Abstract
The relative location of active and repressed genes within the nucleus is becoming recognized as a significant factor in the control of gene expression. We have developed systems to visualize parental and replicated herpes simplex virus type 1 (HSV-1) amplicon genomes in association with PML nuclear bodies (ND10) in live cells. Plasmids containing viral replication and packaging signals, a gene expressing enhanced yellow fluorescent protein linked to the tetracycline repressor DNA binding domain and 14 copies of the tetracycline operator sequence were used to produce amplicon genomes packaged into normal viral particles. The frequency of the juxtaposition of viral genomes and ND10 was substantially increased by inclusion of an active HSV-1 Early gene transcription unit, indicating that the association is neither random nor passive. Furthermore, the ND10-associated genomes preferentially progressed to form viral replication compartments. Thus, active viral transcription contributes to the efficiency of viral genome association with ND10, and this in turn increases the probability that the genome will engage in active DNA replication. These studies in live cells provide a novel insight into virus–ND10 interactions and provide compelling visualization of their functional relevance.
Keywords: HSV-1/live cell microscopy/ND10/PML nuclear bodies/viral DNA replication
Introduction
After the entry of a herpes simplex virus type 1 (HSV-1) virion into a susceptible cell, the tegument and capsid are released into the cytosol, the capsid is transported to the nuclear pore and the viral genome is rapidly released into the nucleoplasm (for reviews, see Fields et al., 1996). Whether the virus is able to initiate productive, lytic infection or instead becomes quiescent (latent) depends on a number of factors, both viral and cellular. VP16 present in the viral tegument forms a tripartite complex with the host proteins HCF and Oct-1 to recognize and stimulate the viral immediate-early (IE) gene promoters. At least three of the five IE proteins are required for the fully efficient expression of the later classes of genes, and if IE gene expression or IE protein function is compromised the parental viral genome can become subject to a cellular repression mechanism. Such repressed parental genomes in cultured cells can attain a stable quiescent state that in some ways resembles latency in animal models (Preston, 2000). Despite the obvious importance of the topic, there is little information available on the chromatin organization or location of quiescent or latent HSV-1 genomes. The available evidence indicates that viral DNA is highly accessible during lytic infection and is not assembled into a regular chromatin structure, whereas latent genomes are more highly chromatinized (Muggeridge and Fraser, 1986; Deshmane and Fraser, 1989).
Recent work has suggested that specific regions of the nucleus may be preferred locations for transcriptional repression and that the relative location of a gene within the nucleus can alter in response to activation or repression (Cockell and Gasser, 1999; Tsukamoto et al., 2000). It is intriguing that parental HSV-1 genomes and those of other viruses are preferentially found close to, or in association with, discrete nuclear structures known as ND10 or PML nuclear bodies during the early stages of lytic infection (Ishov and Maul, 1996; Maul et al., 1996). These previous studies combined in situ hybridization with immunocytochemistry to determine the location of viral genomes. However, this approach is technically difficult because of the very high GC content of the HSV-1 genome, and it is limited to the study of fixed cell samples.
The development of enhanced green fluorescent protein (EGFP) has revolutionized the ability to dissect dynamic cellular processes in vivo. Recent studies have demonstrated the feasibility of using multimerized DNA binding sites and EGFP linked to the cognate DNA binding protein to locate genes within the nucleus (Straight et al., 1996; Belmont, 2001). Several studies have made use of systems based on lac operator–repressor interactions to examine chromatin organization, separation of sister chromatids, chromosome dynamics and even gene expression in living cells (Straight et al., 1997; Belmont and Straight, 1998; Tumbar et al., 1999; Tsukamoto et al., 2000). More recently, the EGFP–lacI/lacO detection method was coupled with a tetracycline operator/repressor (TetO/TetR) transcriptional activation switch to study the location of genes in basal and activated states, and it was found that activated genes (and/or those with many copies of the EGFP–lacI repressor bound to multimerized lacO operator sequences) were often associated with ND10 (Tsukamoto et al., 2000).
In the present study, we show that the principles governing the use of autofluorescent fusion proteins to detect specific genes in live cells are applicable to HSV-1 infection. We have adapted the TetO/TetR specific interaction and enhanced yellow fluorescent protein (EYFP) to visualize the dynamics of parental HSV-1 amplicon genomes and their development into DNA replication compartments by time-lapse microscopy. The large replication compartments formed later in infection were found to comprise discrete territories occupied by replicated DNA derived from different parental genomes, in a situation analogous to the chromosome territories in uninfected cells. The parental amplicon genomes could be associated with ND10, and the frequency of this occurring was increased if the amplicon included active HSV-1 transcription units. Such ND10-associated genomes were preferentially utilized for the initiation of viral replication centres. These results indicate that not all viral genomes in an infected cell nucleus are functionally equivalent and that it is the ND10-associated genomes that preferentially progress to replicate progeny viral DNA. This system allows the study of many facets of lytic viral replication in live cells and opens the possibility of studying the location and accessibility of quiescent and reactivating amplicon viral genomes.
Results
Development of a system to detect HSV-1 amplicon DNA in live cells
HSV-1 amplicon plasmid pSA1 contains minimal viral DNA replication origin and packaging signals (Hodge and Stow, 2001). In the presence of helper virus, amplicon plasmids are replicated into concatemeric molecules that are packaged into otherwise normal progeny viral particles. Each amplicon viral genome contains tandem reiterations of the original amplicon plasmid and thus has multiple copies of any gene or sequence that had been inserted into the original plasmid. These properties allow the system to utilize the concept that specific gene sequences can be detected by the binding of an EGFP– DNA binding domain fusion protein (Belmont, 2001).
