Abstract
The heteromeric amino acid transporters are composed of a type II glycoprotein and a non-glycosylated polytopic membrane protein. System bo,+ exchanges dibasic for neutral amino acids. It is composed of rBAT and bo,+AT, the latter being the polytopic membrane subunit. Mutations in either of them cause malfunction of the system, leading to cystinuria. bo,+AT-reconstituted systems from HeLa or MDCK cells catalysed transport of arginine that was totally dependent on the presence of one of the bo,+ substrates inside the liposomes. rBAT was essential for the cell surface expression of bo,+AT, but it was not required for reconstituted bo,+AT transport activity. No system bo,+ transport was detected in liposomes derived from cells expressing rBAT alone. The reconstituted bo,+AT showed kinetic asymmetry. Expressing the cystinuria-specific mutant A354T of bo,+AT in HeLa cells together with rBAT resulted in defective arginine uptake in whole cells, which was paralleled by the reconstituted bo,+AT activity. Thus, subunit bo,+AT by itself is sufficient to catalyse transmembrane amino acid exchange. The polytopic subunits may also be the catalytic part in other heteromeric transporters.
Keywords: bo,+AT–rBAT exchanger/bo,+AT reconstitution/cystinuria/transport asymmetry/reabsorption
Introduction
The heteromeric amino acid transporters (HATs) are the only known eukaryotic transporters of amino acids composed of two different subunits bound by a disulfide bridge: (i) a type II glycoprotein or heavy subunit (rBAT and 4F2hc), and (ii) a non-glycosylated light subunit with 12 putative transmembrane domains (LAT-1, LAT-2, asc1, y+LAT-1, y+LAT-2, xCT and bo,+AT) (for reviews see Verrey et al., 1999, 2000; Devés and Boyd, 2000; Chillarón et al., 2001; Wagner et al., 2001). The heavy subunit has an ectodomain that is homologous to bacterial glucosidases (reviewed in Chillarón et al., 2001). The light subunit is suspected to be the catalytic part of the holotransporter because it is considered to be a polytopic membrane protein and heterodimers of 4F2hc with different light subunits yield different amino acid transport systems: (i) with LAT-1 or LAT-2, variants of system L (Kanai et al., 1998; Mastroberardino et al., 1998; Pineda et al., 1999; Rossier et al., 1999; Segawa et al., 1999), (ii) with asc1, system asc (Fukasawa et al., 2000; Nakauchi et al., 2000), (iii) with y+LAT-1 or y+LAT-2, variants of system y+L (Torrents et al., 1998; Pfeiffer et al., 1999a; Kanai et al., 2000) and (iv) with xCT, system xc– (Sato et al., 1999, 2000; Bassi et al., 2001).
The rBAT protein is the heavy subunit of bo,+AT in kidney brush border membranes (Fernández et al., 2002), and this heterodimer forms the holotransporter system bo,+ [i.e. exchanger of dibasic amino acids and cystine (influx) for neutral amino acids (efflux)] (Feliubadaló et al., 1999; Chairoungdua et al., 1999; Pfeiffer et al., 1999b; Mizoguchi et al., 2001). The expression of both proteins is needed to express system bo,+ transport at the cell surface (for reviews see Verrey et al., 2000; Chillarón et al., 2001). These two proteins when mutated cause the inherited disease named cystinuria, characterized by hyperexcretion of cystine and dibasic amino acids in urine (for reviews see Palacín et al., 2001a,b): mutations in rBAT cause type I cystinuria (a completely recessive form of the disease) (Calonge et al., 1994), and mutations in bo,+AT cause non-type I cystinuria (in which the heterozygotes show moderate amino acid hyperexcretion) (Feliubadaló et al., 1999; Font et al., 2001). The rBAT cystinuria mutations already studied seem to cause trafficking defects (Chillarón et al., 1997; reviewed in Palacín et al., 2001b), whereas there are no studies demonstrating whether bo,+AT cystinuria mutations cause trafficking defects or inactivate the transporter.
In the present study we reconstituted system bo,+ activity from extracts of cells transfected with bo,+AT. The reconstituted activity is dependent on the expression of a functional bo,+AT protein and the presence of system bo,+ amino acid substrates on the trans-side. In contrast, the expression of rBAT is not necessary to reconstitute the transport activity. Kinetic analysis demonstrated asymmetrical interaction of l-leucine with the reconstituted bo,+AT.
