Abstract
Melatonin, originally discovered as a hormone of the pineal gland, is also produced in other organs and represents, additionally, a normal food constituent found in yeast and plant material, which can influence the level in the circulation. Compared to the pineal, the gastrointestinal tract contains several hundred times more melatonin, which can be released into the blood in response to food intake and stimuli by nutrients, especially tryptophan. Apart from its use as a commercial food additive, supraphysiological doses have been applied in medical trials and pure preparations are well tolerated by patients. Owing to its amphiphilicity, melatonin can enter any body fluid, cell or cell compartment. Its properties as an antioxidant agent are based on several, highly diverse effects. Apart from direct radical scavenging, it plays a role in upregulation of antioxidant and downregulation of prooxidant enzymes, and damage by free radicals can be reduced by its antiexcitatory actions, and presumably by contributions to appropriate internal circadian phasing, and by its improvement of mitochondrial metabolism, in terms of avoiding electron leakage and enhancing complex I and complex IV activities. Melatonin was shown to potentiate effects of other antioxidants, such as ascorbate and Trolox. Under physiological conditions, direct radical scavenging may only contribute to a minor extent to overall radical detoxification, although melatonin can eliminate several of them in scavenger cascades and potentiates the efficacy of antioxidant vitamins. Melatonin oxidation seems rather important for the production of other biologically active metabolites such as N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), which have been shown to also dispose of protective properties. Thus, melatonin may be regarded as a prodrug, too. AMK interacts with reactive oxygen and nitrogen species, conveys protection to mitochondria, inhibits and downregulates cyclooxygenase 2.
Introduction
In several countries, melatonin is sold over the counter; in others its free sale is prohibited. The usefulness of melatonin as a food additive continues to be a matter of debate. Meanwhile, countless people have used melatonin for mitigating the symptoms of jet lag, an application which has been tested and is recommended [1-4]; any person we have spoken to has reported positive experiences. Melatonin has been and is being used in several clinical trials with different therapeutic approaches. In some of these studies, in addition to improvements of sleep, the repeatedly demonstrated antioxidant properties [5-10] were the main reason for testing the pineal hormone. This holds especially for the treatment of neurodegenerative disorders, such as Alzheimer's disease [11-13] and amyotrophic lateral sclerosis [14].
In terms of application it seems necessary to thoroughly analyze the mechanisms of antioxidant actions of melatonin and to distinguish between effects observed at pharmacological or physiological concentrations. These considerations must not be restricted to the melatonin released from the pineal gland into the circulation and to the classic hepatic degradation route of 6-hydroxylation followed by conjugation. On the contrary, we would like to lay emphasis on the significance of tissue melatonin and the alternate oxidative pathways of catabolism leading to different, biologically active products. The relationship between melatonin and nutrition will be discussed, with regard to the presence of the compound as a natural food constituent sometimes affecting circulating levels, to the post-prandial release of melatonin from the gastrointestinal tract, and to interactions with other antioxidants present in food. Finally, a model of mitochondrial protection is reviewed.
Melatonin in food and in the gastrointestinal tract
Melatonin is a natural compound of almost ubiquitous occurrence [15-17]. Its presence was demonstrated in all major taxa of organisms, as far as tested, including bacteria, unicellular eukaryotes, macroalgae, plants, fungi and invertebrate animals. Several studies dealt with melatonin in edible plants [8,18-25]. One can conclude that relevant quantities of melatonin are present in most vegetables, fruit, nuts and cereals. However, the precise melatonin contents are sometimes affected by some uncertainties which result from particular methodological problems arising in material from photoautotrophic organisms. First, melatonin can be easily destroyed by oxidants during extraction [26], and, second, false positive and false negative data are easily obtained due to the presence of secondary plant metabolites, either mimicking melatonin or interfering with it in the assays [16,17,21,22]. It is a strict requirement to apply preservative conditions of extraction, to control the yield by determinations of recovery, and to obtain data by two methodologically different procedures. Although this has not been done in any plant tested, the widespread occurrence of melatonin in plants is beyond doubt. To date, the presence of melatonin was demonstrated in more than 20 dicot and monocot families. Usually, the amounts of melatonin reported varied considerably between species and between plant tissues, from the detection threshold to several hundred pg/g fresh weight. One should, however, be aware that these concentrations frequently greatly exceed avian and mammalian blood levels, which rarely attain more than 200 pg/mL during the nocturnal maximum, and can remain below 10 pg/mL during the day. Intestinal resorption of dietary melatonin should not be a particular problem because the amphiphilic molecule can easily cross any membrane. Therefore, an efficient uptake of the indoleamine from food should be expected to influence the blood plasma concentration (see below). Melatonin was observed to be elevated in alpine and mediterranean plants exposed to strong UV irradiation [25], a finding which may be seen in relation to melatonin's antioxidant properties antagonizing damage by light-induced oxidants. It is particularly worth mentioning the very high levels reported for several seeds and medicinal plants [8,15,24,27,28] (Table 1). The high amounts frequently found in seeds may be interpreted in terms of antioxidative protection within a dormant and more or less dry system, in which enzymes are poorly effective and cannot be upregulated, so that low molecular weight antioxidants such as melatonin are of advantage [20]. Moreover, melatonin's amphiphilicity may favor its accumulation especially in oily seeds.
Table 1.
Particularly high melatonin levels reported for several edible and medicinal plants (selected examples).
| Species | Tissue | Melatonin [ng/g] | References |
| (A) Edible plants | |||
| Lycopersicon esculentum (tomato) | fruit | 0.5 | [18] |
| Raphanus sativus (red radish) | root tuber | 0.6 | [19] |
| Brassica campestris (Japanese radish) | stem, leaves | 0.6 | [19] |
| Brassica nigra (black mustard) | seed | 129 | [24,28] |
| Brassica hirta (white mustard) | seed | 189 | [24,28] |
| Prunus cerasus (tart cherry, Montmorency) | fruit | 15–18 | [23,24] |
| Prunus amygdalus (almond) | seed | 39 | [28] |
| Pimpinella anisum (anise) | seed | 7 | [24,28] |
| Foeniculum vulgare (fennel) | seed | 28 | [24,28] |
| Helianthus annuus (sunflower) | seed | 29 | [24,28] |
| Oryza sativa (rice) | seed | 1 | [19] |
| Zea mays (Indian corn) | seed | 1.3 | [19] |
| Avena sativa (oat) | seed | 1.8 | [19] |
| Festuca arundinacea (tall fescue) | seed | 5 | [19] |
| Elettaria cardamomum (green cardamom) | seed | 15 | [24,28] |
| Zingiber officinale (ginger) | tuber | 0.5 | [19] |
| Musa paradisiaca (banana) | fruit | 0.5 | [18] |
| (B) Officinal plants | |||
| Melissa officinalis (balm mint) | young plant | 16 | [25] |
| Scutellaria baicalensis (huang-qin) | plant | > 2,000 – > 7,000 | [24,25,27] |
| Pimpinella peregrina (-) | dried root | 38 | [25] |
| Hypericum perforatum (St. Johns wort) | leaf | 1,750 | [27] |
| Hypericum perforatum (St. Johns wort) | flower | > 2,400 – > 4,000 | [25,27] |
| Lippia citriodora (lemon verbena) | young plant | 22 | [25] |
| Tanacetum parthenium (feverfew) | leaf (fresh/dried) | > 1,300/> 7,000 | [24,25,27] |
In some of the medicinal plants, interactions or synergisms of melatonin with secondary metabolites may be of importance. In Scutellaria baicalensis, e.g., melatonin is accompanied by acteoside, baicalein, baicalin, wogonin and ganhuangenin, substances with antioxidant, antiinflammatory, sedating and immunomodulatory properties, interfering also with NO synthases and P450 monooxygenases, i.e., functions within the action spectrum of melatonin or affecting melatonin metabolism [17]. Melatonin is also present in fungi and, with regard to nutrition, this may be relevant especially for yeast. In cultures freshly prepared from commercially available cubes of baker's yeast, μmolar concentrations of melatonin were measured, sometimes exceeding 40 μM [29,30].
It is not yet known whether food is the only external source of melatonin in mammals. The presence of melatonin in bacteria including Escherichia coli [31] may suggest a contribution by intestinal bacteria to the high amounts of the indoleamine found in the gut [cf. discussion in ref. [17]]. However, strictly anaerobic bacteria, which predominate in the colon, have not yet been investigated.
The gastrointestinal tract deserves particular attention, not only with regard to melatonin uptake, but, even more, as an extrapineal site of melatonin biosynthesis, where this molecule is present in amounts exceeding those found in the pineal gland by several-hundred-fold, and from where it can be released into the circulation in a post-prandial response, especially under the influence of high tryptophan levels [32-36]. Gastrointestinal melatonin is also released to the lumen and participates in enterohepatic cycling [37-39]. Therefore, nutrition is not only linked to melatonin by uptake, but also by the influences of other food constituents and digestive physiology on melatonin release.
With regard to nutrition, a decisive question is whether the amounts of melatonin present in the food can suffice for changing its level in the blood plasma. This was first indicated by findings of Hattori et al. [19], who observed rises in plasma melatonin after feeding plant material rich in this compound. However, this result allowed a different interpretation, because other substances including its precursor tryptophan might have elicited a post-prandial release of gastrointestinal melatonin. This argument was recently refuted, at least in chicken, because the removal of melatonin from feed caused decreases in plasma levels [40]. The result gave rise to a statement that melatonin may not only be regarded as a hormone and a tissue factor, but also, in a sense, as an antioxidant vitamin.
The redox properties of melatonin may be unfavorable for its preservation in the food. Being an easily oxidizable compound capable of directly detoxifying several free radicals and other oxidants, leads, in turn, to the consequence of non-enzymatic destruction. The experience with melatonin extraction from plant material lets us assume that only a certain fraction of the compound present in food will arrive in the gut and even less in the circulation. Nevertheless, it may be possible that melatonin metabolites, especially substituted kynuramines formed by oxidative pyrrole-ring cleavage, which also possess protective properties and sufficient amphiphilicity [41-43], and/or their derivatives are taken up from the food and will turn out to be beneficial.