Plasmid pSA1.TetO contains a tandem reiteration of the 7-copy TetO array inserted into amplicon plasmid pSA1. We then inserted a gene expressing an EYFP fusion protein linked to a nuclear localization signal (nls) and the TetR DNA binding domain to give plasmid pSA1. TetO.EYFPnlsTetR (Figure 1). Depending on the size of the other components inserted into the amplicon plasmid, the family of plasmids that we have constructed will produce amplicon viral genomes with between nine and 20 plasmid reiterations, containing up to 280 copies of the TetO sequence, and thus sequester sufficient EYFPnlsTetR protein to enable visualization of the parental genomes by autofluorescence. Negative control amplicon plasmids lacking the TetR DNA binding domain (pSA1.TetO. EYFPnls) or the TetO sequences (pSA1.EYFPnlsTetR) were also constructed. Western blotting of Hep-2 cells by a selection of these plasmids indicated that all expressed autofluorescent fusion proteins of the expected sizes (data not shown).
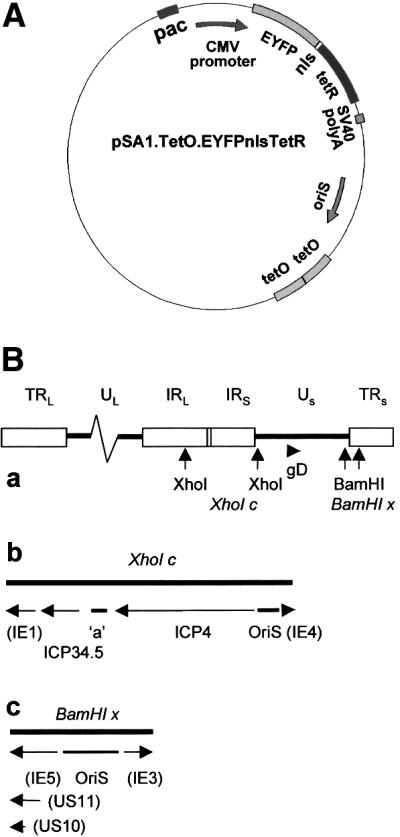
Fig. 1. (A) Schematic representation of the basic amplicon plasmid pSA1.TetO.EYFPnlsTetR containing an HSV-1 OriS replication origin and packaging signals (pac). A CMV promoter drives expression of a fusion protein linking EYFP to a nls and the TetR DNA binding domain. Tandem reiterations of the 7-copy TetO sequence are also included. (B) Maps of the HSV-1 genome showing additional regions incorporated in the various amplicon plasmids: (a) the complete HSV-1 genome with the locations of the gD gene and the restriction sites bounding fragments XhoI c and BamHI x (the boxes depict the repeated sequences); (b) an expansion of the XhoI c region, with the locations of OriS, the included genes and the ‘a’ sequence marked; and (c) an expansion of the BamHI x region, with the locations of OriS and the included genes marked. In (b) and (c), the parentheses indicate that the promoter sequences, but not the complete gene, are included in the restriction fragment.
The amplicon plasmids were transfected into BHK cells in conjunction with helper HSV-1 DNA, or the plasmid-transfected cells were subsequently infected with HSV-1 virus. Virus stocks prepared from such cells contained both helper virus and virus particles containing tandem reiterations of the amplicon plasmid. Initial experiments using antibody staining of cells infected with such stocks indicated that the amplicon viral genomes could replicate in helper virus-infected cells and form replication compartments marked by sequestration of both the EYFP signal and the major HSV-1 DNA binding protein ICP8 (data not shown). The production of the EYFP-labelled structures was dependent on the presence of both the TetR and TetO moieties in the parental amplicon plasmid (data not shown).
Visualization of parental amplicon genomes in live infected cells
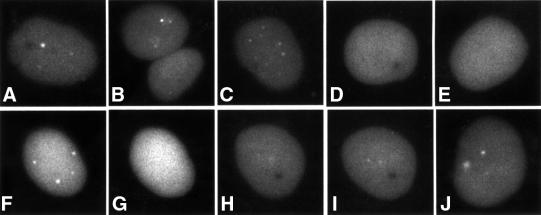
We next extended this approach to study parental amplicon genomes and developing replication centres in live infected cells. The EYFP signal was detectable as early as 90 min after addition of the amplicon stock, and in most amplicon-infected cells there was a small number of tiny bright foci within a more diffuse background signal (Figure 2A–C). These foci were never observed in cells infected with control amplicon stocks lacking either the TetR domain or the TetO binding sites (data not shown). The dots were also absent after infection with EYFPnlsTetR amplicons in the presence of tetracycline, which inhibits binding of TetR to TetO (Figure 2D and E). The addition of tetracycline to amplicon-infected cells resulted in the very rapid loss of the discrete dots (Figure 2F and G), whereas after washing out the tetracycline the dots reappeared over a period of time (Figure 2H–J). Therefore, the discrete foci are the result of TetR binding to DNA. These experiments were conducted in the presence of the DNA replication inhibitor acycloguanosine (ACG), proving that the foci represent parental amplicon viral genomes that can be visualized by these methods early after infection. The number of dots observed increased with the multiplicity of the amplicon virus infection, and since the intensities of the individual dots were similar it is likely that they represent single parental genomes.
Fig. 2. Detection of parental amplicon genomes at early times after infection. Typical examples of EYFPnlsTetR amplicon-infected cells in the presence of DNA replication inhibitor ACG 90 min after infection. Parental amplicon genomes are detectable as distinct foci (A–C) that are not observed in the presence of tetracycline (D and E). The dots formed in the presence of ACG (F) disappear within 10 min of adding tetracycline (G). Washing to remove the tetracycline allows recruitment of EYFPnlsTetR protein to the dots (H and I, same cell; J is another cell in the same sample after the tetracycline removal).
