Abstract
Jet lag is caused by a misalignment between circadian rhythms and local destination time. As humans typically take longer to re-entrain after a phase advance than a phase delay, eastward travel is often more difficult than westward travel. Previous strategies to reduce jet lag have focused on shaping the perceived light-dark cycle after arrival, in order to facilitate a phase shift in the appropriate direction. Here we tested treatments that travelers could use to phase advance their circadian rhythms prior to eastward flight. Thus, travelers would arrive with their circadian rhythms already partially re-entrained to local time. We determined how far the circadian rhythms phase advanced, and the associated side effects related to sleep and mood. Twenty-eight healthy young subjects participated in 1 of 3 different treatments, which all phase advanced each subject’s habitual sleep schedule by 1 h/day for 3 days. The 3 treatments differed in morning light exposure for the 1st 3.5 h after waking on each of the 3 days: continuous bright light (> 3000 lux), intermittent bright light (> 3000 lux, 0.5 h on, 0.5 off, etc.), or ordinary dim indoor light (< 60 lux). Aphase assessment in dim light (< 10 lux) was conducted before and after the treatments to determine the endogenous salivary dim light melatonin onset (DLMO). The mean DLMO phase advances in the dim, intermittent, and continuous light groups were 0.6, 1.5, and 2.1 h, respectively. The intermittent and continuous light groups advanced significantly more than the dim light group (p < 0.01) but were not significantly different from each other. The side effects as assessed with actigraphy and logs were small. A 2-h phase advance may seem small compared to a 6- to 9-h time zone change, as occurs with eastward travel from the USA to Europe. However, a small phase advance will not only reduce the degree of re-entrainment required after arrival, but may also increase postflight exposure to phase-advancing light relative to phase-delaying light, thereby reducing the risk of antidromic re-entrainment. More days of preflight treatment could be used to produce even larger phase advances and potentially eliminate jet lag.
Keywords: bright light, circadian rhythms, jet lag, melatonin, phase-shifts, sleep, travel
Rapid travel across time zones leads to jet lag, which is characterized by symptoms such as fatigue, insomnia, daytime sleepiness, and gastrointestinal problems (Boulos et al., 1995; Boulos, 1998). Jet lag is caused by a misalignment between circadian rhythms and destination time. As the number of time zones crossed increases, the amount of time required for re-entrainment lengthens, thereby lengthening the duration of jet lag. As the human circadian clock has a period slightly longer than 24 h (Wever, 1979; Czeisler et al., 1999), it has a natural tendency to phase delay, which could be one reason why phase delays proceed more rapidly than phase advances (Aschoff et al., 1975; Eastman and Martin, 1999). Traveling east requires a phase advance, and thus jet lag is typically worse after traveling east than west (Boulos et al., 1995; Boulos, 1998). This article will focus on eastward travel.
Antidromic re-entrainment occurs when circadian rhythms re-entrain by phase shifting in the opposite direction to the shift in external time, such as a phase delay instead of a phase advance after eastward travel. If antidromic phase shifts occur following an eastward flight, then the time required for re-entrainment and duration of jet lag will be increased (unless the required phase advance is so large, such as 11 h, that a phase delay achieves re-entrainment as rapidly). Results from studies examining phase shifts in eastward jet-travelers indicate that antidromic shifts are quite common. Early studies suggested that antidromic re-entrainment occurred in 1 of 7 people following an 8-h eastward flight (Arendt et al., 1987) and 4 of 8 people following a 9-h eastward flight (Klein et al., 1977).
More recent work using more reliable circadian phase markers confirms that a significant proportion of eastward travelers experience antidromic shifts (phase delays). In one study, 6 individuals were studied before and after an 8-h eastward flight (Takahashi et al., 1999). By the 5th day in the new time zone, 4 advanced, 1 delayed, and 1 did not shift. An even greater proportion of travelers show antidromic shifts following 10- to 11-h eastward flights. For example, following a 10-h eastward flight, 11 of 12 travelers phase delayed, while only 1 phase advanced (Gundel and Spencer, 1999). Similarly, following an 11-h eastward flight, 7 of 8 phase delayed, while only 1 phase advanced (Takahashi et al., 2001). Thus, the probability of antidromic re-entrainment following eastward travel is likely to increase as the number of time zones crossed grows (Aschoff, 1981). Furthermore, antidromic shifts are more likely if the phase advance required for re-entrainment is greater than ~7 h (Gundel and Wegmann, 1989), although this is likely to vary depending on the pattern of light/dark experienced by the individual as well as individual differences in tau and light sensitivity.
Strategies to ameliorate jet lag will be most effective if they accelerate re-entrainment to the new time zone. Strategies that use artificial bright light, or outdoor light, to ensure that the majority of light coincides with the correct part of the light phase response curve (PRC), are more likely to be effective (and may also reduce the risk of antidromic re-entrainment when it is not desired). Thus, it has been suggested that re-entrainment may be hastened by appropriately timed light exposure and light avoidance in the new destination (Daan and Lewy, 1984; Waterhouse et al., 1997). Eastward travelers should avoid light prior to their temperature minimum (Tmin) and seek light following their Tmin in order to facilitate a phase advance.
