling through chromatin remodeling by displacement of histone H1 (2) and by functional interactions with retinoblastoma protein (3). PELP1 has also been shown to participate in estrogen receptor nongenomic signaling by activation of src kinase (4). PELP1 acts as a coactivator of both estrogen receptor subtypes (estrogen receptor α and β; ref. 5) and its expression is up-regulated by estradiol-estrogen receptor signaling (6). PELP1 expression and localization is deregulated in breast and endometrial tumors (1, 5). Even though a great deal of information has emerged in recent years on the possible role of PELP1 in estrogen receptor signaling, the participation and significance of PELP1 in other cellular signaling pathways remains unknown.
Signal transducers and activators of transcription (STAT) proteins are latent cytoplasmic transcription factors activated through receptors that bind cytokines, growth factors, or peptides (7). Ligand binding triggers intracellular tyrosine phosphorylation and self-dimerization, followed by nuclear translocation, which exerts transcriptional activity through direct binding to the specific target promoters of STAT (8). STAT3 is ubiquitously expressed in most tissues and is activated by various growth factor–mediated signaling pathways, including epidermal growth factor (EGF), and also by numerous oncogenic tyrosine kinases (9). Tyrosine phosphorylation of STAT3 at Tyr705 is required for STAT3 activation and for tumorigenicity (10).
Emerging data suggest that in addition to tyrosine phosphorylation, serine phosphorylation of STAT3 at Ser727 is required for STAT3 to be optimally activated (11–13). In neuronal systems, phosphorylation of STAT3 at Ser727 plays an important role in neuronal migration (14). In macrophages, phosphorylation of STAT3 at Ser727 is implicated in cell survival (15). Constitutive serine phosphorylation has also been reported with B-cell–derived tumors (16). Inhibition of STAT3 serine phosphorylation was reported to reduce transformation by the v-src (17). Together, these findings suggest a significant role for STAT3 phosphorylation at Ser727, both in physiologic and pathologic conditions. The mechanisms and kinases that regulate the serine phosphorylation of STAT3 are elusive and constitute an active area of investigation.
In this study, using a yeast based two-hybrid screen, we identified PELP1 as a novel interacting protein of STAT3. We provide evidence that EGF receptor (EGFR) signaling promotes the association of PELP1 with STAT3 and that PELP1 is required for growth factor–mediated optimal STAT3 Ser727 phosphorylation and activation in breast cancer cells.
Materials and Methods
Cell culture and reagents.
COS-1, HeLa, MCF-7, immortalized mouse fibroblast cells, and immortalized mouse fibroblast cells lacking src kinases (SYF) were purchased from American Tissue Culture Collection (Manassas, VA) and were maintained in DMEM and F-12 (1:1) supplemented with 10% FCS. pcDNA- and PELP1-expressing stable cell lines were earlier described (3). Antibodies against STAT3, phospho-STAT (Ser727), phospho-STAT3 (Tyr705), and phospho-p42/44 mitogen-activated protein kinase (MAPK) were purchased from Cell Signaling Technology, Inc. (Beverly, MA), and antibodies against STAT3 (C-20), EGFR, extracellular signal-regulated kinase 1/2, and lamin B were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-T7 epitope antibody was purchased from Novagen (Madison, WI). Antibodies against paxillin were purchased from Neo-Markers, Inc. (Fremont, CA).
Two-hybrid library screening and plasmid construction.
PELP1 bait was constructed by amplifying amino acids 1 to 600 by PCR and subcloning the products into Gal4 DNA binding domain (GBD) vectors pGBD vector (Clontech, Mountain View, CA). pGBD-PELP1 (1-600) was used as a bait to screen the mammary gland cDNA library fused to Gal4 activation domain (GAD; Clontech) as per instructions of the manufacturer. A total of 2 × 106 clones were screened and positive clones were isolated and sequenced at M.D. Anderson Cancer Center core sequencing facility. Positive clones were also verified by cotransformation using purified plasmids and by selection on agar plates lacking adenine, histidine, tryptophan, and leucine. The wild-type PELP1 and mutant PELP1 (1-880) constructs were described previously (2). GST-STAT 1-320 construct was generated by amplifying the selective region of STAT3 and cloning into pGEX 5× (Amersham, Pitscataway, NJ).
Glutathione S-transferase pulldown assays.
The glutathione S-transferase (GST) pulldown assays were done by incubating equal amounts of GST or GST-STAT3 deletions immobilized on glutathione-sepharose beads (Amersham Biosciences Corp., Piscataway, NJ) with cellular lysates obtained from MCF-7 cells stably expressing T7-tagged PELP1. The mixtures were incubated for 2 hours at 4°C and washed six times with NP40 lysis buffer. Bound proteins were eluted with 2× SDS buffer, separated by SDS-PAGE, and immunoblotted.
Transient transfection, immunoprecipitation, and immunoblotting.
