Abstract
Drosophila Sprouty (dSpry) was genetically identified as a novel antagonist of fibroblast growth factor receptor (FGFR), epidermal growth factor receptor (EGFR) and Sevenless signalling, ostensibly by eliciting its response on the Ras/MAPK pathway. Four mammalian sprouty genes have been cloned, which appear to play an inhibitory role mainly in FGF- mediated lung and limb morphogenesis. Evidence is presented herein that describes the functional implications of the direct association between human Sprouty2 (hSpry2) and c-Cbl, and its impact on the cellular localization and signalling capacity of EGFR. Contrary to the consensus view that Spry2 is a general inhibitor of receptor tyrosine kinase signalling, hSpry2 was shown to abrogate EGFR ubiquitylation and endocytosis, and sustain EGF-induced ERK signalling that culminates in differentiation of PC12 cells. Correlative evidence showed the failure of hSpry2ΔN11 and mSpry4, both deficient in c-Cbl binding, to instigate these effects. hSpry2 interacts specifically with the c-Cbl RING finger domain and displaces UbcH7 from its binding site on the E3 ligase. We conclude that hSpry2 potentiates EGFR signalling by specifically intercepting c-Cbl-mediated effects on receptor down-regulation.
Keywords: c-Cbl/EGFR/RING/sprouty/ubiquitylation
Introduction
Growth factor-induced signalling by receptor tyrosine kinases (RTKs) plays a central role in: embryonic development, signalling in mature cells and regulation of pathogenesis. Various regulatory proteins and feedback mechanisms tightly control these key pathways. Drosophila Sprouty (dSpry) was originally identified as an antagonist of Drosophila development-associated RTK signalling. In dSpry mutants, signalling via the fibroblast growth factor receptor (FGFR) in lung development is deregulated and excessive tracheal branching is observed (Hacohen et al., 1998). dSpry is also expressed in the developing eye and other tissues under the control of epidermal growth factor receptor (EGFR), where it acts to attenuate downstream signalling. Casci et al. (1999) identified dSpry to be an intracellular protein that interacts in vitro with Drk (Drosophila homologue of Grb2) and Gap-1, a Ras GTPase-activating protein, to potentially inhibit Ras signalling. Currently, four mammalian genes have been identified that encode protein homologues of dSpry (de Maximy et al., 1999; Minowada et al., 1999); mSpry2 is ubiquitously expressed in adult tissues such as the brain, kidney, lung, heart and skeletal muscle (Tefft et al., 1999). The various Spry isoforms have variable N-terminal sequences but share considerable cysteine-rich sequence homology in their C-termini. This shared domain appears to play a role in protein targeting to foci in the membrane periphery (ruffles) when cells are stimulated by epidermal growth factor (EGF) or fibroblast growth factor (FGF) (Lim et al., 2000). The relatively poorly conserved N-terminal halves of the proteins are assumed to interact with other effectors to manifest the putative inhibitory role of Spry. The conserved function of dSpry and mammalian Sprys in the negative regulation of growth factor-induced signalling has been documented (Kramer et al., 1999; Reich et al., 1999). Based on genetic evidence, several molecular mechanisms by which Spry proteins might inhibit RTK signalling have been postulated; dSpry acts to inhibit FGF signalling at a point between the activated receptor and Ras (Casci et al., 1999), or at the level of Raf (Reich et al., 1999). Additionally, evidence has been presented that indicates that mSpry4 regulates angiogenesis by inhibition of the FGF pathway upstream of Ras (Lee et al., 2001), and mSpry2 functions as a negative regulator of embryonic lung morphogenesis and development (Mailleux et al., 2001).
The mitogen-activated protein kinase (MAPK) cascade is a central intracellular signalling pathway linking activation of surface receptors to cytoplasmic and nuclear effectors by transducing signals from RTKs to two members of the Ras family of small G-proteins, Ras and Rap1 (Marshall, 1998). Ras interacts directly with, and sequentially activates Raf serine/threonine kinases (c-Raf and B-Raf); phosphorylated Raf then activates MEK, which in turn phosphorylates and activates the MAPKs (ERK1 and ERK2). ERK1/2 have been shown to be essential for cellular proliferation, as well as phenotypic determination (Marshall, 1995). One of the best-studied model systems employed to examine MAPK responses in determining cellular phenotype is the proliferation/differentiation-responsive rat pheochromocytoma (PC12) cell line. These cells respond to EGF treatment by increasing proliferation. In contrast, FGF and nerve growth factor (NGF) stimulation of PC12 cells results in their differentiation into a neuron-like phenotype (Kao et al., 2001). There is well-supported evidence that the amplitude and longevity of MAPK signal governs whether these cells are stimulated to proliferate, or to withdraw from the cell cycle and differentiate. EGF and other mitogens are reported to invoke transient activation of the MAPKs, whereas FGF and NGF treatment results in sustained activation of this signalling pathway.
The ErbB family of RTKs exemplifies the importance of stringent regulation on signalling potency and its impact on human cancers (Yarden and Sliwkowski, 2001). Of the four ErbB family members, ErbB-1 (or EGFR) has the unique property of binding to the negative regulator c-Cbl, and is effectively targeted for lysosomal and proteasomal degradation (Levkowitz et al., 1998). Current concepts regarding c-Cbl function indicate that it is a complex adaptor protein whose phosphorylation leads to formation of activation-dependent complexes (Thien and Langdon, 2001). Despite an incomplete knowledge of its mechanism of action, it is apparent that c-Cbl promotes polyubiquitylation and hence degradation (or ‘down-regulation’) of certain ligand-activated RTKs (e.g. PDGFRα; Miyake et al., 1998), of which extensive studies have been made with EGFR (Levkowitz et al., 1998). The ubiquitin system defines the recruitment of ubiquitin-conjugating enzymes (E2) to specific proteins recognized for degradation through an E3 ubiquitin ligase (Hershko and Ciechanover, 1998). c-Cbl functions as an E3 that docks on to phosphotyrosine sites on activated substrates (via its unique SH2 domain), bringing them into close proximity with an E2 enzyme (bound via its intrinsic RING finger domain) for the relay of activated ubiquitin moieties to target proteins, thereby marking them for later lysosomal/proteasomal destruction and consequent signal attenuation (Joazeiro et al., 1999). The effects of c-Cbl-mediated ubiquitylation on EGFR endocytosis are currently enigmatic. Some groups have demonstrated that c-Cbl-mediated EGFR ubiquitylation does not enhance the endocytic rate of receptors, but expression of inactive RING finger mutants (C381A and ΔRF) inhibits receptor ubiquitylation and subsequent endocytosis (Levkowitz et al., 1998; Waterman et al., 1999). Furthermore, despite possessing intact SH2 domains, two known oncogenic mutants of c-Cbl are both incapable of facilitating ubiquitylation due to their failure to recruit the ubiquitin system to activated RTKs, since they either lack all (v-Cbl) or part (70Z-Cbl) of the RING finger (Levkowitz et al., 1999).
The objective of this study was to investigate the functional effect of hSpry2 on the regulation of MAPK by EGF in relation to the disposition of the ligand-bound receptors, as well as to address the implications of hSpry2-c-Cbl association in mediating this outcome.
Results
hSpry2 prevents internalization of EGFRs into endocytic vesicles
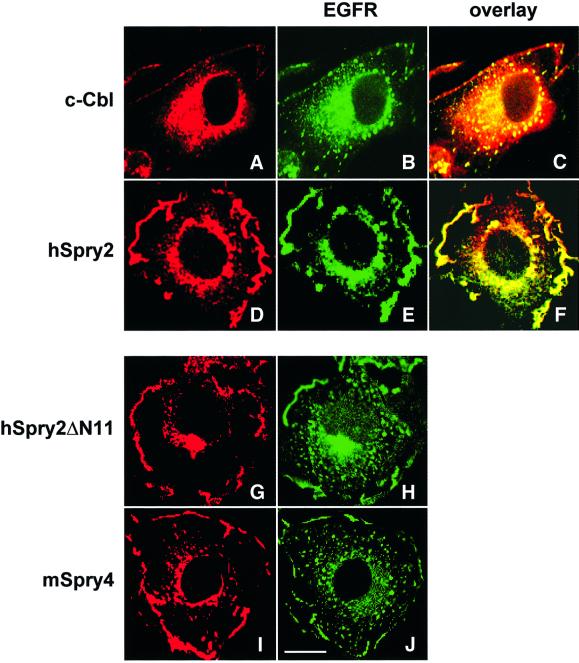
Previously we have demonstrated that a sequence spanning amino acids 11–53 at the N-terminal end of hSpry2 interacts directly with the RING finger domain of c-Cbl (Wong et al., 2001). A preliminary experiment indicated that a corollary of this interaction was increased numbers of EGFRs being retained at the cell surface. Prior to assessing the likely downstream effects of such an event, we sought to apply alternative and more informative techniques to ascertain the extent and nature of EGFR surface retention. Confocal microscopy was employed to observe visually the relationship between EGFR, c-Cbl and isoforms of Spry. We performed a time-dependent (0, 2, 5, 10, 20 and 30 min EGF stimulation) observation on the subcellular localization of EGFRs, in the presence or absence of overexpressed hSpry2 (10 min time-points shown in Figure 1). In unstimulated COS-1 cells, both c-Cbl and EGFR display diffused, cytosolic disposition across the whole cell interior except for the nuclei, with peripheral lining of EGFR at the plasma membrane; the staining pattern of EGFR was unchanged in resting cells transfected with hSpry2 (data not shown). In EGF-stimulated control cells, c-Cbl staining shows a punctate pattern indicative of its incorporation into endocytic vesicles (Figure 1A and C), consistent with the observation that ligand stimulation induces a rapid subcellular relocalization of c-Cbl (Levkowitz et al., 1998). In those same cells, EGFRs can also be observed within endocytic vesicles, as identified by staining with antibodies against EEA1, an established vesicle marker protein (Mu et al., 1995; data not shown), with increased numbers on the cell periphery (Figure 1B and C). With transiently transfected hSpry2, which has been established to translocate to membrane ruffles upon EGF stimulation (Figure 1D), there appears to be a near total inhibition of EGFR endocytosis in that the receptors were mainly retained at the membrane surface and were not apparent in endocytic vesicles throughout the experimental time course of EGF stimulation (10 min time-point shown, Figure 1E and F). Individual N- and C-terminal domains of hSpry2 were found to be incapable of inhibiting receptor endocytosis (data not shown). Hence, the ability of hSpry2 to bind the RING finger of c-Cbl is clearly insufficient to function autonomously as an inhibitor of EGF-induced endocytosis. Seemingly, specific intracellular localization provided by the putative translocation domain in the C-terminal half of hSpry2 (Lim et al., 2000) is necessary for its optimal function. Thus, it would appear that a full-length protein that can bind c-Cbl as well as be targeted to specific membrane locations (ruffles) is essential for withholding the majority of EGFRs at the cell surface and preventing their subsequent transition into endocytic vesicles. We further examined the effects of hSpry2ΔN11 and mSpry4, neither of which binds c-Cbl, on the fate of EGFR localization. In both cases, receptor internalization was not blocked upon EGF stimulation and EGFRs were apparent in punctate endosomal structures, with traceable surface receptor staining (Figure 1G–J).
