Abstract
Background: Increased premalignant epithelial microvascular blood content is a common theme in neoplastic transformation; however, demonstration of this phenomenon in colon carcinogenesis has been stymied by methodological limitations. Our group has recently developed a novel optics technology, four dimensional elastic light scattering fingerprinting (4D-ELF), which allows examination of the colonic mucosal architecture with unprecedented accuracy. In this study, we utilised 4D-ELF to probe the preneoplastic colonic microvasculature.
Methods: Colonic mucosal blood content was assessed by 4D-ELF at serial preneoplastic time points from azoxymethane (AOM) treated Fisher 344 rats and age matched control animals. We also examined the pretumorigenic intestinal mucosa of the MIN mouse, and compared with wild-type mice. Finally, in a pilot study, we examined superficial blood content from the endoscopically normal mid transverse colon in 37 patients undergoing screening colonoscopy.
Results: In the AOM treated rat model, augmentation of superficial mucosal and total mucosal/superficial submucosal blood supply preceded the appearance of aberrant crypt foci (ACF) and temporally and spatially correlated with future ACF occurrence. These findings were replicated in MIN mice. The 4D-ELF based results were corroborated with immunoblot analysis for haemoglobin on mucosal scrapings from AOM treated rats. Moreover, 4D-ELF analysis of normal human colonic mucosa indicated that there was a threefold increase in superficial blood in patients who harboured advanced adenomas.
Conclusion: We report, for the first time, that blood content is increased in the colonic microvasculature at the earliest stages of colon carcinogenesis. These findings may provide novel insights into early biological events in colorectal carcinogenesis and have potential applicability for screening.
Keywords: mucosal blood, biomarker, colon carcinogenesis, four dimensional elastic light scattering fingerprinting, aberrant crypt foci
Elucidating the biological mechanisms involved in neoplastic transformation of the colon is central for designing rational cancer prevention and treatment strategies. Emerging evidence underscores the critical nature of blood supply augmentation in meeting the metabolic demands of the burgeoning tumour. Indeed, tumour angiogenic markers are important independent prognostic indicator in patients with colorectal cancer (CRC).1 Their therapeutic implications are highlighted by demonstration that targeting blood vessel development with the antivascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab resulted in regression in rectal cancer2 and improved survival in patients with metastatic colorectal malignancies.3
While the importance of increased blood supply (for example, angiogenesis) in CRC development is unequivocal, the stage at which it occurs remains unclear. Most studies have reported angiogenesis once the invasive carcinoma has been established. However, even in a premalignant stage, epithelial cells have increased proliferation (as a manifestation of the “field effect”)4 and therefore would be expected to require increased blood supply. Angiogenesis has previously been shown as early as small adenomatous polyp5 or even the aberrant crypt foci (ACF)6 stage. Moreover, abnormalities in the microvasculature of the “transitional mucosa” (histologically normal appearing epithelium adjacent to a CRC)7 suggest that alterations in blood supply may precede macroscopic neoplastic lesions. These reports are consistent with a variety of malignancies (vulva, cervical, lung, skin, pancreas) that show neoangiogenesis at a predysplastic stage.8,9,10,11 However, studies in colon carcinogenesis have been suboptimal because of the utilisation of semi quantitative determination of microvessel density rather than the technically demanding assessment of mucosal blood content.12
Given the pathognomonic light absorption signatures of haemoglobin, biomedical optics represents a robust and practical methodology for accurately evaluating blood content and has been employed in a number of tissue types.13–19 We, in conjunction with others, have pioneered light scattering spectroscopy for identifying atypia in a variety of cell types, including the colon.19–22 In order to enhance the capabilities of light scattering spectroscopy to assay blood content at various tissue depths, we developed a new generation technology, four dimensional elastic light scattering fingerprinting (4D-ELF).19,23,24 This allows us to obtain quantitative information about the microvasculature in tissue samples by analysing the characteristic absorption/reflection spectra of red blood cells (RBC). As discussed below, the accuracy and sensitivity of this technique in determining the blood content far exceeds other non-optic techniques previously utilised. Thus 4D-ELF is perfectly suited to investigate changes in blood content in early carcinogenesis.
In the present study, we used 4D-ELF to probe the microvasculature in the uninvolved colonic mucosa of azoxymethane (AOM) treated rats, a well validated experimental colon carcinogenesis model. We detected, for the first time, an early increase in blood supply (EIBS) at the premalignant stage of colon carcinogenesis. These changes increased in magnitude over time, in a manner consonant with neoplastic transformation. We also utilised immunoblot analysis of mucosal scrapings for haemoglobin to confirm our 4D-ELF findings (albeit with considerably less sensitivity). Furthermore, we replicated these results in another animal model of colon carcinogenesis, the MIN (multiple intestinal neoplasias) mouse. Furthermore, in order to show relevance of EIBS to human colon carcinogenesis, we performed a pilot colonoscopic biopsy study.
METHODS
4D-ELF measurement of blood supply
Biomedical optics has frequently been utilised to measure tissue blood content by exploiting the characteristic absorption spectrum of haemoglobin in the visible range (light absorption at 542 and 577 nm wavelengths). Thus because no other molecules in biological tissue have similar absorption spectra, this provides a unique “spectral fingerprint” allowing remarkably accurate quantitation of RBCs. We have previously described in detail our advanced light scattering instrument utilised for 4D-ELF analysis.19,23,24 Briefly, a collimated polarised beam of cw light from a 75 W Xe lamp (Oriel, Stratford, Connecticut, USA) illuminated a number of sites on the mucosal surface (∼1 mm in diameter). A Fourier lens projected an angular distribution of the light scattered by the specimen onto the slit of the spectrometer, which further projected a two dimensional matrix of wavelength (400–700 nm) versus the angle of backscattering θ (0°–7°) onto a CCD (Roper Scientific, Trenton, New Jersey, USA). The instrument measures two polarisation components of scattered light: polarised along (copolarised component I||) and orthogonally (cross polarised component I⊥) to the polarisation of the incident light, respectively. The instrument was rigorously validated in physical and bioengineered tissue models and in ex vivo tissues.19
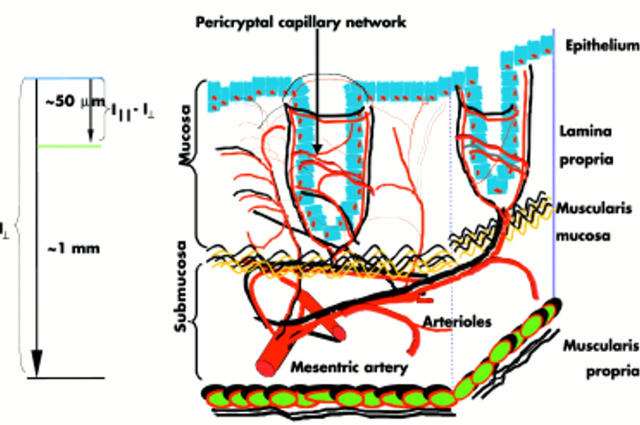
4D-ELF enables us to accurately quantitate RBCs in both the subepithelial and mucosa/submucosa compartments (see fig 2 ▶), which is achieved via polarisation gating. We and others have previously shown that the differential polarisation signal ΔI (λ) = I| |(λ) − I⊥(λ) is primarily generated by scatterers located close to the tissue surface (up to ∼50 μm)—that is, predominantly epithelial cells and the surrounding stroma with mucosal capillary plexus.19 On the other hand, I⊥(λ) contains information about deeper tissues, up to ∼1 mm below the surface.
