Abstract
Clathrin-coated pits at the cell surface select material for transportation into the cell interior. A major mode of cargo selection at the bud site is via the µ2 subunit of the AP-2 adaptor complex, which recognizes tyrosine-based internalization signals. Other internalization motifs and signals, including phosphorylation and ubiquitylation, also tag certain proteins for incorporation into a coated vesicle, but the mechanism of selection is unclear. Disabled-2 (Dab2) recognizes the FXNPXY internalization motif in LDL-receptor family members via an N-terminal phosphotyrosine-binding (PTB) domain. Here, we show that in addition to binding AP-2, Dab2 also binds directly to phosphoinositides and to clathrin, assembling triskelia into regular polyhedral coats. The FXNPXY motif and phosphoinositides contact different regions of the PTB domain, but can stably anchor Dab2 to the membrane surface, while the distal AP-2 and clathrin-binding determinants regulate clathrin lattice assembly. We propose that Dab2 is a typical member of a growing family of cargo-specific adaptor proteins, including β-arrestin, AP180, epsin, HIP1 and numb, which regulate clathrin-coat assembly at the plasma membrane by synchronizing cargo selection and lattice polymerization events.
Keywords: cargo selection/clathrin/Disabled-2/phosphoinositide/receptor-mediated endocytosis
Introduction
Clathrin-coated buds only assemble at the cell surface to convey select macromolecules into the cell interior. To gain entry into the clathrin coat, many transmembrane proteins utilize short endocytic sorting determinants located within the cytosolic domain. The best understood internalization signal employs the tyrosine-based YXXØ sequence, where Ø represents a residue with a bulky hydrophobic side chain (Bonifacino and Dell’Angelica, 1999). This particular class of internalization signals binds directly to the µ2 subunit of the AP-2 adaptor heterotetramer (Ohno et al., 1995). Structurally, the interaction occurs via an extended YXXØ sequence engaging the µ2 β-sandwich in a β-sheet augmentation, with specificity provided by two hydrophobic pockets that accommodate the key tyrosine and bulky hydrophobic side chains (Owen and Evans, 1998). As AP-2 also binds to clathrin directly, these findings explain nicely how the adaptors can efficiently coordinate clathrin lattice assembly with cargo selection.
It is clear that not all cargo molecules cluster into clathrin-coated buds by direct engagement of the adaptor µ2 subunit. Heptahelical G protein-coupled receptors utilize an alternate system centered around β-arrestin (Miller and Lefkowitz, 2001). Normally soluble, β-arrestin translocates onto activated, and thus phosphorylated, heptahelical receptors while synchronously interacting with both clathrin and the β2 subunit of AP-2 (Goodman et al., 1996; Laporte et al., 1999, 2000). The multivalent attachments involving β-arrestin recruit activated receptors into assembling clathrin-coated regions for downregulation. Thus, β-arrestin meshes the receptor with the standard cellular endocytic machinery (Santini et al., 2002; Scott et al., 2002). A role akin to that of β-arrestin for alternate classes of cargo molecules is probably fulfilled by other proteins. Mutagenic inactivation of the µ2 subunit potently blocks transferrin receptor uptake (which uses a YTRF internalization motif), but fails to affect epidermal growth factor (EGF) receptor endocytosis (Nesterov et al., 1999). This indicates that the sorting of transferrin and EGF receptors into clathrin buds is mechanistically distinct. In fact, when one is overexpressed, transferrin, EGF and low density lipoprotein (LDL) receptors do not appear to compete with each other for incorporation into clathrin-coated vesicles, despite all containing tyrosine-based internalization signals (Warren et al., 1998). Dedicated connector proteins, functionally analogous to β-arrestin, were postulated to account for the independent selection of these proteins into clathrin coats at the cell surface (Warren et al., 1998).
Evidence for one additional connector-type protein comes from genetic disruption of UNC-11, the Caeno rhabditis elegans ortholog of AP180 (Nonet et al., 1999). Remarkably, in unc-11 mutant worms, synaptobrevin/VAMP fails to be appropriately recycled together with the synaptic vesicle membrane following exocytosis. Instead, the protein diffuses widely over the presynaptic plasma membrane (Nonet et al., 1999). The size distribution of neuronal clathrin-coated vesicles is also aberrant in these worms, suggesting that UNC-11 could integrate cargo selection temporally with clathrin-coat assembly. The N-terminal ENTH domain of AP180 binds to phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2). With the adjacent AP-2- and clathrin-binding segment of the protein, the ENTH domain facilitates the assembly of highly ordered, invaginated clathrin-coated buds in the presence of soluble AP-2 and clathrin (Ford et al., 2001). We have recently shown that two endocytic accessory proteins, epsin and huntingtin-interacting protein 1 (HIP1), each of which has similar overall domain organization to AP180, can also promote optimal clathrin-coat assembly upon liposome templates (Mishra et al., 2001). These proteins too could be cargo-selective endocytic connectors.
The NPXY internalization motif within the LDL receptor might utilize an alternate connector-type protein because transferrin receptor overexpression does not interfere with LDL receptor internalization (Warren et al., 1998). One candidate connector is Disabled-2 (Dab2). Dab2 contains an N-terminal phosphotyrosine-binding (PTB) domain with a marked preference for non-phosphorylated NPXY motifs, as is found in the LDL receptor and amyloid precursor protein (Morris and Cooper, 2001). At steady state, the intracellular distribution of Dab2 co-localizes well with AP-2 and clathrin. Dab2 binds to AP-2 directly via a central portion that, like epsin, AP180 and HIP1, engages the independently folded appendage domain of the AP-2 α-subunit (Morris and Cooper, 2001). We show here that Dab2 also binds robustly to clathrin, independently of the AP-2 association, and, like other PTB domains, the PTB domain of Dab2 associates directly with phosphoinositides. With synthetic liposome templates, Dab2 drives clathrin-coat assembly in the presence of purified AP-2 and clathrin and, consequently, displays all the properties of a cargo-specific adaptor protein.
Results
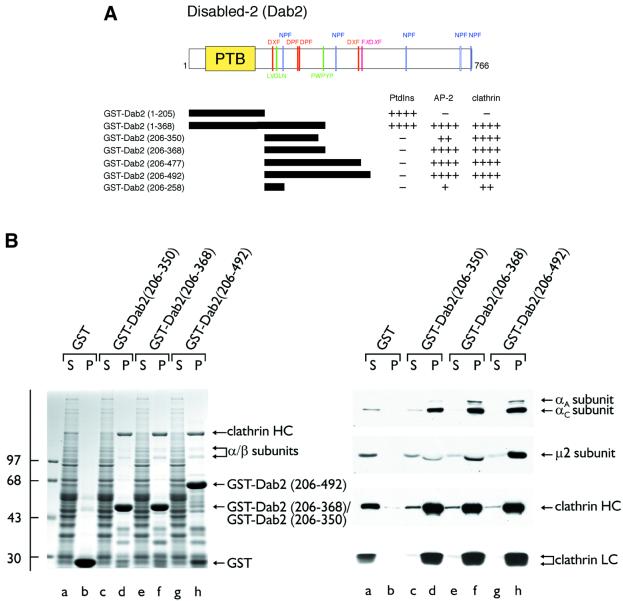
Dab2 binds the AP-2 α-subunit appendage directly using the same interaction surface engaged by epsin, eps15, amphiphysin, HIP1 and AP180 (Morris and Cooper, 2001). Tandem DPF triplets, positioned at 293DPF RDDPF, are potential interaction motifs for the α-appendage, yet amino acids 206–350 of murine Dab2 fused to glutathione S-transferase (GST) bind to soluble AP-2 relatively weakly (Morris and Cooper, 2001) (Figure 1B, lanes d). A GST fusion protein of Dab2 residues 206–492 appears to bind to AP-2 at least 4-fold more avidly (Morris and Cooper, 2001) (Figure 1B, lanes h), indicating that this distal region also contributes to the interaction of Dab2 with AP-2. We recently characterized an alternate α-appendage-binding sequence, the FXDXF motif (Brett et al., 2002), and Dab2 contains the sequence 480FLDLF within the distal segment. To explore the distal AP-2-binding determinant(s) further, we prepared a limited series of truncated Dab2 fusions (Figure 1A). Amino acids 206–368 is the minimal sequence required for optimal AP-2 binding in pull-down-type assays (Figure 1B, lanes f). Surprisingly, the removal of the FXDXF motif has no discernible effect on AP-2 binding, indicating that this sequence is not critical for the α-appendage association measured by these assays. The additional appendage-binding sequences in Dab2 remain to be characterized.