Visualization of amplicon replication compartments in live infected cells
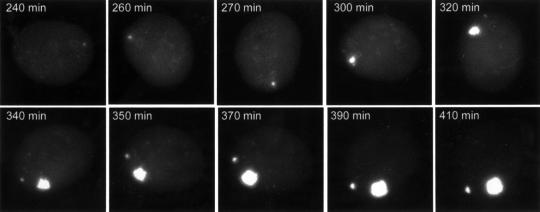
Time-lapse study of individual live cells co-infected with amplicon stocks containing helper virus indicated that a proportion of the parental amplicon genomes went on to expand and develop into much larger structures (Figure 3). The appearance of these large structures was again dependent on the TetR and TetO moieties in the amplicon genome and did not occur in the presence of ACG (data not shown). Such structures were not detected in the presence of tetracycline, but removal of the drug allowed the EYFPnlsTetR protein to bind to the amplicon DNA, such that the amplicon replication compartments that had been formed (but not detected because of the inhibition of DNA binding) became visible only a few minutes after tetracycline withdrawal (Figure 4, upper).
Fig. 3. The dynamics of developing amplicon replication compartments. Selected images of a time-lapse series of Vero cells co-infected with amplicon EYFPnlsTetR and wild-type HSV-1 helper virus are shown. The recruitment of the EYFPnlsTetR fusion protein into the amplicon replication compartment illustrates that replication is very rapid once it has been initiated. A second replication compartment starts developing at later stages of the infection. This particular cell exhibited rapid rotation during the early stages of the infection.
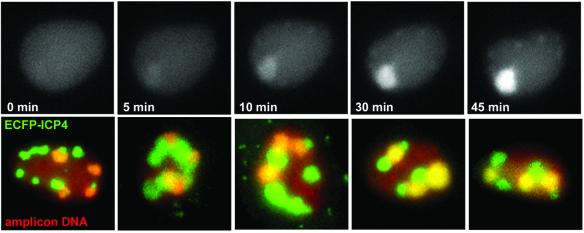
Fig. 4. (Upper panels) Amplicon replication compartments are not detected in the presence of tetracycline but become rapidly detectable after drug removal to allow binding of the EYFPnlsTetR protein onto replicated amplicon DNA. Cells were co-infected with EYFPnlsTetR amplicon and vECFP-ICP4 helper in the presence of tetracycline, and then the drug was removed 8 h after infection. An amplicon replication compartment became detectable only 5 min after removal of the drug and became rapidly brighter as pre-formed EYFPnlsTetR protein was sequestered onto pre-formed replicated amplicon DNA. (Lower panels) Five examples of cells in which both amplicon and helper virus replication compartments had developed between 5 and 6 h after infection. Both amplicon and helper virus replication centres are discrete, giving the concept of replication territories derived from individual parental genomes. The first two images were obtained using the gD amplicon, the third with the XhoI c derivative and the right-hand two with the BamHI x amplicon.
Since the cells containing amplicon replication compartments should also contain helper virus replicating DNA, we investigated the relative locations of these structures by co-infecting cells with amplicon stocks and virus vECFP–ICP4 that expresses the major HSV-1 transcriptional regulator ICP4 linked to ECFP. ICP4 is a DNA binding protein that is known to be recruited into viral replication compartments (Knipe et al., 1987). ICP4 DNA binding sites are present in the amplicon and viral genomes, so the ECFP–ICP4 protein can be recruited into both helper- and amplicon-derived replication compartments. We found that the replication compartments derived from amplicon and helper viral parental genomes were clearly distinguishable, because ECFP–ICP4 was recruited into all compartments whereas EYFPnlsTetR was present only in those derived from amplicon genomes (Figure 4, lower panels; these images were obtained using versions of the amplicon that form replication compartments more efficiently than the original construct; see below). The distinction between the compartments remained even at late times of infection when the replication compartments had apparently fused to fill most of the nucleus. Therefore, replicated viral DNA in large replication compartments is not free to mingle throughout the whole compartment, but rather these structures retain a modular or domain-like structure in which separation between replicated DNA derived from different parental genomes is retained. This concept of ‘replicon territories’ is analogous to the ‘chromosome territories’ of normal interphase cells (Cremer et al., 1993).
An Early gene transcription unit increases the frequency of association of parental amplicon genomes with PML-labelled structures in live cells
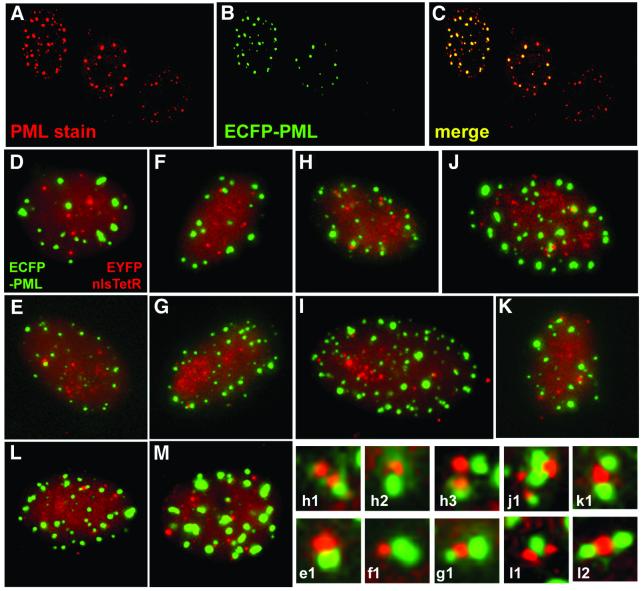
Previous studies using fixed cells have demonstrated that the parental genomes of HSV-1 and several other DNA viruses have a tendency to associate with ND10 (Maul, 1998). To label ND10 in live cells, we constructed a baculovirus with an HCMV promoter driving expression of PML fused at its C-terminus to enhanced cyan fluorescent protein (ECFP). Baculoviruses can enter mammalian cells efficiently (Hofmann et al., 1995; Boyce and Bucher, 1996), and although the insect virus proteins are not expressed (data not shown), PML expression was readily detected in the majority of cells. The expressed ECFP–PML protein was modified by conjugation to SUMO-1 and recruited into punctate foci that also contained endogenous Sp100 (another major ND10 constituent) (data not shown). The increased cellular levels of PML caused by its expression from the baculovirus result in an increase in the size, staining intensity and sometimes the number of ND10 foci (Figure 5A–C). Excessive overexpression of PML resulted in very large accumulations of PML in both the nucleus and the cytoplasm. In the subsequent experiments, only those cells expressing ECFP–PML at the lower end of the range of expression levels were analysed. In such cells, the PML foci behaved in a similar manner to endogenous PML during the onset of mitosis (data not shown).