Figure 2.
Schematic representation of the colonic microvasculature. Superficial (I||−I⊥) light scattering encodes information about structures that are within <50 μm of the tissue surface, such as the pericryptal capillary plexus. Deeper light scattering (I⊥) encompasses the mucosal capillaries along with some submucosal arterioles and venules (structures within ∼1 mm from the tissue surface).
Blood content in superficial tissue (for example, pericryptal capillary plexus) was estimated by spectral analysis of ΔI(λ).19 Firstly, we obtained the scattering maps, ΔIRBC (λ), of RBCs. Because
ΔI(λ) = ΔIS (λ) + α/Ω×ΔIRBC (λ),
where ΔIS(λ) is the signal contributed by non-RBC components of superficial tissue, Ω represents a calibration constant, and RBC concentration in the superficial mucosa was obtained as the value of α that minimises the haemoglobin absorption bands in ΔIS(λ).14,19 Mucosal and superficial submucosal blood supply was assessed via I⊥(λ) using a previously reported and well tested algorithm based on the diffusion approximation.14,16,19,25
Animal studies
These studies were approved by the institutional animal care and use committee of Evanston-Northwestern Healthcare. Fifty eight male Fisher 344 rats (100–150 g) were randomised to receive either two weekly intraperitoneal injections of AOM (15 mg/kg) or saline. Rats were killed 2, 4, 6, 8, 12, and 20 weeks after the second injection. Colons were removed, washed, and divided into proximal and distal segments. These segments were longitudinally bisected, with half undergoing 4D-ELF (within one hour of sacrifice) and the other half either fixed (10% buffered formalin for four hours followed by 70% alcohol) for ACF quantitation or underwent mucosal scrapping for immunoblot analysis.
In each animal, 4D-ELF blood supply measurements were taken from >100 tissue sites (∼1 mm2 each) uniformly distributed throughout the colonic surface. ACF scoring was performed on methylene blue stained mucosa based on criteria by Bird and as reported by us previously.26,27 ACF appeared as collections of elevated thickened crypts with increased staining and pericryptal spaces. Large ACF were defined as foci containing ⩾4 crypts.
In the mice experiments, we used 16 male C57/BL6 mice with either adenomatous polyposis coli (APC) truncations at codon 850 (APCmin) or controls (wild-type APC gene) (Jackson Laboratory, Bar Harbor, Maine, USA). Mice were killed at six weeks of age, the small bowel and colon isolated and opened longitudinally, and subjected to 4D-ELF to assess blood content.
Immunoblot analysis for haemoglobin
Distal colonic mucosa from AOM treated and age matched control rats (eight weeks post injection) were gently scraped with a glass slide as previously described.26,28 Homogenate proteins (25 μg) were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis gel, transferred to polyvinylidene difluoride membranes, probed with a polyclonal antihaemoglobin antibody (1:300 dilution/overnight at 4°C; Santa Cruz Biotechnology, California, USA) or β-actin (Sigma, St Louis, Missouri, USA), and xerograms were developed with enhanced chemiluminescence and quantitated with a laser densitometer.
Human studies
Studies were conducted in accordance with the institutional review board of Evanston-Northwestern Healthcare. Two biopsies from endoscopically normal mid transverse colons were obtained from 37 patients undergoing screening colonoscopy. Patients were excluded if they had a history or endoscopic evidence of colitis or if the biopsy samples were too small for reliable estimation of blood content. Freshly harvested biopsies (within one hour) were subjected to 4D-ELF analysis as previously described.
RESULTS
Accuracy of 4D-ELF measurement of blood content
To test the accuracy of optical measurement of blood content in superficial tissue (that is, predominantly the pericryptal capillary plexus—see methods and fig 2 ▶), we conducted studies with two layer physical tissue models. In these models, the top layer consisted of a 30 μm thick gel containing suspension of 1 μm microspheres (to simulate light scattering in tissue) mixed with a known quantity of rat RBCs. The bottom layer consisted of an optically thick suspension of microspheres. The optical properties of the models were chosen to mimic those of real rat colonic tissue.14 Such models have been widely used in tissue optics. Figure 1 ▶ compares haemoglobin concentrations obtained using the optical measurements with the actual values within the physiological range. As evident from fig 1A ▶, our technique enabled measurement of blood content with excellent accuracy (error <3.6%). To determine the accuracy of the haemoglobin measurement in deeper tissue (that is, all capillaries in the mucosa and superficial submucosa, fig 2 ▶), we constructed physical tissue models that consisted of aqueous suspensions of polystyrene beads and haemoglobin of known concentrations. As demonstrated in fig 1B ▶, our technique provided outstanding accuracy, with error <1.8%. These performance characteristics are superior to all other conventional techniques in measuring blood content in tissue.
Figure 1.