Results
Expression of system bo,+ in rBAT/bo,+AT-transfected cells
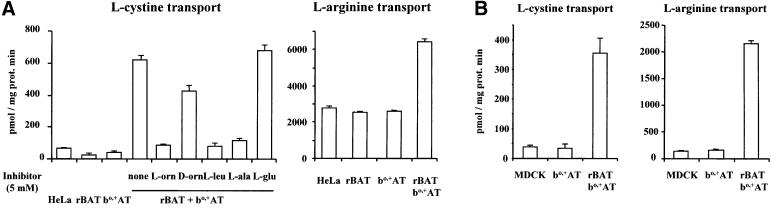
Transient transfection of human rBAT and bo,+AT resulted in the expression of sodium-independent cystine and arginine uptake in HeLa cells with characteristics of system bo,+ (i.e. almost complete inhibition by l-ornithine, l-leucine and l-alanine, only partial inhibition by d-ornithine, and no inhibition by l-glutamate) (Figure 1A). This inhibition pattern of cystine transport is identical to that obtained in rBAT-injected oocytes (Bertran et al., 1992). Kinetic analysis showed an apparent Km of 132 ± 36 and 226 ± 64 µM for cystine and arginine uptake induced in HeLa cells (data not shown). In contrast, transfection of rBAT or bo,+AT alone did not result in the induction of system bo,+ activity (Figure 1A). Similarly, permanent transfection of rBAT and bo,+AT, but not bo,+AT alone, resulted in the expression of sodium-independent cystine and arginine uptake in Madin–Darby canine kidney (MDCK) cells (Figure 1B). This is in full agreement with previous results (Chairoungdua et al., 1999; Feliubadaló et al., 1999; Pfeiffer et al., 1999b; Font et al., 2001) and stresses the need for both subunits to express functional bo,+ transporters at the cell surface. Indeed, expression of rBAT and bo,+AT at the surface of HeLa cells needs cotransfection of both subunits, otherwise each of the proteins remains in an intracellular location (data not shown). The need for bo,+AT for the routing of rBAT to the cell plasma membrane has also been reported in transfected COS cells (Feliubadaló et al., 1999).
Fig. 1. Expression of amino acid transport in cells cotransfected with rBAT and bo,+AT. (A) HeLa cells were transfected with rBAT or bo,+AT alone or together, or were used untransfected (HeLa). Three days later transport of 20 µM l-[35S]cystine or 50 µM l-[3H]arginine was measured in linear conditions. Only cells cotransfected with rBAT and bo,+AT showed amino acid transport above background. For l-[35S]cystine transport the inhibition pattern with the indicated amino acids at 5 mM was studied. (B) MDCK cells were permanently transfected with bo,+AT alone or together with rBAT. The transport of 20 µM l-[35S]cystine or 50 µM l-[3H]arginine was measured in linear conditions in non-transfected (MDCK) or transfected confluent cells. Only cells cotransfected with rBAT and bo,+AT showed amino acid transport above background.