Fig. 1. Dab2 binds to both the AP-2 adaptor complex and clathrin. (A) Schematic illustration of the overall domain organization of Dab2 and the various GST–Dab2 constructs used. Phosphoinositide-, AP-2- and clathrin-binding properties of each fusion protein are indicated qualitatively. (B) Approximately 50 µg of either GST (lanes a and b), GST–Dab2(206–350) (lanes c and d), GST–Dab2(206–368) (lanes e and f) or GST–Dab2(206–492) (lanes g and h) immobilized on GSH–Sepharose were incubated with rat brain cytosol. After centrifugation, aliquots corresponding to 1/60 of each supernatant (S) and 1/5 of each washed pellet (P) were resolved by SDS–PAGE and either stained with Coomassie Blue or transferred to nitrocellulose. Portions of the blots were probed with the anti-AP-2 α-subunit mAb 100/2, anti-AP-2 µ2-subunit antiserum, the anti-clathrin heavy chain (HC) mAb TD.1 or the anti-clathrin light chain (LC) mAb Cl 57.3. The position of the molecular mass standards (in kDa) is indicated on the left and only the relevant portion of each blot is shown.
Dab2 interacts directly with clathrin triskelia
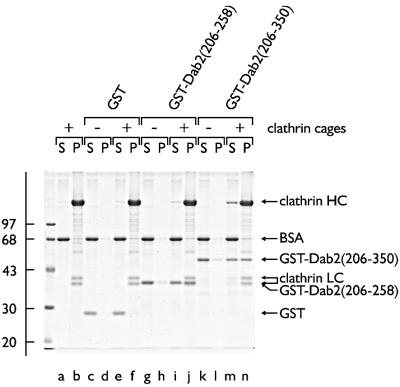
The central region of murine Dab2 also binds to a very prominent ∼180 kDa polypeptide in the pull-down assays (Figure 1B). Appropriate antibodies identify this protein as the clathrin heavy chain (Figure 1B). The smallest GST–Dab2 fusion tested, encoding only residues 206– 258, binds to clathrin (Figure 2, lanes d). Longer Dab2 segments fused to GST bind to soluble clathrin with noticeably greater affinity (Figure 2, lanes h) and remove trimers from cytosol more completely (lanes g compared with a and c). As little AP-2 binds to the GST–Dab2 (206–258) protein (lanes d), the interaction with clathrin is largely independent of the adaptor complex and may well be direct. Indeed, isolated GST–Dab2(206–258) binds to pre-assembled clathrin in vitro and, upon centrifugation, is recovered together with the polyhedral cages in the pellet (Figure 3, lane j). GST (Figure 3, lanes e and f) does not sediment appreciably with the cages (lane f) under the same conditions. In the absence of the clathrin, both GST (Figure 3, lane c) and the GST–Dab2 fusion (lane g) remain soluble and appear in the supernatant fraction, together with the carrier BSA. Similarly, a larger GST– Dab2 fusion (residues 206–350) binds to the assembled cages (Figure 3, lane n), but does not sediment significantly in the absence of clathrin (lane l).
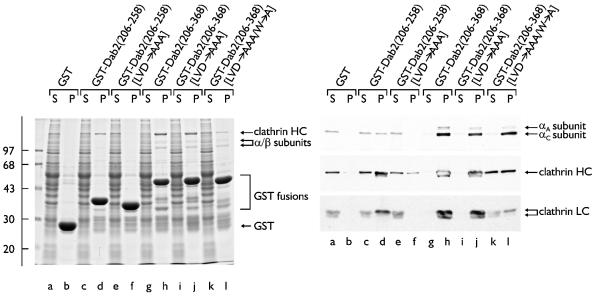
Fig. 2. A clathrin-binding region within Dab2. Approximately 50 µg of either GST (lanes a and b) or GST–Dab2(206–258) (lanes c and d), GST–Dab2(206–258) (LVD→AAA) (lanes e and f), GST–Dab2(206–368) (lanes g and h), GST–Dab2(206–368) (LVD→AAA) (lanes i and j) or GST–Dab2(206–368) (LVD→AAA/W→A) (lanes k and l) immobilized on GSH–Sepharose were incubated with rat brain cytosol. After centrifugation, aliquots corresponding to 1/50 of each supernatant (S) and 1/5 of each washed pellet (P) were resolved by SDS–PAGE and either stained with Coomassie Blue or transferred to nitrocellulose. Portions of the blots were probed with the anti-AP-2 α-subunit mAb 100/2, anti-AP-2 µ2-subunit antiserum, the anti-clathrin HC mAb TD.1 or the anti-clathrin LC mAb Cl 57.3.
Fig. 3. Dab2 associates directly with assembled clathrin cages. Pre-assembled clathrin cages (∼0.5 µM), GST, GST–Dab2(206–258), GST–Dab2(206–350) (each ∼1 µM) or combinations thereof were incubated in MES–OH buffer on ice. After centrifugation, aliquots corresponding to 1/10 of each supernatant (S) or 1/8 of each pellet (P) were analyzed by SDS–PAGE and stained with Coomassie Blue.
Sequence analysis reveals a putative type I clathrin-box sequence, 236LVDLN, located within the minimal segment of Dab2 (residues 206–258) that binds clathrin efficiently. To assess the contribution of this motif to clathrin binding, the first three residues of the sequence were mutated to Ala (LVDLN→AAALN). In the context of GST–Dab2(206– 258), the smallest clathrin-binding fragment, disruption of this type I sequence almost completely ablates clathrin association (Figure 2, lanes f compared with d). Introducing the LVDLN→AAALN substitution within a larger GST– Dab2(206–368) fusion has little effect on clathrin binding, however (Figure 2, lane j). The sequence 363PWPYP is similar to the type II clathrin-binding sequence 381PWDLW found in amphiphysin I and II (Ramjaun and McPherson, 1998; Slepnev et al., 2000). In amphiphysin, substitution of the proximal Trp with Ala completely inactivates the binding properties (Drake and Traub, 2001). Simultaneous mutation (LVD→AAA/W→A) of both the type I LVDLN and putative type II PWPYP sequences in GST–Dab2(206–368) reduces clathrin binding 4- to 5-fold in pull-down assays. The interaction of this Dab2 fusion with AP-2 is not similarly affected (Figure 2, lane l). These experiments clearly indicate that Dab2 contains multiple, tandemly arrayed clathrin-binding sequences. When Dab2 is immunoprecipitated from cytosolic extracts, clathrin trimers do not co-precipitate in significant amounts (not shown). However, we also fail to detect significant co-immunoprecipitation of clathrin with either epsin 1 (not shown) or AP-2 (Arneson et al., 1999), two proteins known to engage clathrin at coat assembly sites using clathrin-binding sequences related to those present in Dab2 (Drake et al., 2000; Dell’Angelica, 2001; Kalthoff et al., 2002).
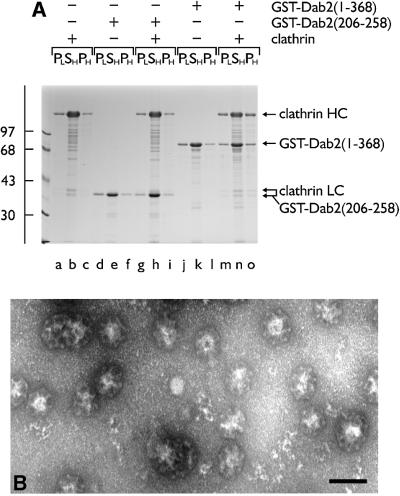
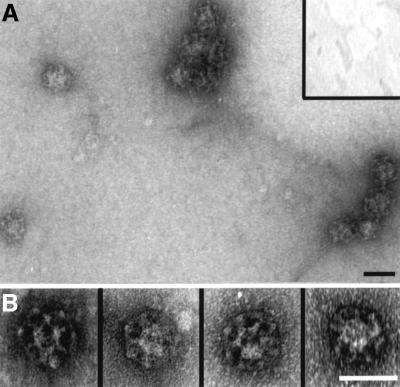
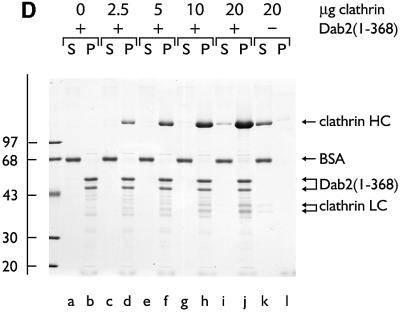
Previously, we have shown that tandemly arranged type I and type II sequences allow amphiphysin to bind to the terminal domain of the clathrin heavy chain and polymerize trimers into sedimentable assemblies (Drake and Traub, 2001). Similarly, if a GST–Dab2(1–368) fusion is mixed with soluble clathrin trimers, a portion of both proteins is recovered in a high-speed pellet after dialysis into either MES–OH buffer (pH 6.8; not shown) or a more physiological assay buffer (pH 7.2; Figure 4A, lane o). Substantially lower levels of the clathrin alone (Figure 4A, lane c) or the GST–Dab2(1–368) alone (lane l) sediment under the same conditions. Electron microscope (EM) examination of the Dab2 assembled population after negative staining reveals regular assemblies of polyhedral clathrin cages (Figure 4B). Despite binding to pre-assembled cages (Figure 3), the minimal clathrin-binding Dab2 fusion GST–Dab2(206–258) does not increase clathrin recovery in the high-speed pellet above background (lane i compared with c). GST is also unable to induce clathrin-coat assembly (data not shown). Compared with the GST–dimer form, thrombin-cleaved monomeric Dab2(206–350) shows a reduced capacity to assemble clathrin cages (not shown). However, monomeric Dab2(1–368) effectively recruits soluble clathrin trimers onto a membrane surface (see Figure 8D). Together, we believe our data show that in addition to AP-2 (Morris and Cooper, 2001), Dab2 binds to clathrin directly, interacts with pre-assembled clathrin cages and, by utilizing multiple binding sites, engages soluble clathrin trimers to productively assemble closed clathrin cages.