In contrast to strategies that focus on shifting circadian rhythms postflight, we propose schemes that shift circadian rhythms preflight. We tested 3 different phase-advancing treatments, designed to phase-advance rhythms prior to eastward jet travel. With a large enough preflight phase advance, jet lag due to eastward travel could theoretically be avoided. Even small advances could be effective in reducing jet lag, especially if the Tmin is advanced far enough such that the majority of light in the new destination falls on the phase advance portion (not the phase delay portion) of the light PRC, and so continues to push the rhythms in the correct direction.
We tested phase-advancing treatments that could be implemented in a real traveler’s home. All treatments included phase advancing each subject’s habitual sleep schedule by 1 h/day for 3 days. The 3 treatments differed in their morning light exposure: ordinary indoor light, intermittent bright light, or continuous bright light. As intermittent light patterns can help produce phase shifts similar in magnitude to those observed following continuous light exposure (e.g., Baehr et al., 1999; Rimmer et al., 2000), we tested intermittent light as a practical and convenient alternative to continuous light. We also assessed the side effects of the treatments by examining sleep and subjective symptoms.
MATERIALS AND METHOD
Subjects
Twenty-eight healthy young adults (14 m, 14 f) between the ages of 22 and 43 years old (mean age ± SD = 27.7 ± 5.0 years) participated. The subjects had normal body mass indices (24.0 ± 3.7 kg/m2), were nonsmokers, did not consume large caffeine doses (had < 500 mg/day), and reported no medical, psychiatric, or sleep disorders as assessed from a telephone interview, in-person interview, medical history, and several screening questionnaires (Minnesota Multiphasic Personality Inventory-2, Pittsburgh Sleep Quality Index [Buysse et al., 1989], and part of a general health questionnaire [Tasto et al., 1978]). Subjects were free from prescription medications, except for 4 female subjects who were taking oral contraceptives. The menstrual phase of the female subjects during the study was not controlled. We excluded individuals who had worked a night shift during the 2 months prior, or individuals who had traveled overseas in the month prior to the start of the study. The protocol was approved by the Rush-Presbyterian-St. Luke’s Medical Center Institutional Review Board, and all subjects gave written informed consent prior to participation. Subjects were reimbursed for their participation.
Protocol
This study was a between-subjects design with 3 groups defined according to the morning light exposure received during an advancing sleep schedule (details below): continuous light, intermittent light, and dim light. Eight subjects were in the continuous light group (5 m, 3 f), 11 in the intermittent light group (5 m, 6 f), and 9 in the dim light group (4 m, 5 f). Assignment to groups was random except that the 3 groups were balanced for sex and Morningness-Eveningness score (Horne and Östberg, 1976).
Each subject adhered to a strict baseline sleep schedule that matched to within 1 h of each subject’s self-reported typical sleep onset and wake times, as confirmed with sleep logs, completed for at least a week before the start of the study. The fixed sleep schedule was designed to stabilize circadian phase and ensure that the subjects were not sleep deprived before the treatment began. The earliest scheduled baseline bedtime (lights out) was 2200 h, and the latest was 0100 h (mean ± SD = 2302 ± 0.8 h). The earliest scheduled wake time was 0530, and the latest was 0800 (0635 ± 0.7 h). The time allotted for sleep was between 7 and 8.5 h, with a mean duration of 7.6 ± 0.5 h. Subjects with matching sleep schedules were run in pairs.
A sample protocol schedule is shown in Figure 1. The phase-advancing treatment was on days 11–13. Time in bed was reduced by 1 h on day 11 but returned to baseline duration on days 12 and 13. Napping was permitted during the 10 days of baseline but only during a 6-h zone centered 12 h from the midpoint of the nocturnal sleep periods. Sleep (dark) at this time should not phase shift circadian rhythms, as neither afternoon sleep/dark episodes (Buxton et al., 2000) nor afternoon bright light episodes (Dumont and Carrier, 1997) shift rhythms.
Figure 1.