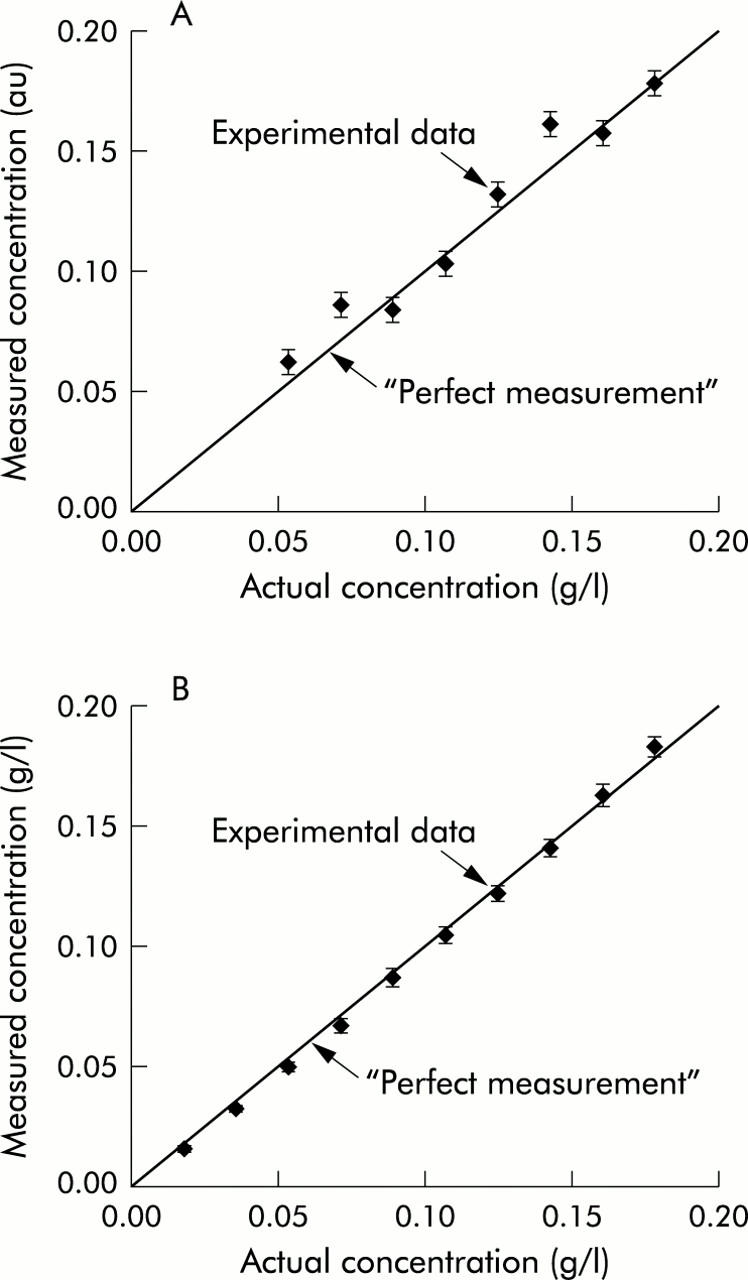
Four dimensional elastic light scattering fingerprinting (4D-ELF) accurately measures mucosal and mucosal/submucosal blood content. Tissue model studies document the accuracy of blood content measurement. As discussed in the results section, physical tissue models were constructed in order to replicate both superficial and mucosal/submucosal tissue depth. A known amount of rat blood was then assessed by 4D-ELF. A would be “perfect” 100% accurate measurement is illustrated by the diagonal line. Measured blood content showed excellent correlation with actual blood content in both the (A) mucosal and (B) mucosal/submucosal models. The error in measurement was <3.6% and <1.8%, respectively.
Blood content is increased at the earliest stage of colon carcinogenesis in the AOM treated rat model
To determine the stage of carcinogenesis when blood supply is augmented, we used the AOM treated rat model, a well validated model that replicates many of the salient morphological, cellular, and genetic changes associated with human sporadic colon carcinogenesis. Following carcinogen injection, ACF typically appear after 5–8 weeks, whereas adenomas and carcinomas require 20 and 40 weeks to develop, respectively.29 We were particularly interested in evaluating blood supply changes at two weeks post AOM when ACF, the earliest conventional marker of colon carcinogenesis, are undetected whereas the non-specific carcinogen effects have dissipated.30 These may best represent subtle preneoplastic changes associated with the “field effect”.
Figure 3A ▶ shows representative spectra obtained from colons of AOM treated or age matched saline treated controls (two weeks post second injection). As shown, the spectra obtained from AOM treated animals showed the signatures of RBC increased absorption. Analysis of the spectra (see methods) revealed a highly significant increase in distal colonic mucosal/submucosal blood content (p value <0.001; fig 3B ▶). On the other hand, in the proximal colon, where the carcinogenic effects are generally minimal, no such increase was noted (fig 3B ▶). When only the superficial (for example, pericryptal capillary plexus) component of blood content was assessed (fig 3C ▶), a very similar picture emerged with a significant increase in the concentration of RBC in the distal (p<0.001) but not the proximal (p = 0.3) colon. Thus EIBS preceded the development of ACF or adenomas, the classical early markers of colon carcinogenesis.
Figure 3.