COS-1 cells were transiently transfected with 2.5 μg of T7-PELP1 and GST-STAT3 vectors or T7-PELP1 and pEBG control vector in a 70% confluent, 60-mm plate by using the FuGENE6 method (Roche Diagnostics Corporation, Indianapolis, IN). After 16 hours of transfection, the cells were serum starved for 24 hours by culturing them in 0% serum–containing medium and then treated with 100 ng/mL of EGF for either 5 or 10 minutes. The cells were washed with PBS, lysed in NP40 buffer, and immunoprecipitated with T7 monoclonal antibody overnight at 4°C. Rabbit anti-mouse antibody sepharose beads were added for 1 hour. After three washes, the NP40 buffer samples were resuspended in 2× SDS loading buffer and separated by 8% SDS-PAGE gels. The proteins were transferred to a nitrocellulose membrane overnight at 4°C and then developed with anti-T7 monoclonal antibody (mouse) or GST antibody (rabbit).
Cell fractionation studies.
HeLa cells were serum starved for 24 hours by culturing in 0% serum–containing medium and then treated with 100 ng/mL EGF for 15 minutes. The cells were washed twice with PBS buffer, and the cytoplasmic and nuclear extracts were prepared according to a previously described procedure (11). Equal amounts of cytoplasmic and nuclear extracts were incubated with STAT3 antibody for 4 hours at 4°C and immunocomplexes were precipitated with protein-A beads. The precipitated proteins were eluted with 2× SDS buffer and analyzed on 8% SDS-PAGE gels. For the immunoblotting, PELP1 antibody was used at 1:500 dilution.
Immunofluorescence and confocal studies.
The cellular colocalization of PELP1/STAT3 was determined by indirect immunofluorescence. The cells were grown in 0% serum for 24 hours and treated with 100 ng/mL EGF for 15 minutes. The cells were then briefly washed with PBS. Cells grown on glass coverslips were fixed in methanol at −20°C for 4 minutes. After being fixed, the cells were incubated with primary antibodies for 1 hour, washed thrice with PBS, and then incubated with secondary antibodies conjugated with Alexa 546 (red), Alexa 633 (blue), or Alexa 488 (green; Molecular Probes, Eugene, OR). The DNA dye Topro-3 (Molecular Probes) was used to costain the DNA (blue). Cells treated only with the secondary antibodies were used as the controls. A confocal scanning analysis was done with a Zeiss laser scanning confocal microscope or with an Olympus FluoView 300 confocal microscope in accordance with established methods using sequential laser excitation to minimize the fluorescent emission bleed-through. Each section was examined for the presence of each stain at two excitations (546 and 488 or 633 nm, as indicated in the text), and the data were compared pixel by pixel. Each image represented z sections at the same cellular level and magnification; a three-dimensional reconstructed image was used to visualize the whole sample. Colocalization of two proteins, shown by red and green or green and blue dyes, was shown by the development of yellow or turquoise colors, respectively.
Reporter gene assays.
We have used 3XLY6E-Gas-luciferase reporter that contained three STAT3 binding sites as a STAT3 reporter (11). Cyclin D1 promoter luciferase reporter was earlier described (3). For the reporter gene transient transfections, the cells were cultured in DMEM supplemented with 10% fetal bovine serum for 24 hours and then cotransfected with 500 ng of the luciferase reporter along with 100 ng of PELP1 wild-type expression plasmid or 100 ng of pCMV vector by using the FuGENE-6 transfection reagent (Roche Diagnostics) according to the instructions of the manufacturer. After 16 hours, the cells were grown in 0% serum for 24 hours and treated with EGF for 6 hours. After a brief washing with PBS, the cells were lysed in passive-lysis buffer, and the luciferase assay was done using a luciferase reporter assay kit (Promega). The activity of the β-galactosidase reporter was used to correct the transfection efficiencies.
Chromatin immunoprecipitation analysis.
Approximately 106 cells were treated with 1% formaldehyde ( final concentration, v/v) for 10 minutes at 37°C. Chromatin immunoprecipitation was done as previously described with STAT3 (C20) antibody (2). The primers used were c-fos: GCCTTGGCGCGTGTCCTAATC (F), GCAGCCCGCGAGCAGTT (R); c-myc: CACAGGACAAGGATGCGGTT (F), CCTCTGCCTCTCGCTGGAAT (R).
Interference with proline-, glutamic acid-, and leucine-rich protein-1 expression by small interfering RNA.
The design and synthesis of PELP1 small interfering RNA (siRNA) were previously described (5). Briefly, MCF-7 cells were seeded at a 30% density the day before transfection in six-well plates. On the day of transfection, the cells were changed to antibiotic-free 10% serum–containing medium. Transfections were done with the oligofectamine reagent (Invitrogen, Carlsbad, CA) according to the instructions of the manufacturer with 12 μL of 20 μmol/L siRNA and 4 μL of oligofectamine reagent per well in six-well plates (final concentration, 50 nmol/L). For the EGF treatment, the cells were cultured in 0% normal serum for 24 hours and then treated for 15 minutes with 100 ng/mL EGF.
Results
Proline-, glutamic acid-, and leucine-rich protein-1 interacts with signal transducers and activators of transcription 3.