Fig. 4. Dab2 assembles soluble clathrin trimers into spherical polyhedral cages. (A) Clathrin (∼0.2 µM), GST–Dab2(206–258) (∼1 µM), GST–Dab2(1–368) (∼1 µM) or combinations thereof were mixed in 0.5 M Tris–HCl pH 7.0 and then dialyzed overnight against assay buffer. The samples were subject to differential centrifugation generating a low-speed pellet (PL) containing aggregated material, a high-speed supernatant (SH) containing soluble protein, and a high-speed pellet (PH) with the sedimentable assemblies. (B) Representative EM micrograph of a negatively stained aliquot of the high-speed pellet obtained from an incubation of clathrin with GST–Dab2(1–368) after resuspension in assay buffer on ice. Bar = 100 nm.
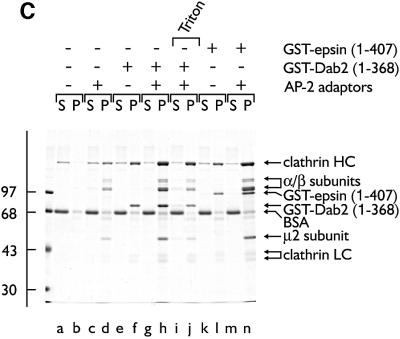
Fig. 8. Dab2 co-operates with AP-2 to drive the assembly of invaginated clathrin buds upon lipid membranes. Clathrin was added to 10% PtdIns(4,5)P2-containing lipid monolayers pre-incubated in the absence (A, inset) or presence (A and B) of GST–Dab2(1–368). An EM grid was used to remove each monolayer and then negatively stained. Bar = 50 nm. (C) Phosphoinositide-containing liposomes were first pre-incubated with AP-2 (lanes c, d, g–j and m, n), GST–Dab2(1–368) (lanes e–j) or GST–epsin 1(1–407) (lanes k–n) at 4°C for 60 min as indicated. After recovery by centrifugation, each liposome pellet was resuspended and then incubated at 4°C for 60 min with purified clathrin trimers in the presence of carrier BSA. After centrifugation, aliquots of 1/40 of each supernatant (S) and 1/4 of each pellet (P) were resolved by SDS–PAGE and stained with Coomassie Blue. Before centrifugation, Triton X-100 (1% final) was added in one reaction (lanes i and j). (D) PtdIns(4,5)P2-containing liposomes were first pre-incubated with (lanes a–j) or without (lanes k and l) thrombin-cleaved Dab2(1–368) at 4°C for 60 min. After recovery by centrifugation, each liposome pellet was resuspended and then incubated at 4°C for 60 min with increasing amounts of purified clathrin trimers in the presence of carrier BSA. After centrifugation, aliquots of 1/25 of each supernatant (S) and 1/5 of each pellet (P) were resolved by SDS–PAGE and stained with Coomassie Blue.
Dab2 binds to phosphoinositides via the N-terminal PTB domain
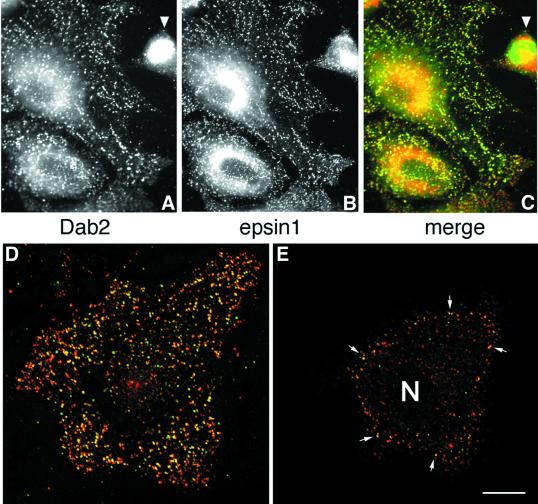
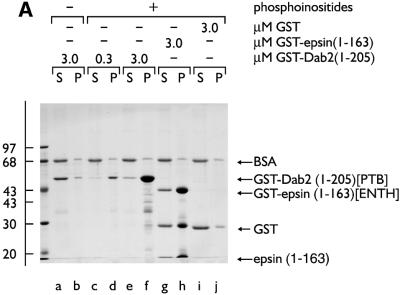
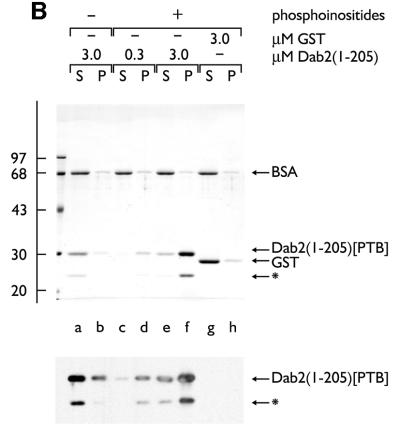
The capacity of the central region of Dab2 to interface with the core endocytic machinery is reminiscent of other so-called endocytic accessory proteins, including AP180, amphiphysin, epsin and HIP1. Indeed, in HeLa cells, epsin 1 and Dab2 show extensive co-localization at steady state (Figure 5), indicating that the two proteins are probably present within common clathrin-coated structures. AP180, epsin and HIP1 utilize an ENTH domain to interact with PtdIns(4,5)P2 and, tethered to a phosphoinositide-containing membrane surface, each protein can assist AP-2 in the assembly of clathrin-coated buds (Ford et al., 2001; Mishra et al., 2001). In Dab2, a PTB domain replaces an ENTH domain (Xu et al., 1995). PTB domains are structurally related to the pleckstrin homology (PH) domain, a known phosphoinositide-binding fold. In fact, the PTB domains of Shc (Ravichandran et al., 1997), IRS-1 (Takeuchi et al., 1998), Dab1 (Howell et al., 1999) and numb (Dho et al., 1999) all bind to phosphoinositides, leading us to test whether the Dab2 PTB domain similarly associates with phosphoinositides. In binding assays with mixed lipid liposomes, the Dab2 PTB domain (amino acids 1–205) fused to GST binds robustly to phosphoinositide-containing membranes in a dose-dependent fashion (Figure 6A, lanes d and f). The extent of the association with phosphoinositides is similar to an epsin 1 ENTH domain–GST fusion (Figure 6A, lane h) (Itoh et al., 2001; Mishra et al., 2001), while PTB-domain binding to liposomes devoid of phosphoinositides (lane b), or GST association with inositide-containing liposomes (lane j), is extremely poor. The monomeric PTB domain, cleaved from GST with thrombin, binds ∼25-fold better to PtdIns(4,5)P2 liposomes, rising from ∼2% without phosphoinositide to >50% bound (Figure 6B, lanes a, b compared with e, f). This suggests that the affinity of the Dab2 PTB domain for phosphoinositides is not massively increased by artificial GST-mediated dimerization. Protein–lipid overlays reveal that the Dab2 PTB domain displays a preference for PtdIns(4,5)P2, although there is binding to several phosphoinositides in this type of assay (Figure 6C).