Reconstitution of arginine/leucine exchange from extracts of bo,+AT-transfected HeLa cells
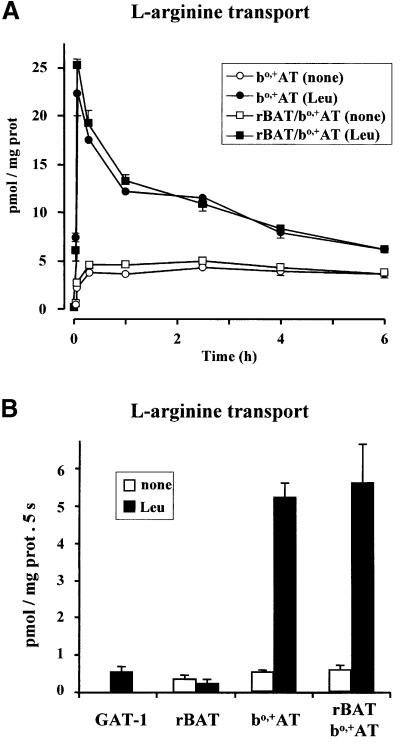
Cells transfected with rBAT, bo,+AT or both together were solubilized with detergent and mixed with phospholipids in order to reconstitute amino acid transporters. Uptake experiments using 0.5 µM l-[3H]arginine were performed for up to 6 h in the absence or presence of 2 mM leucine in the interior of the reconstituted liposomes (Figure 2A). When leucine was present, the uptake of arginine in liposomes obtained from extracts of cells transfected with bo,+AT alone or together with rBAT revealed an overshoot that peaked at 1 min uptake and decayed thereafter to equilibrium values (3.9 ± 0.1 pmol/mg protein; n = 11 preparations) at 6 h (Figure 2A). The uptake in bo,+AT- and rBAT/bo,+AT-reconstituted liposomes at 1 min was, respectively, 4.8 ± 0.5 and 5.4 ± 1.0-fold over the equilibrium value (i.e. overshoot factor) (n = 6 and 3 preparations, respectively, run in triplicate). In contrast, uptake of arginine into liposomes containing leucine and obtained from rBAT-transfected cells or into liposomes containing no leucine and obtained from cells transfected with rBAT and bo,+AT, or bo,+AT alone, were indistinguishable and reached the equilibrium plateau without showing any overshoot (Figure 2A; and data not shown). Similar results were obtained from extracts of cells transfected with the arginine transport-irrelevant GABA amino acid transporter GAT-1 (data not shown).
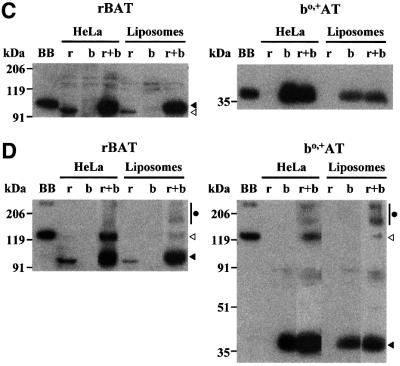
Fig. 2. Transport of arginine in reconstituted systems from HeLa cells. HeLa cells were transfected with rBAT or bo,+AT alone or together (rBAT/bo,+AT), or with the GABA transporter GAT-1. Three days later HeLa extracts were reconstituted into liposomes containing (Leu) or not containing (none) 2 mM leucine in the internal medium. (A) A time course of the transport of 0.5 µM l-[3H]arginine into the indicated liposomes. Transport was measured at 5 s, 1 and 15 min, and at 1, 2.5, 4 and 6 h. Data (mean ± SEM) correspond to a representative experiment run in triplicate. When not visible, error bars are smaller than the symbol. (B) Initial transport rates of 0.5 µM l-[3H]arginine into the indicated liposomes. Data (mean ± SEM) correspond to 12, five, five and one experiments run in triplicate for bo,+AT, rBAT, rBAT/bo,+AT and GAT-1 groups, respectively. (C and D) Western blot of rBAT (left panels) and bo,+AT (right panels) in reducing (C) or non-reducing (D) conditions. One hundred micrograms of protein of HeLa cells extracts (HeLa) or liposomes from the experiments shown in (A) and (B) were subjected to SDS–PAGE in the absence (D) or in the presence (C) of 100 mM DTT. Fifty micrograms of protein of human renal brush border membranes (BB) was loaded as control. Lanes in (C) and (D): rBAT-transfected (r), bo,+AT-transfected (b), and rBAT/bo,+AT-cotransfected (r+b) cells (HeLa) or liposomes. (C) Black arrowhead, rBAT band with mature glycosylation (i.e. endoglucosidase H resistant; data not shown). White arrowhead, rBAT band with core glycosylation (i.e. endoglucosidase H sensistive; data not shown). (D) Black arrowhead, mature and core glycosylation bands of the rBAT monomer (left panel) and bo,+AT monomer (right panel). White arrowhead, rBAT/bo,+AT heterodimer.Black circle, high molecular weight complexes. For (C) and (D) data correspond to two representative experiments. Two additional experiments gave similar results.