To gain insight into the functions of PELP1, we used a yeast two-hybrid screen to identify the proteins that interact with PELP1. Screening of a mammary gland cDNA expression library (10−6 transformants) with the PELP1 NH2-terminal region as the bait resulted in the isolation of several positive clones. One of the positive clone sequences matched with that of STAT3. The specificity of STAT3 and PELP1 interaction in yeast was further confirmed using cotransformation followed by survival assay in selection medium using yeast cells which stably express histidine, tryptophan, and leucine nutrient reporter genes under the control of GAL response elements. The GBD-PELP1 and GAD-STAT3 transformed colonies grew in medium lacking adenosine, histidine, tryptophan, and leucine, whereas the cells cotransformed with the control GBD vector and GAD-STAT3 did not grow (Fig. 1A), suggesting a possible interaction of STAT3 and PELP1 in yeast. To further verify the interaction between STAT3 and PELP1 in mammalian cells, we transiently cotransfected COS-1 cells with T7-PELP1 and GST-STAT3. The cell lysates were immunoprecipitated with GST beads to precipitate GST-STAT3 and subjected to immunoblotting with STAT3 or T7 antibodies, respectively. The immunoprecipitation results showed that PELP1 interacted with STAT3 (Fig. 1B). Next, we examined the physiologic association of PELP1 and STAT3 in MCF7 breast cancer cells. Immunoprecipitation of EGF-treated or untreated MCF-7 cell lysates showed an increased association between PELP1 and STAT3 when stimulated with EGF (Fig. 1C). Collectively, these results suggest that PELP1 interacts with STAT3 in vitro and in vivo.
Figure 1.
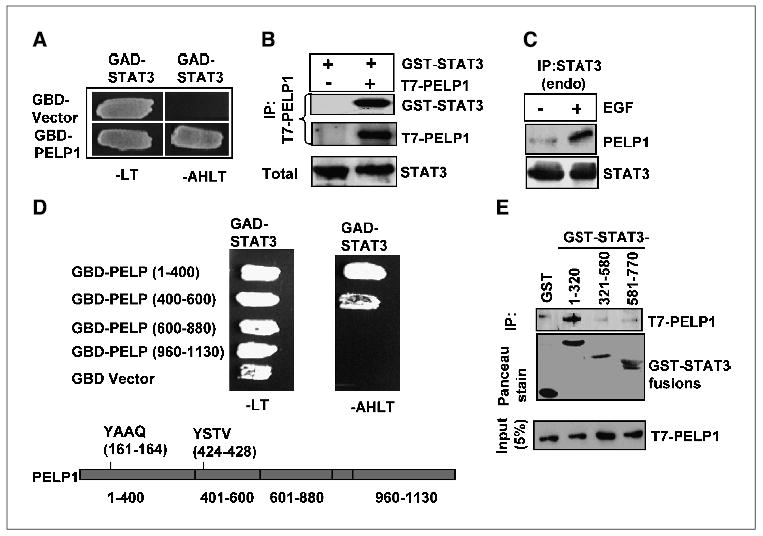
PELP1 interacts with STAT3. A, yeast cells were cotransfected with a control GAD vector or GAD-STAT3, along with a GBD vector, GBD-PELP1. Growth was recorded after 72 hours on selection plates lacking leucine and tryptophan (−LT) or adenosine, histidine, leucine, and tryptophan (−AHLT). B, COS-1 cells were transfected with T7-tagged PELP1 and GST-tagged STAT3. Immunoprecipitation was done with a T7 epitope antibody, after which Western blot analysis was done. C, MCF-7 cells were serum starved and treated with or without EGF (50 ng/mL). Immunoprecipitation was done with a STAT3 antibody. D, GAD fusions of various lengths of PELP1 were used to determine the PELP1 binding region in STAT3. Positive interactors were selected on agar plates lacking either leucine and tryptophan or adenine, histidine, leucine, and tryptophan. Bottom, schematic representation of known STAT3 binding sites in PELP1. E, total cellular lysates from MCF-7 cells expressing T7-tagged PELP1 were incubated with GST-STAT3 fusion proteins of various lengths, and binding was analyzed with a GST pulldown assay.
NH2-terminal region of proline-, glutamic acid-, and leucine-rich protein-1 interacts with signal transducers and activators of transcription 3.
To map the regions of PELP1 that interact with STAT3, we first cotransformed GAD-STAT3 and GBD-PELP1 fusion constructs (amino acids 1-400, 400-600, 600-880, and 960-1,130) into yeast cells. The interaction was assessed by monitoring the growth of the yeast cells on selective media. Yeast cells cotransformed with STAT3 and PELP 1-400 or PELP 401-600 were able to grow on selective medium, whereas yeast cells cotransformed with STAT3 and PELP COOH-terminal regions or GBD vector failed to grow on selective medium. The results suggest that STAT3 interacts with PELP1 at least via two binding sites encompassing amino acids 1 to 400 and amino acids 400 to 600 (Fig. 1D). Next, to determine the PELP1-interacting region in STAT3, we did the GST pulldown assay. We incubated lysates from MCF-7 cells expressing T7-PELP1 with three GST-STAT3 fusion proteins (amino acids 1-320, 320-580, and 580-770). Western blot analysis of eluates from GST pulldown assays showed that the NH2-terminal region amino acids 1 to 320 of STAT3 was the PELP1 interacting site (Fig. 1E).
Proline-, glutamic acid-, and leucine-rich protein-1 is a coactivator of signal transducers and activators of transcription 3–dependent transcription.