Fig. 5. (A–C) Comparison of ND10 in uninfected Vero cells with those expressing ECFP–PML after a 16 h infection with baculovirus Ac.CMV.ECFP-PML. The left-hand two cells have been infected, whereas the right-hand cell is an uninfected control. (D–M) Association of HSV-1 parental amplicon genomes with ND10 (all images were captured between 2 and 3 h after infection). The association between the EYFPnlsTetR amplicon genomes (red) and PML (green) in Vero cells was relatively rare (D and E). Similar frequencies of association were found after infection with the IE3 amplicon containing a model IE transcription unit (F and G). Parental amplicon genomes showed substantially increased juxtaposition with PML in cells infected with an amplicon containing an Early (gD) transcription cassette (H and I). Multiple associations between amplicon DNA and ND10 were also detected using the amplicon containing the 10.5 kb HSV-1 XhoI c fragment (J and K). A similar high incidence of association was observed when the BamHI x fragment containing the complete OriS replication origin region and IE, Early and Late promoters was included in the amplicon (L and M). Enlargement of examples of the juxtaposition of parental amplicon genomes and PML are shown in the lower right-hand panels (the lettering refers to the main panel from which the detail was selected). Apart from the typical association between single genome foci and single ND10 structures, examples of multiple interactions were also observed.
Although endogenous PML is degraded and ND10 disrupted through the activity of the viral IE regulatory protein ICP0 during HSV-1 infection (Everett, 2000), the increased PML expression in the baculovirus-infected cells appeared to slow this process so that the PML foci remained for times sufficient to examine their location with respect to the parental viral genomes.
Given the previous observations on the association of parental viral genomes with ND10, we were surprised to observe in our initial experiments with the EYFPnlsTetR amplicon that the frequency of the association of parental amplicon genomes with the ECFP–PML structures was low (Figures 5D, E and 6). In this and subsequent experiments, the data were analysed by capturing images of cells at random and then analysing them at high magnification. Association was scored only when the ND10 and genome signals were touching. Whereas genome and ND10 numbers varied from cell to cell, analysis of large numbers of cells in each situation evened out these variables. Since all the factors necessary for successful viral infection are provided by the helper, the unexpectedly infrequent association between ND10 and parental genomes derived from our initial amplicon plasmid was more likely to be due to the absence of sequences in the amplicon itself. These genomes do not contain HSV-1 IE, Early or Late transcription units and only the minimal sequences required for DNA replication and packaging. Although the fluorescent fusion protein is expressed from the HCMV promoter, this does not require any trans-acting viral proteins. It is possible that binding of the tripartite VP16–Oct1–HCF complex to IE promoters, virally enhanced recruitment of transcription complexes to Early promoters or the presence of the entire replication origin region could increase the association of amplicon genomes with ND10. These factors were tested in turn.
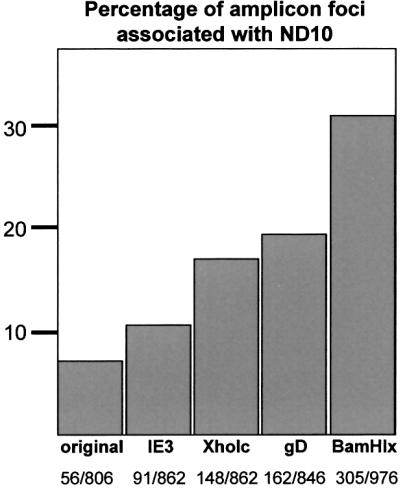
Fig. 6. Quantitation of the frequency of amplicon genome association with ND10. Over 800 individual amplicon foci in cells infected with each of the indicated amplicon stocks were scored for association with PML foci, shown as a percentage of the total. The actual numbers counted are shown in the bottom line.
Amplicon genomes containing a model IE transcription unit showed a slightly greater tendency to associate with ND10 (Figures 5F, G and 6). Inclusion of an Early (gD) gene transcription unit increased the frequency of juxtaposed amplicon genomes and PML structures by ∼2.5-fold (Figures 5H, I and 6). It is striking that the inserted fragment, which contains only the 300 bp gD promoter region, the CAT coding sequences and a short poly(A) signal, caused such a marked increase in genome association with ND10. Transcription from the gD promoter is ICP4 dependent during infection (Preston, 1979; DeLuca and Schaffer, 1985), and the gD promoter region contains two strong binding sites for ICP4 (Tedder et al., 1989). A similar increase in amplicon genome association with PML occurred with amplicons containing the 10 kb HSV-1 XhoI c fragment, encompassing the complete OriS replication origin region, gene IE3 encoding ICP4, the complete ‘a’ sequence (containing the packaging signals), the Late gene ICP34.5 and the 5′ part of the IE1 gene (Figures 1B, 5J, K and 6). The most efficient association of amplicon genomes with ND10 (>4-fold above the frequency of the original amplicon) was observed with the BamHI x amplicon (Figures 5L, M and 6). This contains the complete replication origin and the IE4/5, Late Us11 and Early Us10 promoters (Figure 1B). A large number of cells infected by each amplicon stock were examined and the observations were highly reproducible, indicating that the associations of amplicon genomes with PML are significant and not chance events. Because the XhoI c and BamHI x fragments include several HSV-1 promoters and the complete replication origin, it is not possible to conclude which of these individual factors influences the frequency of ND10 association. However, taken with the results from the gD and IE3 amplicon constructs, we can conclude that the recruitment of parental viral amplicon genomes to the periphery of ND10 is enhanced by the inclusion of cis-acting viral regulatory sequences (such as transcription units or the replication origin) that assemble complexes of trans-acting factors. In the case of the gD amplicon, a simple Early gene transcription unit is sufficient to increase substantially the frequency of ND10 association.