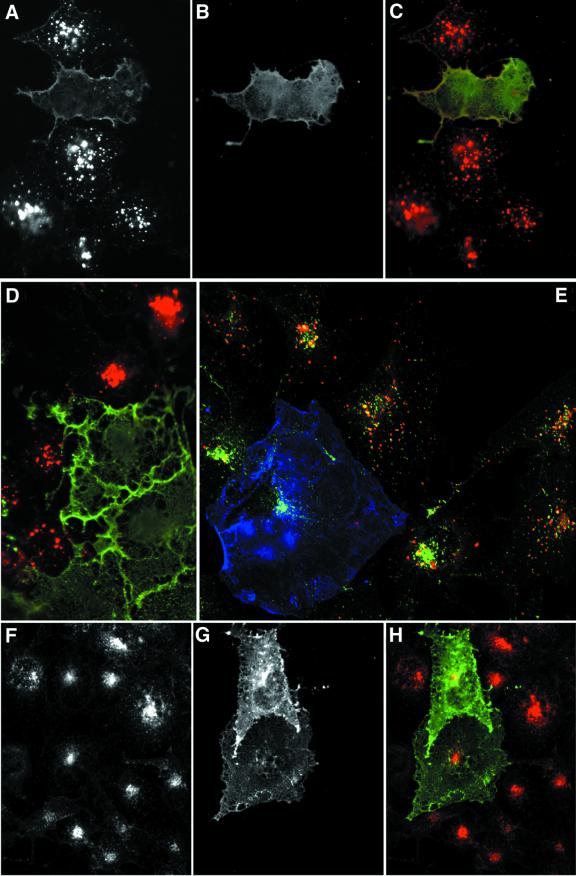
Fig. 5. Dab2 co-localizes with epsin 1 at the plasma membrane. Saponin-permeabilized HeLa cells were fixed and probed with antibodies against Dab2 (A) and epsin 1 (B). The merged color images (C–E) show that areas of overlap are extensive (yellow), but there are clear regions containing each protein alone. Single 0.3 µm confocal sections of the ventral surface (D) and the medial region of the cell (E) through the nucleus (N) show Dab2 co-localized with epsin at the cell surface (arrows). The arrowhead indicates a pre/post-mitotic cell where the Dab2 staining pattern is principally nuclear, although some epsin is still visible at the cell surface; we note that the intracellular distribution of Dab2 appears to alter with the cell cycle. Importantly, the anti-Dab2 mAb used here detects all three splice isoforms of Dab2 (p67, p93 and p96), so we cannot conclude whether the different locations represent distinct Dab2 isoforms. Bar = 25 µm in (A–C) and 10 µm in (D) and (E).
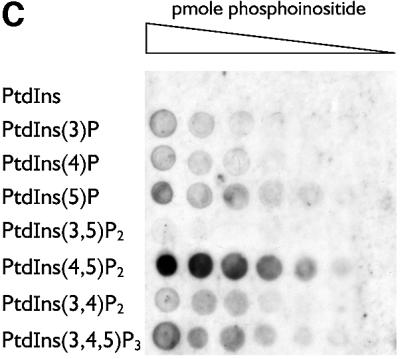
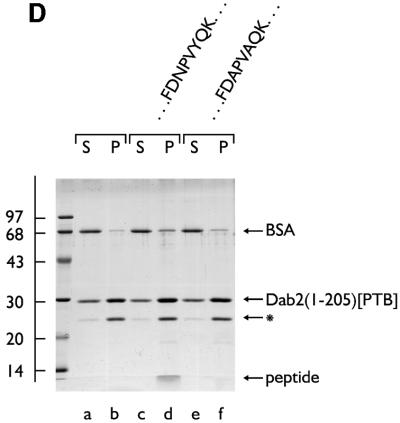
Fig. 6. The PTB domain of Dab2 binds to polyphosphoinositides. (A) GST–Dab2(1–205) (lanes a–f), GST–epsin 1(1–163) (lanes g and h) or GST (lanes i and j), as indicated, were mixed with either control (lanes a and b) or phosphoinositide-containing (lanes c–j) liposomes and incubated together on ice for 60 min. After centrifugation, aliquots of 1/25 of each supernatant (S) or 1/4 of each pellet (P) were analyzed by SDS–PAGE and stained with Coomassie Blue. (B) Thrombin-cleaved Dab2(1–205) (lanes a–f) or GST (lanes g and h) was mixed with either control (lanes a and b) or phosphoinositide-containing (lanes c–j) liposomes and incubated on ice. After centrifugation, aliquots of 1/25 of each supernatant (S) or 1/4 of each pellet (P) were analyzed by SDS–PAGE and stained with Coomassie Blue or transferred to nitrocellulose. The blot was probed with an anti-Dab2 mAb. The asterisk denotes a stable ∼25 kDa thrombin degradation product that binds phosphoinositides and reacts with the anti-Dab2 mAb 52. (C) Nitrocellulose-immobilized phosphoinositides (100, 50, 25, 12.5, 6.2, 3.1, 1.5 pmol/spot) were incubated with GST–Dab2(1–205) PTB domain and then bound protein visualized with GSH-derivatized HRP. (D) The PTB domain of Dab2 binds to phosphoinositides and FXNPXF internalization motifs synchronously. Thrombin-cleaved Dab2(1–205) (PTB domain) was added to 3 µM, together with 0.4 mg/ml phosphoinositide-containing liposomes alone (lanes a and b) or liposomes plus either 15 µM NWRLKNINSIFDNPVYQKTT (lanes c and d) or NWRLKNINSIFDAPVAQKTT (lanes e and f) peptide and incubated on ice for 60 min. After centrifugation, aliquots of 1/25 of each supernatant (S) or 1/4 of each pellet (P) were analyzed by SDS–PAGE and stained with Coomassie Blue. The bound peptide migrates at the dye front and the asterisk denotes the stable ∼25 kDa Dab2 degradation product of the PTB domain.
PTB domains are known to bind directly to the sequence NPXpY (Margolis, 1996), but for Dab1 and Dab2, the PTB designation is a misnomer as both bind to FXNPXY preferentially in the non-tyrosine-phosphorylated state (Howell et al., 1999; Morris and Cooper, 2001). Dab2 PTB association with phosphoinositide-containing mixed liposomes does not preclude NPXY sequence engagement. Addition of a 3- to 5-fold molar excess of a peptide corresponding to residues 2–22 of the LDL receptor cytosolic domain (814NWRLKNINSIFDNPVYQKTT) does not diminish the extent of monomeric PTB domain association with the liposomes (Figure 6D, lane d compared with b). Rather, the wild-type peptide is recovered in the liposome pellet, along with PTB domain (lane d). On standard SDS–PAGE, the bound peptide migrates at the dye front but Tris–Tricine gels confirm the identity of the 2.7 kDa peptide (not shown). The Dab1 PTB domain also binds lipid and NPXY peptides simultaneously (Howell et al., 1999) and, like Dab1, mutation of an invariant Phe (F166V) involved in FXNPXY engagement (Howell et al., 1999; Hocevar et al., 2001) does not prevent phosphoinositide binding (not shown). If an altered peptide, NWRLKNINSIFDAPVAQKTT, is included, negligible amounts of the NPXY-sequence-altered peptide sediment with the liposome/PTB domain pellet (Figure 6D, lane f). Because alteration of the Asn and Tyr side chains in the LDL receptor FXNPXY internalization sequence disrupts endocytic uptake (Chen et al., 1990), the sequence determinants necessary for the LDL receptor peptide to engage the membrane-bound Dab2 PTB domain are the same as those required for LDL receptor internalization.
If the Dab2 PTB domain can engage the FXNPXY internalization sequence in vivo, overexpression of this segment of Dab2 might produce a selective block in LDL receptor internalization. Transient transfection of COS-7 cells with myc epitope-tagged Dab2(1–205) (PTB domain) had little effect on LDL receptor distribution (not shown). The phosphatidylinositol 3-phosphate-binding FYVE domain only targets efficiently to the appropriate intracellular site when expressed in tandem (Gillooly et al., 2000), so we also examined the effect of myc-Dab2 PTBx2 overexpression. Two populations of labeled cells are seen after a 15 min pulse with diI-LDL (Figure 7). The majority of the cells display bright perinuclear punctae, corresponding to endosomes (Figure 7A). A minority of cells display diffuse surface diI-LDL labeling with little or no evidence of accumulation within intracellular structures. These cells invariably express the myc-PTBx2 construct (Figure 7B–D), which also appears, at least in part, associated with the plasma membrane.