The transport rate of 0.5 µM arginine, measured in linear conditions, in bo,+AT- and rBAT/bo,+AT-reconstituted liposomes was 10 times higher when 2 mM leucine was present in the interior of the liposome than when no amino acid was present (Figure 2B). The transport rate of arginine in liposomes obtained from cells transfected with GAT-1 or rBAT alone, irrespective of the presence of leucine in the internal medium, was identical to the transport rate in bo,+AT- or rBAT/bo,+AT-reconstituted liposomes when no amino acid was present in the internal medium. These results demonstrate that transfection of bo,+AT alone in HeLa cells is enough to reconstitute the exchange of arginine and leucine in liposomes. Moreover, reconstitution from bo,+AT- or rBAT/bo,+AT-transfected cells yielded the same bo,+ transport rates (Figure 2B).
The expression of rBAT and bo,+AT in HeLa cells and the reconstitution of these proteins into liposomes was followed by western blot analysis using SDS–PAGE (Figure 2C–D). rBAT or bo,+AT was present in the corresponding cell extracts and liposomes only when transfected (Figure 2C). Transfection of rBAT alone resulted in the expression of the immature core-glycosyl ated form (i.e. endoglucosidase H-sensitive; data not shown) of the protein. This core-glycosylated form has higher electrophoretic mobility than the matured glycosyl ated form (i.e. endoglucosidase H-resistant; data not shown) that was detected in cells cotransfected with rBAT and bo,+AT and in renal brush borders (Figure 2C). These rBAT bands are similar to those detected during biogenesis of the protein in oocytes (Chillarón et al., 1997). Moreover, the level of expression of rBAT was higher when cotransfected with bo,+AT (299 ± 53% of that found when rBAT was transfected alone; n = 4), but the expression of bo,+AT seemed to be independent of the expression of rBAT (83 ± 5% of expressed bo,+AT when transfected alone; n = 4) (Figure 2C). These results suggest that bo,+AT helps the maturation and probably increases the stability of rBAT. In non-reducing conditions, as expected, bands corresponding to the electrophoretic mobility of the rBAT/bo,+AT heterodimers were seen only in cell extracts and liposomes (although to a lesser extent) obtained from cotransfected cells, but most of rBAT and bo,+AT migrated as the monomer (Figure 2D). In all, our results demonstrate that bo,+AT in the absence of rBAT mediates the exchange of arginine and leucine in the reconstituted liposomes.
The cystinuria-specific A354T bo,+AT mutant revealed inactivation of transport in reconstituted systems
To examine whether the exchange of external arginine with internal leucine (l-argo/l-leui) is dependent on the reconstitution of a functional bo,+AT, two cystinuria-specific bo,+AT mutants (A182T and A354T) were studied. Mutation A182T is one of the most common cystinuria mutations found in SLC7A9, whereas mutation A354T has been found in two cystinuria patients (Font et al., 2001; and unpublished result from the International Cystinuria Consortium). We chose these mutations because previous functional analysis showed complete lack of transport activity of A354T, and ∼50% residual transport activity of A182T when cotransfected with rBAT in HeLa cells (Font et al., 2001). Identical results were obtained here (Figure 3A). Kinetic analysis showed an apparent Km of 256 ± 59 µM (mean ± SEM) for arginine in rBAT/A182T bo,+AT-transfected cells (data not shown). This value is similar to that obtained in rBAT/wild-type bo,+AT-transfected cells (see first paragraph of Results). The reconstituted A354T bo,+AT mutant showed <10% of the wild-type bo,+AT transport of arginine trans-stimulated by leucine into liposomes (Figure 3B). In contrast, the reconstituted A182T bo,+AT mutant showed complete arginine transport activity like the wild-type bo,+AT, which was totally dependent on the presence of leucine in the internal medium (Figure 3B). Western blot analysis revealed the presence of the wild-type and the mutated bo,+AT proteins in the cells and reconstituted systems in similar amounts (Figure 3C). Therefore, the defective transport activity of the A354T mutant could not be attributed to lower expression of this protein in the liposomes. These results demonstrate that (i) l-argo/l-leui exchange activity is dependent on the reconstitution of a functional bo,+AT protein, (ii) mutation A354T abolishes bo,+AT transport activity almost completely, and (iii) mutation A182T retains full bo,+AT transport activity.