Reactions of melatonin with oxidants
With regard to the presence of melatonin in food, in medicinal plants and to the use as a food additive, its antioxidant and other protective properties deserve attention. Since the discovery of melatonin oxidation by photocatalytic mechanisms involving free radicals [15,44,45], scavenging by this indoleamine has become a matter of particular interest. Melatonin was also shown to be oxidized by free radicals formed in the absence of light [46], and its capability of scavenging hydroxyl radicals at high rates [47-51] initiated numerous investigations on radical detoxification and antioxidative protection. Melatonin turned out to be considerably more efficient than the majority of its naturally occurring structural analogs [47,50-52], indicating that the substituents of the indole moiety strongly influenced reactivity and selectivity. Rate constants determined for the reaction with hydroxyl radicals were in the range between 1.2 × 1010 and 7.5 × 1010 M-1 s-1, depending on the methods applied [53-57]. Regardless of differences in the precision of determination, melatonin has been shown, independently by different groups, to be a remarkably good scavenger of this radical species. This property can be crucial for antagonizing oxidative damage under pharmacological and other in vitro conditions. To what extent this may contribute to physiological protection remains, however, a matter of debate.
Meanwhile, melatonin has been shown to react with many other oxidants, such as carbonate radicals [58-60], singlet oxygen [15,34,61-65], ozone [15,34], and several biologically occurring aromatic radicals, such as protoporphyrinyl and substituted anthranilyl radicals [15,59,61,62,66,67]. Reactions with other non-biological radicals were also described [15,34], among which the ABTS cation radical [ABTS = 2, 2'-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid)] merits special attention because of its analytical value. This extremely long-lived radical which is stable for many days provides a good example for single-electron donation by melatonin [52,68]. This conclusion was unambiguously confirmed by cyclic voltammetry [69]. Single-electron donation is important for several reasons. Free radicals can react with scavengers in different ways, either by abstraction of an electron, or a hydrogen atom, or by addition. In the case of melatonin, radical addition has been observed or predicted theoretically only for interactions with hydroxyl radicals [69-72] and nitric oxide [69,73-75]. Electron/hydrogen abstraction, however, is a common key step for interactions of melatonin with oxidizing free radicals of both high and low reactivity and, therefore, reflects melatonin's property as a broad spectrum antioxidant. Electron abstraction was also concluded to be a primary step of melatonin oxidation in a pseudoenzymatic reaction catalyzed by oxoferrylhemoglobin [76]. Single-electron transfer reactions are also believed to play a role in detoxification of resonance-stabilized free radicals, such as carbonate and aryl radicals, which are frequently underrated in their destructive potential because of their lower reactivity, compared to the hydroxyl radical. However, due to their longer life-time they can reach more distant sites than the extremely short-lived hydroxyl radical, which exists only for nanoseconds. The capability of melatonin of scavenging carbonate and certain aryl radicals may be of much higher significance and protective value than previously thought. Finally, according to a recently proposed model, single-electron exchange is thought to be the basis for interactions of melatonin with the mitochondrial respiratory chain [77,78] which is assumed to require only very small, quasi-catalytic amounts of melatonin and which would convey antioxidative cell protection by radical avoidance rather than detoxification of radicals already formed (see below).
Reactive nitrogen species represent another category of potentially destructive substances, which react with melatonin. Scavenging of nitric oxide by melatonin in a nitrosation reaction is well documented [9,79-81]. Whether this can be regarded as a detoxification reaction keeping NO from forming the more dangerous peroxynitrite is uncertain because nitrosomelatonin easily decomposes, thereby releasing NO [82], an experience also made with other NO adducts from respective scavengers including NO spin traps [83]. Scavenging of peroxynitrite has also been described [9,80,81,84], although it is sometimes difficult to distinguish betweeen direct reactions with peroxynitrite and with hydroxyl radicals formed by decomposition of peroxynitrous acid. What seems more important than direct scavenging of peroxynitrite is the interaction with products from the peroxynitrite-CO2 adduct (ONOOCO2-), namely, carbonate radicals (CO3•-) and •NO2 [79,85]. In the presence of bicarbonate/CO2, this pathway is favored and the primary interaction of melatonin is that with CO3•- [85], a conclusion in agreement with results from other studies on CO3•- scavenging [58-60]. The mixture of CO3•- and •NO2 represents the physiologically most efficient nitration mixture, because of the high availability of CO2 in biological material. It is worth noting that melatonin can, in fact, decrease 3-nitrotyrosine levels, as shown in guinea pig kidney [86].
Another highly interesting aspect of melatonin's antioxidant actions, which may be particularly important from the nutritional aspect, is its interactions with classic antioxidants. In both chemical and cell-free systems, melatonin was repeatedly shown to potentiate the effects of ascorbate, Trolox (a tocopherol analog), reduced glutathione, or NADH [50,68,69,87]. These findings, which can be clearly distinguished from additive effects, surprisingly indicate multiple interactions via redox-based regeneration of antioxidants transiently consumed. This may, in fact, be of practical importance, since melatonin was also shown to prevent decreases in hepatic ascorbate and α-tocopherol levels in vivo, under conditions of long-lasting experimental oxidative stress induced by a high cholesterol diet [88].
Metabolites of melatonin, a scavenger cascade, and melatonin as a prodrug
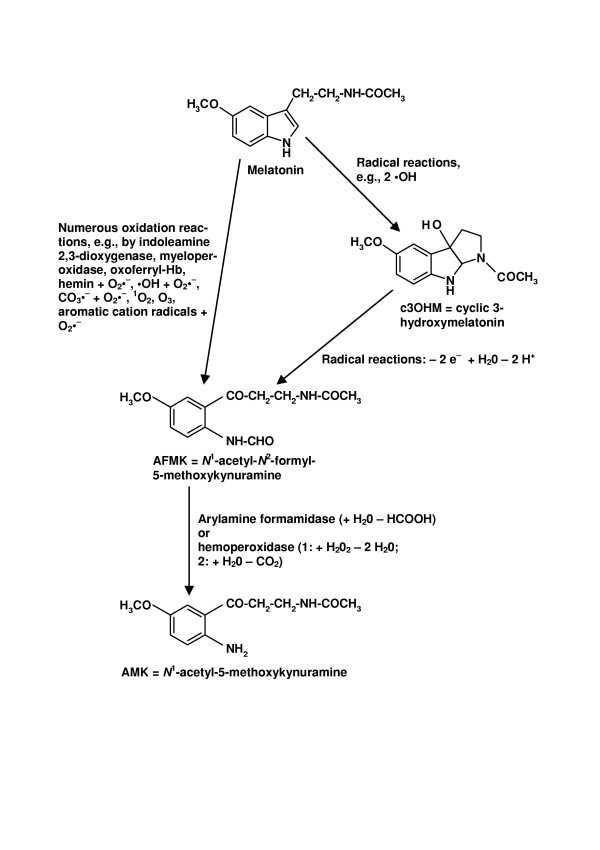
Reactions of melatonin with free radicals and other oxidants are not only a matter of the toxic reactants eliminated, but also of the products formed. It is highly important to distinguish between metabolites formed under physiological or near-physiological conditions from those produced in chemical systems designed for studying reactions with a single radical species in preparations as pure as possible. Disregard of this point has led to several misinterpretations in the past. We have repeatedly emphasized that studies using reaction systems which preferentially generate hydroxyl radicals mainly lead to hydroxylated adducts or their derivatives such as substituted indolinones, whereas biological material usually contains orders of magnitude more superoxide anions than hydroxyl radicals. Therefore, an entirely different product spectrum is obtained as soon as hydroxyl radicals, or other electron-abstracting radicals, act in the presence of an excess of superoxide anions [60,89]. Radicals derived from melatonin by interaction with a first, reaction-initiating radical likely combine with superoxide anions so that the radical reaction chain is readily terminated [15,49]. The product formed by oxidative pyrrole-ring cleavage is a substituted kynuramine, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK; Fig. 1). We have investigated numerous reaction systems and in all those containing sufficient quantities of superoxide anions, AFMK was by far the most abundant product [44,46,58-60,66,89]. Interestingly, a profound and sursprising difference exists between melatonin and other structurally related indoleamines. While substituted kynuramines represent only a limited or small fraction of oxidation products from other indolic compounds, AFMK usually greatly exceeds the total of other substances formed. This indicates a significant contribution not only of the 5-methoxy residue, but also of the N-acetylated side chain to the oxidation chemistry of melatonin, a conclusion corroborated by various scavenging assays and chemiluminescence associated with pyrrole-ring cleavage [52]. Moreover, AFMK was the only melatonin metabolite detected in culture media of various aquatic organisms, unicells and small metazoans, whereas several additional products were found in axenic media incubated for extended periods of time [90]. AFMK formation seems to be a favored pathway of melatonin degradation in these species.
Figure 1.
The kynuric pathway of melatonin metabolism.
These findings do not represent a peculiarity of non-vertebrates, but rather seem to reflect the non-hepatic melatonin catabolism in vertebrates. Contrary to statements in the earlier literature claiming that almost all melatonin is metabolized in the liver to 6-hydroxymelatonin followed by conjugation and excretion, recent estimations attribute about 30 percent of overall melatonin degradation to pyrrole-ring cleavage [91]. The rate of AFMK formation may be considerably higher in certain tissues, since extrahepatic P450 monooxygenase activities are frequently too low for a high turnover via 6-hydroxylation. The high amounts of gastrointestinal melatonin (see above), as far as they are not released unmetabolized, have to enter a pathway different from monooxygenation. AFMK formation is highly likely.
The significance of pyrrole-ring cleavage in oxidative metabolism of tissue melatonin is particularly illustrated in the central nervous system, where a secondary product, N1-acetyl-5-methoxykynuramine (AMK) derived from AFMK by deformylation, was identified as a main metabolite [92]. When melatonin was injected into the cisterna magna, about 35 percent was recovered as AMK. Under the conditions used, AFMK and AMK were the only products formed from melatonin in the brain and no 6-hydroxymelatonin was detected. In this case, the high turnover in the kynuric pathway of melatonin catabolism is the more remarkable as it cannot be explained on the basis of the enzymes capable of catalyzing the formation of AFMK: (i) indoleamine 2, 3-dioxygenase which uses tryptophan as the main substrate, exhibits sufficiently high activities only after inflammatory stimulation of the microglia [93-95]; (ii) myeloperoxidase, which can also catalyze pyrrole-ring cleavage of melatonin [91,96,97], is again associated with activated phagocytes. To assume free radical reactions as the main cause of kynuric melatonin degradation in the brain is, therefore, highly suggestive. Non-enzymatic AFMK formation in other tissues will be a matter for future research.