We previously showed that PELP1 acts as a coactivator of estrogen receptor (1, 2). Because PELP1 interacted with STAT3 in our experiment, we next examined whether PELP1 would act as a coactivator of STAT3-dependent transcription. The luciferase reporter 3XLY6E, which contains three STAT3 response elements (11), was used as a reporter of STAT3 activation. Cotransfection of the STAT3 reporter with PELP1 in HeLa cells enhanced the magnitude of EGF-mediated transactivation of the STAT3 reporter (Fig. 2A). To examine the physiologic significance of PELP1 transactivation of the STAT3 reporter, we examined whether PELP1 would increase the reporter activity of cyclin D1 promoter, a known STAT3 target gene. Cotransfection of PELP1 enhanced the EGF-mediated cyclin D1 promoter activity (Fig. 2B). We then confirmed these findings using previously established MCF-7–based PELP1 model cells which overexpress PELP1 (3). Enhanced transactivation of STAT3 reporter gene was observed in PELP1-overexpressing cells as compared with pcDNA-expressing cells (Fig. 2C). In Northern blot analysis, PELP1-overexpressing cells showed increased induction of cyclin D1 on activation with EGF (Fig. 2D). These results suggest that PELP1 modulates endogenous STAT3 transactivation functions.
Figure 2.
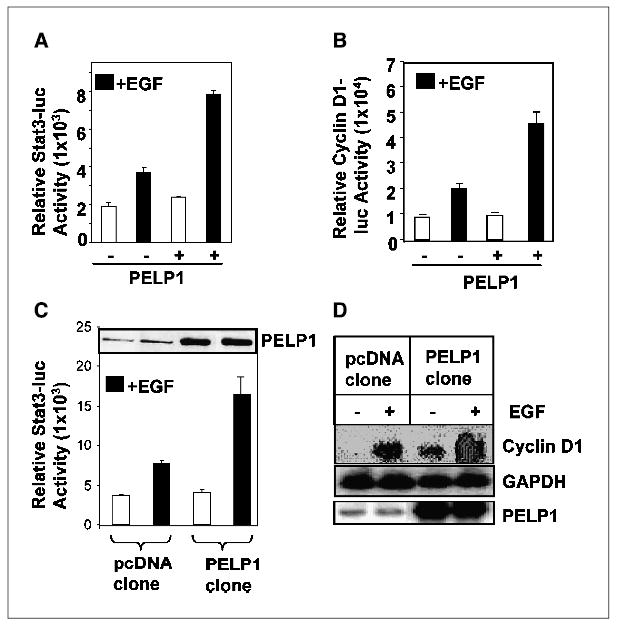
PELP1 is a coactivator of STAT3-dependent transcription. A, HeLa cells were cotransfected with a STAT3 luciferase reporter gene along with or without PELP1. After 24 hours, the cells were serum starved and treated with EGF for 8 hours, and then the STAT3 reporter activity was measured. B, HeLa cells were cotransfected with the cyclin D1 promoter luciferase reporter with or without PELP1. The cells were serum starved and treated with EGF (100 ng/mL) for 8 hours, and then the cyclin D1 reporter activity was measured. C, MCF-7 cells stably expressing pcDNA or PELP1 were transfected with STAT3 luciferase reporter (500 ng/well). After 24 hours, the cells were serum starved, treated with EGF (100 ng/mL) for 8 hours, and luciferase activity was measured. D, MCF-7 cells stably expressing pcDNA or PELP1 were serum starved for 24 hours and treated with EGF (100 ng/mL) for 2 hours. Total RNA was isolated and expression of cyclin D1 was analyzed by Northern blot analysis.
Proline-, glutamic acid-, and leucine-rich protein-1 specifically modulates signal transducers and activators of transcription 3 Ser727 phosphorylation.
To understand the mechanism by which PELP1 modulates STAT3 transactivation functions, we examined the effect of PELP1 overexpression on the phosphorylation status of STAT3 by using phosphospecific antibodies (STAT3 Tyr705; STAT3 Ser727). COS-1 cells were transfected with STAT3 with or without PELP1, and then the status of STAT3 phosphorylation following EGF treatment was determined. PELP1 overexpression had a minimal effect or no effect on EGF-induced STAT3 Tyr705 phosphorylation; however, we noticed a 3- to 4-fold higher induction of STAT3 Ser727 phosphorylation in cells overexpressing PELP1 (Fig. 3A). Similar results of increased STAT3 Ser727 phosphorylation were also observed when PELP1 was overexpressed in murine fibroblast cells (Fig. 3B). To examine whether endogenous PELP1 contributes to Ser727 phosphorylation of STAT3, we knocked down the endogenous PELP1 levels by using PELP1 siRNA. The amount of basal and EGF-stimulated STAT3 Ser727 phosphorylation was remarkably reduced in PELP1 siRNA–transfected cells compared with the amount in cells treated with control siRNA (Fig. 3C). To further examine the biological significance of PELP1 on STAT3 pathway, we determined the expression of STAT3 target genes cyclin D1 and c-fos under conditions in which PELP1 expression was knocked down. Northern blot analysis showed that reduction of endogenous PELP1 substantially reduced growth factor–mediated expression of STAT3 target genes (Fig. 3D and E). These results provide evidence that endogenous PELP1 plays an important role in STAT3 functions presumably by enhancing the phosphorylation at Ser727.