Fig. 7. Dab2 PTB domain overexpression selectively interferes with LDL uptake. COS-7 cells transiently transfected with myc-tagged Dab2 PTBx2 were pulsed for 15 min with 20 µg/ml diI-LDL alone (A–D), both diI-LDL and 25 µg/ml biotinylated transferrin (E) or transferrin alone (F–H) for 15 min prior to fixation. Transfected cells were identified using mAb 9E10 (B–D, G and H, in green in C, D and H) and transferrin with Alexa 488– (E) or Alexa 594–streptavidin (F and H). A single confocal section of triple-labeled cells is shown in (E) (myc epitope, blue; transferrin, green; LDL, red).
Importantly, the PTB domain only affects LDL receptor uptake, and does not produce a global perturbation in endocytic activity of transfected cells. PTBx2-expressing cells, unable to internalize diI-LDL during a 15 min pulse, nevertheless take up transferrin normally compared with the surrounding, untransfected cells (Figure 7E–H). These results verify the capacity of the Dab2 PTB domain to engage the LDL receptor family FXNPXY internalization motif in vivo.
Dab2 as an endocytic adaptor protein
We interpret our findings to indicate that the N-terminal PTB domain of Dab2 can engage both PtdIns(4,5)P2 and select cargo molecules simultaneously. Concurrent AP-2 and clathrin association with the central portion of the protein might promote clathrin-coat assembly and drive cargo incorporation. To investigate this idea further, we used the larger GST–Dab2 fusion encompassing both the PTB and coat machinery-binding segments. Like the PTB domain alone, GST–Dab2(1–368) binds efficiently to mixed liposomes containing low levels of phosphoinositides (see Figure 8C, lane f). Oriented in this way upon a 10% PtdIns(4,5)P2-containing monolayer of mixed lipids (Ford et al., 2001), the protein is able to recruit soluble clathrin and drive the formation of regular polyhedral structures (Figure 8A and B), which, alone, does not associate with the monolayer (Figure 8A, inset, see C, lane b). Similar results are obtained in biochemical assays (Figure 8C, lanes a, b and e, f). Again, the clathrin-recruiting properties of Dab2 do not depend upon GST-mediated dimerization as monomeric Dab2(1–368), tethered to a mixed liposome surface, effectively recruits clathrin in a dose-dependent fashion (Figure 8D). These data confirm the direct clathrin-binding properties of Dab2 (Figures 2–4) and further reveal that the adjacent PTB domain does not negate clathrin interactions. In this type of assay, purified AP-2 adaptors can also bind the liposomes (Ford et al., 2001; Mishra et al., 2001) (Figure 8C, lane d), due to a basic cluster of residues located at the N-terminus of the α-subunit (Gaidarov and Keen, 1999). Clathrin recruitment by the membrane-bound AP-2 is only weak, however (lane d) (Ford et al., 2001; Mishra et al., 2001). Pre-incubating liposomes together with a mixture of AP-2 and the GST–Dab2 fusion increases the recovery of both proteins in the liposome pellet (Figure 8C, lane h), validating the AP-2–Dab2 interactions seen in pull-down assays (Figures 1 and 2) (Morris and Cooper, 2001). When clathrin is then added, substantially more (∼50% of total) assembles onto the sedimenting liposomes (Figure 8C, lane h compared with d and f).
Approximately one-quarter of the liposome-bound coat components are resistant to extraction with 1% Triton X-100 (Figure 8C, lane j), which has been shown by others to correlate well with extensive assembly into curved polyhedral coats (Ford et al., 2001). Both AP-2 and the GST–Dab2 fusion protein remain with the pelleted clathrin after Triton X-100 addition. The behavior of GST–Dab2 closely parallels that of a GST–epsin 1(1–407) fusion in these assays (Figure 8C, lanes k–n), showing that Dab2 can function analogously to known endocytic accessory proteins (Chen et al., 1998; Yamabhai et al., 1998; Drake et al., 2000; Mishra et al., 2001). Importantly, and in contrast to eps15 (Cupers et al., 1998), extensive clathrin recruitment does not disrupt the interaction between AP-2 and either Dab2 or epsin (Figure 8) (Mishra et al., 2001), AP180 (Ford et al., 2001) or HIP1 (Mishra et al., 2001), such that these proteins are now released from the membrane surface. The difference might reflect the fact that eps15 has no known intrinsic clathrin-binding sequence. The common properties of Dab2, epsin, AP180 and HIP1 suggest that they can play functionally similar roles during clathrin-coat assembly, distinct from eps15.
Discussion
There is little question that the EGF, transferrin and LDL receptors all utilize the same basic endocytic elements to enter the cell. All three receptors can be found in common endosomes (Ajioka and Kaplan, 1987) and, if clathrin-mediated endocytosis is grossly perturbed, as in stonin 2 overexpression, which prevents proper recruitment of AP-2 to the plasma membrane, transferrin, EGF and LDL internalization are all impaired (Martina et al., 2001). Yet these three transmembrane proteins each appear to use different mechanisms for selective entry into clathrin-coated buds. Four-fold overexpression of the LDL receptor in HeLa cells does not inhibit the rate of transferrin uptake (Warren et al., 1998) and cells from hypercholesterolemic patients with no mutations in the LDL receptor, apolipoprotein (apo) B or AP-2 µ2-subunit fail to internalize LDL while transferrin endocytosis proceeds normally (Norman et al., 1999). This suggests that LDL receptor uptake might depend on alternate endocytic machinery.
Dab2 and cargo recognition
A large body of data links the NPXY internalization sequence present in LDL receptor family members to PTB domain recognition. The PTB domains of Dab1 (Trommsdorff et al., 1998; Howell et al., 1999; Gotthardt et al., 2000) and the rodent CED-6 ortholog, GULP (Su et al., 2002), bind directly to NPXY sequence-bearing LDL receptor family members, and to amyloid precursor proteins (Trommsdorff et al., 1998; Homayouni et al., 1999; Howell et al., 1999), albeit with differing selectivity. Intact Dab2 associates directly with megalin (Oleinikov et al., 2000) and the isolated Dab2 PTB domain binds to LDL receptor family FXNPXY sequences (Morris and Cooper, 2001) (Figure 6). Compelling genetics ties Dab1, with ∼65% identity to the Dab2 PTB domain, to the very low density lipoprotein (VLDL) receptor, apoE receptor-2 (apoER2), and the extracellular ligand reelin in the process of neural migration and spatial positioning in the brain (Cooper and Howell, 1999; Herz, 2001). Genetic disruption of both apoER2 and VLDL receptors in mice precisely phenocopies the mutant strains reeler (reelin defective) and scrambler (Dab1 defective) (Trommsdorff et al., 1999). The results indicate that a vectorial pathway of reelin→VLDL receptor/apoER2→Dab1 is vital for correct central nervous system formation.
Several types of mutation in the LDL receptor including Class 4, internalization defective due to alterations in the NPXY internalization sequence (Chen et al., 1990), cause familial hypercholesterolemia (Goldstein and Brown, 2001). Naturally occurring mutation of the major ligand, apoB, results in a similar, but milder, clinical phenotype termed familial defective apoB-100 (Goldstein and Brown, 2001). However, unlike the Dab1 signaling pathway paradigm, no mutations in Dab2 have been linked to hypercholesterolemia. This suggests that other PTB domain proteins might compensate or substitute for Dab2 loss, and thus Dab2 would not be singularly responsible for LDL receptor incorporation into clathrin-coated vesicles. This notion is also probable because Dab2 is not expressed ubiquitously, and many ovarian and breast carcinomas fail to express Dab2 at all (Sheng et al., 2000). siRNA knockdown of Dab2 levels by >90% in HeLa cells does not inhibit diI-LDL uptake (not shown). Proteins structurally related to Dab2 are, in fact, already known; numb is a PTB domain-containing protein that, like Dab2, binds to AP-2 and also exhibits several NPF triplets in the distal segment of the protein (Santolini et al., 2000). More interesting is the recent identification of the gene mutated in patients with a rare form of non-LDL receptor-dependent hypercholesterolemia (Garcia et al., 2001). Several different natural mutations in the encoded protein, ARH (autosomal recessive hypercholesterolemia), phenocopy LDL receptor-mutant familial hypercholesterolemia (Garcia et al., 2001). Primary sequence alignments of the ARH PTB domain show that numb, CED-6/GULP and Dab1/2 are the most closely related members of the PTB domain-containing superfamily, predicting that ARH will probably bind preferentially to non-phosphorylated NPXY motifs. It thus appears that several related PTB-type endocytic adaptors might function to cluster the LDL receptor and/or other NPXY-bearing proteins into clathrin-coated buds, possibly in tissue-specific combinations.