Fig. 1. hSpry2 specifically inhibits EGFR endocytosis. COS-1 cells were singly transfected with 1 µg each of HA-tagged c-Cbl alone or FLAG-tagged Sprys, and subjected to serum-deprivation overnight prior to stimulation with 100 ng/ml EGF at 37°C for 10 min. Cells were then fixed, permeabilized and stained for endogenous EGFR using an anti-EGFR monoclonal and FITC-conjugated AffiniPure rabbit anti-mouse IgG (green) secondary antibody. c-Cbl was visualized with an anti-c-Cbl polyclonal, and FLAG-tagged Spry constructs were detected using a polyclonal anti-FLAG, followed by Texas Red dye-conjugated AffiniPure goat anti-rabbit IgG (red). Merged signals are labelled as overlay staining (yellow). Bar = 10 µm.
Increased cell surface retention of EGFRs with hSpry2 expression
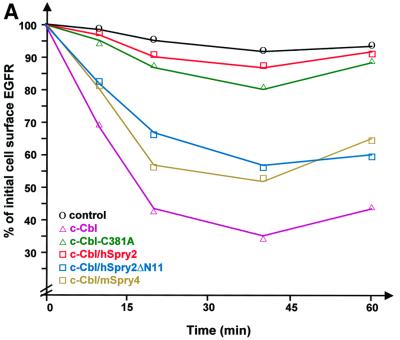
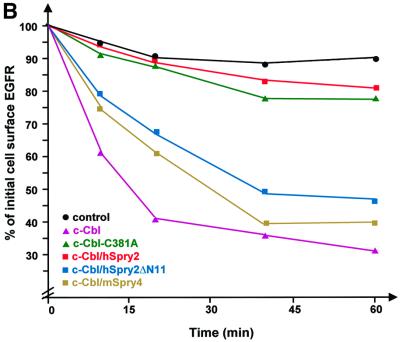
The process of receptor endocytosis has many phases and it was of interest to investigate at which stage EGFR endocytosis was inhibited by hSpry2 overexpression: on the cell surface or at different stages of incorporation into vesicles or internalization of nascent vesicles. We therefore employed an analytical method that measures only cell surface EGFRs following ligand-mediated endocytosis. Initial down-regulation of EGFRs was induced using an unlabelled ligand and status of the remaining surface-associated binding sites was then determined by performing a direct radiolabelled EGF–receptor binding assay. From the graphical representation in Figure 2A, the statistics of external EGFRs in unstimulated cells from various transfection combinations were comparable. However, the patterns of receptor down-regulation exhibited by EGF-treated transfectants were different: whereas c-Cbl accelerates the degree of EGFR down-regulation, the ubquitylation-defective mutant c-Cbl-C381A was unable to mediate such an effect and high levels of EGFRs were retained at the cell surface. A similar phenomenon was noted in hSpry2-transfected cells, but not with the c-Cbl non-binding (hSpry2ΔN11 or mSpry4) transfectants, where the surface EGFR levels were observed to decrease normally, although their effects were not as complete as that exhibited by wild-type c-Cbl.
Fig. 2. hSpry2 enhances cell surface retention of EGFRs. (A) To quantify the surface EGFR population, COS-1 cells were co-transfected with 0.1 µg of an EGFR expression vector, together with 0.5 µg plasmid encoding c-Cbl alone (pink triangles) or with either 0.4 µg FLAG–hSpry2 (red squares), FLAG–hSpry2ΔN11 (blue squares) or FLAG–mSpry4 (yellow squares) cDNA, or with 1.0 µg plasmid encoding the RING finger-defective form of c-Cbl (C381A) alone (green triangles). Forty-eight hours post-transfection, serum-starved culture cells were subjected to stimulation without or with 100 ng/ml EGF at 37°C for the time periods indicated. Bound EGF was removed, and the level of surface receptors relative to the initial number of ligand-binding sites was determined by incubating sister cultures with [125I]EGF (10 ng/ml) at 4°C for 2–4 h, in the absence or presence of a 100-fold excess of unlabelled EGF (1 µg/ml). Control cells were not exposed to EGF (circles). The results are expressed as the average fraction of original binding sites that remained on the cell surface after exposure to the unlabelled ligand at 37°C. The graphical plot shown is derived from a single experiment that is representative of four independent experiments. (B) Similar transfections (closed symbols) and treatment as in (A), except that cells were pre-incubated without or with 100 ng/ml EGF in the presence of 30 µM monensin at 4°C for 2 h before a temperature shift to 37°C for the various time periods. The residual level of surface-bound receptors that did not undergo down-regulation was then assayed by performing a direct binding assay with radiolabelled EGF. The average of duplicate determinations was expressed as the percentage of total radioactivity at time = 0 min. The experiment was repeated twice.
Following an initial phase of receptor down-regulation, the reappearance of EGFRs was observed, presumably due to recycling of endocytosed receptors (see Figure 2A, 40–60 min time-points); that reappearance was completely inhibited by monensin (Figure 2B), a chemical ionophore known to inhibit recycling of transmembrane receptors (Basu et al., 1981) including EGFRs (Gladhaug and Chrisofferson, 1988). Despite monensin treatment, overexpression of hSpry2 effects a high retention of EGFRs at the cell surface (Figure 2B). These results indicate that hSpry2 inhibits EGFR endocytosis at a relatively early stage of the relocation/internalization process, but exerts no apparent effect on the fate of recycling receptors. 4β-phorbol 12-myristate 13-acetate (PMA) has been established previously to divert internalized EGFR molecules from a degradative fate to a recycling pathway (Bao et al., 2000). The dose of inhibitor administered was shown to be effective, where cells exposed to PMA in concert with monensin show significantly reduced levels of surface EGFRs compared with cells without monensin treatment (data not shown).
EGFR ubiquitylation is abrogated in cells expressing hSpry2
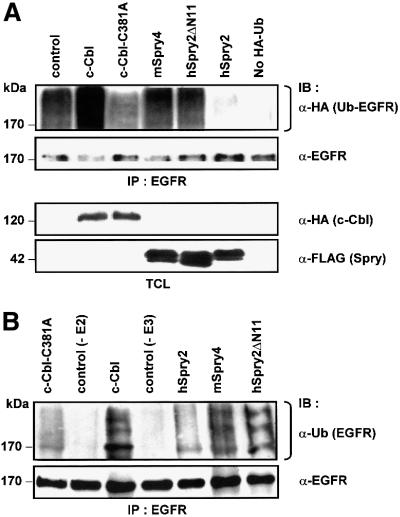
Currently only a few receptor internalization signals have been described. Of these, ubiquitylation has been demonstrated to be a principal trigger for internalization of receptors in yeast cells (Hicke, 1997). Additionally, various effects have been ascribed to mono- and polyubiquitylation of receptors. The endocytosis of EGFRs has been comparatively well studied and c-Cbl, in its role as an E3 ubiquitin ligase, plays a critical role in this process. Since hSpry2 binds directly to the functional RING finger domain on c-Cbl that is necessary for the catalytic transfer of ubiquitin to substrate, it is plausible that hSpry2 affects the ubiquitylation status of EGFRs. We therefore examined whether regulation of the uptake of EGFRs into endosomes correlates with the degree of EGFR ubiquitylation. Ubiquitin cDNA was transfected into COS-1 cells in combination with either vector control, c-Cbl, c-Cbl-C381A mutant (which possesses a crucial disruption in the first cysteine residue of its RING finger and is thus incapable of ubiquitin ligase activity; Waterman et al., 1999) and various Sprys. Cell lysates were precipitated with anti-EGFR and analysed for the extent of ubiquitylation on EGFRs as well as equal expression of the transfected proteins (Figure 3A). Little or no ubiquitylation is detected in resting cells, and the immunoprecipitated EGFR amounts are comparable (data not shown). In the case of EGF-stimulated cells, EGFR ubiquitylation in vector-transfected control cells reflects an amount of ‘background ubiquitylation’ most likely due to endogenous c-Cbl. Overexpressed c-Cbl causes a substantial increase in the amount of ubiquitylated EGFRs, whereas overexpression of c-Cbl-C381A with a defective ubiquitin transferase catalytic site results in significantly less amounts. The levels of EGFR ubiquityl ation in cells co-transfected with mSpry4 or hSpry2ΔN11 are comparable to those in the vector-transfected control, as expected. Only hSpry2 is able to effectively abolish the observed ubiquitylation to a level similar to that induced by the inactive c-Cbl mutant, commensurate with its ability to bind the functional RING finger effector domain of c-Cbl. In the presence of c-Cbl there is enhanced internalization and degradation of EGFRs concomitant with increased ubiquitylation, as indicated by a drop in total immunoprecipitated EGFRs. This is in contrast to the effect obtained with hSpry2, in which much higher levels of residual EGFRs are observed that are not being ubiquitylated.