Development of amplicon replication compartment in juxtaposition with ND10
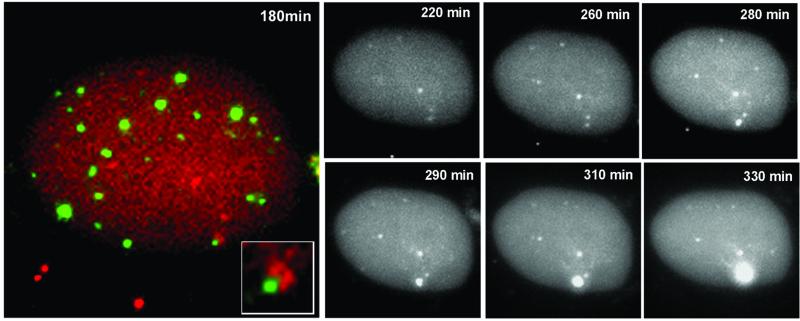
The above experiments illustrate that a proportion of parental amplicon genomes including the appropriate viral sequences are associated with ND10, whereas the remainder are not. Given this differential localization, we asked whether replication compartments could develop preferentially from the ND10-associated genomes. Vero cells displaying such associations in the presence of wild-type helper virus were monitored by time-lapse microscopy (Figure 7). At the early stages of the infection, the cell under study contained several amplicon genomes, two of which were clearly associated with a single PML structure (Figure 7, 180 min). As the infection progressed, only the genomes that were originally associated with ND10 developed into an amplicon replication centre (Figure 7). The other five genomes that were not associated with ND10 at the early times of infection remained as discrete dots and failed to produce replication compartments. In further studies of five other cells using the same protocol, we observed that replication compartments developed only from ND10-associated genomes, although not all such associated genomes began replication during the time course of the experiments. None of the 25 amplicon genomes that were not associated with ND10 in these cells developed into replication compartments. These findings illustrate that there is functional variation between the parental viral genomes in the nucleus and that the spatial organization of these genomes to the peripheries of ND10 confers a functional advantage. These findings provide solid evidence that ND10 represent preferred sites where the early steps of viral replication take place.
Fig. 7. The development of an amplicon replication compartment in association with ND10. A cell containing several parental amplicon genomes (red) (co-infected with wild-type helper virus) was selected early during infection, and a double-labelled image was captured to show two genomes initially juxtaposed with a PML body (green) in the lower right-hand portion of the cell. The inset shows this region of the cell at a higher magnification. The greyscale panels show the EYFP-labelled amplicon genomes in the same cell during the course of the infection. A replication compartment developed from the genomes initially associated with the ND10 site, but not from other genomes that were not associated with PML foci. This is a typical example; several other cells were examined in other independent experiments, and in all cases replication compartments developed only from ND10-associated genomes.
ND10 association increases the replication efficiency of the amplicon genome
A prediction from the above observation is that amplicon genomes that include sequences that increase the frequency of ND10 association should replicate more efficiently than our original construct. To test this hypothesis, we constructed an ECFPnlsTetR version of the original amplicon and used it in parallel and mixed infections with the EYFPnlsTetR/gD amplicon. The progeny stocks from these initial infections were serially passaged twice more, with total cellular DNA and supernatant progeny virus harvested at each stage. The EYFP gene has an additional PstI restriction site, allowing the distinction between the two amplicon genomes by Southern blotting. Analysis of total cell DNA from each passage showed that bands derived from the gD amplicon increased in abundance to a greater degree than those of the ECFPnlsTetR amplicon. Thus, the gD amplicon replicated more efficiently than the original ECFP version (Figure 8), confirming the implication from Figures 5–7 that the association of viral genomes with ND10 increases the probability of initiating a replication compartment. The Southern blot results were consistent with the titres of the ECFP and EYFP amplicons in the passaged stocks (estimated by counting infected cells) and the ability of the gD, XhoI c and BamHI x amplicons to form multiple repli cation compartments far more readily than the original (Figure 4; data not shown).
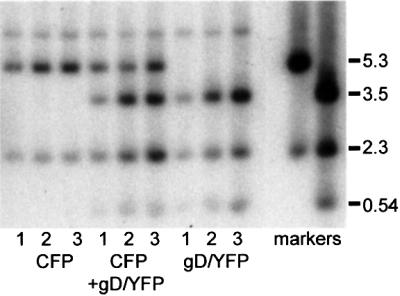
Fig. 8. Preferential replication of amplicons with increased frequency of ND10 association. Stocks of amplicons expressing ECFPnlsTetR without additional sequences and EYFPnlsTetR containing the gD promoter cassette were prepared. Cells were infected with equalized amounts of the two amplicons either separately or together, and then the progeny supernatant virus was serially passaged twice more. DNA prepared from the cells of the three passages (as indicated) was cut with PstI and analysed by Southern blotting with markers derived from the amplicon plasmids. The 5.3 kb band identifies the ECFPnlsTetR amplicon, and the 3.5 and 0.54 kb bands identify the EYFPnlsTetR/gD amplicon. The 2.3 kb band is common to both. The EYFPnlsTetR/gD amplicon shows enhanced replication over the three passages.
Discussion
We have developed a method to observe parental HSV-1 amplicon DNA and developing replication compartments in live cells. Earlier evidence from experiments using fixed cells demonstrated that the genomes of HSV-1 and several other DNA viruses have a tendency to localize at sites in close proximity to, or at the periphery of, ND10 (reviewed by Maul, 1998; Everett, 2001). These prior studies brought new insights to the field of HSV-1 DNA localization and replication, but fixed-cell experiments leave open the questions of sequence of events and functional differences between individual genomes. Our aim was to establish a robust methodology that could be adapted to the study of these aspects of viral infection in live cells.