The phenotype of Dab2 nullizygous mice also supports a role for Dab2 in endocytosis. Targeted disruption of the Dab2 gene is lethal, but if expression is disrupted only in the embryo, the animals are viable and survive to adulthood (Morris et al., 2002b). These mice have a prominent kidney sorting dysfunction that mimics, in a milder form, megalin gene disruption (Morris et al., 2002b). The mice exhibit proteinuria and secrete several vitamin-binding proteins normally efficiently reabsorbed in the kidney proximal tubule by internalization of the scavenger receptor megalin within clathrin-coated vesicles (Morris et al., 2002b). Proximal tubules of Dab2–/– mice, like those of megalin-deficient animals, display decreased numbers of apical clathrin-coated pits and a generally diminished subapical endosome compartment (Leheste et al., 1999; Morris et al., 2002b). This links Dab2 to megalin endocytosis in the kidney in vivo. An additional link between Dab2 and clathrin-mediated endocytosis comes from the recent identification of myosin VI as a Dab2 interaction partner (Inoue et al., 2002; Morris et al., 2002a). Myosin VI is a component of some clathrin-coated vesicles (Buss et al., 2001) and Dab2 probably plays an important role in targeting this motor to the vesicle.
Overexpression of Dab1 in CHO cells results in ∼2-fold more LDL receptor at the cell surface without affecting the rate of uptake (Gotthardt et al., 2000). The increased residency of the LDL receptor at the plasma membrane could be due to Dab1 PTB-mediated interference with receptor incorporation into endocytic coated buds, as Dab1 does not interface with the clathrin-coat machinery. This is in full accord with our demonstration that the PTBx2 potently, but selectively, prevents LDL uptake in COS cells. In addition to two NPXY motifs, LRP also contains a YXXØ sequence within the cytosolic domain that confers more rapid endocytosis than either the LDL, megalin, VLDL or apoER2 receptor cytosolic domain (Li et al., 2001). This suggests that NPXY-driven internalization might be augmented by also engaging the AP-2 complex at the bud site.
Dab2 as a member of a class of monomeric clathrin adaptors
As overexpression of a single Dab2 PTB domain has negligible effect on LDL uptake, we believe that Dab2 normally encounters FXNPXY-bearing cargo in the context of an assembling coated bud. The PtdIns(4,5)P2-, AP-2- and clathrin-binding properties will facilitate Dab2 placement at the bud site, as do analogous determinants in AP180, epsin and HIP1 (Ford et al., 2001; Mishra et al., 2001). Although able to assemble clathrin cages in vitro, we do not contend that Dab2 plays an indispensable role in clathrin lattice assembly. Rather, like epsin and AP180, it potentiates AP-2-driven coat formation, perhaps also determining vesicle size. Each of these proteins is likely to be found within a single forming bud (Figure 5). This distinguishes this group of proteins from β-arrestin, which, despite also containing PtdIns(4,5)P2-, AP-2- and clathrin-binding determinants, appears to accompany heptahelical receptors into existing coated buds (Santini et al., 2002; Scott et al., 2002).
If the overall domain organization and binding properties of epsin, AP180, HIP1 and Dab2 predict a common function as dedicated endocytic sorting adaptors, what cargo might the other proteins be selecting? AP180 could actively cluster synaptobrevin/VAMP (Nonet et al., 1999). A potential cargo for HIP1 is huntingtin, which interacts with the N-terminal segment of HIP1 (Wanker et al., 1997) and has been localized to clathrin-coated structures and endosomes (Velier et al., 1998). Epsin might be part of the long-sought-after machinery that recognizes ubiquitin as an endocytic signal (Polo et al., 2002). Importantly, our identification of AP180, epsin, HIP1 and Dab2 as putative adaptors, each dedicated to selection of certain cargo to the bud site, clarifies our understanding of clathrin-coat assembly; we believe that these proteins can now be viewed as a general class of functionally similar cargo sorters.
Like epsin, Dab2 also contains multiple NPF triplets, the EH domain ligand (de Beer et al., 2000). Mammalian EH domain-containing proteins include eps15, intersectin, POB-1 and EHD1 (Santolini et al., 1999). Dab2 is, therefore, capable of establishing a complex web of protein–protein interactions at the clathrin bud site. These additional functional sequences argue that, like numb (Santolini et al., 2000), Dab2 probably accompanies the clathrin-coated vesicle during budding and fission. These multifaceted connections may be a critical aspect of ensuring the fidelity of cargo incorporation into assembling coated buds.
Finally, Dab2 may participate in more sorting events than only those involving LDL receptor family members. The protein was initially identified as a prominent phosphoprotein accompanying colony-stimulating factor-1 (CSF-1) receptor activation (Xu et al., 1995). The CSF-1 receptor utilizes ubiquitin as an endocytic signal (Lee et al., 1999), but the NPF triplets in Dab2 could recruit eps15, which, like epsin, can bind ubiquitin directly (Polo et al., 2002). Alternatively, the observed phosphorylation of Dab2 might possibly prevent incorporation into coated buds, favoring epsin/eps15 inclusion and possibly enhancing CSF-1 receptor internalization. Dab2 is also implicated in transforming growth factor β (TGF-β) receptor activation and downstream Smad activation (Hocevar et al., 2001). Direct association of the Dab2 PTB domain with Smad2/3 has been reported (Hocevar et al., 2001). Intriguingly, Smad2 and Smad3 activation by liganded TGF-β receptors occurs only post-endocytic internalization (Penheiter et al., 2002), raising the possibility that Dab2, acting at the coated vesicle, coordinates aspects of TGF-β signaling.
Materials and methods
DNA constructs
Plasmids encoding murine Dab2 residues 206–350 and 206–492, each in pGSTag, were generously provided by Shelli Morris and Jonathon Cooper. The PTB domain of mouse Dab2 residues 1–205 [GST– Dab2(1–205)] was amplified using EST uf85g06.y1 (Research Genetics). GST–Dab2(1–368) was produced using the GST–Dab2(1–205) and GST–Dab2(206–492) plasmids as templates. The GST–epsin 1(1–163) ENTH domain was prepared using the rat epsin 1 cDNA provided by Pietro de Camilli and preparation of GST–epsin 1(1–407) has been described previously (Mishra et al., 2001). All mutations were introduced into the appropriate vectors using the QuikChange (Stratagene) system with the required mutagenic primer pairs. All clones and mutations were verified by automated sequencing.
Antibodies
The monoclonal antibody (mAb) directed against a peptide from within the PTB domain of Dab2 (p96) was purchased from Transduction Laboratories. Anti-clathrin mAb TD.1 and Cl57.3, the AP-2 α-subunit mAb 100/2 and the affinity-purified rabbit anti-epsin antibodies have been described previously (Drake et al., 2000). The rabbit anti-AP-2 µ2-subunit serum was generously provided by Juan Bonifacino and the anti-myc mAb 9E10 was from Covance.
Protein and peptide preparation
Synthetic peptides corresponding to amino acids 814–834 (NWRLKN INSIFDNPVYQKTT) of the murine LDL receptor and a related peptide, NWRLKNINSIFDAPVAQKTT, were synthesized by the University of Pittsburgh peptide synthesis facility and both peptides purified by HPLC on a C-18 column to >98% purity. GST and the various GST fusion proteins, rat brain cytosol and purified AP-2 and clathrin were prepared exactly as described previously (Mishra et al., 2001). Clathrin cages were prepared by first concentrating aliquots of the clathrin pool from the Sepharose CL-4B column by precipitation with 50% ammonium sulfate followed by dialysis overnight against 100 mM MES–OH pH 6.2, 0.5 mM magnesium chloride, 0.05% sodium azide and 3 mM calcium chloride. Assembled cages were collected by centrifugation at 42 000 r.p.m. in a TLA-55 rotor for 20 min at 4°C and resuspended gently in MES buffer on ice before use.
Binding assays
Pull-down-type assays, in 300 µl total volume containing 25 mM HEPES–KOH pH 7.2, 125 mM potassium acetate, 5 mM magnesium acetate, 2 mM EDTA, 2 mM EGTA, 1 mM DTT (assay buffer), were as described previously (Drake et al., 2000; Drake and Traub, 2001). PTB domain binding to liposomes was performed using either 10% cholesterol, 45% PtdEth and 45% PtdCho, or 10% cholesterol, 30% PtdEth, 30% PtdCho and 30% phosphoinositides (Sigma), or 10% cholesterol, 35% PtdEth, 35% PtdCho 10% PtdSer and 10% PtdIns(4,5)P2 prepared as described previously (Zhu et al., 1999), at a final concentration of 0.4 mg/ml. Proteins were added in assay buffer with 0.1 mg/ml BSA as carrier and, after incubation at 4°C for 60 min, the tubes centrifuged at 20 000 gmax for 15 min at 4°C. Aliquots of each supernatant and pellet were analyzed by SDS–PAGE and immunoblotting where indicated. The two-stage liposome-binding assays were performed similarly, as described previously (Mishra et al., 2001). Data quantification was with NIH Image 1.62.