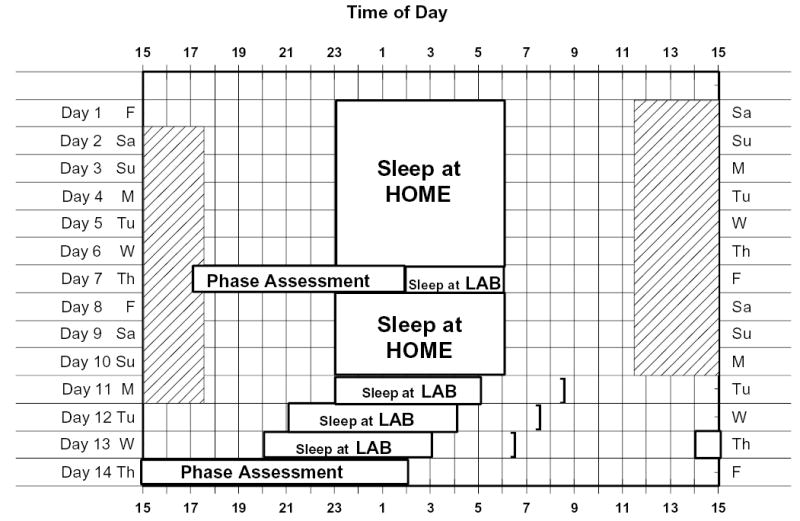
A diagram of the study protocol, for a 2300 to 0600 sleeper. This was the most common sleep schedule in the study. During the phase assessment sessions, saliva was sampled every 30 min in dim light (< 10 lux) for later determination of melatonin levels. On days 11–13, subjects received the phase-advancing treatment, their wake time was advanced by 1 h each morning, and they received continuous, intermittent, or dim light in the morning. The end of the treatment on each day is indicated by the brackets. The shaded area represents time within which subjects were permitted to nap; for this schedule, it was from 1130 to 1730.
Phase-Advancing Treatment
On days 11–13, subjects slept in the lab in individual, dark, temperature-controlled bedrooms. The 3 morning light treatments were continuous 3.5 h bright light exposure, intermittent bright light exposure for 3.5 h (0.5 h on, 0.5 h off, etc.), or dim ordinary room light (< 60 lux) for 3.5 h. The bright light was ~3000–11000 lux depending on angle of gaze (mean intensity 6322 ± 783 lux, as measured periodically with an Extech 401025 Light Meter, Waltham, MA). We used a duration of 3.5 h because it is a feasible amount of time to sit in front of a light box, and a longer duration would have less of an additional phase-advancing effect because it would be further from the crossover point on the light PRC. The light was produced by a single light box (61 × 61 × 10 cm, Enviro-Med, WA) placed on a desk, about 40 cm in front of the subject’s eyes. Each light box had a diffuser screen and contained four 54 cm long 40 watt fluorescent horizontal tubes (Philips PL-L40W/41/RS/IS, 4100K). Subjects were permitted to eat and read during the light sessions, providing the reading material remained flat in front of them. Each bedroom had 1 ceiling fixture containing 3 fluorescent tubes, and it was controlled by a dimmer switch inaccessible to subjects. The light during the dim light condition and in between the light pulses in the intermittent condition was produced by this ceiling fixture and was < 60 lux in the direction of gaze.
Phase Assessments
During the phase assessments, subjects remained awake in dim light (< 10 lux), as verified at the level of the subjects’ eyes and in the direction of gaze, with a Minolta TL-1 light meter (Ramsey, NJ). The subjects sat in recliners in a semirecumbent position and only changed posture for trips to the nearby washroom. The washroom and hall to washroom were also dimly lit (< 10 lux). Washroom trips were discouraged during the 10 min before each saliva sample. Subjects gave a 2 ml saliva sample every 30 min using Salivettes (Sarstedt, NC). To avoid contamination of the samples, no caffeine, chocolate, bananas, or lipstick were allowed in the 5 h before and during the phase assessment. No toothpaste or mouthwash were allowed during the phase assessment. Small snacks and fluids were permitted, except in the 10 min before each sample, and subjects were required to rinse and brush their teeth with water 10 min before each sample if they had consumed food or drink. Saliva samples were centrifuged immediately after collection and frozen. The samples were later shipped to Pharmasan Labs (Osceola, WI) in dry ice to be radioimmuno-assayed for melatonin. All samples from an individual subject were assayed in the same batch. The sensitivity of the assay was 0.7 pg/ml, and intra- and interassay coefficient of variabilities were 12.1% and 13.2%, respectively.
Measures of Sleep
Bedtimes, sleep onset times, wake times of greater than 5 min during sleep, final morning awakening time, and naps were reported by subjects using daily sleep logs. Subjects also wore an actigraphy monitor (Actiwatch 64, Mini Mitter, Bend, OR) on their nondominant wrist, except during showers and baths. The wrist monitor collected data that were later analyzed to estimate sleep parameters (see below).
Symptom Questionnaires
Two different 1-page questionnaires were completed by subjects during every day of the study. The 1st questionnaire was entitled “How Are You Feeling Right Now?” and was completed 5 times/day: wake time, 4 h after wake time, in the middle of the waking period, 4 h before bedtime, and at bedtime. Subjects were provided with programmed timers that reminded them of when to complete this questionnaire. This questionnaire consisted of the Stanford Sleepiness Scale (SSS) (Hoddes et al., 1973), where 1 of 7 descriptors is circled that most closely matches the subject’s subjective level of sleepiness. Following the SSS, on the same page, were 6 additional questions relating to physical fatigue, mental fatigue, sadness, anxiety, irritability, and gastrointestinal problems. Subjects reported their symptoms by circling a number from 1 (very little) to 10 (very much).