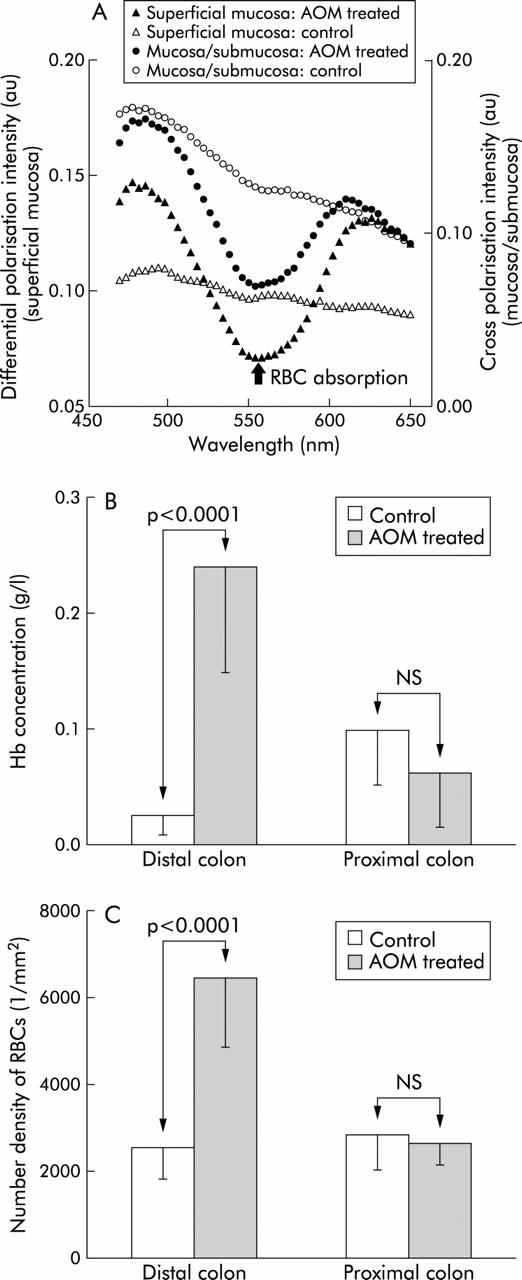
Increase in blood content is one of the earliest events in neoplastic transformation in the azoxymethane (AOM) treated rat model. (A) Representative light scattering spectra recorded from colonic superficial mucosa and mucosa/submucosa of rats treated with AOM (two weeks post-AOM treatment). The spectra obtained from the colonic tissue of AOM treated rats showed clear signatures of increased RBC content. (B) Mucosal/submucosal blood content was increased in the distal colon at two weeks post-AOM injection, a time point that precedes aberrant crypt foci or other conventional markers of neoplasia. Augmentation of the blood supply was seen in the distal but not in the proximal colon consistent with the marked distal predilection for colon carcinogenesis in this model. Hb, haemoglobin. (C) Superficial blood content. The number density of superficial red blood cells (RBCs) (1/mm2) mirrored the increase in total (mucosal/submucosal) content in that it was noted in the distal but not in the proximal colon.
Blood content temporally and spatially correlates with carcinogenesis
In order for increased blood content to serve as an intermediate biomarker in the AOM treated rat model, we reasoned that it should occur at an early time point, increase in magnitude over time, and take place predominantly in the distal colon. Numerous studies, including ours, have demonstrated that AOM induced neoplastic biomarkers appear in a distinct chronology. As previously mentioned, following AOM initiation, ACF typically occur after 5–8 weeks and increase in number and complexity (that is, size and dysplasia).31 We confirmed this in a subset of animals by assaying for the occurrence of large ACF (⩾4 crypts per foci), a more robust marker of neoplasia. Two salient features of the model were evident from this assay. From a temporal perspective, large ACF were detectable at six weeks and increased in number over time (fig 4A ▶). Spatially, the vast majority of ACF (∼90%) were located in the distal colon, consistent with the known regional neoplastic effects of AOM.
Figure 4.
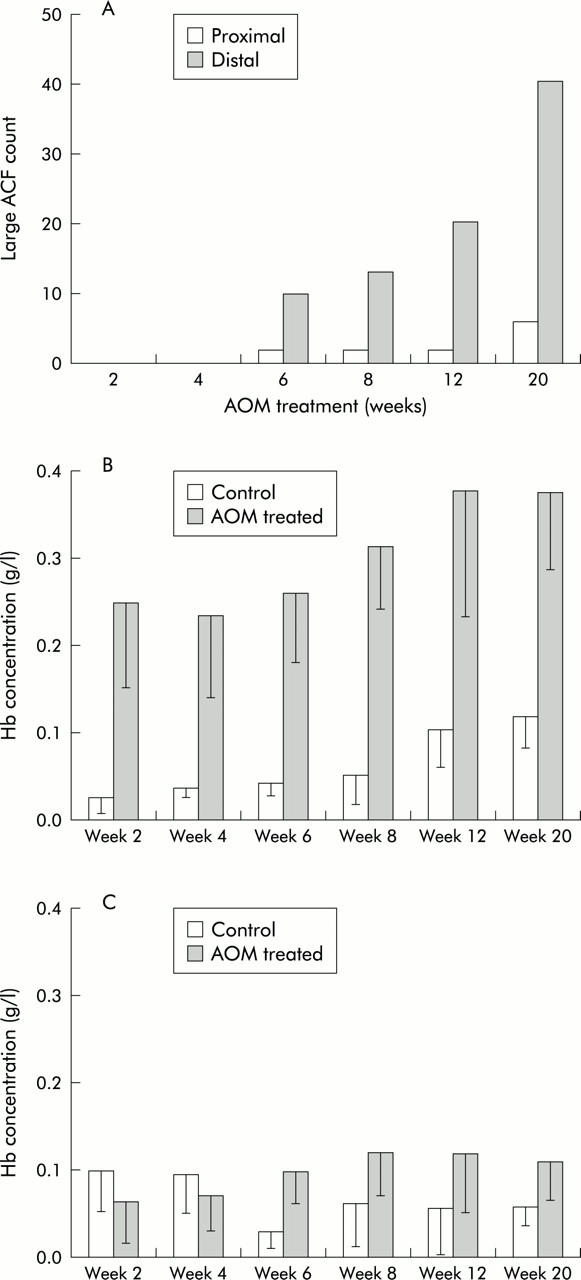
Temporal and spatial nature of augmentation of colonic mucosal/submucosal blood content is consonant with progression of carcinogenesis in the azoxymethane (AOM) treated rat model. (A) Aberrant crypt foci (ACF) analysis was performed using the technique described in the methods section. Large (>4 crypts per foci) ACF were scored. The temporal and spatial nature of ACF development is clearly evident. ACF were first detectable at week 6 and progressed over time. However, the vast majority of ACF occurred in the distal colon, with minimal carcinogenesis in the proximal colon. (B) In the distal colon, there was a progressive and statistically highly significant increase in blood content over time (ANOVA, p value <0.0001). While the blood content in saline treated (control) animals was also increased over time, it was fairly modest and failed to reach statistical significance. Hb, haemoglobin. (C) In the proximal colon, there was a marginal increase in blood content (p = 0.12), paralleling the minimal carcinogenic effect of AOM in this region of the colon, as noted in (A).