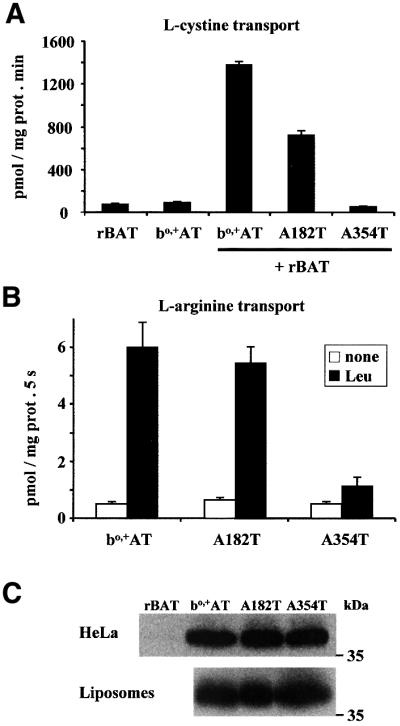
Fig. 3. Transport activity of cystinuria-specific bo,+AT mutants in HeLa cells and reconstituted systems. HeLa cells were transfected with rBAT or bo,+AT alone, or with rBAT plus wild-type bo,+AT, A182T- or A354T-mutated bo,+AT. (A) Three days later transport rates of 20 µM l-[35S]cystine into cells were measured. Data (mean ± SEM) from a representative experiment. Three additional experiments gave similar results. (B) Transport of arginine in reconstituted systems. Cells were transfected with wild-type bo,+AT, A182T- or A354T-mutated bo,+AT. Three days later, cells were used for reconstitution into liposomes containing (Leu) or not containing (none) 2 mM leucine in the internal medium, and the transport rates of 0.5 µM l-[3H]arginine were measured. Transport of arginine trans-stimulated by leucine was significant in the three groups. Data (mean ± SEM) correspond to a representative experiment run in triplicate. A second experiment gave similar results. (C) Western blot of bo,+AT in reconstituted systems. Cell extracts (HeLa) and liposomes (100 µg protein) from the experiment shown in (B) were used for SDS–PAGE in the presence of 100 mM DTT. As a negative control, extracts from cells transfected with rBAT are shown.
Reconstituted bo,+AT amino acid transport activity shows substrate specificity and electrogenicity characteristic of system bo,+
Next, we attempted the reconstitution of system bo,+ transport activity from MDCK cells permanently transfected with bo,+AT alone or together with rBAT. In these reconstituted systems similar results were obtained to those shown from HeLa transfected cells (Figure I in Supplementary data). Thus, bo,+AT-reconstituted liposomes showed an overshoot of 0.5 µM l-[3H]arginine transport generated by 2 mM leucine in the internal medium. The transport at the peak of the overshoot was 4.9 ± 0.7 times (i.e. overshoot factor) higher than the equilibrium values (3.4 ± 0.3 pmol/mg protein) (n = 7 preparations). Similar results were obtained from rBAT/bo,+AT-reconstituted liposomes with an average overshoot factor of ∼6 in two independent experiments. In contrast, reconstitution into liposomes of extracts from untransfected MDCK cells did not show any overshoot of arginine and uptake increased with time approaching equilibrium (data not shown). The transport rate of 0.5 µM l-[3H]arginine in the presence of 2 mM leucine in the internal medium of bo,+AT- and rBAT/bo,+AT-reconstituted liposomes was 10 times higher than the background transport rates (i.e. transport in liposomes obtained from non-transfected MDCK cells or in bo,+AT- and rBAT/bo,+AT-reconstituted liposomes with no amino acids inside) (Figure I in Supplementary data). Similarly, no differences were observed between bo,+AT- and rBAT/bo,+AT-reconstituted liposomes when transport of l-[3H]arginine was measured at saturating concentrations (5 and 10 µM) (data not shown).
Western blot analysis showed an identical situation (Figure I in Supplementary data) to that described in the HeLa experiments. (i) rBAT and bo,+AT proteins were expressed only in the liposomes obtained from the corresponding transfected cells. (ii) In non-reducing conditions, heterodimers of rBAT and bo,+AT were visible only in cells expressing rBAT and bo,+AT. (iii) These heterodimers were not detected in cells transfected with bo,+AT alone. These results demonstrate that bo,+AT, which does not form heterodimers with rBAT, can also mediate the exchange of arginine and leucine in liposomes reconstituted from bo,+AT-expressing MDCK cells.