The specificity of the Dab2 PTB domain was assayed using nitrocellulose-immobilized phospholipids (PIP-array; Echelon Research Laboratories). After blocking the nitrocelluose with 3% BSA in 10 mM Tris–HCl pH 7.5, 150 mM sodium chloride, 1 mM EDTA and 0.05% Tween-20, 1 µg/ml GST–Dab2(1–205) was added and, following a 30 min incubation at 25°C, 1 µg/ml GSH-derivatized horseradish peroxidase (HRP; Sigma) was added and the incubation continued for an additional 60 min. After thorough washing, the bound GST fusion was visualized by enhanced chemiluminescence. The monolayer binding assay upon a lipid mixture of 10% PtdIns(4,5)P2, 10% PtdSer, 10% cholesterol, 35% PtdEth and 35% PtdCho was performed as described previously (Ford et al., 2001).
Immunofluorescence analysis
HeLa cells were cultured in DMEM supplemented with 10% fetal calf serum and 2 mM l-glutamine. Cells on glass coverslips were placed into assay buffer with 0.03% saponin at 25°C for 1 min before fixation in 3.7% formaldehyde in PBS. After permeabilization in 0.2% saponin, 10% normal goat serum in PBS, cells were incubated for 2–3 h at 25°C with anti-Dab2 and anti-epsin antibodies, followed by appropriate secondary reagents. Transfection of COS-7 cells with DEAE–dextran was as described previously (Mishra et al., 2001), but with incubation in DMEM supplemented with 8% lipoprotein-deficient serum and 2 mM l-glutamine for ∼40 h before LDL and transferrin uptake assays. After fixation, cells were permeablized in 0.05% saponin, 10% normal goat serum in PBS.
Acknowledgments
Acknowledgements
We are very grateful to Shelli Morris and Jonathon Cooper for generously providing us with the GST–Dab2 fusion plasmids, to Ken Dunn for generously providing the diI-LDL, to Donna Stolz for expert guidance with the EM work, to Barbara Pearse for assistance with the monolayer technique, and to Juan Bonifacino, Goujun Bu and Stuart Kornfeld for thoughtful comments on the manuscript. We also thank our many colleagues for kindly providing important antibodies. This work was supported in part by NIH grant R01 DK53249 (L.M.T.).
References
- Ajioka R.S. and Kaplan,J. (1987) Characterization of endocytic compartments using the horseradish peroxidase–diaminobenzidine density shift technique. J. Cell Biol., 104, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneson L.S., Kunz,J., Anderson,R.A. and Traub,L.M. (1999) Coupled inositide phosphorylation and phospholipase D activation initiates clathrin-coat assembly on lysosomes. J. Biol. Chem., 274, 17794–17805. [DOI] [PubMed] [Google Scholar]
- Bonifacino J.S. and Dell’Angelica,E.C. (1999) Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol., 145, 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett T.J., Traub,L.M. and Fremont,D.H. (2002) Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure, 10, 797–809. [DOI] [PubMed] [Google Scholar]
- Buss F., Arden,S.D., Lindsay,M., Luzio,J.P. and Kendrick-Jones,J. (2001) Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J., 20, 3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Fre,S., Slepnev,V.I., Capua,M.R., Takei,K., Butler,M.H., Di Fiore,P.P. and De Camilli,P. (1998) Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature, 394, 793–797. [DOI] [PubMed] [Google Scholar]
- Chen W.J., Goldstein,J.L. and Brown,M.S. (1990) NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem., 265, 3116–3123. [PubMed] [Google Scholar]
- Cooper J.A. and Howell,B.W. (1999) Lipoprotein receptors: signaling functions in the brain? Cell, 97, 671–674. [DOI] [PubMed] [Google Scholar]
- Cupers P., Jadhav,A.P. and Kirchhausen,T. (1998) Assembly of clathrin coats disrupts the association between Eps15 and AP-2 adaptors. J. Biol. Chem., 273, 1847–1850. [DOI] [PubMed] [Google Scholar]
- deBeer T., Hoofnagle,A.N., Enmon,J.L., Bowers,R.C., Yamabhai,M., Kay,B.K. and Overduin,M. (2000) Molecular mechanism of NPF recognition by EH domains. Nat. Struct. Biol., 7, 1018–1022. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica E.C. (2001) Clathrin-binding proteins: got a motif? Join the network! Trends Cell Biol., 11, 315–318. [DOI] [PubMed] [Google Scholar]
- Dho S.E., French,M.B., Woods,S.A. and McGlade,C.J. (1999) Characterization of four mammalian numb protein isoforms. Identification of cytoplasmic and membrane-associated variants of the phosphotyrosine binding domain. J. Biol. Chem., 274, 33097–33104. [DOI] [PubMed] [Google Scholar]
- Drake M.T. and Traub,L.M. (2001) Interaction of two structurally-distinct sequence types with the clathrin terminal domain β-propeller. J. Biol. Chem., 276, 28700–28709. [DOI] [PubMed] [Google Scholar]
- Drake M.T., Downs,M.A. and Traub,L.M. (2000) Epsin binds to clathrin by associating directly with the clathrin-terminal domain: evidence for cooperative binding through two discrete sites. J. Biol. Chem., 275, 6479–6489. [DOI] [PubMed] [Google Scholar]
- Ford M.G., Pearse,B.M., Higgins,M.K., Vallis,Y., Owen,D.J., Gibson,A., Hopkins,C.R., Evans,P.R. and McMahon,H.T. (2001) Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science, 291, 1051–1055. [DOI] [PubMed] [Google Scholar]
- Gaidarov I. and Keen,J.H. (1999) Phosphoinositide–AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J. Cell Biol., 146, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C.K. et al. (2001) Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science, 292, 1394–1398. [DOI] [PubMed] [Google Scholar]
- Gillooly D.J., Morrow,I.C., Lindsay,M., Gould,R., Bryant,N.J., Gaullier,J.M., Parton,R.G. and Stenmark,H. (2000) Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J., 19, 4577–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.L. and Brown,M.S. (2001) Molecular medicine. The cholesterol quartet. Science, 292, 1310–1312. [DOI] [PubMed] [Google Scholar]
- Goodman O.B. Jr, Krupnick,J.G., Santini,F., Gurevich,V.V., Penn,R.B., Gagnon,A.W., Keen,J.H. and Benovic,J.L. (1996) β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature, 383, 447–450. [DOI] [PubMed] [Google Scholar]
- Gotthardt M., Trommsdorff,M., Nevitt,M.F., Shelton,J., Richardson,J.A., Stockinger,W., Nimpf,J. and Herz,J. (2000) Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem., 275, 25616–25624. [DOI] [PubMed] [Google Scholar]
- Herz J. (2001) The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron, 29, 571–581. [DOI] [PubMed] [Google Scholar]
- Hocevar B.A., Smine,A., Xu,X.X. and Howe,P.H. (2001) The adaptor molecule Disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J., 20, 2789–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayouni R., Rice,D.S., Sheldon,M. and Curran,T. (1999) Disabled-1 binds to the cytoplasmic domain of amyloid precursor-like protein 1. J. Neurosci., 19, 7507–7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.W., Lanier,L.M., Frank,R., Gertler,F.B. and Cooper,J.A. (1999) The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell. Biol., 19, 5179–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A., Sato,O., Homma,K. and Ikebe,M. (2002) DOC-2/DAB2 is the binding partner of myosin VI. Biochem. Biophys. Res. Commun., 292, 300–307. [DOI] [PubMed] [Google Scholar]
- Itoh T., Koshiba,S., Kigawa,T., Kikuchi,A., Yokoyama,S. and Takenawa,T. (2001) Role of the ENTH domain in phosphatidyl inositol-4,5-bisphosphate binding and endocytosis. Science, 291, 1047–1051. [DOI] [PubMed] [Google Scholar]
- Kalthoff C., Alves,J., Urbanke,C., Knorr,R. and Ungewickell,E.J. (2002) Unusual structural organization of the endocytic proteins AP180 and epsin 1. J. Biol. Chem., 277, 8209–8216. [DOI] [PubMed] [Google Scholar]
- Laporte S.A., Oakley,R.H., Zhang,J., Holt,J.A., Ferguson,S.S., Caron,M.G. and Barak,L.S. (1999) The β2-adrenergic receptor/β-arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl Acad. Sci. USA, 96, 3712–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte S.A., Oakley,R.H., Holt,J.A., Barak,L.S. and Caron,M.G. (2000) The interaction of β-arrestin with the AP-2 adaptor is required for the clustering of β2-adrenergic receptor into clathrin-coated pits. J. Biol. Chem., 275, 23120–23126. [DOI] [PubMed] [Google Scholar]