The second questionnaire was “The Columbia Jet Lag Scale” (Spitzer et al., 1999), which was completed just before bedtime. It consisted of 9 items relating to sleepiness, fatigue, daytime alertness, concentration, lethargy, light-headedness, weakness, clumsiness, and memory. Subjects indicated how much these symptoms had bothered them during that day by circling a number from 0 (not at all) to 4 (extremely). The items on each day were summed to yield an overall jet lag score, with a possible range of 0–36.
Other Procedures
During their sleep periods, subjects were instructed to lie in bed in the dark and try to sleep. They were not permitted to read, watch TV, listen to music, or talk on the telephone at this time. The wrist actigraphy monitor used to estimate sleep parameters also helped to monitor compliance. Additionally, subjects were required to wear a photosensor around their neck as a medallion (Actiwatch-L, Mini Mitter, Bend, OR), except during showers and baths. During sleep periods, the light medallion was placed on their nightstand, but they continued to wear the wrist actigraph. Subjects were also required to call the lab voice mail (time and date of call was recorded) before turning out their lights at night and when they woke in the morning each day. Every 1–3 days, subjects came to the lab and the data from their wrist actigraph, photosensor, and sleep logs were examined and compared in their presence. Thirty-two subjects began the study, but 4 were dropped: 1 for noncompliance, 1 because of low melatonin levels, and 2 for technical reasons.
Subjects were only permitted to consume caffeine (up to 300 mg) in the first 3 h after their wake time, and up to 2 standard drinks of alcohol per day on days 1–4, after which alcohol was not permitted. Subjects were breathalyzed on nights they came to the lab. Nonsteroidal anti-inflammatory drugs were permitted on days 1–3 but thereafter were not allowed, as these drugs suppress melatonin (Murphy et al., 1996).
Data Analysis
Circadian Phase
The threshold used to determine each subject’s DLMO was calculated based on the Kennaway method (Voultsios et al., 1997). The threshold was equal to the mean + 2 SDs of 5 low daytime values. Each DLMO was the point in time when the melatonin concentration exceeded and remained above the threshold, as determined with linear interpolation. For each subject, the same threshold was used for both phase assessments, and in our sample it ranged from 1.0 to 4.4 pg/ml (mean ± SD = 2.4 ± 0.9 pg/ml).
Sleep Parameters
Sleep was estimated by wrist actigraphy and sleep logs. Sleep parameters from days 2–6 were averaged to produce baseline values that were compared to treatment days 11, 12, and 13. Total sleep time on each day was the sleep time during the nighttime sleep period, plus any sleep during the naps.
The wrist monitors collected activity counts in 1-min bins. Sleep times were estimated by using the Actiware-Sleep 3.1 analysis program (Mini Mitter, Bend, OR), which analyzed the sleep period from 1 h before to 1 h after the subjects’ self-reported bed and wake times. The analysis program was set at the highest sensitivity for detecting movement, which may underestimate sleep, as any movement during sleep is considered to be wake. The program provides objective estimates of total sleep time, movement time, sleep onset latency, and sleep efficiency (sleep time/sleep period time).
We also determined total sleep time and sleep onset latency times from the sleep logs. Total sleep time equaled sleep duration reported for nighttime sleep plus naps minus awakenings of > 5 min. Sleep onset latency was the time between bedtime, recorded each night, and sleep onset time, estimated each morning.
Questionnaires
Each item on the 5 “How Are You Feeling Right Now” questionnaires that were completed each day were averaged into 1 value per day. For this questionnaire and for the Columbia Jet Lag Scale, the 5 baseline days were averaged together so they could be compared to each of the three 3-treatment days.
Statistical Analysis
The phase advance in the DLMO (from baseline to final phase assessment) in the 3 light groups (dim, intermittent, and continuous) was analyzed with a 1-way ANOVA. The sleep parameters (from wrist actigraphy and sleep logs) were analyzed with a 2-way MANOVA with 1 within-subjects factor SECTION OF STUDY (baseline, treatment days 11, 12, and 13) and 1 between-subjects factor LIGHT GROUP (dim, intermittent, or continuous). When significant, Greenhouse-Geisser corrected degrees of freedom were used for univariate analyses, although the original degrees of freedom are reported. Similarly, the Columbia Jet Lag score and SSS were each analyzed with 2-way ANOVAs (SECTION OF STUDY × LIGHT GROUP), and the remaining 6 How Are You Feeling Right Now items were analyzed with a 2-way MANOVA (SECTION OF STUDY × LIGHT GROUP). Any significant interaction in these ANOVAs indicates that changes in the variable of interest from baseline to treatment days 11, 12, and 13 were different among the 3 light groups. A significant interaction was explored with a Winer simple main effects analysis. Data are presented as means ± SDs unless otherwise specified. Statistical significance was determined at p < 0.05.