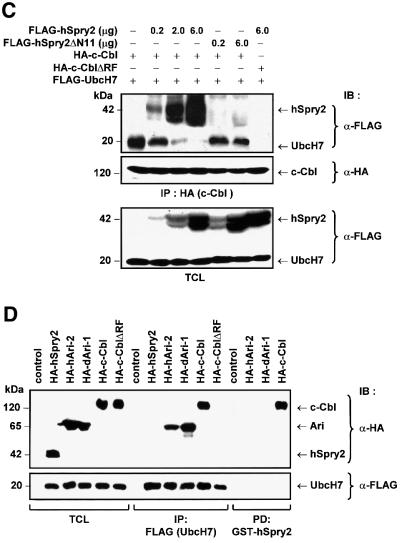
Fig. 3. hSpry2 abrogates c-Cbl-dependent ubiquitylation of EGFRs. (A) COS-1 cells were co-transfected with 1.0 µg HA-tagged ubiquitin and 3.0 µg each of either vector control, c-Cbl, c-Cbl-C381A or the different FLAG-tagged Spry forms per 100 mm culture dish. Forty-eight hours later, cell monolayers were treated with 100 ng/ml EGF at 37°C for 10 min. Total cell lysates (TCL) were subjected to immunoprecipitation (IP) using anti-EGFR–agarose-conjugated beads, immunoblotted (IB) with anti-HA to distinguish ubiquitin-conjugated EGFR, and anti-EGFR to assess the amounts of immunoprecipitated EGFR. TCL samples were analysed with anti-HA or anti-FLAG to show relative expression levels of the various constructs. The bracket indicates the position of high molecular weight species of ubiquitin-positive EGFRs. (B) Immunoprecipitated EGFR proteins were subjected to an in vitro ubiquitylation assay in the presence of purified UbcH7 (or no added UbcH7 as control without E2), eluted c-Cbl protein alone (or no added c-Cbl as control without E3), recombinant c-Cbl with either GST–hSpry2, GST–mSpry4 or GST–hSpry2ΔN11 fusion proteins, or c-Cbl-C381A fusion protein alone, plus the essential components in the ubiquitylation system. The reaction products were analysed following western blotting protocol with anti-ubiquitin. The bracket highlights the position of high molecular species of ubiquitin-positive EGFRs. (C) Competitive binding between hSpry2 and UbcH7 for the RING finger domain of c-Cbl. COS-1 cells (100 mm dishes) were co-transfected with 3.0 µg each of FLAG-tagged UbcH7, HA–c-Cbl or HA–c-Cbl-ΔRF mutant, and varying amounts of FLAG–hSpry2 or the c-Cbl non-binding truncation mutant FLAG–hSpry2ΔN11, as indicated. Serum-deprived cells were stimulated with 100 ng/ml EGF at 37°C for 10 min. TCL were subjected to precipitation using anti-HA and immunoblotted (IB) with anti-FLAG to detect associated hSpry2 or UbcH7, and anti-HA to show the relative amounts of immunoprecipitated c-Cbl. A TCL blot was probed with anti-FLAG to demonstrate quantitatively the expression levels of exogenous hSpry2 and UbcH7 proteins. (D) COS-1 cells (100 mm dishes) were untransfected (control), or co-transfected with 3.0 µg each of FLAG-tagged UbcH7 and either HA–hSpry2, HA–human Ariadne-2 (hARI-2), HA–Drosophila Ariadne-1 (dAri-1), HA–c-Cbl or HA–c-CblΔRF constructs. Immunocomplexes of FLAG–UbcH7 or ‘pull-downs’ using GST–hSpry2 were detected using anti-HA. Total cell lysates were analysed for equivalent levels of protein expression and normalized sample loading using antibodies as indicated. An immunoblot (with TCL loaded alongside) was probed with anti-HA to detect hSpry2-binding proteins, and with anti-FLAG to verify equal amounts of immunoprecipitated UbcH7.
To substantiate our observation that hSpry2 prevents c-Cbl-mediated ubiquitylation of EGFRs, an in vitro ubiquitylation assay was performed to demonstrate a defined impediment to c-Cbl activity as an E3 ubiquitin ligase. In this experiment, a ubiquitylation reaction was reconstituted wherein EGFRs serve as substrates of c-Cbl in an in vitro enzyme mixture containing the essential ubiquitin system components: ubiquitin, E1, E2 (UbcH7), E3 (c-Cbl) and ATP, with addition of eluted glutathione S-transferase (GST)–hSpry2, GST–mSpry4 or GST– hSpry2ΔN11 proteins. As a negative control, recombinant protein of c-Cbl-C381A ubiquitylation-defective mutant was added in place of wild-type c-Cbl. From Figure 3B, heavy polyubiquitylation of substrate EGFRs was ob served with c-Cbl alone, and in the presence of mSpry4 and hSpry2N11; but not with hSpry2, which shows only a faint background smear comparable to the negative control (c-Cbl-C381A).
hSpry2 competes with UbcH7 for specific binding to the c-Cbl RING finger
Ubiquitylation is a post-translation modification in which ubiquitin chains or single ubiquitin molecules are appended to target proteins, giving rise to poly- or mono- ubiquitylation, respectively (Laney and Hochstrasser, 1999; Hicke, 2001). There is currently no direct evidence that c-Cbl is involved in monoubiquitylation. From the data presented, it is conceivable that hSpry2, by binding to the RING finger of c-Cbl (or another unidentified ubiquitin E3 ligase), is competing against the binding of ubiquitin E2 proteins and hence preventing subsequent polyubiquitylation of substrate proteins by c-Cbl. To investigate likely competition between a prospective E2 and hSpry2, COS-1 cells were transfected with a constant amount of UbcH7 (human E2) and HA–c-Cbl, together with an increasing dosage of hSpry2 or the c-Cbl non-binding hSpry2ΔN11 as indicated. HA–c-CblΔRF (with RING finger domain deleted) was included as a negative binding control for hSpry2 and UbcH7. Analysis of anti-HA immunoprecipitates revealed that the binding of UbcH7 to the c-Cbl RING finger is competed off by increasing amounts of hSpry2 (Figure 3C). It thus appears that when present in sufficient concentrations, hSpry2 has the capacity to inhibit receptor ubiquitylation by hindering the interaction between the E2 and E3 ubiquitin transferases.
We also addressed whether the interaction between hSpry2 and the RING finger of c-Cbl is specific, as RING domains are well conserved in structure and have a commonality of function in the ubiquitylation process (Borden, 2000). Proteins other than c-Cbl, such as the Ariadne family of RING-containing proteins, recruit UbcH7 in a similar RING-dependent manner. HHAri, the human homologue of Drosophila Ariadne, has been reported to interact and co-localize with UbcH7 in mammalian cells via its RING finger domain (Ardley et al., 2001). Additionally, an interaction between the orthologues of HHAri and UbcH7 has been demonstrated in Drosophila (Aguilera et al., 2000). To investigate the specificity of hSpry2 binding and inhibitory effect on c-Cbl, we tested the abilities of dAri-1 and hAri-2 (a protein closely related to HHAri that can also interact with UbcH7) to substitute for c-Cbl binding to hSpry2. COS-1 cells were co-transfected with FLAG–UbcH7 and the various known binding or non-binding partners, as indicated in Figure 3D. Immunoprecipitated UbcH7 binds only to RING finger-containing proteins, indicating that the various constructs were functional in vivo. The binding specificity of hSpry2 to the RING finger-containing constructs was assessed in GST ‘pull-down’ assays (Figure 3D); hSpry2 interacts specifically with c-Cbl, but fails to bind either dAri-1 or hAri-2. Our findings are in accord with Ardley et al. (2001), who reported that the highly homologous UbcH7-interacting RING finger structures of HHAri and c-Cbl could not be functionally interchanged. The binding specificity of hSpry2 for the c-Cbl RING finger was further affirmed by its lack of in vitro and in vivo binding with Hakai, a recently identified c-Cbl-like E3 ubiquitin ligase (Fujita et al., 2002; data not shown).