- Lee P.S., Wang,Y., Dominguez,M.G., Yeung,Y.G., Murphy,M.A., Bowtell,D.D. and Stanley,E.R. (1999) The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis and attenuates macrophage proliferation. EMBO J., 18, 3616–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leheste J.R. et al. (1999) Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol., 155, 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Lu,W., Marzolo,M.P. and Bu,G. (2001) Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J. Biol. Chem., 276, 18000–18006. [DOI] [PubMed] [Google Scholar]
- Margolis B. (1996) The PI/PTB domain: a new protein interaction domain involved in growth factor receptor signaling. J. Lab. Clin. Med., 128, 235–241. [DOI] [PubMed] [Google Scholar]
- Martina J.A., Bonangelino,C.J., Aguilar,R.C. and Bonifacino,J.S. (2001) Stonin 2: an adaptor-like protein that interacts with components of the endocytic machinery. J. Cell Biol., 153, 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.E. and Lefkowitz,R.J. (2001) Expanding roles for β-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr. Opin. Cell Biol., 13, 139–145. [DOI] [PubMed] [Google Scholar]
- Mishra S.K., Agostinelli,N.R., Brett,T.J., Mizukami,I., Ross,T.S. and Traub,L.M. (2001) Clathrin- and AP-2-binding sites in HIP1 uncover a general assembly role for endocytic accessory proteins. J. Biol. Chem., 276, 46230–46236. [DOI] [PubMed] [Google Scholar]
- Morris S.M. and Cooper,J.A. (2001) Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic, 2, 111–123. [DOI] [PubMed] [Google Scholar]
- Morris S.M., Arden,S.D., Roberts,R.C., Kendrick-Jones,J., Cooper,J.A., Luzio,J.P. and Buss,F. (2002a) Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic, 3, 331–341. [DOI] [PubMed] [Google Scholar]
- Morris S.M., Tallquist,M.D., Rock,C.O. and Cooper,J.A. (2002b) Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J., 21, 1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterov A., Carter,R.E., Sorkina,T., Gill,G.N. and Sorkin,A. (1999) Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant µ2 subunit and its effects on endocytosis. EMBO J., 18, 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M.L. et al. (1999) UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol. Biol. Cell, 10, 2343–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman D., Sun,X.M., Bourbon,M., Knight,B.L., Naoumova,R.P. and Soutar,A.K. (1999) Characterization of a novel cellular defect in patients with phenotypic homozygous familial hypercholesterolemia. J. Clin. Invest., 104, 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H.J.S., Fournier,M.-C., Bosshart,H., Rhee,I., Miyatake,S., Saito,T., Galluser,A., Kirchhausen,T. and Bonifacino,J.S. (1995) Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science, 269, 1872–1875. [DOI] [PubMed] [Google Scholar]
- Oleinikov A.V., Zhao,J. and Makker,S.P. (2000) Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem. J., 347 Pt 3, 613–621. [PMC free article] [PubMed] [Google Scholar]
- Owen D.J. and Evans,P.R. (1998) A structural explanation for the recognition of tyrosine-based endocytotic signals. Science, 282, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter S.G., Mitchell,H., Garamszegi,N., Edens,M., Dore,J.J.,Jr and Leof,E.B. (2002) Internalization-dependent and -independent requirements for transforming growth factor β receptor signaling via the Smad pathway. Mol. Cell. Biol., 22, 4750–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S., Sigismund,S., Faretta,M., Guidi,M., Capua,M.R., Bossi,G., Chen,H., De Camilli,P. and Di Fiore,P.P. (2002) A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature, 416, 451–455. [DOI] [PubMed] [Google Scholar]
- Ramjaun A.R. and McPherson,P.S. (1998) Multiple amphiphysin II splice variants display differential clathrin binding: identification of two distinct clathrin-binding sites. J. Neurochem., 70, 2369–2376. [DOI] [PubMed] [Google Scholar]
- Ravichandran K.S., Zhou,M.M., Pratt,J.C., Harlan,J.E., Walk,S.F., Fesik,S.W. and Burakoff,S.J. (1997) Evidence for a requirement for both phospholipid and phosphotyrosine binding via the Shc phosphotyrosine-binding domain in vivo. Mol. Cell. Biol., 17, 5540–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F., Gaidarov,I. and Keen,J.H. (2002) G protein-coupled receptor/arrestin3 modulation of the endocytic machinery. J. Cell Biol., 156, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolini E., Salcini,A.E., Kay,B.K., Yamabhai,M. and Di Fiore,P.P. (1999) The EH network. Exp. Cell Res., 253, 186–209. [DOI] [PubMed] [Google Scholar]
- Santolini E., Puri,C., Salcini,A.E., Gagliani,M.C., Pelicci,P.G., Tacchetti,C. and Di Fiore,P.P. (2000) Numb is an endocytic protein. J. Cell Biol., 151, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M.G., Benmerah,A., Muntaner,O. and Marullo,S. (2002) Recruitment of activated G protein-coupled receptors to pre-existing clathrin-coated pits in living cells. J. Biol. Chem., 277, 3552–3559. [DOI] [PubMed] [Google Scholar]
- Sheng Z., Sun,W., Smith,E., Cohen,C. and Xu,X.X. (2000) Restoration of positioning control following Disabled-2 expression in ovarian and breast tumor cells. Oncogene, 19, 4847–4854. [DOI] [PubMed] [Google Scholar]
- Slepnev V.I., Ochoa,G.C., Butler,M.H. and De Camilli,P. (2000) Tandem arrangement of the clathrin and AP-2 binding domains in amphiphysin 1 and disruption of clathrin coat function mediated by amphiphysin fragments comprising these sites. J. Biol. Chem., 275, 17583–17589. [DOI] [PubMed] [Google Scholar]
- Su H.P., Nakada-Tsukui,K., Tosello-Trampont,A.C., Li,Y., Bu,G., Henson,P.M. and Ravichandran,K.S. (2002) Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells, with CED-1 and CD91/LRP. J. Biol. Chem., 277, 11772–11779. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Matsuda,M., Yamamoto,T., Kanematsu,T., Kikkawa,U., Yagisawa,H., Watanabe,Y. and Hirata,M. (1998) PTB domain of insulin receptor substrate-1 binds inositol compounds. Biochem. J., 334, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M., Borg,J.P., Margolis,B. and Herz,J. (1998) Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem., 273, 33556–33560. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M., Gotthardt,M., Hiesberger,T., Shelton,J., Stockinger,W., Nimpf,J., Hammer,R.E., Richardson,J.A. and Herz,J. (1999) Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell, 97, 689–701. [DOI] [PubMed] [Google Scholar]
- Velier J., Kim,M., Schwarz,C., Kim,T.W., Sapp,E., Chase,K., Aronin,N. and DiFiglia,M. (1998) Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Exp. Neurol., 152, 34–40. [DOI] [PubMed] [Google Scholar]
- Wanker E.E., Rovira,C., Scherzinger,E., Hasenbank,R., Walter,S., Tait,D., Colicelli,J. and Lehrach,H. (1997) HIP-I: a huntingtin interacting protein isolated by the yeast two-hybrid system. Hum. Mol. Genet., 6, 487–495. [DOI] [PubMed] [Google Scholar]
- Warren R.A., Green,F.A., Stenberg,P.E. and Enns,C.A. (1998) Distinct saturable pathways for the endocytosis of different tyrosine motifs. J. Biol. Chem., 273, 17056–17063. [DOI] [PubMed] [Google Scholar]
- Xu X.X., Yang,W., Jackowski,S. and Rock,C.O. (1995) Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J. Biol. Chem., 270, 14184–14191. [DOI] [PubMed] [Google Scholar]
- Yamabhai M., Hoffman,N.G., Hardison,N.L., McPherson,P.S., Castagnoli,L., Cesareni,G. and Kay,B.K. (1998) Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J. Biol. Chem., 273, 31401–31407. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Drake,M.T. and Kornfeld,S. (1999) ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc. Natl Acad. Sci. USA, 96, 5013–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]