In our longitudinal studies, we observed a highly significant increase in the blood supply in the distal colon over time (ANOVA; p value <0.0001; fig 4B ▶). In comparison, the proximal colon showed much less dramatic increase than the distal colon (p = 0.12; fig 4C ▶). EIBS therefore mirrors both the temporal (increase over time) as well as the spatial (distal dominance over proximal) progression of carcinogenesis. We also observed that the superficial blood content continued to be elevated over age matched saline treated controls (p<0.0001) (data not shown). Furthermore, in age matched controls, there was no significant increase in blood content over time (fig 4B ▶, C).
Non-optics corroboration of EIBS
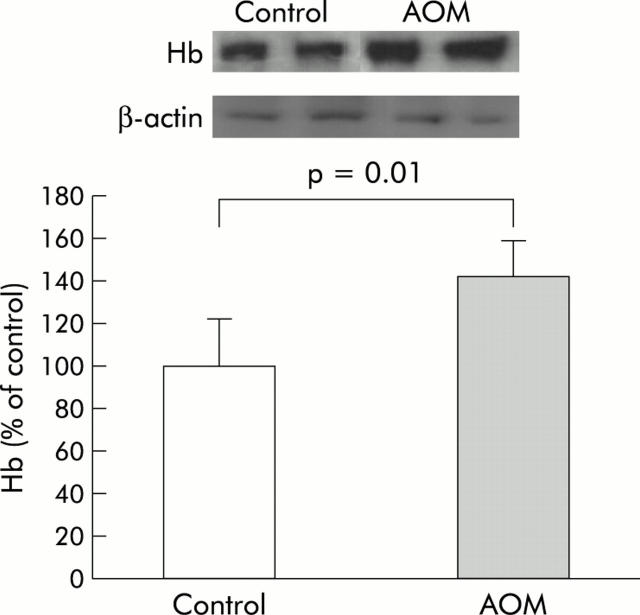
Immunoblot analysis of distal colonic mucosal scrapings was used as an additional methodology to assess haemoglobin content. One clear band at the appropriate molecular weight was noted (68 kDa) which was absent in negative controls (including lysates of two colon cancer cell lines HT-29 and HCT-116 and rat samples probed with secondary antibody alone, data not shown). At week 8 (fig 5 ▶), there was a marked increase in haemoglobin (142.4 (16.2)% of control, p = 0.01). While the magnitude of EIBS determined immunoblot analysis was considerably less than noted with 4D-ELF, these data provide important non-optics corroboration of the EIBS phenomenon.
Figure 5.
Immunoblot analysis for haemoglobin (Hb) in distal colonic mucosal scrapings from animals sacrificed eight weeks after the second injection corroborated early increase in blood supply. The inset shows a representative immunoblot. Equal amounts (25 μg) of protein were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis gel, as described in the methods section. Membranes were initially probed with an antibody to haemoglobin which revealed a single band at the appropriate molecular weight (68 kDa). Negative controls included secondary antibody alone and human colon cancer cell lines (HT-29 and HCT-116) which failed to reveal a band (data not shown). Membranes were stripped and probed with β-actin. As shown by densitometric analysis, azoxymethane (AOM) treated rat colon scrapings had a significant increase in blood content compared with saline treated controls (p = 0.01).
Blood content is increased in the preneoplastic MIN mouse
In order to demonstrate that EIBS is not model specific, we assessed blood content in the preneoplastic intestinal mucosa of the MIN mouse, another major model of experimental colon carcinogenesis. In this model, there is a germline mutation in the APC tumour suppressor gene, replicating the initiating genetic event in most human sporadic colon carcinogenesis.29 This leads to spontaneous and progressive development of intestinal adenomas. However, typically ∼90% of the adenomas are located in the small bowel with the colon being minimally involved. We analysed animals that were six weeks old, an age which precedes the occurrence of frank adenomatous polyps, thus being comparable with the premalignant stage (that is, two weeks post carcinogen) in our AOM model. We noted a statistically significant increase in microvascular blood content in the small bowel but not in the colon, paralleling the location of future tumours (fig 6A ▶). The superficial blood supply was also significantly increased compared with age matched wild-type mice in the small bowel but not in the colon (fig 6B ▶).
Figure 6.
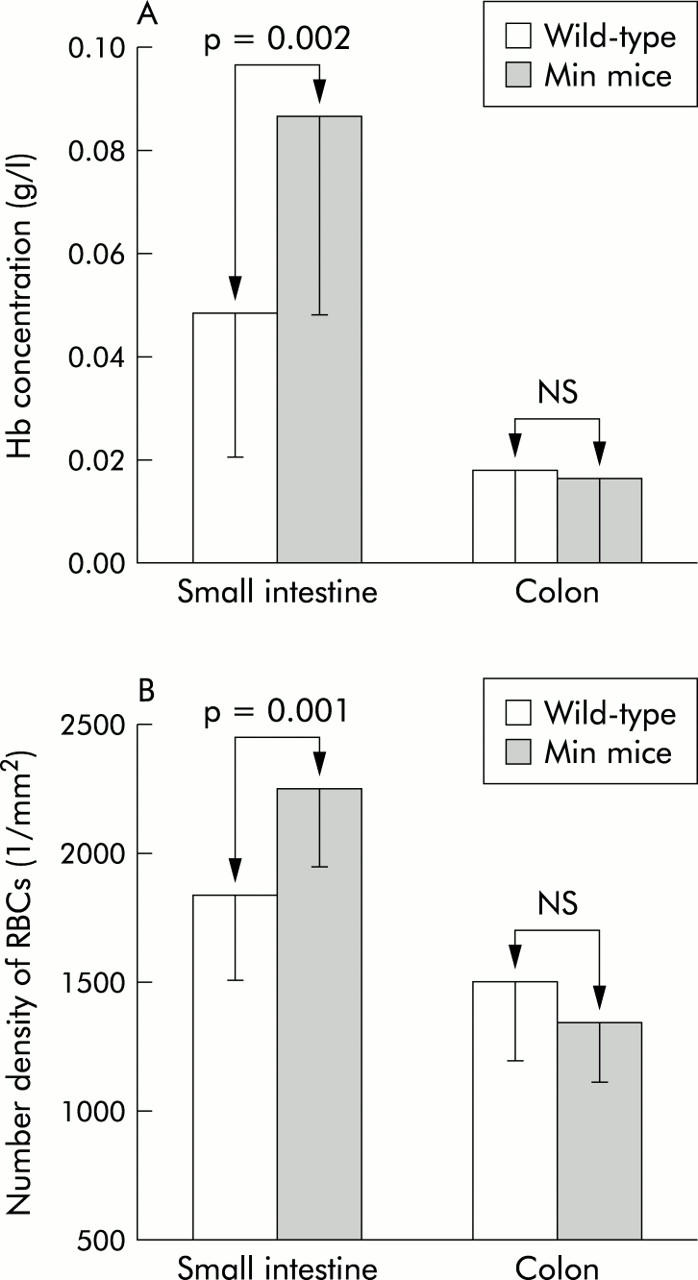
Blood content increase in the MIN (multiple intestinal neoplasias) mouse model. Blood content was increased in the small bowel but not in the colon of MIN mice at an age prior to the occurrence of adenomatous polyps (six weeks). Blood content paralleled the subsequent development of tumours (which were predominantly in the small bowel and only minimal in the colon). (A) Mucosal/submucosal blood content was significantly increased in the small bowel but not in the colon. Hb, haemoglobin. (b) The number density of superficial red blood cells (RBCs) (1/mm2) paralleled findings in the mucosa/submucosa in that there was a significant increase in the small bowel but not in the colon.