Figure 3.
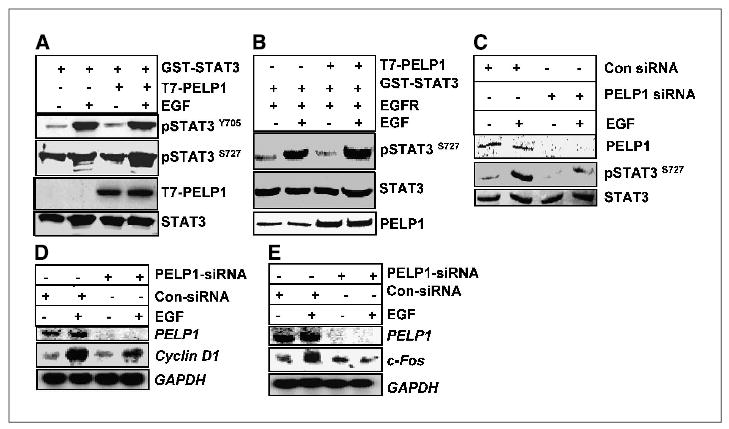
PELP1 selectively modulates STAT3 phosphorylation at Ser727. COS-1 cells (A) and murine fibroblast cells (B) were cotransfected with STAT3 with or without PELP1. After 24 hours, the cells were serum starved for an additional 24 hours and then treated with EGF for 30 minutes. The status of STAT3 phosphorylation was analyzed with phosphospecific antibodies. C, MCF-7 cells were transfected with the control or PELP1-specific siRNA and then treated with EGF for 30 minutes. Total cell lysates were subjected to Western blotting with phospho-STAT3 Ser727–specific antibody. D, MCF-7 cells were transfected with control or PELP1-specific siRNA. After 48 hours, the cells were serum starved for 24 hours and treated with EGF (100 ng/mL) for 2 hours. Total RNA was isolated and expression of cyclin D1 was analyzed by Northern blot analysis. E, HeLa cells were transfected with control or PELP1-specific siRNA. After 48 hours, the cells were serum starved for 24 hours and treated with EGF (100 ng/mL) for 2 hours. Total RNA was isolated and expression of c-fos was analyzed by Northern blot analysis.
Proline-, glutamic acid-, and leucine-rich protein-1 up-regulates signal transducers and activators of transcription 3 Ser727 phosphorylation via activation of src-mitogen-activated protein kinase pathway.
Recent evidence has suggested that PELP1 participates in estradiol-mediated nongenomic signaling via activation of the src-MAPK pathway (4). To determine whether PELP1 is a component of the EGF-mediated MAPK signaling cascade to STAT3, COS-1 cells were cotransfected with or without PELP1. Activation of MAPK was analyzed with phosphospecific antibodies. Overexpression of PELP1-potentiated EGF induced MAPK activation (Fig. 4A and B) and activation was observed for extended periods of time (Fig. 4B). Interestingly, PELP1-mediated enhancement of MAPK activation translated into increased STAT3 Ser727 phosphorylation (Fig. 4C). Conversely, blockage of MAPK activation by a specific inhibitor, PD98059, substantially reduced the ability of PELP1 to enhance STAT3 Ser727 phosphorylation (Fig. 4C). Similarly, treatment of cells with PD98059 reduced the PELP1-mediated increase in STAT3 reporter gene activity, suggesting a mechanistic role of MAPK activation in PELP1-mediated stimulation of STAT3 transactivation functions (Fig. 4D).
Figure 4.
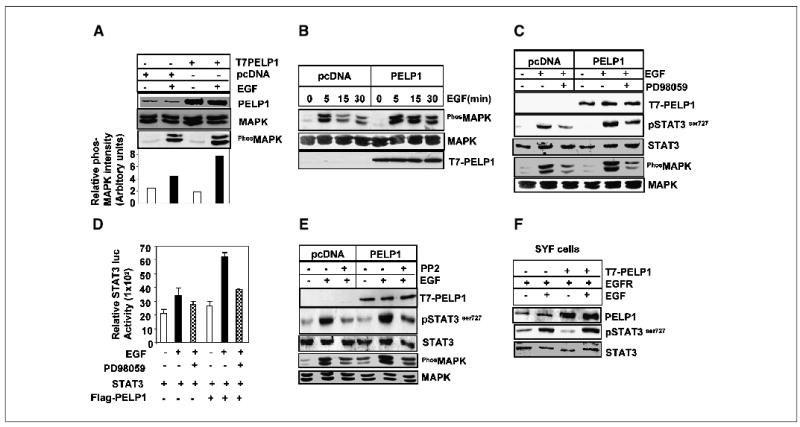
PELP1 regulates STAT3 Ser727 phosphorylation via the src-MAPK pathway. A, COS-1 cells were cotransfected with or without PELP1 and treated with EGF for 15 minutes. The total lysates were immunoblotted with a phospho-MAPK–specific antibody. B, MCF-7 cells stably expressing pcDNA or PELP1 were treated with EGF for various periods of time, and MAPK activation was analyzed by Western blot analysis with a phosphospecific antibody. C, MCF-7 cells stably expressing pcDNA or PELP1 were pretreated with or without the MAPK inhibitor PD98059 for 60 minutes and then treated with EGF for 15 minutes. MAPK activation was analyzed by Western blotting. D, MCF-7 cells were cotransfected with the STAT3 luciferase reporter gene with or without PELP1. The cells were pretreated with or without PD98059 and stimulated with EGF for 8 hours. The STAT3 reporter gene activity was determined. E, MCF-7 cells stably expressing pcDNA or PELP1 were pretreated with or without the src inhibitor PP2 for 60 minutes and then treated with EGF for 15 minutes. The status of MAPK activation and STAT3 Ser727 phosphorylation was analyzed by immunoblotting. F, murine fibroblast cells deficient in src kinase (SYF cells) were transfected with or without PELP1 and treated with or without EGF, and STAT Ser727 phosphorylation was measured by immunoblotting.