Decreased EGFR ubiquitylation upon expressing hSpry2 correlates with sustained ERK phosphorylation
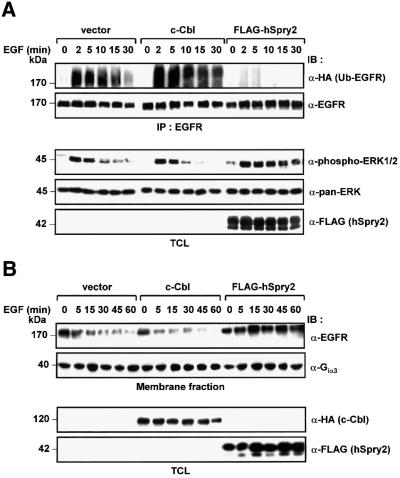
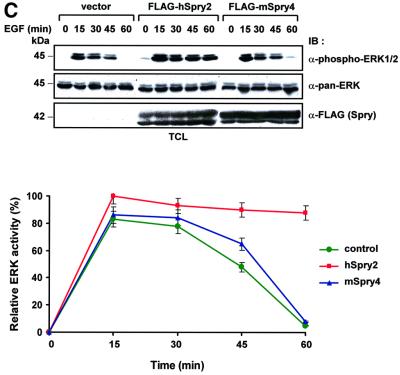
The well-characterized Ras/MAPK pathway is stimulated by a number of activated receptors including EGFRs (Gonzalez et al., 1991; Moghal and Sternberg, 1999). There has been considerable debate centred on whether receptor internalization occurs to propagate or terminate ERK signalling. In our experimental system we had a useful tool in which to address the correlation between receptor ubiquitylation and ERK activity, since c-Cbl instigates EGFR polyubiquitylation and internalization, while hSpry2 seems predominantly to inhibit this function. We and others have observed that ubiquitylation of EGFRs occurs rapidly and robustly within 2 min of EGF stimulation (Waterman and Yarden, 2001). A 30 min time course of EGF stimulation was carried out to monitor the corresponding ERK activation profile concurrent with the receptor ubiquitylation event. Ubiquitin and EGFR cDNAs were transfected into 293T cells in combination with vector control, c-Cbl or hSpry2. Cell lysates were subjected to immunoprecipitation with anti-EGFR and analysed for degrees of ubiquitylation (characterized by an up-smearing of receptor), phospho-ERK activities of normalized, endogenous ERK proteins and equal expression of hSpry2 (Figure 4A). Lysates containing vector control show a basal level of ubiquitylation due to endogenous c-Cbl. A significant increase in ubiquitylated EGFRs was observed upon c-Cbl overexpression, with the level gradually diminishing towards 30 min. Lysates from hSpry2-transfected cells exhibit profoundly diminished ubiquitylation of EGFRs throughout the 30 min period. In vector-transfected control cells, the phospho-ERK1/2 (p44/42) blot reflects a transient ERK activity that was detected at 2 min, and essentially diminishes from 10 min and thereafter towards basal levels. There appears to be a greater extent of decrease of phospho-ERK signals in c-Cbl-overexpressing cells compared with control cells. Significantly, as a consequence of hSpry2 transient expression, an enhanced and sustained activation of phospho-ERK was observed until at least 30 min (Figure 4A), a phenomenon distinct to the vector control or c-Cbl transfectants. Such an observation is in contrast to the postulated role of hSpry2 as a general RTK inhibitor (Kramer et al., 1999). To correlate the surface receptor levels with ubiquitylation, a time course experiment was performed in which cell membrane fractions were extracted and the surface EGFR levels were compared. From an EGFR immunoblot shown in Figure 4B, c-Cbl-transfected cells show enhanced EGFR disappearance (with correspondingly lower amounts of surface EGFRs) over time compared with vector control cells; while hSpry2 transfectants were shown to retain comparatively high receptor levels on the membrane across the same period of stimulation, in line with its blockade of ubiquitylation of EGFRs. To serve as a loading control, a parallel blot was probed with an antibody specific for the heterotrimeric G-protein Giα3 subunit, a membrane-bound protein was shown to be unaffected by EGF treatment. Furthermore, to verify that this effect on extended ERK signalling is indeed c-Cbl mediated, we tested hSpry2 against a c-Cbl non-binding isoform over a time course of EGF stimulation (Figure 4C). ERK activity is similarly up-regulated by hSpry2, with an elevated phospho-ERK signal detectable up to 60 min; whereas ERK activation (phosphorylation) pattern obtained with c-Cbl non-binding mSpry4 appears similar to the vector-transfected control, which diminishes in activity after 15 min of EGF stimulation. The resulting ERK activation levels from three independent sets of experiments were quantified and the mean values are presented graphically as shown.
Fig. 4. hSpry2 elicits sustained ERK activation in response to EGF induction. (A) 293T cells (60 mm dishes) were co-transfected with plasmids encoding EGFR (0.2 µg) and HA-tagged ubiquitin (0.2 µg), together with an empty vector control, HA–c-Cbl (1.0 µg) or FLAG–hSpry2 (0.8 µg). Forty-eight hours post-transfection, cell monolayers were incubated with 100 ng/ml EGF at 37°C for the indicated time periods. Total cell lysates (TCL) were subjected to precipitation using anti-EGFR–agarose-conjugated beads, and receptor ubiquitylation was detected with an anti-HA probe against HA–Ub-conjugated EGFR. An anti-EGFR blot demonstrates equal amounts of immunoprecipitated EGFR. Total cell extracts were immunoblotted (IB) with anti-phospho-ERK1/2 (p44/42) to detect corresponding activated endogenous ERK levels; anti-pan-ERK to ascertain equivalent protein loading; and anti-FLAG to assess equality of expression of FLAG–hSpry2. (B) Cell membrane extracts of a similar set of transfections to that depicted in (A) were isolated and immunoblots of one-tenth sample volumes loaded were assayed with anti-EGFR to assess the levels of surface EGFRs; and with anti-Giα3 to ascertain equivalent amounts of loaded membrane fraction samples. TCL were analysed for protein expression of transfected c-Cbl and hSpry2 by immunoblotting (IB) with anti-HA and anti-FLAG, respectively. (C) 293T cells (60 mm dishes) co-transfected with 0.2 µg EGFR and 0.8 µg of either vector control, FLAG–hSpry2 or FLAG–mSpry4 were treated with 100 ng/ml EGF at 37°C for the different time periods, as indicated. TCL were immunoblotted (IB) with anti-phospho-ERK1/2 to assess the activation profiles of endogenous ERK. Anti-pan-ERK and anti-FLAG were used to reveal the equality of protein loading and expression. Phospho-ERK1/2 signals were quantified with the aid of a densitometer, and represented as a percentage of maximal ERK activity (ERK activation, for hSpry2, at 15 min stimulation treated as 100%) in the graphical plot. Shown are the averages of three separate experiments ± SEM.
Sustained EGF-induced ERK activation leads to neurite outgrowth in PC12 cells expressing hSpry2
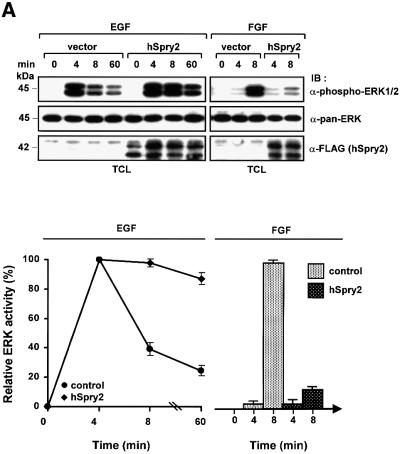
To ascertain whether such prolonged stimulation of EGF-induced ERK signalling observed upon hSpry2 overexpression is general, and that it affects endogenous, longer-term EGF signalling, we enlisted another cell system that expresses high intrinsic amounts of EGFRs. As alluded to earlier, transient expression of hSpry2 in PC12 cells also causes an increased and extended activation of ERK1/2 over a 60 min duration, compared with control cells, upon EGF stimulation (Figure 5A). In parallel, FGF stimulation of the same set of hSpry2 transfectants demonstrated a definite inhibition on phospho-ERK activation at the 8 min time-point compared with the vector control as expected, and in agreement with other reports (Impagnatiello et al., 2001; Sasaki et al., 2001; Yusoff et al., 2002). Quantification of phospho-ERK signals was performed and the average values of three separate experiments are represented either as line graphs (induction with EGF) or as bar charts (FGF stimulation) as shown.
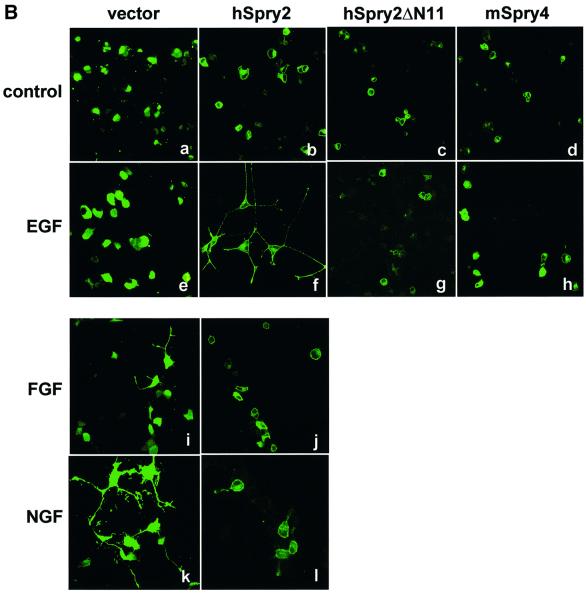
Fig. 5. (A) hSpry2 potentiates EGF- but inhibits FGF-induced ERK activation. PC12 cells (60 mm dishes) were transiently transfected with 2.0 µg each of FLAG vector or FLAG–hSpry2. Forty-eight hours post-transfection, cells were adjusted to low serum condition (1.0% HBS, 0.5% FBS) for 3–5 h before stimulation with 100 ng/ml EGF or 10 ng/ml FGF at 37°C for the time periods indicated. TCL were resolved by immunoblotting (IB) analysis to detect activated ERK (p42/44) using anti-phospho-ERK1/2, and probed with anti-pan-ERK and anti-FLAG to show similar protein loading and expression of the transfected Spry proteins. The amount of activated ERK1/2 signals was measured on a PhosphorImager, and presented as a percentage of maximal ERK activity at 4 min (EGF) and 8 min (FGF) stimulation in the respective line or bar chart. Shown are the averages of three independent experiments ± SEM. (B) EGF-induced neurite extensions are observed in PC12 cells expressing hSpry2. PC12 cells transfected with 2.0 µg plasmids encoding either GFP–vector control, GFP–hSpry2, GFP–hSpry2ΔN11 or GFP–mSpry4 were grown on poly-lysine-coated cover slips, and fixed after 4 days incubation in low serum media without added growth factor (control), supplemented with 100 ng/ml EGF, 10 ng/ml FGF or 10 ng/ml NGF. Panels show GFP fluorescence (green) of representative cell clones from each experimental treatment.
hSpry2/EGF-induced, sustained ERK phosphorylation was observed over a relatively short duration (60 min), as compared with dSpry-induced inhibition of RTK pathways during Drosophila development (Hacohen et al., 1998; Casci et al., 1999). Previously it has been shown that prolonged activation of ERK pathways (by FGF or NGF) in cultured PC12 cells would lead to their differentiation, as opposed to a shorter duration stimuli (EGF-induced), which leads to proliferation (Marshall, 1995; Kao et al., 2001). Hence, the use of PC12 cells to demonstrate whether the sustained MAPK signal induced by EGF upon hSpry2 overexpression has biological consequences on cell fate is appropriate.