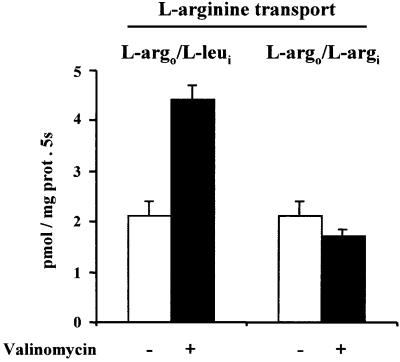
System bo,+ induced by rBAT in Xenopus oocytes has been shown to be electrogenic when a neutral and a dibasic amino acid are exchanged through the transporter (Busch et al., 1994; Coady et al., 1994, 1996; Chillarón et al., 1996). For this reason all the experiments in reconstituted systems in this study were performed with an imposed membrane potential (negative inside) generated by 120 mM KPi in the internal medium of the liposomes and the addition of valinomycin. The effect of the membrane potential on the exchange of external arginine for internal leucine (l-argo/l-leui) or external arginine for internal arginine (l-argo/l-argi) was tested in bo,+AT-reconstituted liposomes from MDCK transfected cells. As shown in Figure 4, the imposed membrane potential (negative inside) doubled the transport rate of arginine in the l-argo/l-leui exchange mode, whereas it did not affect the transport rate of arginine in the l-argo/l-argi exchange mode. This demonstrates that the exchange l-argo/l-leui in the bo,+AT-reconstituted system depends on the membrane potential, whereas the exchange l-argo/l-argi is independent, as expected for system bo,+ transport activity.
Fig. 4. Effect of membrane potential on the amino acid transport activity elicited by bo,+AT in reconstituted systems. bo,+AT-expressing MDCK cells were used for reconstitution in liposomes. Initial transport rates of 0.5 µM l-[3H]arginine (l-argo) into liposomes in the exchange mode l-argo/l-leui or l-argo/l-argi were measured. Liposomes containing no amino acids or 2 mM leucine (l-leui) or arginine (l-argi) in the internal medium were used. Transport in exchange with the internal amino acid was calculated by subtracting transport in the absence of amino acid in the internal medium from transport in its presence. Valinomycin was added when indicated to liposomes containing 120 mM KPi in the internal medium to impose a membrane potential (negative inside). Data (mean ± SEM) correspond to a representative experiment run in triplicate.
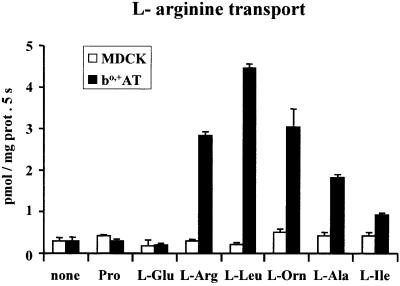
In order to characterize further the amino acid transport activity of the reconstituted bo,+AT, several amino acids were analysed for their capacity to trans-stimulate the transport of arginine. Substrates of the rBAT/bo,+AT-induced system bo,+, such as arginine, ornithine, alanine, leucine or isoleucine, produced trans-stimulation of arginine transport in bo,+AT-reconstituted liposomes, whereas proline or glutamate, which are not substrates of system bo,+, did not trans-stimulate arginine transport (Figure 5). The level of trans-stimulated arginine transport elicited by these system bo,+ substrates parallels the transport of these amino acids induced in cells expressing rBAT and bo,+AT (Chairoungdua et al., 1999; Pfeiffer et al., 1999b). No trans-stimulation was detected in liposomes from non-transfected cells (Figure 5).
Fig. 5. Trans-stimulation of arginine transport by amino acids in bo,+AT-reconstituted systems. bo,+AT-expressing MDCK cells (filled bars) or non-transfected cells (open bars) were used for reconstitution in liposomes. Initial transport rates of 0.5 µM l-[3H]arginine into the liposomes were measured in the absence (none) or presence of the amino acids indicated at 2 mM concentration inside the liposomes. Data (mean ± SEM) from a representative experiment run in triplicate.