Our data are pertinent to the intriguing question of how and why parental viral genomes have a tendency to become associated with ND10. Previous work in fixed cells has shown that transfected plasmids containing SV40 sequences become associated with ND10 as long as an active origin of DNA replication and large T antigen are present, and analogous observations have been made using papillomavirus DNA and proteins (Swindle et al., 1999; Tang et al., 2000). In both these examples, ND10 associa tion was increased when viral proteins involved in both replication and transcription were expressed, findings that are consistent with our results using HSV-1. However, a chromosomally integrated gene cassette containing in total ∼3500 lacO and TetO binding sites was found to become associated with ND10 when either the cognate EGFP–lacI or VP16–TetR fusion proteins were expressed (Tsukamoto et al., 2000). It was suggested that ND10 in some way sense and sequester exogenous or ‘foreign’ DNA–protein complexes (Tsukamoto et al., 2000). This conclusion is not totally consistent with our data because the amplicon plasmids without the HSV-1 transcription units still have the multiple TetO sites that will be binding many copies of the EYFPnlsTetR protein. Thus, in our system, assembly of ‘foreign’ DNA–protein complexes is insufficient to impart efficient ND10 localization. Rather than being the non-specific or passive process that this model implies, it seems that ND10 association is an active process, dependent on functional characteristics and with functional consequences. The differences between our observations and those of Tsukamoto et al. (2000) may be because our experiments use >10-fold fewer repeat sequences and are performed transiently over a few hours rather than in stably transformed cells.
Our results suggest that the assembly of virally directed nucleoprotein complexes, such as those involved in transcription and replication, increases parental viral genome association with ND10. In particular, an ICP4-responsive gene cassette in the gD amplicon increased the frequency of association by 2- to 3-fold. Although our amplicon plasmids contain only one or a small number of ICP4-responsive promoters, reiteration of the plasmid sequences in the amplicon genomes will increase this number substantially. Once associated with ND10, the parental viral genomes have an increased probability of initiating DNA replication and spawning a replication compartment. Thus, not all parental viral genomes are functionally equivalent, and a critical factor in this distinction between different genomes is their localization within the nucleus. This hypothesis is consistent with the observations that genomes of the γ-herpesvirus Epstein– Barr virus in the latent state are not associated with ND10, but induction of reactivation leads to increased ND10 association (Bell et al., 2000). A similar implication can be made from partly indirect data regarding the location of latent KSHV (Kaposi’s sarcoma-associated herpes virus; HHV-8) DNA and the assembly of plasmid-derived KSHV replication complexes at sites associated with ND10 (Wu et al., 2001).
Our observations raise the question of the location of parental viral genomes and developing replication compartments in cells lacking PML. Unfortunately, preliminary experiments indicated that HSV-1 does not replicate efficiently in either the PML–/– mouse primary fibroblasts or the corresponding PML+/+ control cells (R.D.Everett and A.Orr, unpublished data). Whatever the potential role of PML itself, the situation is complicated by the many other cellular proteins that can be associated with ND10. In addition, the HSV-1 regulatory protein ICP0 is also localized at ND10 at the early stages of infection. ICP0 induces extensive structural and biochemical changes to ND10, and this activity correlates with the ability of ICP0 to stimulate viral gene expression (Everett, 2000). These factors may play a role in the efficiency of viral genome association with ND10 and the functional consequences of this interaction.
The advantage of a live cell system over experiments utilizing fixed cells is that it is possible to study the dynamics of changes in viral genomes and replication compartments in response to various stimuli. Our experimental model is very versatile in that the genotypes of both helper and amplicon are easily modified independently. The use of baculoviruses to express in high proportions of cells other exogenous proteins with minimal manipulation allows yet greater flexibility. This approach opens the exciting possibility of studying the dynamics of viral genomes during the establishment and maintenance of quiescent infection and during the crucial process of reactivation of quiescent genomes.
Materials and methods
Viruses and cells
Amplicon vectors and virus were grown and titrated in BHK cells propagated in Glasgow modified Eagle’s medium (GMEM) containing 100 U/ml penicillin and 100 µg/ml streptomycin, and supplemented with 10% newborn calf serum and 10% tryptose phosphate broth. Vero cells and Hep-2 cells were grown in GMEM or Dulbecco’s modified Eagle’s medium (DMEM), respectively, supplemented with 10% fetal calf serum and antibiotics as above. Virus vECFP–ICP4 was derived from HSV-1 strain 17+ and expresses the viral IE protein ICP4 as a fusion with ECFP from the normal ICP4 genomic loci. Tetracycline was used at a concentration of 1 µg/ml and ACG at 5 µg/ml. The times of infection given in the figures relate to times after addition of the virus, rather than after an absorption period.
Baculovirus Ac.CMV.ECFP-PML construction
Plasmid pECFP-PML contains the cDNA of the 633 residue PML isoform IV [excised from plasmid pPML(F); Kastner et al., 1992] inserted in-frame into plasmid pECFP-N1. The CMV promoter region of the vector plus the ECFP–PML coding region were inserted into plasmid pFastBacHTa of the Bac-to-Bac baculovirus construction system (Life Technologies). A recombinant baculovirus (Ac.CMV.ECFP-PML) that expressed the ECFP–PML fusion protein from the CMV promoter in both insect and mammalian cells was isolated. Preliminary experiments showed that infection of Hep-2 or Vero cells with Ac.CMV.ECFP-PML at a multiplicity of 50 plaque-forming units (p.f.u.) per cell led to expression of PML located in ND10 in a high percentage of cells by 16 h after infection.