Green fluorescent protein (GFP)-tagged versions of Spry are ideal for the purpose of observing the effects and disposition of Spry isoforms in living cells. For this purpose, GFP-tagged versions of Spry proteins were constructed. Preliminary experiments indicated that the resultant GFP fusion protein products of these constructs were capable of membrane targeting, as assessed by EGF-stimulated translocation assays (data not shown). GFP–hSpry2, GFP–mSpry4 and GFP–hSpry2ΔN11 were singly transfected into PC12 cells to monitor any morphological changes with respect to long-term EGF stimulation. When cells were incubated in low serum growth medium, all three Spry constructs were found to be located at the cell periphery of rounded, undifferentiated PC12 cells compared with the GFP vector control (Figure 5B, compare a with b, c and d). This result was expected, as each Spry protein comprises an intact membrane translocation domain and endogenous growth factors were present in the serum medium. With EGF supplement for 4 days it was observed that neurite extensions were apparent in the hSpry2-overexpressing cells (Figure 5B, f), but not in those cells transfected with GFP vector (Figure 5B, e), hSpry2ΔN11 mutant (Figure 5B, g) or mSpry4 (Figure 5B, h). As positive controls, a similar set of transfectants was stimulated with either FGF or NGF to demonstrate that under the same experimental conditions, hSpry2 inhibits both FGF- (Figure 5B, compare i and j) and NGF- (compare k and l) induced neurite outgrowth in PC12 cells.
In summary, we have established a correlation between the degree of inhibition of EGFR ubiquitylation and endocytosis, MAPK activation and neurite outgrowth morphology observed with PC12 cells, with the differential abilities of Spry isoforms to bind the c-Cbl RING finger. The data portrayed also provide evidence for ubiquitylation of EGFRs via c-Cbl acting as a prerequisite for receptor internalization, as little or no EGFR endocytosis occurs when the RING finger of c-Cbl is blocked or rendered non-functional by hSpry2.
Discussion
hSpry2 abrogates c-Cbl-mediated EGFR ubiquitylation and endocytosis
We have shown previously that transient transfection of hSpry2 in cells resulted in increased numbers of EGFRs being retained on the cell surface (Wong et al., 2001). There are at least two potential mechanisms that could explain this observation: (i) there is inhibition of EGFR internalization, or (ii) there is accelerated recycling of receptors from internal sorting compartments back to the cell surface. Interestingly, c-Cbl-mediated ubiquitylation has been postulated to play a role in either or both of these activities with certain receptors such as EGFR (Levkowitz et al., 1998; de Melker et al., 2001). Precisely where EGFR is ubiquitylated, and what are the corollaries of this ubiquitylation are sources of debate. We therefore set about analysing the relationship between EGFR, hSpry2 and c-Cbl with respect to ubiquitylation of the signalling receptors.
The means by which receptor ubiquitylation influences protein trafficking remain obscure, including the role of mono- and polyubiquitylation (Waterman and Yarden, 2001). The consensus view is that monoubiquitin or short ubiquitin chains are sufficient to direct internalization of cell surface proteins, whereas the proteasomal machinery recognizes polyubiquitylated proteins in Saccharomyces cerevisiae (Terrell et al., 1998; Shih et al., 2000; Hicke, 2001). In mammalian cells however, the situation is not as clear because a number of plasma membrane proteins that are ubiquitylated appear to be degraded through both the proteasomal and lysosomal pathways. Other classical endocytic signals include reversible modification such as phosphorylation, damage to the protein, genetically encoded sequence motifs (e.g. YXXΦ, where Φ is a bulky hydrophobic amino acid; MPXY or di-leucine), as well as sorting events that are coupled to clathrin-dependent or -independent routes (Laney and Hochstrasser, 1999; Waterman and Yarden, 2001; Soubeyran et al., 2002). There are currently disparate views on how and where c-Cbl ubiquitylates its target RTKs; evidence derived from studies with yeast, growth hormone receptor and inhibition of ErbB-1/EGFR (and other diverse receptors) uptake into internalized vesicles using dynamin mutants suggest that ubiquitylation may be associated with sorting at the plasma membrane (Damke et al., 1994; Govers et al., 1997; Stang et al., 2000). In a recent publication, it was demonstrated that c-Cbl-mediated ubiquitylation of EGFRs occurs at the plasma membrane, which then facilitates recruitment of activated EGFRs into clathrin-coated pits and the complex remains associated throughout the endocytic route (de Melker et al., 2001). Our results further support the notion that c-Cbl is likely to act on EGFR at the cell surface, and inhibition of this interaction by hSpry2 attenuates early stages of receptor internalization. Our data concur with previous evidence pertaining to the endocytic events governing mCSF-1R, where internalization of the macrophage receptor is retarded in c-Cbl-defective cells (Lee et al., 1999); and yeast membrane receptor regulation (Hicke, 1997); but is seemingly at odds with reports on EGFR endocytosis where c-Cbl has been arguably implicated as an endosomal sorting protein with signalling potential (Burke et al., 2001).
In recent studies involving the analysis of crystal structures, it was demonstrated that UbcH7 interacts closely with both the RING finger domain and the N-terminal 70Z linker region of c-Cbl (Zheng et al., 2000), apparently initiated upon tyrosine phosphorylation on residue 371 on the linker sequence by activated EGFR (Yokouchi et al., 2001). Our present study provides additional insights into the mechanism of c-Cbl’s mediatory effect on receptor ubiquitylation, in that the binding of UbcH7 can be successfully competed off by hSpry2. Much remains to be elucidated regarding the specific details of c-Cbl-dependent ubiquitylation, such as: resolving the identity of the candidate lysine residue on c-Cbl that becomes ubiquitylated, elucidating the structural conformation of phosphorylated c-Cbl (on Y371) and determining whether dimerization of c-Cbl (Bartkiewicz et al., 1999) might be important in its function as a ubiquitin ligase; all of which will advance our understanding as to how hSpry2 intercepts and disrupts the functional role of its E3-binding partner.
hSpry2 sustains EGFR signal transduction
A principal goal of this study was to investigate the effects of Spry isoforms on the Ras/MAPK pathway via the EGFR signalling cascade. The enhanced surface retention of EGFRs and blockade of their entry into endosomes led us to pursue a possible downstream effect. There are a number of reports indicating that activation of the Ras/MAPK pathway is dependent on receptor endocytosis (Vieira et al., 1996; Haugh et al., 1999); whereas others contend that it occurs at the cell membrane and is independent of receptor endocytosis (Lee et al., 1999; Johannessen et al., 2000; de Melker et al., 2001). Our observations clearly support the tenet that EGFR instigates ERK activation while at the cell surface. Superficially, the data on sustained ERK elevation by hSpry2 seems at odds with the genetic observations in other systems where dSpry, mSpry4 and mSpry2 were shown to inhibit the activation of FGF signalling (Hacohen et al., 1998; Lee et al., 2001; Mailleux et al., 2001). In Drosophila, dSpry inhibits FGF and EGF signal transduction by suppressing the Ras/MAPK pathway, either at the level of Ras during eye development (Casci et al., 1999), or at the level of Raf or MEK during wing development (Reich et al., 1999). We have shown that hSpry2 inhibits FGF-mediated ERK activation at the level of Raf (Yusoff et al., 2002), while mSpry4 has been shown to regulate angiogenesis via inhibition of RTK signalling upstream of Ras (Lee et al., 2001). However, several groups have failed to observe inhibition of EGFR signalling by endogenous hSpry2 and hSpry4, or overexpressed mSpry2 (Impagnatiello et al., 2001; Sasaki et al., 2001), despite their inhibitory effect on both FGF- and VEGF-induced ERK activation. On the other hand, dominant-negative Sprys enhanced FGF-, but not EGF-induced ERK activation (Sasaki et al, 2001). Taken together, these suggest the possibility that mammalian Sprys are not general inhibitors of RTK-induced ERK signalling, but rather specific inhibitors of certain RTKs. Besides, the genetic system employs a knock-out copy of dSpry in comparison with exogenously introduced Spry proteins engaged in our approach; while dSpry seems to function solely as a down-regulator of EGFR/MAPK signalling, our results with hSpry2 instead show that it enhances EGFR signalling to ERK. However, these observations are probably confined to RTKs where their down-regulation is via c-Cbl-mediated ubiquitylation, as our data clearly indicate that the c-Cbl-binding property of hSpry2 is essential, coupled with a translocating C-terminus, to mediate its positive modulator effect on EGF-dependent MAPK activation. Additionally, we observed that overexpression of c-Cbl indeed accelerates the kinetics of EGF-induced ERK signalling. Although we have previously reported that the c-Cbl-binding region is conserved across invertebrate and vertebrate species, and dSpry was demonstrated also to interact with Drosophila Cbl (dCbl), as well as to exhibit heterologous binding with c-Cbl (Wong et al., 2001), disparity between the Cbl-binding abilities and the functional properties of both Spry species remains to be elucidated. An obvious explanation is that the mode of interaction between Spry and Cbl differs in both the mammalian and Drosophila systems. Indeed, it has not been shown that dCbl functions to ubiquitylate and down-regulate Drosophila EGF receptor (DER); and interestingly, a recent study suggested a role for de-ubiquitylation in initiating receptor internalization in the invertebrate context (Cadavid et al., 2000). Furthermore, the N-terminal domains of Spry isoforms are highly divergent amongst the four mammalian proteins, suggesting that each family member possess a non-redundant function (i.e. mSpry4 behaves differently from hSpry2 in that it fails to inhibit c-Cbl action). Since there exists only a single Spry isoform in Drosophila, and none identified in Caenorhabditis elegans, one might well infer a unique function to the invertebrate counterpart.