It is a remarkable fact that AFMK is formed by many different mechanisms [summarized in refs. [15,41,59,66,89]]. Apart from the enzymes mentioned, pseudoenzymatic catalysis by oxyferrylhemoglobin or by hemin, interactions with free radicals, e.g., combinations of •OH and O2•-, or CO3•- and O2•-, or organic cation radicals and O2•-, oxidation by singlet oxygen, by ozone, or by O2 under photoexcitation of melatonin all lead to AFMK. Even another product formed from melatonin by interactions with free radicals, cyclic 3-hydroxymelatonin [70], can be further metabolized by free radicals to AFMK [68]. All these findings indicate that AFMK is a central metabolite of melatonin oxidation especially in non-hepatic tissues.
As already mentioned, AFMK is easily deformylated to AMK. To date two enzymes capable of catalyzing this reaction have been identified, arylamine formamidase and hemoperoxidase [49,89,98]. The two methoxylated kynuramines, AFMK and AMK, are of particular interest because of their own radical-scavenging and protective properties. In any case, kynuramines, a separate class of biogenic amines, exhibit various biological activities [99], which are, however, rarely investigated. With regard to antioxidative protection, AFMK was shown to reduce 8-hydroxy-2-deoxyguanosine formation [42] and lipid peroxidation, and to rescue hippocampal neurons from oxidotoxic cell death [41]. Although AFMK interacts, not surprisingly, with the highly reactive hydroxyl radicals, it is otherwise relatively inert towards radicals of lower or intermediate reactivity [43,89]. This is convincingly explained by its preference for two-electron transfer reactions as demonstrated by cyclic voltammetry [41].
The deformylated product AMK, easily formed from AFMK [92], appears to be a highly interesting substance, for several reasons: first, it is a radical scavenger of considerably higher reactivity than AFMK because it easily undergoes single-electron transfer reactions [43,89,100] and, second, it acts as a cyclooxygenase (COX) inhibitor that is much more potent than acetylsalicylic acid [101] and has relative specificity for COX-2 (B Poeggeler, pers. commun.). Moreover, AMK was recently shown to downregulate COX-2 expression in macrophages [102]. AMK might, therefore, contribute to the attenuation of oxidative stress both directly and indirectly by interference with inflammatory responses. A third, mitochondrial effect will be discussed below. Unfortunately, the precise tissue levels of AMK are still unknown, partially because of a lack of specific assays, partially due to its high reactivity which readily leads to other products. Since AMK can be recovered from the urine after a melatonin load [92], sufficient amounts may be present in the tissues, at least after administration of pharmacological doses. Therefore, melatonin seems to act not only directly, but, additionally, as a prodrug of AMK.
It is a remarkable fact that the kynuric pathway of melatonin metabolism includes a series of radical scavengers, which may be regarded as a scavenger cascade [68], with a possible sequence of melatonin → cyclic 3-hydroxymelatonin → AFMK → AMK, where melatonin can be alternately converted to AFMK directly. From melatonin to AFMK, up to 4 free radicals can be consumed [68]; recent determinations [Rosen J, Hardeland R, unpubl. data] have shown that even higher numbers of free radicals can be eliminated, and other, previously unknown products are being characterized. The potent scavenger AMK consumes further radicals in primary and secondary reactions. Interestingly, AMK not only interacts with reactive oxygen but also with reactive nitrogen species and several products have been structurally characterized in Göttingen [[103]; manuscript in preparation]. Neither the end of the kynuric pathway of melatonin nor that of the scavenger cascade is in sight.
Multiple levels of antioxidative protection by melatonin
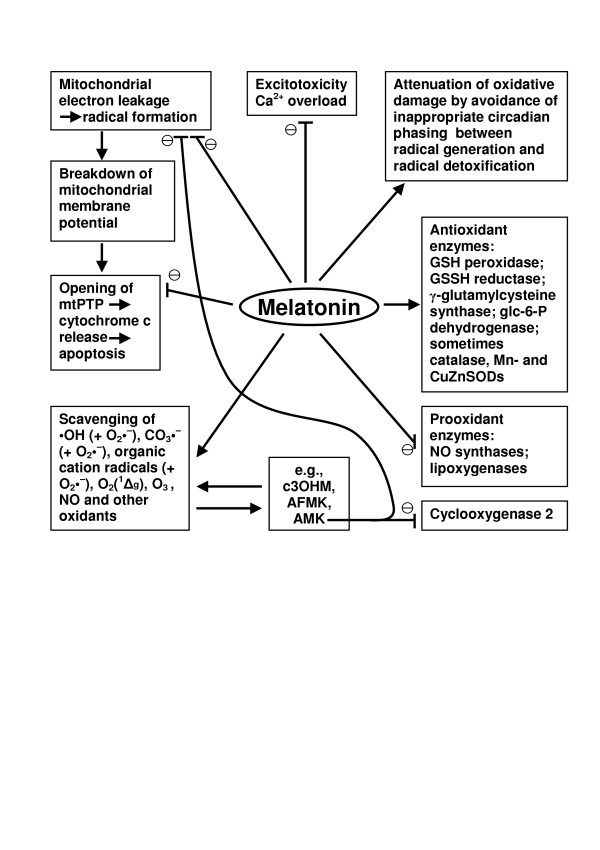
Antioxidative protection by melatonin is not just a matter of direct radical scavenging (Fig. 2), as becomes immediately evident from stoichiometry. Although tissue levels of melatonin can be considerably higher than those in the circulation, the quantities of free radicals generated in its metabolism would still be too high for the available amounts of the indoleamine. Our understanding is that direct scavenging by physiological concentrations of melatonin by a non-enzymatic contribution to the kynuric pathway and the subsequent actions of the metabolites formed becomes important. Signaling effects of melatonin, however, are always possible at physiological levels.
Figure 2.
Overview of the pleiotropic actions of melatonin and some of its metabolites in antioxidative protection.
Melatonin upregulates several antioxidant enzymes. Most frequently, this has been demonstrated for glutathione peroxidase [7,77,88,104-118] and sometimes glutathione reductase [7,108,112,119], presumably indirectly via GSSG. In some tissues Cu, Zn- and/or Mn-superoxide dismutases [7,108-112,117-123] and, rarely, catalase [112,118,123,124] are upregulated. Stimulation of glutathione peroxidase seems to be widely distributed among tissues and is observed quite regularly in both mammalian and avian brain; upregulations in other organs were more variable. The action of melatonin on glutathione metabolism seems to exceed the effects mentioned. Stimulation of glucose-6-phosphate dehydrogenase [108] and γ-glutamylcysteine synthase [10,112] indirectly supports the action of glutathione peroxidase by providing reducing equivalents (NADPH) for the action of glutathione reductase and by increasing the rate of glutathione synthesis, respectively.
Contrary its effect on the enzymes of glutathione metabolism, the effect of melatonin on superoxide dismutase subforms and catalase strongly depends on organs and species. Stimulation was observed in some tissues, but not in others; in some cases, even decreases were reported. This may not only be a matter of differences in responsiveness of cell types. The complexity in the regulation of the respective enzymes has to be considered. Frequently, they exhibit compensatory rises in response to oxidative stress. When melatonin is counteracting experimentally induced stress, the result may be a normalization of enzyme activity, i.e., lower values, compared to animals treated with oxidotoxins, rather than inductions. Such normalizations were, in fact, described [114,125]. However, in cases of stronger oxidative stress, active centers of enzymes may be destroyed by the free radicals generated and normalization of enzyme activities by melatonin administration appears as an increase [110,124,125].
An additional aspect of melatonin's actions on antioxidant enzymes deserves future attention: In two neuronal cell lines, physiological concentrations of melatonin not only induced glutathione peroxidase and superoxide dismutases at the mRNA level, but concomitantly increased the life-time of these mRNAs [117].
Melatonin also contributes to the avoidance of radical formation in several independent ways. It downregulates prooxidant enzymes, in particular 5- and 12-lipoxygenases [112,126-128] and NO synthases [9,34,77,108,112,129-134]. The widely observed attenuation of NO formation is particularly important in terms of limiting rise in the strongly prooxidant metabolite peroxynitrite and of the free radicals derived from this compound, namely, •NO2, carbonate (CO3•-) and hydroxyl (•OH) radicals. Suppressions of both lipoxygenase and NO synthase may additionally set limits to inflammatory responses, although the immunomodulatory actions of melatonin are certainly more complex and may involve additional effects of melatonin and AMK, too.
Another widely unexplored but potentially important signaling effect of melatonin in antioxidative protection concerns quinone reductase 2 [77,135,136]. This enzyme, which is implicated in the detoxification of potentially prooxidant quinones, binds melatonin at upper physiological concentrations, so that it had originally been presumed to represent a melatonin receptor. Although its precise function under the influence of melatonin is not yet fully understood, the relationship to the indoleamine may become of future interest from the standpoint of nutrition, since quinones are taken up with food, especially, vegetables.
Although less relevant from a nutritional point of view, melatonin also contributes indirectly to radical avoidance, e.g., by its antiexcitatory effects in the central nervous system, and as an endogenous regulator molecule controlling rhythmic time structures. This last action may be particularly important for well-timed alimentary melatonin supplementation in the elderly, who exhibit a strongly reduced amplitude in the circadian melatonin rhythm. The significance of appropriate timing for maintaining low levels of oxidative damage has been overlooked for quite some time. However, temporal perturbations as occurring in short-period or arrhythmic circadian clock mutants lead to enhanced oxidative damage, effects observed in organisms as different as Drosophila and the Syrian Hamster [77,137,138].
In the last few years, mitochondrial effects of melatonin have been discovered which may turn out to be even more important than the protective actions described above. Mitochondria are the main source of free radicals in the majority of animal cells and are implicated in aging processes. The importance of mitochondrial diseases is increasingly perceived. Mitochondria play a key role in apoptosis. Notably, several of the mitochondrial effects of melatonin were obtained at low pharmacological doses in drinking water [116,139,140] or even at near-physiological concentrations down to 1 nM [141].