RESULTS
There were no differences among the 3 light groups in Morningness-Eveningness score (1-way ANOVA: F[2,27] = 0.28, p = 0.76) or sex (χ2[2] = 0.70, p = 0.70). The average Morningness-Eveningness score was 52.1 ± 8.5, and there were 7 moderate morning, 18 neither, and 3 moderate evening types. The 3 light groups were similar in age (continuous = 32.5 ± 6.4 y; intermittent = 26.7 ± 4.0 y; dim = 25.4 ± 2.6 y), although the dim light group was statistically significantly younger than the continuous light group (Tukey’s HSD, p = 0.025, 1-way ANOVA, F[2,27] = 4.36, p = 0.024).
Circadian Phase
Figure 2 shows that the magnitude of the phase advance increased from the dim light group to the intermittent group to the continuous light group (see Table 1 for means). The ANOVA was significant, [F(2,27) = 15.00, p < 0.001], and Tukey’s HSD post hoc test revealed that the phase advances in the continuous and intermittent groups were not significantly different (p = 0.13), but that the continuous (p < 0.001) and intermittent light groups (p = 0.003) phase advanced significantly more than the dim light group. There were no differences in circadian phase among the 3 groups at baseline. The baseline DLMOs of the 3 groups were not significantly different (1-way ANOVA: F[2,27] = 0.45, p = 0.64, see Table 1). The time between the baseline DLMO and the start of the 1st morning bright light pulse was 8.6 ± 1.5 h in the continuous light group and 9.2 ± 1.2 h intermittent light group, but these were not significantly different, [t(17) = –0.97, p = 0.35]. Several studies have shown that the average interval between the DLMO and the Tmin is 7 h (e.g., Cagnacci et al., 1996; Brown et al., 1997; Eastman et al., 2000). Thus, we estimate that the interval between the baseline Tmin and the start of the 1st bright light pulse was 1.5 to 2 h (remember wake time on 1st day of treatment was 1 h earlier than baseline).
Figure 2.

Mean salivary melatonin profiles for the dim (n = 9), intermittent (n = 11), and continuous light groups (n = 8). Error bars represent SEs. In each graph; the mean melatonin profile during the baseline phase assessment is represented by the lighter line; the bold line represents the mean melatonin profile during the final phase assessment. Horizontal lines indicate the average DLMO threshold for each group. The mean melatonin profiles were constructed referencing each individual subject’s data to the time of their DLMO in the baseline phase assessment.
Table 1.
Measures of circadian phase and scheduled bed and wake times in the 3 light groups
|
Dim Light |
Intermittent Light |
Continuous Light |
||||
|---|---|---|---|---|---|---|
| Mean | SD (h) | Mean | SD (h) | Mean | SD (h) | |
| Phase advance of DLMO (h) | 0.6 | 0.3 | 1.5 | 0.8 | 2.1 | 0.5 |
| Baseline | ||||||
| DLMO | 20:43 | 1.1 | 20:37 | 1.1 | 21:07 | 1.5 |
| Bedtime | 23:07 | 0.6 | 23:11 | 1.0 | 22:45 | 0.6 |
| Wake time | 06:37 | 0.4 | 06:38 | 0.8 | 06:30 | 0.9 |
| Estimated Tmina | 03:43 | 1.1 | 03:37 | 1.1 | 04:07 | 1.5 |
| Tmin to Wake interval (h) | 2.9 | 1.2 | 3.0 | 1.2 | 2.4 | 1.5 |
| Third treatment day | ||||||
| Bedtime | 20:07 | 0.5 | 20:11 | 1.0 | 19:45 | 0.6 |
| Wake time | 03:37 | 0.4 | 03:38 | 0.8 | 03:30 | 0.8 |
| Estimated Tminb | 03:20 | 1.1 | 02:36 | 0.9 | 02:46 | 1.2 |
| Tmin to Wake interval (h) | 0.3 | 1.2 | 1.0 | 1.1 | 0.7 | 1.3 |
| Final phase assessment | ||||||
| DLMO | 20:09 | 1.1 | 19:06 | 0.9 | 19:04 | 1.1 |
| Estimated Tmina | 03:09 | 1.1 | 02:06 | 0.9 | 02:04 | 1.1 |
Calculated by adding 7 h to the time of the DLMO (see Results).
Calculated with linear interpolation between the baseline and final Tmin.
Sleep
The average scheduled bed and wake times during baseline in each of the 3 groups were similar (see Table 1). The actigraph data from 2 subjects (1 in continuous light group, 1 in dim light group) were collected with a different epoch length than the remaining subjects. So while these data were able to confirm the subjects’ compliance to the sleep schedule, their actigraph data were not included in the final analysis.