Proliferation and differentiation of cells in response to extracellular signals is influenced by the differential regulation of MAPKs (Marshall et al., 1995). The rat pheochromocytoma PC12 cells have been widely used as a cell system for the study of growth factor-stimulated cell functions, whereby the intensity and duration of activation of the MAPKs (ERK1/2) have been proposed to govern a distinct switch between cell proliferation and differentiation (Kao et al., 2001). Our data show that, at least in cultured mammalian cells, priming with EGF causes neurite outgrowth from hSpry2-transfected PC12 cells, presumably correlated with sustained ERK1/2 activation. The pro-differentiation effect obtained with the hSpry2–EGFR combination was found to be restricted to the EGFR signalling cascade. This result supports the postulated threshold theory that differentiation is determined by the duration of ERK activation; such that EGF induction of sustained ERK activation can, in a similar manner to FGF and NGF, effect differentiation of PC12 cells. Furthermore, the readout of ERK1/2 phosphorylation was found to be specifically mediated by activation of EGFR channelled via the Ras/MAPK cascade, as it was abolished by treatment with a selective EGFR inhibitor, PD1(8393), and by U0126, a specific MEK1/2 inhibitor (data not shown). In addition, hSpry2ΔN11 fails to exhibit such effects, which suggests that binding to the RING finger domain of c-Cbl is crucial for hSpry2 to induce differentiation. Overexpression of mSpry4 is also incapable of augmenting neurite outgrowth, in line with predictions based on our previous finding that mSpry4 could not bind the RING finger of c-Cbl (Wong et al., 2001). Recently, Ho et al. (2001) demonstrated that in addition to NGF, EGF is also involved in determining the threshold level of ERK activation required for directional migration of PC12 cells. It would be of interest to explore whether overexpression of hSpry2 can alter a switch in cell migration patterns by regulating the extent and duration of ERK signalling in response to EGF stimulation.
EGFR-mediated signalling is critical for the growth and development of multicellular organisms (Moghal and Sternberg, 1999). As ascribed to in this study, the potentiating effects of hSpry2 on EGF-induced MAPK activation may extend to physiological scenarios where EGFR is deregulated. It has been reported that constitutive activation of ERK is observed in many human tumour cell lines due to disordered regulation of Ras, Raf-1 or some signalling molecules upstream of Ras (Hoshino et al., 1999), which coincides with the regulatory mode of Spry proteins. Interestingly, Ozaki et al. (2001) have recently demonstrated that induction of Spry gene expression is positively regulated by the ERK pathway, and found the expression levels of Spry1 and Spry2 genes to be significantly elevated in selected tumorigenic cells. Moreover, varying expression of each Spry gene isoform was detected in different cell types. In addition to hSpry2, we found that mSpry1, which binds c-Cbl, was also able to enhance and sustain ERK activity, and block receptor endocytosis when overexpressed (data not shown). Therefore, we speculate that mSpry1 and hSpry2 may potentially be pro-oncogenic due to their inhibitory binding to the RING finger of c-Cbl and enhanced mitogenic response to EGF induction.
Attenuation of growth factor signalling is essential for the regulation of developmental processes and tissue homeostasis in most organisms. Timed destruction or turnover of many short-lived cellular regulators or abnormal proteins by the ubiquitin–proteosomal/lysosomal pathway plays a critical role in ensuring normal cellular processes. Although still speculative, recent advances in the area of ubiquitylation have opened new avenues in the treatment of some proliferative and autoimmune diseases and of inflammation. Hence, pharmacological interventions that will alter the half-lives of important regulatory proteins might have wide therapeutic potential. The novel role of hSpry2 in inhibiting receptor ubiquitylation and down-regulation is analogous to the effect of the class of de-ubiquitylating enzymes (Kalderon, 1996). Previous studies by Fischer-Vize and colleagues (Huang et al., 1995) have provided an example in which the loss of function of a de-ubiquitylating enzyme is associated with a specific developmental phenotype, prompting a re-examination of how regulation and specificity are achieved in ubiquitin-mediated events. A physiological consequence may well be a concerted event stemming from an intricate interplay between the dual roles of hSpry2 as a positive or negative regulator of Ras/MAPK in the EGF versus FGF context. It is also likely that the cellular effects of hSpry2 are both temporally and spatially regulated.
Our work with hSpry2 reveals a novel perspective in understanding the complex mechanism(s) whereby c-Cbl functions, and how hSpry2 impinges on this. c-Cbl interacts directly with an assortment of proteins via its other signalling domains with variously ascribed outcomes (Thien and Langdon, 2001), and has also been reported to target and ubiquitylate a number of non-RTK molecules (Yokouchi et al., 2001). Furthermore, Yarden and colleagues (Waterman et al., 2002) recently uncovered a surrogate route for c-Cbl-mediated lysosomal degradation of EGFRs involving recruitment of the adapter molecule, Grb2, which has also been found to bind hSpry2 (Glienke et al., 2000). In addition, our data also reinforced the notion that c-Cbl-dependent ubiquitylation dictates endocytosis and sorting of selected receptors, thus priming them for further protein modification. It would be of interest to determine whether hSpry2 also inhibits such targeting and what potential role(s) it may play in the modification of other signalling pathways under various stimulatory influences.
Materials and methods
Materials
Monoclonal antibodies against the HA and FLAG epitope tags, and anti-ubiquitin were obtained from Roche Molecular Biochemicals (Indianapolis, IN) and Sigma Aldrich (St Louis, MO), respectively; polyclonal anti-FLAG was from Affinity Bioreagents, Inc. (Golden, CO); Texas Red-conjugated AffiniPure goat anti-rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated AffiniPure rabbit anti-mouse IgG were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA); monoclonal anti-phospho-ERK1/2 (p42/44) was from Cell Signalling Technology (Beverly, MA). Horseradish peroxidase-conjugated anti-phosphotyrosine (PY20), monoclonal antibodies to EGFR (E12020) and anti-pan-ERK were purchased from Transduction Laboratories (Lexington, KY); mouse anti-EGFR (528), anti-c-Cbl (C-15) and anti-GFP polyclonals were from Santa Cruz Biotechnology (Santa Cruz, CA); protein G/A–agarose was from Calbiochem (San Diego, CA). Monoclonal anti-ubiquitin, secondary anti-mouse and anti-rabbit conjugated to horseradish peroxidase were from Sigma Aldrich; anti-Giα3 rabbit antiserum and human recombinant EGF were from Upstate Biotechnology (Lake Placid, NY); FGF was purchased from Sigma Aldrich; and human NGF from Calbiochem. Ubiquitin, ubiquitin-activating enzyme E1 (mammalian) and E2 ubiquitin-conjugating enzyme (UbcH7, human recombinant) were purchased from Affiniti Research (Devon, UK). 125I-labelled human recombinant EGF was from Amersham Pharmacia Biotech (Buckinghamshire, UK). Monensin sodium salt was obtained from Biomol Research Laboratories, Inc (Plymouth, PA).
Plasmid constructs
Human Sprouty2 (hSpry2), hSpry2ΔN11 and mSpry4 cDNAs were PCR-amplified from pXJ40FLAG-hSpry2, pXJ40FLAG-hSpry2ΔN11 and pXJ40FLAG-mSpry4, respectively (Lim et al., 2000; Wong et al., 2001) and subcloned into pEGFP-C3 vector (Clontech Laboratories, Inc., Palo Alto, CA) at EcoRI–BamHI sites. The EGFR cDNA was a gift from Dr A.Ullrich (Max-Plank-Institut fur Biochemie), and was subcloned into pMyc (vector obtained from Dr B.L.Tang, IMCB, Singapore) at XhoI–XbaI sites. HA–ubiquitin was obtained from Dr D.Bohmann (EMBL-Heidelberg, Germany) and Dr Y.Yarden (Weizmann Institute of Science, Israel). UbcH7 cDNA in pET-3a was kindly provided by Dr M.Scheffner (Institut fuer Biochemie I Med. Fakultaet, Universitaet Koeln, Germany) and subcloned into pXJ40FLAG. dAri-1 cDNA was courtesy of Dr A.Ferrus (Cajal Institute, Spain), and subcloned into pXJ40HA at BamHI–SmaI sites. Human Ari-2 was PCR-amplified from an adult human brain library (Clontech) and subcloned into pXJ40HA at BamHI–XhoI sites. Full-length Hakai in pcDNA3 and pGEX-Hakai-N (N-terminal construct containing the RING finger domain) were generous gifts of Dr Y.Fujita (Max-Delbruck-Center for Molecular Medicine, Germany).
Cell culture, immunoprecipitation, western blotting and immunofluorescence microscopy
Maintenance and transfection of 293T and COS-1 cells, and the various immuno-based purification and visualization techniques employed were carried out as described previously (Wong et al., 2001). Confocal laser scanning microscopy and immunofluorescence analysis were performed using Bio-Rad MRC1024 confocal laser optics attached to a microscope (Zeiss, Oberkochen, Germany) interfaced with an argon/krypton laser. Simultaneous double fluorescence acquisitions were performed using the 488 and 568 nm laser lines to excite FITC and Texas Red dyes using a 40× oil immersion, 1.4 numerical aperture bright-field objective and fluorescein filter sets. Under similar acquisition parameters, confocal images were captured from a basal plane of the cells under study, just above the basal membrane, unless otherwise indicated. PC12 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 4500 mg glucose, 10% inactivated horse serum (Sigma Aldrich), 5% fetal bovine serum (FBS; HyCloneLaboratories, Inc., Logan, UT) and 1% glutamine. Cells were seeded on poly-d-lysine (Iwaki, Chiba, Japan)-coated coverslips and transfected using the GenePorter Transfection Reagent from Gene Therapy Systems, Inc. (San Diego, CA). 293T cells were used in overexpression studies due to their ease of handling and susceptibility to transfections; COS-1 cells were chosen for morphological studies due to their large and well-defined cellular structures, and PC12 cells for their differentiation phenotype.