Several studies of mitochondrial effects revealed attenuation of mitochondrial lipid peroxidation, prevention of oxidative protein and DNA modifications, preservation of ultrastructure, resistance against toxins etc., findings which were widely in line with previous concepts of protection [10,113,142-146]. Moreover, melatonin was shown to affect redox-active compounds in mitochondria, in particular, to decrease NO [143,147] and to restore normal levels of reduced glutathione [113,144] and coenzyme Q10 [148].
More importantly, beyond these rather conventional findings, with few exceptions, melatonin was found to increase mitochondrial respiration and ATP synthesis, in conjunction with rises in complex I and IV activities [112,141-143,146,147,149,150]. Complex I and IV activities were also found to be increased by melatonin in hepatic mitochondria of senescence-accelerated mice [116,140,151]. Moreover, melatonin was found to enhance gene expression of complex IV components [147].
The improvements of ATP formation and O2 consumption are presumably not decisive for protection, but can serve as good indicators for the reduction of electron leakage from the respiratory chain. Electron transfer to molecular oxygen at the matrix side, largely at iron-sulfur cluster N2 of complex I [152], is a major source of free radicals. This process also diminishes electron flux rates and, therefore, the ATP-generating proton potential. Processes affecting the mitochondrial membrane potential such as calcium overload, either due to overexcitation, to protein misfolding or to damage by free radicals, are antagonized by melatonin. In cardiomyocytes, astrocytes and striatal neurons, melatonin prevented calcium overload [153,154], counteracted the collapse of the mitochondrial membrane potential induced by H2O2 [153], doxorubicin [155] or oxygen/glucose deprivation [154], and also inhibited the opening of the mitochondrial permeability transition pore (mtPTP), thereby rescuing cells from apoptosis. In addition to the antioxidant actions, melatonin directly diminished mtPTP currents, with an IC50 of 0.8 μM [154], a concentration which would require mitochondrial accumulation of melatonin, something which is possible again due to the amphiphilicity of melatonin.
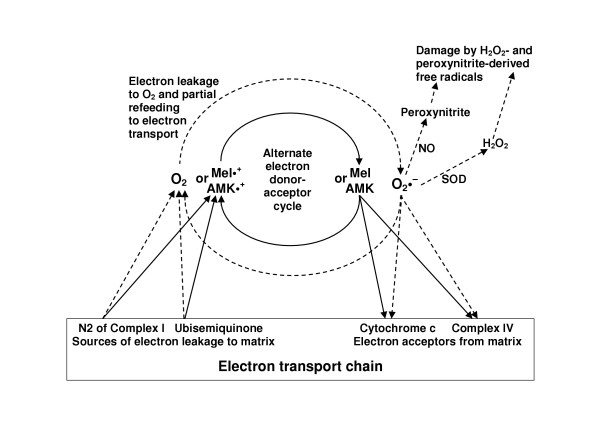
The effects of melatonin on the respiratory chain open new perspectives for diminishing radical formation, instead of seeking only antioxidant effects for the elimination of radicals already formed. We have proposed a model of radical avoidance (Fig. 3) in which electron leakage is reduced by single-electron exchange reactions betwen melatonin and components of the electron transport chain [77,78]. In fact, mitochondrial H2O2 formation was found to be reduced by melatonin [156]. The basic idea of the model is that of a cycle of electron donation to the respiratory chain, eventually to cytochrome c [78], followed by reduction of the formed melatonyl cation radical by electron transfer from N2 of complex I. The cation radical is assumed to act as alternate electron acceptor competing with molecular oxygen, thereby decreasing the rate of O2•- formation. In addition to the electrons being largely recycled, most of the melatonin is also. Therefore, such a mechanism would only require very low, quasi-catalytic amounts of melatonin, in accordance with the effects demonstrated with nanomolar concentrations. Because the recycled electrons are not lost for the respiratory chain, this would also lead to improvements in complex IV activity, oxygen consumption and ATP production. Alternately, the melatonin metabolite AMK, which is also highly reactive and can undergo single-electron transfer reactions [43], may act in the same way. The prediction of our model of mitochondrial protection by AMK was confirmed by other investigators [147]: AMK was shown to exert effects on electron flux through the respiratory chain and ATP synthesis very similar to those observed with melatonin.
Figure 3.
A model of mitochondrial radical avoidance and support of electron flux by melatonin and its metabolite AMK. The potent electron donors melatonin and AMK are thought to feed electrons into the respiratory chain, thereby forming resonance-stabilized cation radicals which may efficiently compete with molecular oxygen for electrons leaking from iron-sulfur cluster N2 or from ubisemiquinone. The competition reduces superoxide anion formation and, thereby, the generation of secondary radicals; at the same time, electrons re-fed to the electron transport chain contribute to the maintenance of the proton potential and, thus, to ATP synthesis. The model is partially hypothetical, but might explain observations of reductions in electron leakage and oxidant formation as well as an enhancement of ATP formation.
A highly attractive aspect of mitochondrial protection results from the small quantities required: experimentally induced mitochondrial damage in rat fetuses was even prevented by maternally administered melatonin [146]. The mechanism as outlined, requiring only low amounts of melatonin or its metabolite AMK, would make these compounds even more interesting from a nutritional point of view. The amounts present in selected food, such as some vegetables, but even more nuts and cereals, could suffice for maintaining tissue levels of the indoleamine capable of safeguarding mitochondrial function, particularly in elderly persons whose nocturnal melatonin maxima in pineal gland and circulation have substantially declined with age. Transient moderate rises in blood melatonin during the day resulting from direct uptake or postprandrial release from the gastrointestinal tract should not be regarded as a problem in terms of circadian timing. The circadian system responds to melatonin according to a phase response curve [157,158]: the so-called silent zone, during which no substantial phase shifts are induced, extends throughout the largest part of the day.
Safety of melatonin
Can all these findings on antioxidant and radical-avoiding actions of melatonin justify its intake as a food additive or as a medication? The idea of substitution therapy may seem especially attractive for the elderly who have more or less lost the nocturnal peak of circulating melatonin. Nevertheless, the use as a food additive is still a matter of controversy. The argument for a naturally occurring compound, which is a normal food constituent, cannot suffice alone, since commerical preparations would always lead to at least transient pharmacological concentrations in the blood, and the immunomodulatory actions of melatonin may not be desired in every case. Therefore, experience will have to answer the question of its usefulness. Without any doubt, melatonin is remarkably well tolerated. Of course, one can find in any large statistical sample of melatonin users some individuals who complain about side effects, scientifically understandable or not. In a currently running study on ALS, patients receiving daily very high doses of melatonin (30 or even 60 mg per day), we did not see any harmful side effects [14] and have not to date. In patients with rheumatoid arthritis, some symptoms were suspected to be associated with immunomodulatory actions of melatonin [159], so that caution is due in this group of individuals. More research will be required on melatonin in different diseases and disorders, but there is no good reason to assume that melatonin, at moderate or even at high doses, is dangerous to a healthy person or to patients with types of oxidative stress phenomena not caused by (auto-)-immune responses. One might also suspect that melatonin could exert unfavorable effects by increasing the blood pressure, due to downregulation of NO synthase and NO scavenging by the indoleamine itself or by AMK. Melatonin was tested in clinical trials on hypertension and was reported to decrease blood pressure in one study [160], but to interfere with nifedipine [161], whereas a combination of lacidipine with melatonin was recommended in another investigation [162]. Therefore, interaction with other medication has to be considered.
Problems of dosage and side effects may also arise from impurities in the melatonin preparations sold over the counter. Contaminants have repeatedly been detected in such material, including our own experience of that kind. As long as the contaminant is only AFMK, this may be less serious, but one should be aware that the pharmacology of kynuramines is only partially known. Moreover, manufacturers must consider that an easily oxidizable compound like melatonin can undergo reactions under air exposure. On large surfaces, such as silica gels, we see this every day in the laboratory.
Another important aspect for the use of melatonin as a food additive is timing. As soon as the substance is given as a pill or as a preparation from a medicinal plant causing relatively high pharmacological blood levels, the situation is entirely different from the uptake with normal food or from the postprandial gastrointestinal release. Since circulating melatonin peaks at night, pharmaceutical preparations should be strictly given at the same time of day in the evening. The usual recommendation „at bed time" may be insufficient since this could mean in practice different hours of the day. Here, one has to consider the chronobiological functions of melatonin. When given during the day, a high dose of melatonin would cause mild narcotic effects, drowsiness etc. and the practice is not recommended for this reason. It would not shift the circadian oscillator much, because of the silent zone of the phase response curve for melatonin, in which phase shifts are negligibly small. This is the same reason that a postprandial release of gastrointestinal melatonin does not shift the circadian oscillator. Advance shifts of the endogenous clock by melatonin are much larger at late afternoon and early night [157,158]. Therefore, melatonin should be given relatively precisely at the same hour, to avoid phase shifts differing in extent and pushing of the circadian oscillator back and forth. As mentioned above, pertubations of the internal time structure can also cause oxidative stress [77].
Conclusion
In terms of nutrition, melatonin is interesting both as a natural constituent of food, and as a food additive. Its use for the latter purpose can be recommended only with some caution, given the present state of our knowledge, although the risks by melatonin appear remarkably low, compared to other medications and food additives. Melatonin's antioxidant capacity is based not only on direct radical detoxification, but comprises manifold effects. Some of the most promising areas, modulation of mitochondrial metabolism by melatonin and actions of its kynuric metabolites, deserve particular attention in the future and may change our view of the value of these compounds profoundly.
List of abbreviations
ABTS: 2, 2'-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid)
AFMK: N1-acetyl-N2-formyl-5-methoxykynuramine
ALS: amyotrophic lateral sclerosis
AMK: N1-acetyl-5-methoxykynuramine
c3OHM: cyclic 3-hydroxymelatonin
COX-2: cyclooxygenase 2
GSSG: oxidized glutathione
Competing interests
Authors declare that they have no competing interests concerning the use of melatonin or melatonin-containing preparations as a food additive.
Authors' contributions
This review was initiated by SRP-P; a first version by RH was jointly revised.
Contributor Information
Rüdiger Hardeland, Email: rhardel@gwdg.de.