Evidence of EIBS in humans
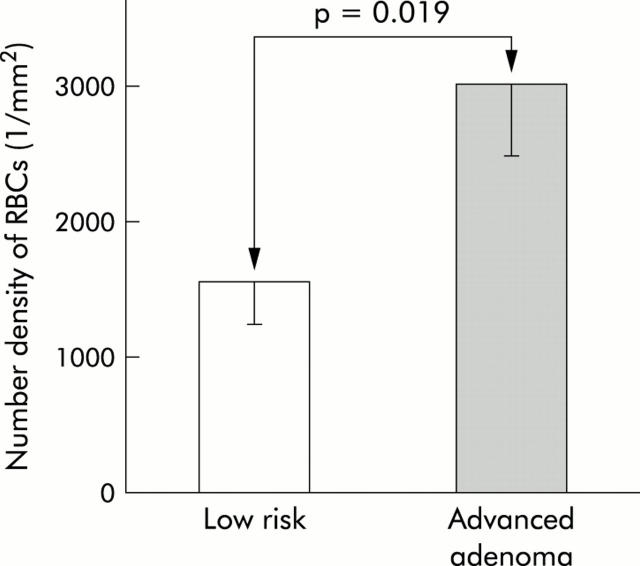
We compared the blood content from endoscopically normal mid trans colonic mucosa from patients with advanced adenomas (adenoma ⩾1 cm, high grade dysplasia or >25% villus component) versus those deemed to be at low risk for CRC (no history or present evidence of adenomas, colitis, or family history of CRC). There were no significant differences in age or sex between the low risk group and those that harboured advanced neoplasia. Importantly, none of the adenomas were located in the transverse colon (all lesions were located in the rectum, sigmoid colon, or caecum). Our data (fig 7 ▶) demonstrated marked augmentation of the blood content in the uninvolved (endoscopically normal) colonic mucosa in patients who harboured advanced neoplasia compared with those who were neoplasia free. Indeed, while our patient numbers were modest, this ∼3-fold increase was highly statistically significant (p<0.001). Limitations related to small biopsy size precluded accurate assessment of deeper blood content. While analysis of blood content in the distal colon would be most relevant to screening, the erythema/oedema associated with the phosphate based bowel preparatory regimen confounded blood content measurements in the rectum.
Figure 7.
Evidence of early increase in blood supply in human colon carcinogenesis. Two endoscopically normal biopsies from the mid transverse colons of each patient (out of 37 patients undergoing colonoscopy) were collected. Subjects were divided into two groups: one with advanced adenomas (adenoma ⩾1 cm) (high risk) and the other with no personal or family history of colonic neoplasia (either adenoma or carcinoma) (low risk group). Freshly harvested biopsies (within one hour) were subjected to four dimensional elastic light scattering fingerprinting analysis, as previously described. Number density of red blood cells (RBCs) was significantly higher in the uninvolved mucosa of patients with advanced adenomas compared with the low risk group (p<0.019).
DISCUSSION
We report herein, for the first time, that microvascular blood content increased very early in colon carcinogenesis, prior to development of adenomas or even ACF. The carcinogenic relevance is underscored by demonstration that blood content temporally and spatially mirrored future development of neoplastic lesions such as large ACF. To demonstrate that this novel observation was not model specific, we replicated our studies in a genetic model of colon carcinogenesis, the MIN mouse. Moreover, our pilot colonoscopic data provide an important affirmation of the relevance of EIBS to humans.32
Our ability to detect these changes is largely related to the major technological advance of 4D-ELF. Previous studies have suggested that increased blood supply may occur as early as the small adenomatous polyp33 and ACF stage6 although blood content/flow was not directly ascertained. Biomedical optics provides a powerful and well validated tool for detecting subtle changes in blood supply. The combination of remarkable sensitivity with depth selectivity enables 4D-ELF to quantitate subtle changes in mucosal microvascular blood content with heretofore unattainable accuracy. We confirmed this phenomenon with immunoblot analysis of haemoglobin of intestinal scrapings in the AOM treated rat. However, the magnitude of EIBS noted by immunoblot analysis was much more modest than with 4D-ELF (142% versus ∼750% of control, respectively), which is probably related to the deeper vasculature sampled in the mucosal scrapings. As we postulate that EIBS is predominantly a microvascular phenomena, contamination with larger submucosal blood vessels may obscure these subtle changes in superficial tissue. However, given that the scraping were obtained in an identical fashion from AOM and saline treated animals, this provides an important corroboration for EIBS in the premalignant colonic mucosa.
There is compelling precedence in other types of malignancy for augmentation of blood content in the premalignant mucosa. For instance, Folkman and colleagues34 have demonstrated that angiogenesis occurred at the hyperplasia-neoplasia transition in models of neoplastic transformation. Activation of β-catenin signalling, one of the initiating genetic events in colon carcinogenesis,35 has been recently demonstrated to increase VEGF.36 Additionally, Hoffman and colleagues11 used phage display of homing peptides to convincingly demonstrate that blood vessels from normal versus premalignant stages of epidermal carcinogenesis were molecularly distinct. Furthermore, Joyce and colleagues,10 using similar methodology, demonstrated early stage specific alterations in vascular markers in a mouse model of pancreatic islet carcinogenesis. Thus the early abnormalities in the microvessels noted in other malignancies provide, by analogy, biological support for our observed increase in blood supply in early colon carcinogenesis.