Amplicon plasmids
Amplicon plasmids were based on pSA1, which contains the HSV-1 OriS replication origin and packaging signals derived from the ‘a’ sequence (Hodge and Stow, 2001). PCR primers were used to amplify the 7-copy TetO repeat sequence from pUHD10-3 (Gossen and Bujard, 1992), and a tandem reiteration of the 7-copy sequence was inserted into pSA1 to give plasmid pSA1.TetO. A fragment containing the TetR DNA binding domain coding region was generated by PCR from plasmid pUHD15-1 (Gossen and Bujard, 1992) and inserted downstream of the GFP coding region in pEGFP-C1. A nls coding sequence, based on that of SV40 large T antigen, was then inserted between the EGFP and TetR moieties to create plasmid pEGFPnlsTetR. The entire promoter, coding region and 3′ processing signal region of pEGFPnlsTetR were excised and inserted into pSA1.TetO to create pSA1.TetO.EGFPnlsTetR. Derivatives of this plasmid lacking either the TetR coding sequence or the TetO binding sites, and spectral variants expressing ECFP or EYFP fusion proteins, were also constructed. Further derivatives including model IE and Early transcription cassettes were constructed by inserting fragments derived from reporter plasmids pIE3CAT and pgDCAT, respectively (Everett, 1986; Paterson and Everett, 1988). Another derivative of the amplicon was constructed in which the minimal OriS replication origin was replaced by a larger fragment (BamHI x) containing the complete OriS region and flanking IE and Early promoters. Finally, plasmid pAT.TetO.EYFPnlsTetR.XhoIc contains the TetO and EYFPnlsTetR segments as above, but in place of the fragments containing minimal replication and packaging signals it includes HSV-1 genomic fragment XhoI c, a 10 kb region including most of gene IE4, the complete OriS replication origin region, gene IE3 encoding ICP4, the complete ‘a’ sequence (containing the packaging signals), the Late gene ICP34.5 and the 5′ part of the IE1 gene. The basic structures of these plasmids are shown in Figure 1.
Transfection and packaging of HSV-1 amplicons
Amplicon plasmids (200 ng) were transfected into BHK cells in 24-well dishes using Lipofectamine Plus (Life Technologies) in accordance with the manufacturer’s instructions. Amplicon-transfected cells were infected the following day with HSV-1 helper virus (10 p.f.u./cell). When the cell monolayers showed extensive cytopathic effect, they were harvested by scraping the cells into the supernatant medium, sonicating the mixture and removing cellular debris by low-speed centrifugation and filtration through a 0.45 µm membrane. The amplicon and helper virus particle mixtures were centrifuged at 20 000 r.p.m. for 45 min, and the pellet was resuspended in fresh medium and aliquoted for storage at –80°C. The amplicon and helper virus yields of each stock were determined by counting fluorescent amplicon-infected Vero cells and by plaque assay of infectious virus on BHK cells, respectively. All the amplicon stocks used in these experiments had equivalent titres of helper (1–2 × 108 p.f.u./ml) and amplicon (∼5 × 106 focus-forming units/ml).
Time-lapse microscopy of live cells
Live cells were observed using a Zeiss Axiovert S100 inverted fluorescence microscope equipped with a Hamamatsu Orca 2 digital camera. Excitation wavelength was controlled by mercury lamp illumination and a motorized filter wheel equipped with filters specific for ECFP, EGFP and EYFP (Chroma Technology). Dichroic and emission filter combinations (Chroma Technology) suitable for dual ECFP/EYFP and single EGFP fluorophores were housed in a static filter block. The filter wheel, shutters on fluorescence and DIC illumination light paths, and camera image acquisition were controlled by AQM software (Imaging Associates and Kinetic Imaging). Single images or timed image sequences were exported as bitmap or TIFF files from the AQM software and then transferred to the programme FLEX running in the KS300 environment (Imaging Associates) for the selection of regions of interest from image sequences. Individual frames were prepared for presentation using Adobe Photoshop.
An essential part of the system was the ability to maintain the cells for long periods in a controlled environment with constant temperature, CO2 and humidity. The main body of the microscope, excluding the manual controls, was housed in a humidified gas-tight Perspex box equipped with temperature-controlled air circulation and a CO2 detection and maintenance device (supplied by EMBL Workshops, Heidelberg, Germany). The conditions within the box were maintained at 37°C, 5% CO2, 90% humidity, such that the cells remained healthy indefinitely. The cells were seeded into two-well, chambered coverglass units with coverslip quality glass bottoms (Lab-Tek; Nunc) at a density of 2–4 × 105 cells per well the day before each experiment, and the samples were examined with a ×63 oil immersion objective lens.
Confocal microscopy
Vero cells seeded onto coverslips were infected with Ac.CMV. ECFP-PML. The following day the cells were fixed, stained for immunofluorescence with anti-PML rabbit serum r8 and examined by confocal microscopy as described previously (Everett et al., 1999).
Comparative replication of amplicon stocks
Amplicon stocks expressing ECFPnlsTetR in the original background and EYFPnlsTetR in the amplicon containing the gD transcription unit were prepared. Aliquots giving equal numbers of positive cells were used to infect wells of Vero cells both individually and in combination. After 2 days, progeny virus was recovered from the supernatant and total cell DNA made from the cells. The procedure was repeated twice more to give three serially passaged DNA samples from each of the individual and mixed infections. Equal amounts of DNA were digested with PstI, and then the fragments were analysed by Southern blotting using a probe derived from an amplicon plasmid derivative lacking any HSV-1 DNA sequences.
Acknowledgments
Acknowledgements
The authors would like to thank Duncan McGeoch for his encouragement and helpful comments on the manuscript, Nigel Stow for plasmid pSA1, Chris Boutell and Melanie Lambotin for help with baculovirus experiments and provision of unpublished data, Jens Rietdorf and Andreas Girod (EMBL) for help with the live cell management system, Paul Sheppard (Imaging Associates) for help with the automated microscopy and Dagmar Mörsdorf for anti-baculovirus antibodies. This work was supported by the Medical Research Council. G.S. is a postdoctoral researcher funded by a Marie Curie Fellowship of the European Community Framework 5 programme (contract number HPMF-CT-2000-01078).