SR Pandi-Perumal, Email: pandiperumal@gmail.com.
References
- Arendt J, Deacon S. Treatment of circadian rhythm disorders – melatonin. Chronobiol Int. 1997;14:185–204. doi: 10.3109/07420529709001155. [DOI] [PubMed] [Google Scholar]
- Arendt J. Jet-lag. Lancet. 1998;351:293–294. doi: 10.1016/S0140-6736(05)78236-2. [DOI] [PubMed] [Google Scholar]
- Herxheimer A, Waterhouse J. The prevention and treatment of jet lag. Br Med J. 2003;326:296–297. doi: 10.1136/bmj.326.7384.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug GF, Requintina PJ. Melatonin and jet lag syndrome: experimental model and clinical applications. CNS Spectr. 2003;8:139–148. doi: 10.1017/s109285290001837x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Oxidative damage in the central nervous system: Protection by melatonin. Prog Neurobiol. 1998;56:359–384. doi: 10.1016/S0301-0082(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan D-X, Cabrera J, D'Arpa D, Sainz RM, Mayo JC, Ramos S. The oxidation/antioxidant network: role of melatonin. Biol Signals Recept. 1999;8:56–63. doi: 10.1159/000014569. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan D-X, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7:444–458. doi: 10.1159/000025480. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan D-X, Burkhardt S, Manchester LC. Melatonin in plants. Nutr Rev. 2001;59:286–290. doi: 10.1111/j.1753-4887.2001.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan D-X, Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech Ageing Dev. 2002;123:1007–1019. doi: 10.1016/S0047-6374(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan D-X, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- Pappolla MA, Chyan Y-J, Poeggeler B, Frangione B, Wilson G, Ghiso J, Reiter RJ. An assessment of the antioxidant and antiamyloidogenic properties of melatonin: implications for Alzheimer's disease. J Neural Transm. 2000;107:203–231. doi: 10.1007/s007020050018. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Brusco LI, Liberczuk C, Furio AM. The use of melatonin in Alzheimer's disease. Neuroendocrinol Lett. 2002;23:20–23. [PubMed] [Google Scholar]
- Singer C, Tractenberg RE, Kaye J, Schafer K, Gamst A, Grundman M, Thomas R, Thal LJ. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Poeggeler B, Weishaupt JH, Sirén A-L, Hardeland R, Bähr M, Ehrenreich H. Melatonin as a candidate compound for neuroprotection in amyotrophic lateral sclerosis (ALS): High tolerability of daily oral melatonin administration in ALS patients. J Pineal Res. 2002;33:186–187. doi: 10.1034/j.1600-079X.2002.02943.x. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Fuhrberg B. Ubiquitous melatonin – Presence and effects in unicells, plants and animals. Trends Comp Biochem Physiol. 1996;2:25–45. [Google Scholar]
- Hardeland R. Melatonin and 5-methoxytryptamine in non-metazoans. Reprod Nutr Dev. 1999;39:399–408. doi: 10.1051/rnd:19990311. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Poeggeler B. Non-vertebrate melatonin. J Pineal Res. 2003;34:233–241. doi: 10.1034/j.1600-079X.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HP, Schloot W. Melatonin in edible plants identified by radioimmunoasay and by high performance liquid-chromatography-mass spectrometry. J Pineal Res. 1995;18:28–31. doi: 10.1111/j.1600-079x.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int. 1995;35:627–634. [PubMed] [Google Scholar]
- Balzer I, Hardeland R. Melatonin in algae and higher plants – Possible new roles as a phytohormone and antioxidant. Bot Acta. 1996;109:180–183. [Google Scholar]
- Van Tassel DL, O'Neill SD. Putative regulatory molecules in plants: evaluating melatonin. J Pineal Res. 2001;31:1–7. doi: 10.1034/j.1600-079X.2001.310101.x. [DOI] [PubMed] [Google Scholar]
- Van Tassel DL, Roberts N, Lewy A, O'Neill SD. Melatonin in plant organs. J Pineal Res. 2001;31:8–15. doi: 10.1034/j.1600-079X.2001.310102.x. [DOI] [PubMed] [Google Scholar]
- Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus) J Agric Food Chem. 2001;49:4898–4902. doi: 10.1021/jf010321+. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan D-X. Melatonin: an antioxidant in edible plants. Ann NY Acad Sci. 2002;957:341–344. doi: 10.1111/j.1749-6632.2002.tb02938.x. [DOI] [PubMed] [Google Scholar]
- Conti A, Tettamanti C, Singaravel M, Haldar C, Pandi-Perumal RS, Maestroni GJM. Melatonin: An ubiquitous and evolutionary hormone. In: Haldar C, Singaravel M, Maitra SK, editor. Treatise on Pineal Gland and Melatonin. Enfield, NH: Science Publishers; 2002. pp. 105–143. [Google Scholar]
- Poeggeler B, Hardeland R. Detection and quantification of melatonin in a dinoflagellate, Gonyaulax polyedra. Solutions to the problem of methoxyindole destruction in non-vertebrate material. J Pineal Res. 1994;17:1–10. doi: 10.1111/j.1600-079x.1994.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Murch SJ, Simmons CB, Saxena PK. Melatonin in fever few and other medicinal plants. Lancet. 1997;350:1598–1599. doi: 10.1016/S0140-6736(05)64014-7. [DOI] [PubMed] [Google Scholar]
- Manchester LC, Tan D-X, Reiter RJ, Park W, Monis K, Qi W. High levels of melatonin in the seeds of edible plants. Possible function in germ tissue protection. Life Sci. 2000;67:3023–3029. doi: 10.1016/S0024-3205(00)00896-1. [DOI] [PubMed] [Google Scholar]
- Sprenger J, Hardeland R, Fuhrberg B. Methoxyindoles in yeast: Variability and effects of growth conditions. In: Hardeland R, editor. Biological Rhythms and Antioxidative Protection. Göttingen: Cuvillier; 1997. pp. 116–118. [Google Scholar]
- Sprenger J, Hardeland R, Fuhrberg B, Han S-Z. Melatonin and other 5-methoxylated indoles in yeast: Presence in high concentrations and dependence on tryptophan availability. Cytologia. 1999;64:209–213. [Google Scholar]
- Balzer I, Höcker B, Kapp H, Bartolomaeus B. Occurrence and comparative physiology of melatonin in evolutionary diverse organisms. In: Vanden Driessche T, Guisset J-L, Petiau-de Vries GM, editor. The Redox State and Circadian Rhythms. Boston – London: Kluwer; 2000. pp. 95–119. [Google Scholar]
- Huether G, Poeggeler B, Reimer A, George A. Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 1992;51:945–53. doi: 10.1016/0024-3205(92)90402-B. [DOI] [PubMed] [Google Scholar]
- Huether G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia. 1993;49:665–670. doi: 10.1007/BF01923948. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Melatonin: Multiple functions in signaling and protection. In: Altmeyer P, Hoffmann K, Stücker M, editor. Skin Cancer and UV Radiation. Berlin – Heidelberg: Springer; 1997. pp. 186–198. [Google Scholar]
- Bubenik GA. Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol Signals Recept. 2001;10:350–366. doi: 10.1159/000046903. [DOI] [PubMed] [Google Scholar]
- Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. doi: 10.1023/A:1020107915919. [DOI] [PubMed] [Google Scholar]
- Messner M, Hardeland R, Rodenbeck A, Huether G. Tissue retention and subcellular distribution of continuously infused melatonin in rats under near physiological conditions. J Pineal Res. 1998;25:251–259. doi: 10.1111/j.1600-079x.1998.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Bubenik GA, Hacker RR, Brown GM, Bartos L. Melatonin concentrations in the luminal fluid, mucosa and muscularis of the bovine and porcine gastrointestinal tract. J Pineal Res. 1999;26:56–63. doi: 10.1111/j.1600-079x.1999.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Tan D-X, Manchester LC, Reiter RJ, Qi W, Hanes MA, Farley NJ. High physiological levels of melatonin in the bile of mammals. Life Sci. 1999;65:2523–2529. doi: 10.1016/S0024-3205(99)00519-6. [DOI] [PubMed] [Google Scholar]
- Tan D-X, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Melatonin – a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res. 2003;34:75–78. doi: 10.1034/j.1600-079X.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- Tan D-X, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, Shohami E, Huo Y-S, Hardeland R, Reiter RJ. N 1-Acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001;15:2294–2296. doi: 10.1096/fj.01-0309fje. [DOI] [PubMed] [Google Scholar]
- Burkhardt S, Reiter RJ, Tan D-X, Hardeland R, Cabrera J, Karbownik M. DNA oxidatively damaged by chromium(III) and H2O2 is protected by melatonin, N 1-acetyl-N2-formyl-5-methoxykynuramine, resveratrol and uric acid. Int J Biochem Cell Biol. 2001;33:775–783. doi: 10.1016/S1357-2725(01)00052-8. [DOI] [PubMed] [Google Scholar]
- Ressmeyer A-R, Mayo JC, Zelosko V, Sáinz RM, Tan D-X, Poeggeler B, Antolín I, Zsizsik BK, Reiter RJ, Hardeland R. Antioxidant properties of the melatonin metabolite N 1-acetyl-5-methoxykynuramine (AMK): scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003;8:205–213. doi: 10.1179/135100003225002709. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Poeggeler B, Balzer B, Behrmann G. Common basis of photoperiodism in phylogenetically distant organisms and its possible origins. J Interdiscipl Cycle Res. 1991;22:122–123. [Google Scholar]
- Hardeland R, Poeggeler B, Balzer I, Behrmann G. Chronobiology & Chronomedicine. Gutenbrunner C, Hildebrandt G, Moog R. Frankfurt: Lang; 1993. A hypothesis on the evolutionary origins of photoperiodism based on circadian rhythmicity of melatonin in phylogenetically distant organisms; pp. 113–120. [Google Scholar]