Because PELP1 has been shown to activate MAPK via its interactions with src kinase (15), we next examined whether PELP1-mediated MAPK stimulation in EGF-treated cells also requires src kinase. Treatment of cells with a specific src kinase inhibitor, PP2, completely abolished PELP1-mediated stimulation of STAT3 Ser727 phosphorylation, as well as MAPK activation (Fig. 4E). Similarly, overexpression of PELP1 failed to increase STAT3 Ser727 phosphorylation in murine fibroblast cell lines which lack src kinase expression (SYF cells; Fig. 4F). These results suggest that PELP1-mediated nongenomic signaling events, such as enhanced src-MAPK activation, contribute to the previously noted STAT3 Ser727 phosphorylation.
Proline-, glutamic acid-, and leucine-rich protein-1 functionally interacts with signal transducers and activators of transcription 3 and they colocalize in the nucleus.
STATs translocate into the nucleus when they are activated in response to different ligands by means of phosphorylation at Tyr705 (9). We recently showed that a substantial amount of PELP1 resides in the nuclear compartment of cells in addition to its presence in the cytosol (2). To examine the possibility that PELP1 and STAT3 interact in the nucleus, we did a confocal scanning analysis. In the absence of EGF treatment, STAT3 was mainly seen in the cytoplasm. EGF treatment for 15 minutes induced a substantial relocalization of STAT3 to the nuclear compartment and colocalization with PELP1 and STAT3 was also observed in some areas (Fig. 5A).
Figure 5.
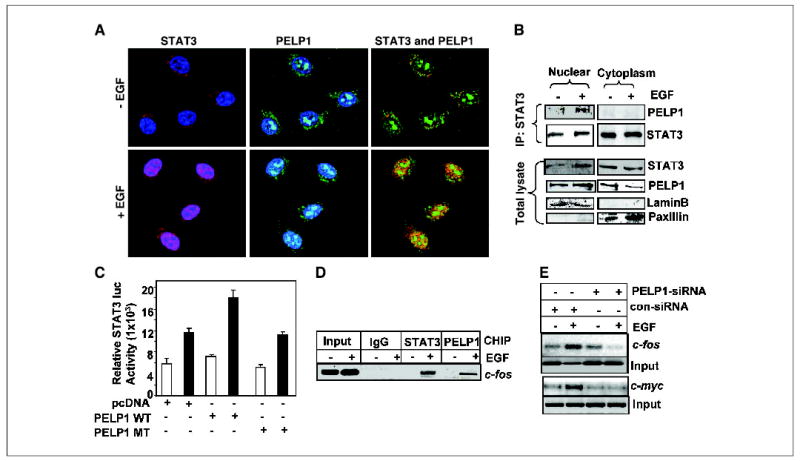
PELP1 and STAT3 colocalize in the nuclear compartment. A, MCF-7 cells were treated with or without EGF (100 ng/mL). The cells were then fixed with methanol and costained with antibodies against PELP1 (green) and STAT3 (red). Blue, DNA stained with Topo-3. The images were analyzed by confocal microscopy. Yellow, colocalization of PELP1 and STAT3. B, MCF-7 cells were treated with or without EGF and biochemically fractionated into nuclear and cytoplasm extracts. The total lysates from each compartment were subjected to immunoprecipitation with a STAT3 antibody followed by immunoblotting with PELP1. Lamin B and paxillin were used as markers of the nuclear and cytoplasm compartments. C, MCF-7 cells were transfected with the STAT3 reporter gene along with wild-type PELP1 or mutant PELP1, which lacks a histone binding domain. After 24 hours, the cells were serum starved and treated with EGF, and the STAT3 reporter activity was determined. D, MCF-7 cells expressing T7-PELP1 were treated with EGF and cross-linked with formaldehyde, and the chromatin was immunoprecipitated with control immunoglobulin G (IgG), STAT3, and T7 epitope antibodies. DNA eluates from the chromatin precipitates were used to determine the recruitment of STAT3 and PELP1 to the STAT3-responsive element–containing region of the c-fos promoter by PCR. E, MCF-7 cells were transfected with control or PELP1-specific siRNA. After 48 hours, the cells were serum starved for 24 hours and treated with EGF (100 ng/mL) for 1 hour. Chromatin was isolated and immunoprecipitated with STAT3 antibody. STAT3 recruitment to the target gene promoters (c-fos and c-myc) was analyzed by PCR.