Total sleep time from sleep logs and actigraphy is shown in Figure 3, top 2 panels. Total sleep time decreased by less than an hour from baseline to the 1st day of treatment (day 11) and thereafter returned to close to baseline levels. The decrease from baseline to treatment was statistically significant (main effect of section of study, sleep logs F[3,69] = 15.20, p < 0.001, actigraphy F[3,69] = 15.10, p < 0.001), but there was no significant main effect of light group and no significant interaction. Sleep onset latency (Fig. 3, bottom panels) did not significantly change from baseline to treatment; the main effects of section of study were not significant. However, there was a significant interaction for the sleep logs, [F(6,69) = 2.99, p = 0.02]. The simple main effects analysis indicated that sleep onset latency lengthened during the treatment in both the continuous and dim light groups but not in the intermittent light group. Actigraphy yielded movement time, which on average decreased by 0.1 ± 0.2 h from baseline to treatment (section of study: F[3,69] = 12.81, p < 0.001). There were no significant changes in sleep efficiency from actigraphy. Total sleep times as estimated from sleep logs and actigraphy were significantly correlated (r = 0.56, p < 0.001), as was sleep onset latency (r = 0.50, p < 0.001).
Figure 3.

Total sleep time and sleep onset latency, from sleep logs and from wrist actigraphy during baseline (days 2–6) and during the phase-advancing treatment (days 11–13). Values are means ± SEs.
Questionnaires
The Columbia Jet Lag scores showed a gradual increase from baseline through the 3 treatment days for the dim and intermittent light groups, but remained at about baseline levels for the continuous light group. The highest mean score that occurred was ~11 (out of a possible 36), and this occurred in the dim light group on the 3rd treatment day and in the intermittent light group on the 2nd and 3rd treatment days. Thus, the increase in this jet lag scale was fairly small. There was a significant main effect of section of study, [F(3,75) = 9.62, p < 0.001], and a significant section of study by light group interaction, [F(6,75) = 2.56, p = 0.044], but no significant main effect of group. The simple main effects analysis confirmed that the jet lag score significantly increased from baseline to treatment days in the dim and intermittent group but not in the continuous light group.
In general, the ratings on the SSS and the other items on the How Are You Feeling Right Now questionnaire during the treatment days were similar to baseline levels, or there was a slight change. The only group that showed much of a change in the SSS was the intermittent light group, but the highest mean ratings were only ~3 out of a possible 7, which corresponds to “relaxed, awake, not at full alertness, responsive.” The 2 items on the rest of the How Are You Feeling Right Now questionnaire that had the highest average ratings during treatment were physical and mental fatigue, and these were also low, always less than 4 out of a possible 10. The highest ratings were seen in the dim light group followed by the intermittent light group; the continuous light group showed the least change from baseline. Statistics revealed that for all 3 groups combined, sleepiness (SSS), physical fatigue, and mental fatigue increased during the treatment days as compared to baseline (there were significant main effects of section of study: [F(3,75) = 3.72, p = 0.02], [F(3,75) = 10.27, p < 0.001], [F(3,75) = 5.92, p = 0.004]. In addition, 2 items showed a significant section of study by light group interaction: anxiety and irritability, [F(6,75) = 2.54], [p = 0.03], [F(6,75) = 3.12], [p = 0.017], respectively. Simple main effects analysis revealed that anxiety levels in the continuous light group significantly decreased from baseline to treatment, but remained unchanged in the dim and intermittent light groups. Irritability significantly increased from baseline to treatment in the intermittent light group but remained unchanged in the dim and continuous light group.
DISCUSSION
We showed that a 3-day preflight treatment with bright morning light, which could be easily self-administered at home, phase-advanced circadian rhythms with little “cost” in terms of impaired sleep and alertness. Thus, this would be a feasible method to reduce jet lag for eastward travel. Continuous bright light and the gradually advancing sleep schedule produced a slightly greater advance than when the bright light was intermittent (by about 0.6 h), but the difference was not statistically significant. The slightly greater advance in the continuous light group may have been due to the greater duration of light, or to the bright light on the 1st treatment day beginning at a slightly earlier circadian phase. Regardless of the cause, a power analysis (1-tailed test) revealed that to obtain a significant difference between continuous and intermittent light, we would need 18 subjects in each group. While intermittent light is a more feasible method of administering bright light (people can temporarily move from the light box while they shower, dress, etc.), our results do suggest that the phase advances may increase slightly in magnitude with less time away from the light box.
Was the 2-h advance we produced in the continuous light group large enough to really help eastward travelers? It is possible that had we measured the entire melatonin profile, we may have found larger phase advances in the offset of the melatonin rhythm (DLMOff), as this phase marker can show larger phase advances than the DLMO, in response to morning light (Buresova et al., 1991). In any case, a key determinant of the speed and direction of re-entrainment is the amount and timing of light exposure on arrival. To promote phase advances, light (especially outdoor light) prior to the traveler’s Tmin needs to be minimized, and light after the Tmin needs to be maximized. It is the overall 24-h light-dark pattern relative to the Tmin that is important in determining subsequent phase shifts (e.g., Eastman and Martin, 1999). A traveler’s preflight circadian baseline phase is also important, as “early birds” with earlier circadian phases will receive even less delaying light (before their Tmins) following eastward travel. We believe that our 3-day treatment could help eastward travelers, particularly those who travel across multiple time zones and who arrive in the morning, because the phase-advancing treatment could lead to less light falling on the phase delay portion of the PRC. Clearly, however, the actual light exposure travelers receive depends on many factors, such as the weather, time of year, and their behavior in the destination—including time outside, use of sunglasses, exposure to indoor light, and sleeping or napping behavior.