Several lines of evidence support the notion that EIBS is necessary to meet the increased metabolic requirements of the premalignant mucosa (that is, the “field effect”). For instance, increased cell proliferation has been noted shortly after carcinogen treatment.37 In humans, increased proliferation in the uninvolved mucosa has been correlated with the risk of neoplasia throughout the colon.4 Indeed, we have demonstrated that mucosal NADH, a sensitive marker of the increased metabolism in early carcinogenesis,38 was elevated two weeks after AOM treatment.39 Chen et al have observed dramatic alterations in gene expression profiles in the uninvolved mucosa of patients with CRC compared with those without neoplasia, providing insights into the genetic underpinnings of the “field effect”.40 They also demonstrated similar genetic abnormalities in the preneoplastic mucosa of the MIN mouse compared with wild-type controls. Intriguingly, the magnitude of dysregulation of gene expression in the “field” appeared to be intermediate between normal and neoplastic tissue, supporting relevance to carcinogenesis.40 Moreover, several of the genes assayed may have relevance to cellular metabolism, including growth related oncogene α, macrophage inflammatory protein 2, and CXC cytokine receptor 2.40 Thus there is strong biological plausibility to support the need for the blood supply to be augmented early during colon carcinogenesis.
While our studies do not address mechanisms of EIBS, the most likely possibilities are neoangiogenesis and vasodilation. As discussed above, there is ample precedence for early angiogenesis. With regard to vasodilation, nitric oxide generation through early overexpression of inducible nitric oxide synthase may be important.41 Indeed, targeting inducible nitric oxide synthase has been shown to be an effective chemopreventive strategy.42 Future studies will be conducted to understand the mechanisms of EIBS during colon carcinogenesis.
The phenomenon of EIBS lends itself to potential applications in CRC screening and prevention. From a screening perspective, our data show that even at the earliest time point (two weeks post AOM injection) increased blood supply was able to detect carcinogen exposure with a sensitivity of 93.8%, specificity of 95.8%, and a positive predictive value of 96.8%. Our human data support the clinical relevance of the early increase in blood supply although it needs to be emphasised that the modest number of patients serves purely as “proof of concept”. In the future, we are planning larger scale human studies to assess its diagnostic abilities.
We are cognisant that blood content may be altered by a variety of factors such as physiological stimuli (for example, feeding) and inflammation, potentially making conclusions about correlation as a neoplastic biomarker suspect. However, the use of identically matched controls (both in animal and human studies) mitigates this issue. Moreover, interindividual variability was minimal in both the animal and human studies, arguing against their significance. Furthermore, there was no evidence of inflammation in the AOM treated rats, MIN mouse, or humans. These lines of evidence strongly suggest that EIBS is not related to any confounding factors. As outlined above, it is biologically plausible to speculate that EIBS is important in colon carcinogenesis; however, our data can not exclude the possibility that this may simply be an epiphenomenon. Even in the unlikely case that the latter is correct, our observation may still have ramifications for screening.
In summary, development of 4D-ELF analysis has enabled us to demonstrate increased microvascular blood content very early at the premalignant stage in experimental and human colon carcinogenesis. The potential accuracy of blood supply as an intermediate biomarker is supported by spatial and temporal correlation with subsequent ACF occurrence in the AOM treated model. The concordance of the findings in the AOM treated rat, MIN mouse, and pilot human data supports the validity of EIBS in colon carcinogenesis. Our novel demonstration of EIBS may provide important insights into the biological events of early colon carcinogenesis. It may also find potential application in colon cancer screening and detection. Future studies will be conducted to elucidate the biological and clinical implications of EIBS.
Acknowledgments
Supported by research grants from the National Institutes of Health (1R21CA102750-01, 1RO3CA10549-01 and 1U01CA11125-01), National Science Foundation (BES-0238903), and American Cancer Society-Illinois Division.
Abbreviations
ACF, aberrant crypt foci
EIBS, early increase in blood supply
4D-ELF, four dimensional elastic light scattering fingerprinting
AOM, azoxymethane
VEGF, vascular endothelial growth factor
CRC, colorectal cancer
APC, adenomatous polyposis coli
MIN, multiple intestinal neoplasias
RBC, red blood cells
Conflict of interest: None declared.
Presented in part in abstract form at the 105th Digestive Disease Week Meetings, 15–19 May 2004, New Orleans, LA, USA.
REFERENCES
- 1.De Vita F, Orditura M, Lieto E, et al. Elevated perioperative serum vascular endothelial growth factor levels in patients with colon carcinoma. Cancer 2004;100:270–8. [DOI] [PubMed] [Google Scholar]
- 2.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004;10:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- 4.Ahnen DJ, Byers T. Proliferation happens. JAMA 1998;280:1095–6. [DOI] [PubMed] [Google Scholar]
- 5.Aotake T, Lu CD, Chiba Y, et al. Changes of angiogenesis and tumor cell apoptosis during colorectal carcinogenesis. Clin Cancer Res 1999;5:135–42. [PubMed] [Google Scholar]
- 6.Shpitz B, Gochberg S, Neufeld D, et al. Angiogenic switch in earliest stages of human colonic tumorigenesis. Anticancer Res 2003;23:5153–7. [PubMed] [Google Scholar]
- 7.Sun XF, Zhang H, Wu XC, et al. Microvascular corrosion casting of normal tissue, transitional mucosa and adenocarcinoma in the human colon. Acta Oncol 1992;31:37–40. [DOI] [PubMed] [Google Scholar]
- 8.Teo NB, Shoker BS, Martin L, et al. Angiogenesis in pre-invasive cancers. Anticancer Res 2002;22:2061–72. [PubMed] [Google Scholar]
- 9.Sharma RA, Dalgleish AG, Steward WP, et al. Angiogenesis and the immune response as targets for the prevention and treatment of colorectal cancer (review). Oncol Rep 2003;10:1625–31. [PubMed] [Google Scholar]
- 10.Joyce JA, Laakkonen P, Bernasconi M, et al. Stage-specific vascular markers revealed by phage display in a mouse model of pancreatic islet tumorigenesis. Cancer Cell 2003;4:393–403. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman JA, Giraudo E, Singh M, et al. Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma. Cancer Cell 2003;4:383–91. [DOI] [PubMed] [Google Scholar]
- 12.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med 2003;9:713–25. [DOI] [PubMed] [Google Scholar]
- 13.Ishimaru A . Wave propagation and scattering in random media. Piscataway: Wiley IEEE Computer Society Press, 1999.