To confirm whether PELP1 interacts with STAT3 in the nuclear compartment, we have used biochemical fractionation followed by immunoprecipitation. The results from these studies showed that PELP1 preferentially interacts with STAT3 in the nuclear compartment when treated with EGF (Fig. 5B). In a recent study, we found that PELP1 may play an important role in estrogen receptor genomic functions because of its ability to interact with histones and by chromatin remodeling (2). We next examined whether the histone binding domain of PELP1 is important for STAT3 coactivation functions. The wild-type PELP1 but not the mutant PELP1 (PELP1 1-880), which lacks a histone binding domain, enhanced the activation of the STAT3 reporter gene (Fig. 5C). Because PELP1 interacted with STAT3 in the nuclear compartment and requires histone binding region for STAT3 transactivation functions, we examined whether PELP1 is recruited to the promoters of STAT3 target genes using chromatin immunopecipitation assay. Our results show PELP1 recruitment on the promoter of c-fos, which contains a STAT3 response element (Fig. 5D). To further examine the significance of endogenous PELP1 in STAT3 nuclear functions, we determined STAT3 recruitment to the target gene promoters (c-fos and c-myc) under conditions of PELP1 knockdown. Reduction of PELP1 endogenous levels substantially affected STAT3 recruitment to the target gene promoter (Fig. 5E). Taken together, these results suggest that PELP1 associates with STAT3 in the nuclear compartment and that such interaction plays an important role in the nuclear functions of STAT3.
Discussion
Posttranslational modifications of STAT3 are essential for achieving the full transcriptional activation of STAT3 (7, 18). Previous studies have shown that STAT3 phosphorylation at Tyr705 plays a role in dimerization and nuclear localization and that phosphorylation at Ser727 is required for maximal activation of STAT3 (11). The mechanisms and kinases that regulate the phosphorylation of STAT3 are elusive. In the present study, using a yeast two-hybrid screen, we identified PELP1 as a novel interacting protein of STAT3 and found evidence that PELP1 interacts with STAT3 both in vitro and in vivo and enhances STAT3 transcriptional activation functions. Our results show that the interaction of PELP1 with STAT3 is essential for growth factor–mediated optimal serine phosphorylation of STAT3.
Accumulating evidence suggests that for STAT3 to achieve its maximal transcriptional activity and/or DNA binding, serine phosphorylation is required (10). For example, inhibition of STAT3 serine phosphorylation resulted in reduced transformation by v-src (17) and increased apoptosis (19). Earlier studies have identified MAPK to be one of the kinases responsible for serine phosphorylation of STAT3 (20). Our results suggest that PELP1 selectively enhances STAT3 Ser727 phosphorylation in growth factor–stimulated cells and such increase requires functional src-MAPK pathway. Our preliminary studies also indicate that PELP1 has a potential to interact with phosphatidylinositol 3-kinase;1 therefore, it is possible that growth factors may also use PELP1 to couple phosphatidylinositol 3-kinase to STAT3. Using PELP1 siRNA, we showed that PELP1 is essential for growth factor–mediated optimal STAT3 Ser727 phosphorylation and for expression of STAT3 target genes. Our study extends earlier studies that showed that PELP1 activates the src-MAPK pathway on estrogen stimulation (4) and further implicates PELP1 in the growth factor signaling axis, which indicates that PELP1 plays a role in augmenting growth factor–mediated STAT3 transactivation functions.
Earlier studies suggested that Ser727 phosphorylation likely increases STAT activity through the association of STAT with other proteins (21). It seems that increased transcription factor activity of STATs could be due to protein interactions occurring preferentially after the phosphorylation of Ser727 (22). It is therefore possible that PELP1-mediated increase in STAT3 Ser727 phosphorylation may facilitate unique coregulator recruitment and thus contribute to increased STAT3 activity. In addition, PELP1-STAT3 interactions in the nucleus suggest additional functions in the nuclear compartment. In chromatin immunoprecipitation assays, we observed that PELP1 associates with STAT3 target promoter regions on growth factor stimulation. PELP1 mutant lacking a histone-interacting motif failed to promote STAT3 transcriptional activation. When expression of endogenous PELP1 was reduced by siRNA, recruitment of STAT3 to its target gene promoter was substantially affected. Because PELP1 is implicated in the modulation of chromatin via its interactions with cAMP-responsive element binding protein (CREB) binding protein (CBP)/p300 (1) and histones (2), it is possible that PELP1-STAT3 nuclear interactions may facilitate STAT3 recruitment or its retention in the target gene promoters by facilitating chromatin modifications. Collectively, these findings strongly suggest that PELP1 facilitates STAT3-mediated transcription both by chromatin modifications (genomic functions) and by STAT3 Ser727 modification (nongenomic functions).
PELP1 contains several unique structural motifs that facilitate protein-protein interactions. First, PELP1 interacts with estrogen receptor through LXXLL motifs and with src kinase through PXXP motifs, both of which are present in the NH2-terminal region of PELP1 (23). In our yeast two-hybrid system mapping studies, we found that PELP1 interacts with STAT3 using two binding regions (NH2-terminal amino acids 1-400 and amino acids 400-600). Examination of these two regions suggested the presence of two potential, known STAT3 interacting motifs, YXXQ (amino acids 161-164: YAAQ) and YXXV (amino acids 424-428: YSTV). Previous studies showed that these two motifs facilitate STAT3 binding to other signaling proteins (24, 25). The presence of these STAT3 binding motifs in the NH2-terminal region of PELP1 and the ability of PELP1 to interact with a number of proteins, including src (4), CBP/p300 (1), estrogen receptor (1), and phosphatidylinositol 3-kinase,1 raise an interesting possibility that PELP1 functions as an adaptor, forming a multiprotein signaling complex with STAT3.