References
- Bell P., Lieberman,P.M. and Maul,G.G. (2000) Lytic but not latent replication of Epstein–Barr virus is associated with PML and induces sequential release of nuclear domain 10 proteins. J. Virol., 74, 11800–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont A.S. (2001) Visualizing chromosome dynamics with GFP. Trends Cell Biol., 11, 250–257. [DOI] [PubMed] [Google Scholar]
- Belmont A.S. and Straight,A.F. (1998) In vivo visualization of chromosomes using lac operator–repressor binding. Trends Cell Biol., 8, 121–124. [DOI] [PubMed] [Google Scholar]
- Boyce F.M. and Bucher,N.L. (1996) Baculovirus-mediated gene transfer into mammalian cells. Proc. Natl Acad. Sci. USA, 93, 2348–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell M. and Gasser,S.M. (1999) Nuclear compartments and gene regulation. Curr. Opin. Genet. Dev., 9, 199–205. [DOI] [PubMed] [Google Scholar]
- Cremer T. et al. (1993) Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb. Symp. Quant. Biol., 58, 777–792. [DOI] [PubMed] [Google Scholar]
- DeLuca N.A. and Schaffer,P.A. (1985) Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol. Cell. Biol., 5, 1997–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane S.L. and Fraser,N.W. (1989) During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J. Virol., 63, 943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R.D. (1986) The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J. Gen. Virol., 67, 2507–2513. [DOI] [PubMed] [Google Scholar]
- Everett R.D. (2000) ICP0, a regulator of herpes simplex virus during lytic and latent infection. BioEssays, 22, 761–770. [DOI] [PubMed] [Google Scholar]
- Everett R.D. (2001) DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene, 20, 7266–7273. [DOI] [PubMed] [Google Scholar]
- Everett R.D., Earnshaw,W.C., Findlay,J. and Lomonte,P. (1999) Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J., 18, 1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B.N., Knipe,D.M. and Howley,P.M. (eds) (1996) Virology. Vol. 2, 3rd edn. Lippincott-Raven, Philadelphia, PA. [Google Scholar]
- Gossen M. and Bujard,H. (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl Acad. Sci. USA, 89, 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge P.D. and Stow,N.D. (2001) Effects of mutations within the herpes simplex virus type 1 DNA encapsidation signal on packaging efficiency. J. Virol., 75, 8977–8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C., Sandig,V., Jennings,G., Rudolph,M., Schlag,P. and Strauss,M. (1995) Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc. Natl Acad. Sci. USA, 92, 10099–10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov A.M. and Maul,G.G. (1996) The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J. Cell Biol., 134, 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P., Perez,A., Lutz,Y., Rochette-Egly,C., Gaub,M.P., Durand,B., Lanotte,M., Berger,R. and Chambon,P. (1992) Structure, localization and transcriptional properties of two classes of retinoic acid receptor α fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J., 11, 629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D.M., Senechek,D., Rice,S.A. and Smith,J.L. (1987) Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J. Virol., 61, 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G.G. (1998) Nuclear domain 10, the site of DNA virus transcription and replication. BioEssays, 20, 660–667. [DOI] [PubMed] [Google Scholar]
- Maul G.G., Ishov,A.M. and Everett,R.D. (1996) Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology, 217, 67–75. [DOI] [PubMed] [Google Scholar]
- Muggeridge M.I. and Fraser,N.W. (1986) Chromosomal organization of the herpes simplex virus genome during acute infection of the mouse central nervous system. J. Virol., 59, 764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson T. and Everett,R.D. (1988) Mutational dissection of the HSV-1 immediate-early protein Vmw175 involved in transcriptional transactivation and repression. Virology, 166, 186–196. [DOI] [PubMed] [Google Scholar]
- Preston C.M. (1979) Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J. Virol., 29, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C.M. (2000) Repression of viral transcription during herpes simplex virus latency. J. Gen. Virol., 81, 1–19. [DOI] [PubMed] [Google Scholar]
- Straight A.F., Belmont,A.S., Robinett,C.C. and Murray,A.W. (1996) GFP tagging of budding yeast chromosomes reveals that protein–protein interactions can mediate sister chromatid cohesion. Curr. Biol., 6, 1599–1608. [DOI] [PubMed] [Google Scholar]
- Straight A.F., Marshall,W.F., Sedat,J.W. and Murray,A.W. (1997) Mitosis in living budding yeast: anaphase A but no metaphase plate. Science, 277, 574–578. [DOI] [PubMed] [Google Scholar]
- Swindle C.S., Zou,N., Van Tine,B.A., Shaw,G.M., Engler,J.A. and Chow,L.T. (1999) Human papillomavirus DNA replication compartments in a transient DNA replication system. J. Virol., 73, 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Bell,P., Tegtmeyer,P. and Maul,G.G. (2000) Replication but not transcription of simian virus 40 DNA is dependent on nuclear domain 10. J. Virol., 74, 9694–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder D.G., Everett,R.D., Wilcox,K.W., Beard,P. and Pizer,L.I. (1989) ICP4-binding sites in the promoter and coding regions of the herpes simplex virus gD gene contribute to activation of in vitro transcription by ICP4. J. Virol., 63, 2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T., Hashiguchi,N., Janicki,S.M., Tumbar,T., Belmont,A.S. and Spector,D.L. (2000) Visualization of gene activity in living cells. Nat. Cell Biol., 2, 871–878. [DOI] [PubMed] [Google Scholar]
- Tumbar T., Sudlow,G. and Belmont,A.S. (1999) Large-scale chromatin unfolding and remodeling induced by VP16 acidic activation domain. J. Cell Biol., 145, 1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F.Y., Ahn,J.H., Alcendor,D.J., Jang,W.J., Xiao,J., Hayward,S.D. and Hayward,G.S. (2001) Origin-independent assembly of Kaposi’s sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J. Virol., 75, 1487–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]