- 14.Zonios G, Perelman LT, Backman V, et al. Diffuse reflectance spectroscopy of human adenomatous colon polyps in vivo. Appl Optics 1999;38:6628–37. [DOI] [PubMed] [Google Scholar]
- 15.Muller MG, Valdez TA, Georgakoudi I, et al. Spectroscopic detection and evaluation of morphologic and biochemical changes in early human oral carcinoma. Cancer 2003;97:1681–92. [DOI] [PubMed] [Google Scholar]
- 16.Georgakoudi I, Sheets EE, Muller MG, et al. Trimodal spectroscopy for the detection and characterization of cervical precancers in vivo. Am J Obstet Gynecol 2002;186:374–82. [DOI] [PubMed] [Google Scholar]
- 17.Georgakoudi I, Motz J, Backman V, et al. Quantitative characterization of biological tissue using optical spectroscopy. In: Vo-Dinh T, ed. Biomedical photonics handbook. New York: CRC Press, 2003.
- 18.Perelman LT, Backman V, Wallace MB, et al. Observation of periodic fine structure in reflectance from biological tissue: A new technique for measuring nuclear size distribution. Phys Rev Lett 1998;80:627. [Google Scholar]
- 19.Kim Y, Liu Y, Wali RK, et al. Simultaneous measurement of angular and spectral properties of light scattering for characterization of tissue microarchitecture and its alteration in early precancer. IEEE J Sel Top Quantum Electron 2003;9:243–55. [Google Scholar]
- 20.Backman V, Wallace MB, Perelman LT, et al. Detection of preinvasive cancer cells. Nature 2000;406:35–6. [DOI] [PubMed] [Google Scholar]
- 21.Gurjar RS, Backman V, Perelman LT, et al. Imaging human epithelial properties with polarized light-scattering spectroscopy. Nat Med 2001;7:1245–8. [DOI] [PubMed] [Google Scholar]
- 22.Wallace MB, Perelman LT, Backman V, et al. Endoscopic detection of dysplasia in patients with Barrett’s esophagus using light-scattering spectroscopy. Gastroenterology 2000;119:677–82. [DOI] [PubMed] [Google Scholar]
- 23.Roy HK, Liu Y, Wali RK, et al. Four-dimensional elastic light-scattering fingerprints as preneoplastic markers in the rat model of colon carcinogenesis. Gastroenterology 2004;126:1071–81. [DOI] [PubMed] [Google Scholar]
- 24.Roy HK, Iversen P, Hart J, et al. Downregulation of SNAIL suppresses MIN mouse tumorigenesis: modulation of apoptosis, proliferation and fractal dimension. Mol Cancer Ther 2004;3:1159–65. [PubMed] [Google Scholar]
- 25.Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 2001;120:1620–9. [DOI] [PubMed] [Google Scholar]
- 26.Roy HK, Karolski WJ, Ratashak A. Distal bowel selectivity in the chemoprevention of experimental colon carcinogenesis by the non-steroidal anti-inflammatory drug nabumetone. Int J Cancer 2001;92:609–15. [DOI] [PubMed] [Google Scholar]
- 27.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett 1987;37:147–51. [DOI] [PubMed] [Google Scholar]
- 28.Roy HK, Karoski WJ, Ratashak A, et al. Chemoprevention of intestinal tumorigenesis by nabumetone: induction of apoptosis and Bcl-2 downregulation. Br J Cancer 2001;84:1412–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee A, Quirke P. Experimental models of colorectal cancer. Dis Colon Rectum 1998;41:490–505. [DOI] [PubMed] [Google Scholar]
- 30.Jackson PE, O’Connor PJ, Cooper DP, et al. Associations between tissue-specific DNA alkylation, DNA repair and cell proliferation in the colon and colon tumor yield in mice treated with 1,2-dimethylhydrazine. Carcinogenesis 2003;24:527–33. [DOI] [PubMed] [Google Scholar]
- 31.Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett 1995;93:55–71. [DOI] [PubMed] [Google Scholar]
- 32.Roy HK, Wali RK, Koetsier J, et al. Increased mucosal blood supply is an early preneoplastic marker in colon neoplasia. Gastroenterology 2004;126 (suppl 2).
- 33.Akagi K, Ikeda Y, Sumiyoshi Y, et al. Estimation of angiogenesis with anti-CD105 immunostaining in the process of colorectal cancer development. Surgery 2002;131:S109–13. [DOI] [PubMed] [Google Scholar]
- 34.Folkman J, Watson K, Ingber D, et al. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989;339:58–61. [DOI] [PubMed] [Google Scholar]
- 35.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell 2000;103:311–20. [DOI] [PubMed] [Google Scholar]
- 36.Easwaran V, Lee SH, Inge L, et al. Beta-catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res 2003;63:3145–53. [PubMed] [Google Scholar]
- 37.Barnes CJ, Lee M, Hardman WE, et al. Aspirin, age, and proximity to lymphoid nodules influence cell proliferation parameters in rat colonic crypts. Cell Prolif 1995;28:59–71. [DOI] [PubMed] [Google Scholar]
- 38.Georgakoudi I, Jacobson BC, Muller MG, et al. NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes. Cancer Res 2002;62:682–7. [PubMed] [Google Scholar]
- 39.Wali RK, Roy HK, Kim Y, et al. Increased mucosal blood flow is an early marker of colon carcinogenesis. Gastroenterology 2003;124 (suppl) :A4. [Google Scholar]
- 40.Chen LC, Hao CY, Chiu YS, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res 2004;64:3694–700. [DOI] [PubMed] [Google Scholar]
- 41.Xu MH, Deng CS, Zhu YQ, et al. Role of inducible nitric oxide synthase expression in aberrant crypt foci-adenoma-carcinoma sequence. World J Gastroenterol 2003;9:1246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao CV, Indranie C, Simi B, et al. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res 2002;62:165–70. [PubMed] [Google Scholar]