Our study had a unique combination of gradually advancing sleep/dark and morning bright light. A gradually advancing sleep/dark schedule has been used in studies in which subjects live in artificially short light-dark (T) cycles in temporal isolation (e.g., 20 h day, Wyatt et al., 1999; 23 h 40 min day, Miyazaki et al., 2001). However, those subjects were kept in dim light (< 15 lux) when awake and were not exposed to bright light on awakening or outdoor light as were our subjects. Other laboratory studies examined phase shifts following various intensities of light combined with a large abrupt advance of sleep/dark (e.g., Honma et al., 1995; Boivin et al., 1996) and thus are less relevant to our design with small gradual advances of sleep/dark. Nonetheless, the results from these studies show (as we did) that when appropriately timed, bright light can increase the magnitude of the phase advance compared to dim light.
What side effects did the subjects experience preflight to potentially reduce jet lag postflight? The loss of sleep was minimal (on average less than 1 h) and occurred primarily on the 1st day of treatment when the sleep opportunity was shortened by 1 h. In the continuous light group (the group that got the most light and phase advanced the most), the various subjective symptoms remained near baseline levels on all 3 treatment days. Thus, the 2-h phase advance was achieved with minimal side effects, and only a slight reduction in sleep duration.
We would expect more negative consequences if the sleep schedule advanced much more than the circadian rhythms, such that the Tmin occurred after the scheduled wake times. Table 1 shows the average time from the estimated Tmin to wake during baseline and on the 3rd treatment day for the 3 groups. For the bright light groups, on the 3rd treatment day this interval was 1.0 and 0.7 h. Thus, the Tmin fell within the sleep period for most of these subjects. However, for the dim light group, which phase advanced the least, the average Tmin occurred only 0.3 h before wake time on the 3rd treatment day. Hence, the dim light group was most at risk for serious misalignment. Nonetheless, none of our groups reported large increases in negative subjective symptoms, probably because the misalignment, if any, was small and our subjects were young and probably quite “phase tolerant” (Dawson and Campbell, 1991).
Although our treatment has not yet been tested in the field, it seems likely that many travelers will view the treatment as worthwhile. Vacationers who have invested a substantial sum will want every day to be as free from jet lag as possible. Diplomats, businesspeople, politicians, athletes, and the military are also likely to want to have less jet lag on arrival if they are traveling to an event that requires them to perform well. Our results suggest that more than 3 days of treatment would be necessary to effectively prepare for eastward flight across several time zones. More days of treatment would not be a problem in the morning hours for many individuals, as desk work and computer work can be done in front of a light box. A greater deterrent could be the early bedtimes, as some may be unwilling to give up evening social time. It would be up to the individual to decide if it is worthwhile to give up a few evenings in order to reduce or eliminate jet lag.
More research is needed to measure the phase advance that could be produced with more days of treatment and the associated side effects. Adding exogenous melatonin (timed to phase advance) may increase the phase advance. Further work also needs to test if greater phase advances can be produced by advancing the sleep schedule at a faster rate (e.g., by 2 h/day) without producing a meaningful increase in side effects. The elderly should also be studied, as despite their more advanced circadian phase (Duffy et al., 2002), they too find it harder to phase advance than phase delay (Monk et al., 2000).
Acknowledgments
This work was supported by a grant to C. Eastman: NINR R01 NR07667. We thank Caroline Kang, Natalie Stroupe, and Barbara Trzop for their assistance with data collection. We thank Clara Lee for help with the figures. We thank Enviro-Med, Vancouver, WA, for donating the light boxes.
References
- Arendt J, Aldhous M, English J, Marks V, Arendt JH. Some effects of jet-lag and their alleviation by melatonin. Ergonomics. 1987;30:1379–1393. [Google Scholar]
- Aschoff J (1981) Circadian rhythms: interference with and dependence on work-rest schedules. In Biological Rhythms, Sleep and Shift Work, LC Johnson, DI Tepas, WP Colquhoun, and MJ Colligan, eds, pp 11–34, Spectrum, New York.
- Aschoff J, Hoffmann K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase shifts of the zeitgeber. Chronobiologia. 1975;2:23–78. [PubMed] [Google Scholar]
- Baehr EK, Fogg LF, Eastman CI. Intermittent bright light and exercise to entrain human circadian rhythms to night work. Am J Physiol. 1999;277:R1598–R1604. doi: 10.1152/ajpregu.1999.277.6.R1598. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- Boulos Z (1998) Bright light treatment for jet lag and shift work. In Seasonal Affective Disorder and Beyond Light Treatment for SAD and Non-SAD conditions, RW Lam, ed, pp 253–287, American Psychiatric Press, Washington DC.
- Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk DJ, Eastman CI. Light treatment for sleep disorders: consensus report: VII. Jet lag. J Biol Rhythms. 1995;10:167–176. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- Brown EN, Choe Y, Shanahan TL, Czeisler CA. A mathematical model of diurnal variations in human plasma melatonin levels. Am J Physiol. 1997;272:E506–E516. doi: 10.1152/ajpendo.1997.272.3.E506. [DOI] [PubMed] [Google Scholar]
- Buresova M, Dvorakova M, Zvolsky P, Illnerova H. Early morning bright light phase advances the human circadian pacemaker within one day. Neurosci Lett. 1991;121:47–50. doi: 10.1016/0304-3940(91)90646-b. [DOI] [PubMed] [Google Scholar]
- Buxton OM, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol. 2000;278:R373–R382. doi: 10.1152/ajpregu.2000.278.2.R373. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Soldani R, Laughlin GA, Yen SSC. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol. 1996;80:25–29. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Daan S, Lewy AJ. Scheduled exposure to daylight: a potential strategy to reduce “jet lag” following transmeridian flight. Psychopharmacol Bull. 1984;20:566–568. [PubMed] [Google Scholar]
- Dawson D, Campbell SS. Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep. 1991;14:511–516. doi: 10.1093/sleep/14.6.511. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol. 2002;282:E297–E303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- Dumont M, Carrier J. Daytime sleep propensity after moderate circadian phase shifts induced with bright light exposure. Sleep. 1997;20:11–17. doi: 10.1093/sleep/20.1.11. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31:87–98. doi: 10.3109/07853899908998783. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Martin SK, Hebert M. Failure of extraocular light to facilitate circadian rhythm reentrainment in humans. Chronobiol Int. 2000;17:807–826. doi: 10.1081/cbi-100102116. [DOI] [PubMed] [Google Scholar]
- Gundel A, Spencer MB. A circadian oscillator model based on empirical data. J Biol Rhythms. 1999;14:516–523. doi: 10.1177/074873099129000849. [DOI] [PubMed] [Google Scholar]
- Gundel A, Wegmann HM. Transition between advance and delay responses to eastbound transmeridian flights. Chronobiol Int. 1989;6:147–156. doi: 10.3109/07420528909064625. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Honma K-I, Honma S, Nakamura K, Sasaki M, Endo T, Takahashi T. Differential effects of bright light and social cues on reentrainment of human circadian rhythms. Am J Physiol. 1995;268:R528–R535. doi: 10.1152/ajpregu.1995.268.2.R528. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Klein KE, Herrmann R, Kuklinski P, and Wegmann HM (1977) Circadian performance rhythms: experimental studies in air operations. In Vigilance: Theory, Operational Performance and Physiological Correlates, RR Mackie, ed, pp 111–131, Plenum, New York.
- Miyazaki T, Hashimoto S, Masubuchi S, Honma S, Honma KI. Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. Am J Physiol. 2001;281:R197–R205. doi: 10.1152/ajpregu.2001.281.1.R197. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Carrier J, Kupfer DJ. Inducing jet-lag in older people: directional asymmetry. J Sleep Res. 2000;9:101–116. doi: 10.1046/j.1365-2869.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–139. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol. 2000;279:R1574–R1579. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Terman M, Williams JB, Terman JS, Malt UF, Singer F, Lewy AJ. Jet lag: clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am J Psychiatry. 1999;156:1392–1396. doi: 10.1176/ajp.156.9.1392. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Sasaki M, Itoh H, Sano H, Yamadera W, Ozone M, Obuchi K, Nishimura H, Matsunaga N. Re-entrainment of circadian rhythm of plasma melatonin on an 8-h eastward flight. Psychiatry Clin Neurosci. 1999;53:257–260. doi: 10.1046/j.1440-1819.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Sasaki M, Itoh H, Yamadera W, Ozone M, Obuchi K, Matsunaga N, Sano H, Hayashida K. Re-entrainment of the circadian rhythms of plasma melatonin in an 11-h eastward bound flight. Psychiatry Clin Neurosci. 2001;55:275–276. doi: 10.1046/j.1440-1819.2001.00857.x. [DOI] [PubMed] [Google Scholar]
- Tasto DL, Colligan MJ, Skjei EW, and Polly SJ (1978) Health Consequences of Shift Work, NIOSH Publication #78–154, Cincinnati, OH.
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Reilly T, Atkinson G. Jet-lag. Lancet. 1997;350:1611–1616. doi: 10.1016/S0140-6736(97)07569-7. [DOI] [PubMed] [Google Scholar]
- Wever RA (1979) The Circadian System of Man, Springer-Verlag, New York.
- Wyatt JK, Ritz-DeCecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]