- Hardeland R, Fuhrberg B, Behrmann G, Balzer I. Sleep-latency reducing pineal hormone melatonin as a scavenger of free radicals: hemin-catalysed formation of N1-acetyl-N2-formyl-5-methoxykynuramine. Sleep Res. 1993;22:621. [Google Scholar]
- Tan D-X, Chen L-D, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- Reiter RJ, Poeggeler B, Tan D-X, Chen L-D, Manchester LC, Guerrero JM. Antioxidant capacity of melatonin: A novel action not requiring a receptor. Neuroendocrinol Lett. 1993;15:103–116. [Google Scholar]
- Hardeland R, Reiter RJ, Poeggeler B, Tan D-X. The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci Biobehav Rev. 1993;17:347–357. doi: 10.1016/s0149-7634(05)80016-8. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Reiter RJ, Hardeland R, Sewerynek E, Melchiorri D, Barlow-Walden LR. Melatonin, a mediator of electron transfer and repair reactions, acts synergistically with the chain-breaking antioxidants ascorbate, trolox and glutathione. Neuroendocrinol Lett. 1995;17:87–92. [Google Scholar]
- Poeggeler B, Reiter RJ, Hardeland R, Tan D-X, Barlow-Walden LR. Melatonin and structurally-related, endogenous indoles act as potent electron donors and radical scavengers in vitro. Redox Rep. 1996;2:179–184. doi: 10.1080/13510002.1996.11747046. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Thuermann S, Dose A, Schoenke M, Burkhardt S, Hardeland R. Melatonin's unique radical scavenging properties – Roles of its functional substituents as revealed by a comparison with its structural analogs. J Pineal Res. 2002;33:20–30. doi: 10.1034/j.1600-079X.2002.01873.x. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Saarela S, Reiter RJ, Tan D-X, Chen L-D, Manchester LC, Barlow-Walden LR. Melatonin – a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann NY Acad Sci. 1994;738:419–420. doi: 10.1111/j.1749-6632.1994.tb21831.x. [DOI] [PubMed] [Google Scholar]
- Matuszak Z, Reszka KJ, Chignell CF. Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic Biol Med. 1997;23:367–372. doi: 10.1016/S0891-5849(96)00614-4. [DOI] [PubMed] [Google Scholar]
- Stasica P, Ulanski P, Rosiak JM. Reactions of melatonin with radicals in deoxygenated aqueous solution. J Radioanal Nucl Chem. 1998;232:107–113. [Google Scholar]
- Chyan Y-J, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA. Potent neuroprotective properties against the Alzheimer β-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem. 1999;274:21937–21942. doi: 10.1074/jbc.274.31.21937. [DOI] [PubMed] [Google Scholar]
- Mahal HS, Sharma HS, Mukherjee T. Antioxidant properties of melatonin: a pulse radiolysis study. Free Radic Biol Med. 1999;26:557–565. doi: 10.1016/S0891-5849(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Niebergall R, Schoenke M, Poeggeler B. Carbonate radicals as initiators of melatonin oxidation: Chemiluminescence and formation of oxidation products. In: Hardeland R, editor. Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites. Göttingen: Cuvillier; 2001. pp. 49–55. [Google Scholar]
- Hardeland R, Poeggeler B, Burkhardt S, Zelosko V, Niebergall R, Tomás-Zapico C, Thuermann S, Schoenke M, Dose A, Gruetzner T, Coto-Montes A. Oxidation chemistry of melatonin: New aspects of radical reactions. In: Haldar C, Singh SS, editor. Neuroendocrine System and Pineal Gland with Special Reference to Lifestock. Varanasi: Banaras Hindu University; 2003. pp. 27–35. [Google Scholar]
- Hardeland R, Poeggeler B, Niebergall R, Zelosko V. Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. J Pineal Res. 2003;34:17–25. doi: 10.1034/j.1600-079X.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Uría H, Fuhrberg B, Poeggeler B, Hardeland R, Menéndez-Peláez A. Photooxidation of melatonin. In: Hardeland R, editor. Cell Biological Problems in Chronobiology. Göttingen: University of Göttingen; 1994. pp. 89–99. [Google Scholar]
- Hardeland R, Balzer I, Poeggeler B, Fuhrberg B, Uría H, Behrmann G, Wolf R, Meyer TJ, Reiter RJ. On the primary functions of melatonin in evolution: Mediation of photoperiodic signals in a unicell, photooxidation and scavenging of free radicals. J Pineal Res. 1995;18:104–111. doi: 10.1111/j.1600-079x.1995.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Cagnoli CM, Atabay C, Kharlamova E, Manev H. Melatonin protects neurons from singlet oxygen-induced apoptosis. J Pineal Res. 1995;18:222–226. doi: 10.1111/j.1600-079x.1995.tb00163.x. [DOI] [PubMed] [Google Scholar]
- Matuszak Z, Bilska MA, Reszka KJ, Chignell CF, Bilski P. Interaction of singlet molecular oxygen with melatonin and related indoles. Photochem Photobiol. 2003;78:449–455. doi: 10.1562/0031-8655(2003)078<0449:IOSMOW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- de Almeida EA, Martinez GR, Klitzke CF, de Medeiros MHG, Di Mascio P. Oxidation of melatonin by singlet molecular oxygen (O2(1Δg)) produces N 1-acetyl-N2-formyl-5-methoxykynurenine. J Pineal Res. 2003;35:131–137. doi: 10.1034/j.1600-079X.2003.00066.x. Remark: according to formula and mechanism, authors obviously mean N1-acetyl-N2-formyl-5-methoxykynuramine = AFMK, not the corresponding kynurenine. [DOI] [PubMed] [Google Scholar]
- Burkhardt S, Poeggeler B, Tan D-X, Rosner C, Gruetzner T, Nitzki F, Schoenke M, Thuermann S, Reiter RJ, Hardeland R. Oxidation products formed from melatonin in various radical-generating systems. In: Hardeland R, editor. Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites. Göttingen: Cuvillier; 2001. pp. 9–22. [Google Scholar]
- Tomás-Zapico C, Hardeland R, Poeggeler B, Coto-Montes A. 3-Hydroxyanthranilic acid and 3-hydroxykynurenine as photooxidants: Catalysis of N 1-acetyl-N2-formyl-5-methoxykynuramine formation from melatonin. In: Hardeland R, editor. Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites. Göttingen: Cuvillier; 2001. pp. 136–141. [Google Scholar]
- Tan D-X, Hardeland R, Manchester LC, Poeggeler B, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J Pineal Res. 2003;34:249–259. doi: 10.1034/j.1600-079X.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- Tan D-X, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- Tan D-X, Manchester LC, Reiter RJ, Plummer BF, Hardies LJ, Weintraub ST, Vijayalaxmi , Shepherd AMM. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: A biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun. 1998;253:614–620. doi: 10.1006/bbrc.1998.9826. [DOI] [PubMed] [Google Scholar]
- Stasica P, Paneth P, Rosiak JM. Hydroxyl radical reaction with melatonin molecule: a computational study. J Pineal Res. 2000;29:125–127. doi: 10.1034/j.1600-079X.2000.290209.x. [DOI] [PubMed] [Google Scholar]
- Agozzino P, Avellone G, Bongiorno D, Ceraulo L, Filizzola F, Natoli MC, Livrea MA, Tesoriere L. Melatonin: structural characterization of its non-enzymatic mono-oxygenate metabolite. J Pineal Res. 2003;35:269–275. doi: 10.1034/j.1600-079X.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Turjanski AG, Chaia Z, Rosenstein RE, Estrin DA, Doctorovich F, Piro O. N-nitrosomelatonin. Acta Crystallogr. 2000;C56:682–683. doi: 10.1107/S010827010000456X. [DOI] [PubMed] [Google Scholar]
- Turjanski AG, Leonik F, Rosenstein RE, Estrin DA, Doctorovich F. Scavenging of NO by melatonin. J Am Chem Soc. 2000;122:10468–10469. doi: 10.1021/ja002006u. [DOI] [Google Scholar]
- Turjanski AG, Saenz DA, Doctorovich F, Estrin DA, Rosenstein RE. Nitrosation of melatonin by nitric oxide: a computational study. J Pineal Res. 2001;31:97–101. doi: 10.1034/j.1600-079x.2001.310201.x. [DOI] [PubMed] [Google Scholar]
- Tesoriere L, Avellone G, Ceraulo L, D'Arpa D, Allegra M, Livrea MA. Oxidation of melatonin by oxoferryl hemoglobin: a mechanistic study. Free Radic Res. 2001;35:633–642. doi: 10.1080/10715760100301161. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Coto-Montes A, Poeggeler B. Circadian rhythms, oxidative stress and antioxidative defense mechanisms. Chronobiol Int. 2003;20:921–962. doi: 10.1081/CBI-120025245. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Poeggeler B. Melatonin: New aspects of its protective actions and novel metabolites. In: Haldar C, Singaravel M, Pandi-Perumal SR, Cardinali DP, editor. Experimental Endocrinology and Reproductive Biology. Enfield, NH: Science Publishers; 2005. [Google Scholar]
- Blanchard B, Pompon D, Ducrocq C. Nitrosation of melatonin by nitric oxide and peroxynitrite. J Pineal Res. 2000;29:184–192. doi: 10.1034/j.1600-079X.2000.290308.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan D-X, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan D-X, Manchester LC, Lopez-Burillo S, Sainz RM, Mayo JC. Melatonin: detoxification of oxygen and nitrogen-based toxic reactants. Adv Exp Med Biol. 2003;527:53–548. doi: 10.1007/978-1-4615-0135-0_62. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fillion B, Servy C, Ducrocq C. 1-Nitrosomelatonin is a spontaneous NO-releasing compound. Free Radic Res. 2001;35:857–866. doi: 10.1080/10715760100301351. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Saverschek N. The NO adduct of spin trap TMA-PTIO [= 2-(trimethylammonio-phenyl)-4, 4, 5, 5-tetramethyl-imidazoline-1-oxyl-3-oxide] acts as an NO donor and stimulator of nitrosation reactions. In: Hardeland R, editor. Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites. Göttingen, Germany: Cuvillier; 2001. pp. 131–135. [Google Scholar]