Receptor down-regulation assay and monensin inhibition of receptor recycling
COS-1 cells (1.0 × 105) were cultured in 24-well plates, in duplicates per transfection per time-point. Cells were incubated at 37°C with unlabelled EGF (100 ng/ml) in a DMEM/0.5% BSA binding buffer for various time periods. Receptor-bound EGF remaining on the cell surface was removed by a series of ice-cold washes: thrice with binding buffer, twice with 150 mM acetic acid + 150 mM NaCl, and twice again with binding buffer. Fresh DMEM/0.5% BSA binding medium was added and kept on ice for 5 min. The residual level of surface receptor that did not undergo down-regulation was determined by performing a direct binding assay with 10 ng/ml [125I]EGF (+ 100-fold excess unlabelled EGF to account for non-specific binding). Incubations were proceeded at 4°C for 2–4 h, after which reaction was stopped by removal of unbound ligand. Thereafter, cell monolayers were rinsed twice with cold binding buffer to remove non-sequestrated radioactivity, and solubilized at 37°C for 15 min with 500 µl 0.1 M NaOH + 0.1% SDS. Resultant solutions were pipetted into a 2 ml screw cap tube and placed in counter vials for analysis on a LKB-Wallac 1282 CompuGamma counter. To quantify the receptor levels following inhibition of receptor recycling, cells were pre-incubated at 4°C for 2 h with unlabelled EGF (100 ng/ml) in the absence or presence of 30 µM monensin, followed by a temperature shift to 37°C for stimulation by EGF for various time periods, and the same procedure to determine surface EGFR was used. For induction of EGFR recycling, cells were pre-treated with 100 nM PMA for 20 min prior to EGF stimulation at 37°C.
Membrane fractionation
Cells stimulated without or with EGF (100 ng/ml) were washed twice with ice-cold PBS, before being scraped off the plate surface, and pelleted by centrifugation at 500 g for 5 min at 4°C. Supernatant was removed and cell pellets were frozen at –80°C as a freeze–thaw step for easy lysis of cells. Frozen cells were thawed on ice, resuspended in 200 µl homogenizing buffer (1 mM EDTA, 5 mM HEPES pH 7.5, 50 mM sucrose) and disrupted by Dounce homogenization (∼25 stokes for 3–4 min with twisting). Nuclei and cell debris were removed by centrifugation at 500 g for 5 min at 4°C. The supernatant was transferred to a fresh Eppendorf and spun at 16 000 g for 30 min at 4°C. The final pellet containing the membranal fraction was resuspended in 100 µl TE buffer (10 mM Tris + 0.1 mM EDTA pH 7.4) and one-tenth sample volumes were analysed by SDS–PAGE and western blotting.
In vitro ubiquitylation assay
Endogenous EGFR was immunoprecipitated from COS-1 cells using agarose-conjugated EGFR fusion protein beads. Aliquots (2 µg) of the protein-bound beads were incubated in 50 µl reaction mixture containing the following: 1× reaction buffer [50 mM Tris–HCl pH 7.5, 2.5 mM MgCl, 2 mM dithiothreitol (DTT), 2 mM ATP], 10 µg ubiquitin, 100 ng E1, 200 ng E2 and either 1 µg eluted GST–c-Cbl-C381A (negative control), 1 µg eluted GST–c-Cbl (positive control) alone or in combination with 0.5 µg eluted GST–hSpry2, GST–mSpry4 or GST–hSpry2ΔN11 recombinant proteins. Control samples without added E2 or E3 (c-Cbl) were included. Enzymic reactions were incubated at 37°C for 2 h, after which the treated EGFR beads were washed thrice with 1× reaction buffer. Sample beads were boiled with 2× Laemmli buffer containing 2-mercaptoethanol, and the reaction products were resolved on a 7.5% SDS–PAGE gel and western blotted against anti-ubiquitin.
Acknowledgments
Acknowledgements
We thank Drs Axel Ullrich, Dirk Bohmann, Yosef Yarden, Martin Scheffner, Albert Ferrus and Yasuyuki Fujita for their generous gifts of plasmid constructs. Appreciation also extends to Ms Sumana Chandramouli for contribution of the experimental data on hSpry2/Hakai, and Ms Hwei Fen Leong for technical assistance. This work was supported by the Agency for Science, Technology and Research (A*STAR).
Note added in proof
While this manuscript was under review, Egan et al. (2002) [Proc. Natl Acad. Sci. USA, 99, 6041–6046] reported the dual modulatory functions of human Spry proteins on EGFR signalling, which supports some of the main concepts discussed herein.
References
- Ardley H.C., Tan,N.G., Rose,S.A., Markham,A.F. and Robinson,P.A. (2001) Features of the Parkin/Ariadne ubiquitin ligase, HHARI, which regulates its interaction with the ubiquitin-conjugating enzyme, UbcH7. J. Biol. Chem., 276, 19640–19647. [DOI] [PubMed] [Google Scholar]
- Aguilera M., Oliveros,M., Martinez-Padron,M., Barbas,J.A. and Ferrus,A. (2000) Ariadne-1: a vital Drosophila gene is required in development and defines a new conserved family of RING Finger proteins. Genetics, 155, 1231–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J., Alroy,I., Waterman,H., Schejter,E.D., Brodie,C., Gruenberg,J. and Yarden,Y. (2000) Threonine phosphorylation diverts internalized epidermal growth factor receptors from a degradative pathway to the recycling endosome. J. Biol. Chem., 275, 26178–26186. [DOI] [PubMed] [Google Scholar]
- Bartkiewicz M., Houghton,A. and Baron,R. (1999) Leucine zipper-mediated homo-dimerization of the adaptor protein c-Cbl. A role in c-Cbl’s tyrosine phosphorylation and its association with epidermal growth factor receptor. J. Biol. Chem., 274, 30887–30895. [DOI] [PubMed] [Google Scholar]
- Basu S.K., Goldstein,J.L., Anderson,R.G. and Brown,M.S. (1981) Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell, 24, 493–502. [DOI] [PubMed] [Google Scholar]
- Borden K.L. (2000) RING domains: master builders of molecular scaffolds? J. Mol. Biol., 295, 1103–1112. [DOI] [PubMed] [Google Scholar]
- Burke P., Schooler,K. and Wiley,H.S. (2001) Regulation of epidermal growth factor receptor signalling by endocytosis and intracellular trafficking. Mol. Biol. Cell, 12, 1897–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid A.L., Ginzel,A. and Fischer,J.A. (2000) The function of the Drosophila fat facets de-ubiquitinating enzyme in limiting photoreceptor cell number is intimately associated with endocytosis. Development, 127, 1727–1736. [DOI] [PubMed] [Google Scholar]
- Casci T., Vinos,J. and Freeman,M. (1999) Sprouty, an intracellular inhibitor of Ras signalling. Cell, 96, 655–665. [DOI] [PubMed] [Google Scholar]
- Damke H., Baba,T., Warnock,D.E. and Schmid,S.L. (1994) Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol., 127, 915–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maximy A.A., Nakatake,Y., Moncada,S., Itoh,N., Thiery,J.P. and Bellusci,S. (1999) Cloning and expression pattern of a mouse homologue of Drosophila Sprouty in the mouse embryo. Mech. Dev., 81, 213–216. [DOI] [PubMed] [Google Scholar]
- de Melker A.A., van der Horst,G., Calafat,J., Jansen,H. and Borst,J. (2001) c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains associated throughout the endocytic route. J. Cell Sci., 114, 2167–2178. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Krause,G., Scheffner,M., Zechner,D., Leddy,H.E.M., Behrens,J., Sommer,T. and Birchmeier,W. (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol., 4, 222–231. [DOI] [PubMed] [Google Scholar]
- Gladhaug I.P. and Christoffersen,T. (1988) Rapid constitutive internalization and externalization of epidermal growth factor receptors in isolated rat hepatocytes. Monensin inhibits receptor externalization and reduces the capacity for continued endocytosis of epidermal growth factor. J. Biol. Chem., 263, 12199–12203. [PubMed] [Google Scholar]
- Glienke J., Fenten,G., Seemann,M., Sturz,A. and Thierauch,K.H. (2000) Human Spry2 inhibits FGF2 signalling by a secreted factor. Mech. Dev., 96, 91–99. [DOI] [PubMed] [Google Scholar]
- Gonzalez F.A., Raden,D.L. and Davis,R.J. (1991) Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J. Biol. Chem., 266, 22159–22163. [PubMed] [Google Scholar]
- Govers R., van Kerkhof,P., Schwartz,A.L. and Strous,G.J. (1997) Linkage of the ubiquitin-conjugating system and the endocytic pathway in ligand-induced internalization of the growth hormone receptor. EMBO J., 16, 4851–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen N., Kramer,S., Sutherland,D., Hiromi,Y. and Krasnow,M.A. (1998) Sprouty encodes a novel antagonist of FGF signalling that patterns apical branching of the Drosophila airways. Cell, 92, 253–263. [DOI] [PubMed] [Google Scholar]
- Haugh J.M., Huang,A.C., Wiley,H.S., Wells,A. and Lauffenburger,D.A. (1999) Internalized epidermal growth factor receptors participate in the activation of p21(Ras) in fibroblasts. J. Biol. Chem., 274, 34350–34360. [DOI] [PubMed] [Google Scholar]
- Hershko A. and Ciechanover,A.L. (1998) The ubiquitin system. Annu. Rev. Biochem., 67, 425–479. [DOI] [PubMed] [Google Scholar]