STAT3 protein is shown to play an important role in oncogenesis (7, 18). Constitutive STAT3 Ser727 phosphorylation was observed in several human tumors, including leukemias, lymphomas, and breast cancer (7, 16, 18, 22), suggesting a role of STAT3 serine phosphorylation in oncogenesis. PELP1 is widely expressed in a variety of tumors, and its expression is deregulated in a number of them, including breast and endometrial (1, 5) and prostrate tumors.2 The possible interaction of PELP1 with STAT3 and the ability of PELP1 to potentiate STAT3 Ser727 phosphorylation suggest the possibility that deregulation of PELP1 expression up-regulates STAT3 signaling in these tumors. Future studies are needed to formally test this possibility and the possibility that PELP1-STAT3 interactions have functional consequences in other tumor types.
Acknowledgments
Grant support: NIH grants CA 109379, CA 098823 (R. Kumar), and CA 095681 (R.K. Vadlamudi).
We thank Diep Nguyen for assistance in two-hybrid screening and DNA sequencing. We are grateful to Dr. J.E. Darnell, Jr. (The Rockefeller University, New York, NY) for providing wild-type STAT3 and 3XLY-GAS-STAT3-Luc constructs; Dr. Yoshihisa Kataoka (Osaka University, Osaka, Japan) for GST-STAT3 321-580 and GST-STAT3 581-770 constructs; and Dr. Xinmin Cao (Signal Transduction Laboratory, Institute of Molecular and Cell Biology, Singapore) for pXJ40 GST-STAT3 full-length mammalian expression vector.
Footnotes
Vadlamudi R.K. and Kumar R., unpublished data.
S. Nair and R.K. Vadlamudi, unpublished data.
References
- 1.Vadlamudi RK, Wang RA, Mazumdar A, et al. Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor α. J Biol Chem. 2001;276:38272–9. doi: 10.1074/jbc.M103783200. [DOI] [PubMed] [Google Scholar]
- 2.Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res. 2004;64:6416–23. doi: 10.1158/0008-5472.CAN-04-1786. [DOI] [PubMed] [Google Scholar]
- 3.Balasenthil S, Vadlamudi RK. Functional interactions between the estrogen receptor coactivator PELP1/MNAR and retinoblastoma protein. J Biol Chem. 2004;278:22119–27. doi: 10.1074/jbc.M212822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci U S A. 2002;99:14783–8. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Vadlamudi RK, Balasenthil S, Broaddus R, Gustafson J, Kumar R. Deregulation of estrogen receptor coactivator proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor in human endometrial tumors. J Clin Endocrinol Metab. 2004;89:6130–8. doi: 10.1210/jc.2004-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra SK, Balasenthil S, Nguyen D, Vadlamudi RK. Cloning and functional characterization of PELP1/MNAR promoter. Gene. 2004;330:115–22. doi: 10.1016/j.gene.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Darnell JE. Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–9. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 8.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 9.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 10.Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE. Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–8. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–4. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 13.O’Rourke L, Shepherd PR. Biphasic regulation of extracellular-signal-regulated protein kinase by leptin in macrophages: role in regulating STAT3 Ser727 phosphorylation and DNA binding. Biochem J. 2002;364:875–9. doi: 10.1042/BJ20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu AK, Fu WY, Ng AK, et al. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc Natl Acad Sci U S A. 2004;101:6728–33. doi: 10.1073/pnas.0307606100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Ma Y, Cole SM, et al. Serine phosphorylation of STAT3 is essential for Mcl-1 expression and macrophage survival. Blood. 2003;102:344–52. doi: 10.1182/blood-2002-11-3396. [DOI] [PubMed] [Google Scholar]
- 16.Frank DA, Mahajan S, Ritz J. B lymphocytes from patients with chronic lymphocytic leukemia contain signal transducer and activator of transcription (STAT) 1 and STAT3 constitutively phosphorylated on serine residues. J Clin Invest. 1997;100:3140–8. doi: 10.1172/JCI119869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol Cell Biol. 1999;19:7519–28. doi: 10.1128/mcb.19.11.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu CL, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Schlessinger K, Zhu X, et al. Essential role of STAT3 in postnatal survival and growth revealed by mice lacking STAT3 serine 727 phosphorylation. Mol Cell Biol. 2004;24:407–19. doi: 10.1128/MCB.24.1.407-419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17:6508–16. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JJ, Zhao Y, Chait BT, et al. Ser727-dependent recruitment of MCM5 by Stat1α in IFN-γ-induced transcriptional activation. EMBO J. 1998;17:6963–71. doi: 10.1093/emboj/17.23.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–37. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 23.Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol. 2004;18:1096–108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- 24.Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE, Jr, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–53. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 25.Fukada T, Hibi M, Yamanaka Y, et al. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–60. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]