- Zhang H, Squadrito GL, Uppu R, Pryor WA. Reaction of peroxynitrite with melatonin: a mechanistic study. Chem Res Toxicol. 1999;12:626–534. doi: 10.1021/tx980243t. [DOI] [PubMed] [Google Scholar]
- Zhang H, Squadrito GL, Pryor WA. The reaction of melatonin with peroxynitrite: formation of melatonin radical cation and absence of stable nitrated products. Biochem Biophys Res Commun. 1998;251:83–87. doi: 10.1006/bbrc.1998.9426. [DOI] [PubMed] [Google Scholar]
- Cimen B, Turkozkan N, Unlu A, Erbil MK. Effects of melatonin on 3-nitrotyrosine formation and energy charge ratio in guinea pig kidney in LPS-induced stress. Cell Biochem Funct. 2005;23:273–277. doi: 10.1002/cbf.1151. [DOI] [PubMed] [Google Scholar]
- Gitto E, Tan D-X, Reiter RJ, Karbownik M, Manchester LC, Cuzzocrea S, Fulia F, Berberi I. Individual and synergistic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J Pharm Pharmacol. 2001;53:1393–1401. doi: 10.1211/0022357011777747. [DOI] [PubMed] [Google Scholar]
- Balkan J, Sener G, Cevikbas U, Keyser-Uysal M, Uysal M. Melatonin improved the disturbances in hepatic prooxidant and antioxidant balance and hepatotoxicity induced by a cholesterol diet in C57BL/6J mice. Int J Vitam Nutr Res. 2004;74:349–254. doi: 10.1024/0300-9831.74.5.349. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Ressmeyer A-R, Zelosko V, Burkhardt S, Poeggeler B. Metabolites of melatonin: Formation and properties of the methoxylated kynuramines AFMK and AMK. In: Haldar C, SS Singh SS, editor. Recent Advances in Endocrinology and Reproduction: Evolutionary, Biotechnological and Clinical Applications. Varanasi: Banaras Hindu University; 2004. pp. 21–38. [Google Scholar]
- Poeggeler B, Hardeland R. Observations on melatonin oxidation and metabolite release by unicellular organisms and small aquatic metazoans. In: Hardeland R, editor. Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites. Göttingen: Cuvillier; 2001. pp. 66–69. [Google Scholar]
- Ferry G, Ubeaud C, Lambert PH, Bertin S, Cogé F, Chomarat P, Delagrange P, Serkiz B, Bouchet JP, Truscott RJ, Boutin JA. Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan. Investigation on both indoleamine-2, 3-dioxygenase and myeloperoxidase. Biochem J. 2005;388:205–215. doi: 10.1042/BJ20042075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F, Hayaishi O, Tokuyama T, Senoh S. In vitro and in vivo formation of two new metabolites of melatonin. J Biol Chem. 1974;249:1311–1313. [PubMed] [Google Scholar]
- Alberati-Giani D, Ricciardi-Castagnoli P, Köhler C, Cesura AM. Regulation of the kynurenine pathway by IFN-γ in murine cloned macrophages and microglial cells. Adv Exp Med Biol. 1966;398:171–175. doi: 10.1007/978-1-4613-0381-7_28. [DOI] [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2, 3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/S0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Kwidzinski E, Bunse J, Kovac AD, Ullrich O, Zipp F, Nitsch R, Bechmann U. IDO (indolamine 2, 3-dioxygenase) expression and function in the CNS. Adv Exp Med Biol. 2003;527:113–118. doi: 10.1007/978-1-4615-0135-0_13. [DOI] [PubMed] [Google Scholar]
- Ximenes VF, Catalani LH, Campa A. Oxidation of melatonin and tryptophan by an HRP cycle involving compound III. Biochem Biophys Res Commun. 2001;287:130–134. doi: 10.1006/bbrc.2001.5557. [DOI] [PubMed] [Google Scholar]
- Rodrigues MR, Rodriguez D, Catalani LH, Russo M, Campa A. Interferon-gamma independent oxidation of melatonin by macrophages. J Pineal Res. 2003;34:69–74. doi: 10.1034/j.1600-079X.2003.02944.x. [DOI] [PubMed] [Google Scholar]
- Tan D-X, Manchester LC, Reiter RJ, Qi W-B, Karbownik M, Calvo JR. Significance of melatonin in antioxidative defense system: Reactions and products. Biol Signals Recept. 2000;9:137–159. doi: 10.1159/000014635. [DOI] [PubMed] [Google Scholar]
- Balzer I, Hardeland R. Action of kynuramine in a dinoflagellate: stimulation of bioluminescence in Gonyaulax polyedra. Comp Biochem Physiol. 1989;94C:129–132. [Google Scholar]
- Behrends A, Hardeland R, Ness H, Grube S, Poeggeler B, Haldar C. Photocatalytic actions of the pesticide metabolite 2-hydroxyquinoxaline: destruction of antioxidant vitamines and biogenic amines – implications of organic redox cycling. Redox Rep. 2004;9:279–288. doi: 10.1179/135100004225006759. [DOI] [PubMed] [Google Scholar]
- Kelly RW, Amato F, Seamark RF. N-Acetyl-5-methoxy kynurenamine, a brain metabolite of melatonin, is a potent inhibitor of prostaglandin biosynthesis. Biochem Biophys Res Commun. 1984;121:372–379. doi: 10.1016/0006-291x(84)90732-0. [DOI] [PubMed] [Google Scholar]
- Mayo JC, Sainz RM, Tan D-X, Hardeland R, Leon J, Rodriguez C, Reiter RJ. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005;165:139–149. doi: 10.1016/j.jneuroim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Guenther AL, Schmidt SI, Laatsch H, Fotso S, Ness H, Ressmeyer A-R, Poeggeler B, Hardeland R. Reactions of the melatonin metabolite AMK (N 1-acetyl-5-methoxykynuramine) with reactive nitrogen species: Formation of novel compounds, 3-acetamidomethyl-6-methoxycinnolinone and 3-nitro-AMK. J Pineal Res. 2005 doi: 10.1111/j.1600-079X.2005.00242.x. online ahead publ.: DOI 10.1111/j.1600-079X.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- Barlow-Walden LR, Reiter RJ, Abe M, Pablos M, Menendez-Pelaez A, Chen LD, Poeggeler B. Melatonin stimulates brain glutathione peroxidase activity. Neurochem Int. 1995;26:497–502. doi: 10.1016/0197-0186(94)00154-M. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow-Walden L, Chuang J, Ortiz GG, Acuña-Castroviejo D. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995;18:1–11. doi: 10.1111/j.1600-079x.1995.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Pablos MI, Agapito MT, Gutierrez R, Recio JM, Reiter RJ, Barlow-Walden L, Acuña-Castroviejo D, Menendez-Pelaez A. Melatonin stimulates the activity of the detoxifying enzyme glutathione peroxidase in several tissues of chicks. J Pineal Res. 1995;19:111–115. doi: 10.1111/j.1600-079x.1995.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Pablos MI, Chuang J, Reiter RJ, Ortiz GG, Daniels WM, Sewerynek E, Melchiorri D, Poeggeler B. Time course of the melatonin-induced increase in glutathione peroxidase activity in chick tissues. Biol Signals. 1995;4:325–30. doi: 10.1159/000109459. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Garcia JJ, Pie J. Oxidative toxicity in models of neurodegeneration: responses to melatonin. Restor Neurol Neurosci. 1998;12:135–42. [PubMed] [Google Scholar]
- Kotler M, Rodríguez C, Sáinz RM, Antolín I, Menéndez-Peláez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res. 1998;24:83–89. doi: 10.1111/j.1600-079x.1998.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Ustundag B, Kazez A, Demirbag M, Canatan H, Halifeoglu I, Ozercan IH. Protective effect of melatonin on antioxidative system in experimental ischemia-reperfusion of rat small intestine. Cell Physiol Biochem. 2000;10:229–236. doi: 10.1159/000016354. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Kaneda C. Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J Pineal Res. 2000;28:89–96. doi: 10.1034/j.1600-079X.2001.280204.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann NY Acad Sci. 2001;939:200–215. [PubMed] [Google Scholar]
- Wakatsuki A, Okatani Y, Shinohara K, Ikenoue N, Kaneda C, Fukaya T. Melatonin protects fetal rat brain against oxidative mitochondrial damage. J Pineal Res. 2001;30:22–28. doi: 10.1034/j.1600-079X.2001.300103.x. [DOI] [PubMed] [Google Scholar]
- Gürdöl F, Genç S, Öner-Ýyidogan Y, Süzme R. Coadministration of melatonin and estradiol in rats: effects on oxidant status. Horm Metab Res. 2001;33:608–611. doi: 10.1055/s-2001-17908. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Shinohara K, Kaneda C, Fukaya T. Melatonin stimulates glutathione peroxidase activity in human chorion. J Pineal Res. 2001;30:199–205. doi: 10.1034/j.1600-079X.2001.300402.x. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Reiter RJ. Melatonin protects hepatic mitochondrial respiratory chain activity in senescence-accelerated mice. J Pineal Res. 2002;32:143–148. doi: 10.1034/j.1600-079x.2002.1o106.x. [DOI] [PubMed] [Google Scholar]
- Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci. 2002;59:1706–1713. doi: 10.1007/PL00012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez M, Esparza JL, Nogués MR, Giralt M, Cabré M, Domingo JL. Pro-oxidant activity of aluminum in the rat hippocampus: gene expression of antioxidant enzymes after melatonin administration. Free Radic Biol Med. 2005;38:104–111. doi: 10.1016/j.freeradbiomed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Liu F, Ng TB. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem Cell Biol. 2000;78:447–453. doi: 10.1139/bcb-78-4-447. [DOI] [PubMed] [Google Scholar]
- Antolín I, Rodríguez C, Sáinz RM, Mayo JC, Uría H, Kotler ML, Rodríguez-Colunga MJ, Tolivia D, Menéndez-Peláez A. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J. 1996;10:882–890. doi: 10.1096/fasebj.10.8.8666165. [DOI] [PubMed] [Google Scholar]
- Öztürk G, Coşkun S, Erbaş D, Hasanoğlu E. The effect of melatonin on liver superoxide dismutase activity, serum nitrate and thyroid hormone levels. Jap J Physiol. 2000;50:149–153. doi: 10.2170/jjphysiol.50.149. [DOI] [PubMed] [Google Scholar]