- Hicke L. (1997) Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J., 11, 1215–1226. [DOI] [PubMed] [Google Scholar]
- Hicke L. (2001) Protein regulation by monoubiquitin. Nature Rev. Mol. Cell Biol., 2, 195–201. [DOI] [PubMed] [Google Scholar]
- Ho W., Uniyal,S., Meakin,S.O., Morris,V.L. and Chan,B.M. (2001) A differential role of extracellular signal-regulated kinase in stimulated PC12 pheochromocytoma cell movement. Exp. Cell Res., 263, 254–264. [DOI] [PubMed] [Google Scholar]
- Hoshino R. et al. (1999) Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signalling pathway in human tumours. Oncogene, 18, 813–822. [DOI] [PubMed] [Google Scholar]
- Huang Y., Baker,R.T. and Fischer-Vize,J.A. (1995) Control of cell fate by a de-ubiquitinating enzyme encoded by the fat facets gene. Science, 270, 1828–1831. [DOI] [PubMed] [Google Scholar]
- Impagnatiello M.A., Weitzer,S., Gannon,G., Compagni,A., Cotten,M. and Christofori,G. (2001) Mammalian Sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signalling in endothelial cells. J. Cell Biol., 152, 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro C.A.P., Wing,S.S., Huang,H.K., Leverson,J.D., Hunter,T. and Liu,Y.C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin–protein ligase. Science, 286, 309–312. [DOI] [PubMed] [Google Scholar]
- Johannessen L.E., Ringerike,T., Molnes,J. and Madshus,I.H. (2000) Epidermal growth factor receptor efficiently activates mitogen-activated protein kinase in HeLa cells and Hep2 cells conditionally defective in clathrin-dependent endocytosis. Exp. Cell Res., 260, 136–145. [DOI] [PubMed] [Google Scholar]
- Kalderon D. (1996) Protein degradation: de-ubiquitinate to decide your fate. Curr. Biol., 6, 662–665. [DOI] [PubMed] [Google Scholar]
- Kao S.C., Jaiswal,R.K., Kolch,W. and Landreth,G.E. (2001) Identification of the mechanisms regulating the differential activation of the MAPK cascade by epidermal growth factor and nerve growth factor in PC12 cells. J. Biol. Chem., 276, 18169–18177. [DOI] [PubMed] [Google Scholar]
- Kramer S., Okabe,M., Hacohen,N., Krasnow,M.A. and Hiromi,Y. (1999) Sprouty: a common antagonist of FGF and EGF signalling pathways in Drosophila. Development, 126, 2515–2525. [DOI] [PubMed] [Google Scholar]
- Laney J.D. and Hochstrasser,M. (1999) Substrate targeting in the ubiquitin system. Cell, 97, 427–430. [DOI] [PubMed] [Google Scholar]
- Lee P.S.W., Wang,Y., Dominguez,M.G., Yeung,Y.G., Murphy,M.A., Bowtell,D.D.L. and Stanley,E.R. (1999) The Cbl protooncogene stimulates CSF-1 receptor multi-ubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J., 18, 3616–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Schloss,D.J., Jarvis,L., Krasnow,M.A. and Swain,J.L. (2001) Inhibition of angiogenesis by a mouse Sprouty protein. J. Biol. Chem., 276, 4128–4133. [DOI] [PubMed] [Google Scholar]
- Levkowitz G., Waterman,H., Zamir,E., Kam,Z., Oved,S., Langdon,W.Y., Beguinot,L., Geiger,B. and Yarden,Y. (1998) c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev., 12, 3663–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G. et al. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signalling by c-Cbl/Sli-1. Mol. Cell, 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- Lim J., Wong,E.S.M., Ong,S.H., Yusoff,P., Low,B.C. and Guy,G.R. (2000) Sprouty proteins are targeted to membrane ruffles upon growth factor receptor tyrosine kinase activation: identification of a novel translocation domain. J. Biol. Chem., 275, 32837–32845. [DOI] [PubMed] [Google Scholar]
- Mailleux A.A., Tefft,D., Ndiaye,D., Itoh,N., Thiery,JP., Warburton,D. and Bellusci,S. (2001) Evidence that Sprouty2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech. Dev., 102, 81–94. [DOI] [PubMed] [Google Scholar]
- Marshall C.J. (1995) Specificity of receptor tyrosine kinase signalling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Marshall C.J. (1998) Signal transduction. Taking the Rap. Nature, 392, 553–554. [DOI] [PubMed] [Google Scholar]
- Minowada G., Jarvis,L.A., Chi,C.L., Neubuser,A., Sun,X., Hacohen,N., Krasnow,M.A. and Martin,G.R. (1999) Vertebrate Sprouty genes are induced by FGF signalling and can cause chondrodysplasia when overexpressed. Development, 126, 4465–4475. [DOI] [PubMed] [Google Scholar]
- Miyake S., Lupher,M.L.,Jr, Druker,B. and Band,H. (1998) The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor α. Proc. Natl Acad. Sci. USA, 95, 7927–7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghal N. and Sternberg,P.W. (1999) Multiple positive and negative regulators of signalling by the EGF receptor. Curr. Opin. Cell Biol., 11, 190–196. [DOI] [PubMed] [Google Scholar]
- Mu F.T. et al. (1995) EEA1, an early endosome-associated protein. EEA1 is a conserved α-helical peripheral membrane protein flanked by cysteine ‘fingers’ and contains a calmodulin-binding IQ motif. J. Biol. Chem., 270, 13503–13511. [DOI] [PubMed] [Google Scholar]
- Ozaki K., Kadomoto,R., Asato,K., Tanimura,S., Itoh,N. and Kohno,M. (2001) ERK pathway positively regulates the expression of Sprouty genes. Biochem. Biophys. Res. Commun., 285, 1084–1088. [DOI] [PubMed] [Google Scholar]
- Reich A., Sapir,A. and Shilo,B. (1999) Sprouty is a general inhibitor of receptor tyrosine kinase signalling. Development, 126, 4139–4147. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Taketomi,T., Wakioka,T., Kato,R. and Yoshimura,A. (2001) Identification of a dominant negative mutant of Sprouty that potentiates fibroblast growth factor- but not epidermal growth factor-induced ERK activation. J. Biol. Chem., 276, 36804–36808. [DOI] [PubMed] [Google Scholar]
- Shih S.C., Sloper-Mould,K.E. and Hicke,L. (2000) Mono-ubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J., 19, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran P., Kowanetz,K., Szymkiewicz,I., Langdon,W.Y. and Dikic,I. (2002) Cbl–CIN85–endophilin complex mediates ligand-induced down-regulation of EGF receptors. Nature, 416, 183–187. [DOI] [PubMed] [Google Scholar]
- Stang E., Johannessen,L.E., Knardal,S.L. and Madshus,I.H. (2000) Poly-ubiquitination of the epidermal growth factor receptor occurs at the plasma membrane upon ligand-induced activation. J. Biol. Chem., 275, 13940–13947. [DOI] [PubMed] [Google Scholar]
- Tefft J.D., Lee,M., Smith,S., Leinwand,M., Zhao,J.S., Bringas,P.,Jr, Crowe,D.L. and Warburton,D. (1999) Conserved function of mSpry-2, a murine homolog of Drosophila Sprouty, which negatively modulates respiratory organogenesis. Curr. Biol., 9, 219–222. [DOI] [PubMed] [Google Scholar]
- Terrell J., Shih,S., Dunn,R. and Hicke,L. (1998) A function for mono-ubiquitination in the internalization of a G protein-coupled receptor. Mol. Cell, 1, 193–202. [DOI] [PubMed] [Google Scholar]
- Thien C.B.F. and Langdon,W.Y. (2001) Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell. Biol., 2, 294–305. [DOI] [PubMed] [Google Scholar]
- Vieira A.V., Lamaze,C. and Schmid,S.L. (1996) Control of EGF receptor signalling by clathrin-mediated endocytosis. Science, 274, 2086–2089. [DOI] [PubMed] [Google Scholar]
- Waterman H. and Yarden,Y. (2001) Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett., 490, 142–152. [DOI] [PubMed] [Google Scholar]
- Waterman H., Levkowitz,G., Alroy,I. and Yarden,Y. (1999) The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J. Biol. Chem., 274, 22151–22154. [DOI] [PubMed] [Google Scholar]
- Waterman H., Katz,M., Rubin,C., Shtiegman,K., Lavi,S., Elson,A., Jovin,T. and Yarden Y. (2002) A mutant EGF receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signalling. EMBO J., 21, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.S.M., Lim,J., Low,B.C., Chen,Q. and Guy,G.R. (2001) Evidence for direct interaction between Sprouty and Cbl. J. Biol. Chem., 276, 5866–5875. [DOI] [PubMed] [Google Scholar]
- Yarden Y. and Sliwkowski,M.X. (2001) Untangling the ErbB signalling network. Nat. Rev. Mol. Cell. Biol., 2, 127–137. [DOI] [PubMed] [Google Scholar]
- Yokouchi M., Kondo,T., Sanjay,A., Houghton,A., Yoshimura,A., Seturo,K., Zhang,H. and Baron,R. (2001) Src-catalyzed phosphorylation of c-Cbl leads to the inter-dependent ubiquitination of both proteins. J. Biol. Chem., 276, 35185–35193. [DOI] [PubMed] [Google Scholar]
- Yusoff P., Lao,D.H., Ong,S.H., Wong,E.S.M., Lim,J., Lo,T.L., Leong,H.F., Fong,C.W. and Guy,G.R. (2002) Sprouty2 inhibits the Ras/MAP kinase pathway by inhibiting the activation of Raf. J. Biol. Chem., 277, 3195–3201. [DOI] [PubMed] [Google Scholar]
- Zheng N., Wang,P., Jeffrey,P.D. and Pavletich,N.P. (2000) Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin–protein ligases. Cell, 102, 533–539. [DOI] [PubMed] [Google Scholar]