- Wang H, Wei W, Shen YX, Dong C, Zhang LL, Wang NP, Yue L, Xu SY. Protective effect of melatonin against liver injury in mice induced by Bacillus Calmette-Guerin plus lipopolysaccharide. World J Gastroenterol. 2004;10:2690–2696. doi: 10.3748/wjg.v10.i18.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079X.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Tomás-Zapico C, Coto-Montes A, Martínez-Fraga J, Rodríguez-Colunga MJ, Hardeland R, Tolivia D. Effects of δ-aminolevulinic acid and melatonin in the Harderian gland of female Syrian hamster. Free Radic Biol Med. 2002;32:1197–1204. doi: 10.1016/S0891-5849(02)00812-2. [DOI] [PubMed] [Google Scholar]
- Erten SF, Kocak A, Ozdemir I, Aydemir S, Colak A, Reeder BS. Protective effect of melatonin on experimental spinal cord ischemia. Spinal Cord. 2003;41:533–538. doi: 10.1038/sj.sc.3101508. [DOI] [PubMed] [Google Scholar]
- Uz T, Manev H. Circadian expression of pineal 5-lipoxygenase mRNA. Neuroreport. 1998;9:783–786. doi: 10.1097/00001756-199803300-00003. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T, Qu T. Early upregulation of hippocampal 5-lipoxygenase following systemic administration of kainate to rats. Restor Neurol Neurosci. 1998;12:81–85. [PubMed] [Google Scholar]
- Zhang H, Akbar M, Kim HY. Melatonin: an endogenous negative modulator of 12-lipoxygenation in the rat pineal gland. Biochem J. 1999;344:487–493. doi: 10.1042/0264-6021:3440487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo D, Reiter RJ, Calvo JR, Guerrero JM. Physiological concentrations of melatonin inhibit nitric oxide synthase in rat cerebellum. Life Sci. 1994;55:PL455–PL460. doi: 10.1016/0024-3205(94)00532-X. [DOI] [PubMed] [Google Scholar]
- Bettahi I, Pozo D, Osuna C, Reiter RJ, Acuña-Castroviejo D, Guerrero JM. Melatonin reduces nitric oxide synthase activity in rat hypothalamus. J Pineal Res. 1996;20:205–210. doi: 10.1111/j.1600-079x.1996.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Gilad E, Wong HR, Zingarelli B, Virag L, O'Connor M, Salzman AL, Szabo C. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: role of inhibitions of NFκB activation. FASEB J. 1998;12:685–693. doi: 10.1096/fasebj.12.9.685. [DOI] [PubMed] [Google Scholar]
- Chang HM, Ling EA, Chen CF, Lue H, Wen CY, Shieh JY. Melatonin attenuates the neuronal NADPH-d/NOS expression in the nodose ganglion of acute hypoxic rats. J Pineal Res. 2002;32:65–73. doi: 10.1034/j.1600-079x.2002.1816.x. [DOI] [PubMed] [Google Scholar]
- Rogério F, de Souza Queiroz L, Teixeira SA, Oliveira AL, de Nucci G, Langone F. Neuroprotective action of melatonin on neonatal rat motoneurons after sciatic nerve transection. Brain Res. 2002;926:33–41. doi: 10.1016/S0006-8993(01)03286-3. [DOI] [PubMed] [Google Scholar]
- Storr M, Koppitz P, Sibaev A, Saur D, Kurjak M, Franck H, Schusdziarra V, Allescher HD. Melatonin reduces non-adrenergic, non-cholinergic relaxant neurotransmission by inhibition of nitric oxide synthase activity in the gastrointestinal tract of rodents in vitro. J Pineal Res. 2002;33:101–108. doi: 10.1034/j.1600-079X.2002.02909.x. [DOI] [PubMed] [Google Scholar]
- Nosjean O, Ferro M, Cogé F, Beauverger P, Henlin J-M, Lefoulon F, Fauchère J-L, Delagrange P, Canet E, Boutin JA. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- Nosjean O, Nicolas JP, Klupsch F, Delagrange P, Canet E, Boutin JA. Comparative parmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. Biochem Pharmacol. 2001;61:1369–1379. doi: 10.1016/S0006-2952(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Coto-Montes A, Hardeland R. Diurnal rhythm of protein carbonyl as an indicator of oxidative damage in Drosophila melanogaster : Influence of clock gene alleles and deficiencies in the formation of free-radical scavengers. Biol Rhythm Res. 1999;30:383–391. doi: 10.1076/brhm.30.4.383.1414. [DOI] [Google Scholar]
- Coto-Montes A, Tomás-Zapico C, Rodríguez-Colunga MJ, Tolivia-Cadrecha D, Martínez-Fraga J, Hardeland R, Tolivia D. Effects of the circadian mutation 'tau' on the Harderian glands of Syrian hamsters. J Cell Biochem. 2001;83:426–434. doi: 10.1002/jcb.1240. [DOI] [PubMed] [Google Scholar]
- Sharman EH, Bondy SC. Effects of age and dietary antioxidants on cerebral electron transport chain activity. Neurobiol Aging. 2001;22:629–634. doi: 10.1016/S0197-4580(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Reiter RJ, Miyahara Y. Hepatic mitochondrial dysfunction in senescence-accelerated mice: correction by long-term, orally administered physiological levels of melatonin. J Pineal Res. 2002;33:127–133. doi: 10.1034/j.1600-079X.2002.02109.x. [DOI] [PubMed] [Google Scholar]
- Martín M, Macías M, León J, Escames G, Khaldy H, Acuña-Castroviejo D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol. 2002;34:348–357. doi: 10.1016/S1357-2725(01)00138-8. [DOI] [PubMed] [Google Scholar]
- Acuña-Castroviejo D, Martin M, Macias M, Escames G, Leon J, Khaldy H, Reiter RJ. Melatonin, mitochondria, and cellular bioenergetics. J Pineal Res. 2001;30:65–74. doi: 10.1034/j.1600-079X.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- Escames G, León J, Macías M, Khaldy H, Acuña-Castroviejo D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003;17:932–934. doi: 10.1096/fj.02-0692fje. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Reiter RJ, Enzan H, Miyahara Y. Protective effect of melatonin against mitochondrial injury induced by ischemia and reperfusion of rat liver. Eur J Pharmacol. 2003;469:145–152. doi: 10.1016/S0014-2999(03)01643-1. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Wakatsuki A, Shinohara K, Ikenoue N, Yokota K, Fukaya T. Maternally administered melatonin protects against ischemia and reperfusion-induced oxidative mitochondrial damage in premature fetal rat brain. J Pineal Res. 2004;37:276–280. doi: 10.1111/j.1600-079X.2004.00167.x. [DOI] [PubMed] [Google Scholar]
- León J, Acuña-Castroviejo D, Escames G, Tan D-X, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res. 2005;38:1–9. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- Acuña-Castroviejo D, Escames G, León J, Carazo A, Khaldy H. Mitochondrial regulation by melatonin and its metabolites. Adv Exp Med Biol. 2003;527:549–557. doi: 10.1007/978-1-4615-0135-0_63. [DOI] [PubMed] [Google Scholar]
- Yalcin A, Kilinc E, Kocturk S, Resmi H, Sozmen EY. Effect of melatonin cotreatment against kainic acid on coenzyme Q10, lipid peroxidation and Trx mRNA in rat hippocampus. Int J Neurosci. 2004;114:1085–1097. doi: 10.1080/00207450490475535. [DOI] [PubMed] [Google Scholar]
- Martín M, Macías M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuña-Castroviejo D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res. 2000;28:242–248. doi: 10.1034/j.1600-079X.2000.280407.x. [DOI] [PubMed] [Google Scholar]
- León J, Acuña-Castroviejo D, Sáinz RM, Mayo JC, Tan D-X, Reiter RJ. Melatonin and mitochondrial function. Life Sci. 2004;75:765–790. doi: 10.1016/j.lfs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Reiter RJ, Miyahara Y. Acutely administered melatonin restores hepatic mitochondrial physiology in old mice. Int J Biochem Cell Biol. 2003;35:367–375. doi: 10.1016/S1357-2725(02)00260-1. [DOI] [PubMed] [Google Scholar]
- Genova ML, Merlo Pich M, Bernacchia A, Bianchi C, Biondi A, Bovina C, Falasca AI, Formiggini G, Parenti Castelli G, Lenaz G. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann NY Acad Sci. 2004;1011:86–100. doi: 10.1196/annals.1293.010. [DOI] [PubMed] [Google Scholar]
- Jou M-J, Peng T-I, Reiter RJ, Jou S-B, Wu H-Y, Wen S-T. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J Pineal Res. 2004;37:55–70. doi: 10.1111/j.1600-079X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004;18:869–871. doi: 10.1096/fj.03-1031fje. [DOI] [PubMed] [Google Scholar]
- Xu M, Ashraf M. Melatonin protection against lethal myocyte injury induced by doxorubicin as reflected by effects on mitochondrial membrane potential. J Mol Cell Cardiol. 2002;34:75–79. doi: 10.1006/jmcc.2001.1485. [DOI] [PubMed] [Google Scholar]
- Poeggeler B. Neuroprotection by indole and nitrone compounds acting as mitochondrial metabolism modifiers with potent antioxidant activity [abstract] 9th International Conference on Alzheimer's Disease and Related Disorders, 17-22 July 2004; Philadelphia. pp. P4–398.
- Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Ahmed S, Sack RL. Phase shifting the human circadian clock using melatonin. Behav Brain Res. 1996;73:131–134. doi: 10.1016/0166-4328(96)00084-8. [DOI] [PubMed] [Google Scholar]
- Sulli A, Maestroni GJ, Villaggio B, Hertens E, Craviotto C, Pizzorni C, Briata M, Seriolo B, Cutolo M. Melatonin serum levels in rheumatoid arthritis. Ann NY Acad Sci. 2002;966:276–283. doi: 10.1111/j.1749-6632.2002.tb04227.x. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- Lusardi P, Piazza E, Fogari R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: a 24-hour study. Br J Clin Pharmacol. 2000;49:423–427. doi: 10.1046/j.1365-2125.2000.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escames G, Khaldy H, León J, González L, Acuña-Castroviejo D. Changes in iNOS activity, oxidative stress and melatonin levels in hypertensive patients treated with lacidipine. J Hypertens. 2004;22:629–635. doi: 10.1097/00004872-200403000-00027. [DOI] [PubMed] [Google Scholar]