Asymmetry of bo,+AT
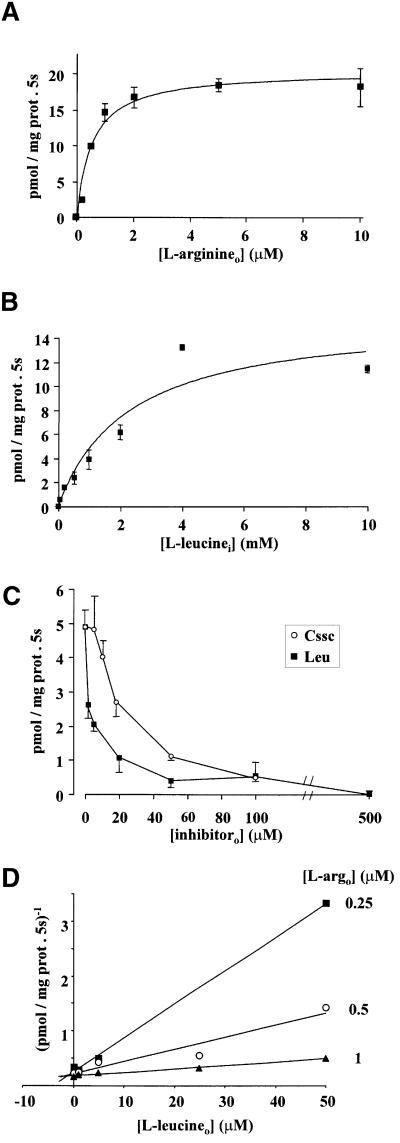
Kinetic analysis of arginine uptake into bo,+AT-reconstituted liposomes revealed a high-affinity (apparent Km ∼0.5 µM) exchange with 2 mM leucine in the internal medium (Figure 6A). Next, we studied the effect of the variation of the leucine concentration in the internal medium on the exchange with 0.5 µM l-[3H]arginine in the external medium. Internal leucine trans-stimulated transport of external arginine with saturation kinetics, and the concentration of internal leucine needed to trans-stimulate half-maximally the transport of arginine was estimated to be ∼2.5 mM (Figure 6B). In order to check the interaction of leucine with bo,+AT at the external face of the liposomes, the exchange of external 0.5 µM l-[3H]arginine with 2 mM internal leucine was measured at different leucine concentrations in the external medium. External leucine almost abolished the exchange at 50 µM; similarly, 100 µM external cystine also almost abolished the exchange (Figure 6C). To characterize the cis-inhibition elicited by leucine, the exchange l-argo/l-leui was measured at three different external arginine concentrations and five different external leucine concentrations. Dixon plots of these studies showed convergent lines crossing at negative values of the x-axis, demonstrating competitive inhibition by external leucine of the l-argo/l-leui exchange, with an apparent Ki of 1.6 µM. In summary, internal leucine trans-stimulated transport of arginine with a semi-maximal concentration in the mM range, whereas external leucine cis-inhibited the exchange l-argo/l-leui with a Ki in the µM range. These results demonstrate that the reconstituted bo,+AT transporter interacts asymmetrically with leucine.
Fig. 6. Kinetic analysis of system bo,+ amino acid transport in bo,+AT-reconstituted liposomes. bo,+AT-expressing MDCK cells were used for reconstitution in liposomes. (A) Kinetic analysis of the leucine-trans-stimulated l-[3H]arginine transport rates. The arginine transport trans-stimulated by leucine was calculated by subtracting transport into liposomes containing no amino acids from the transport into liposomes containing 2 mM leucine. The estimated apparent Km for arginine outside (l-arginineo) was 0.53 ± 0.16 µM (GraphPad Prism program). Data (mean ± SEM) correspond to a representative experiment run in triplicate. An additional experiment gave similar results. (B) Kinetic analysis of the leucine in the internal medium (l-leucinei) trans-stimulating the transport rate of 0.5 µM l-[3H]arginineo. The estimated apparent Km for leucinei was 2.5 ± 0.6 mM (GraphPad Prism program). Data (mean ± SEM) correspond to a representative experiment run in triplicate. An additional experiment gave similar results. (C) Inhibition curve of the leucine-trans-stimulated arginineo transport by the indicated concentrations of cystine (Cssc) and leucine (Leu) outside. The 0.5 µM l-[3H]arginineo transport trans-stimulated by 2 mM leucine in the internal medium was calculated as in (A). Data (mean ± SEM) correspond to a representative experiment run in triplicate. (D) Dixon plot of leucine-trans-stimulated arginine transport inhibited by different leucine concentrations outside (l-leucineo). The transport of 0.25, 0.5 and 1 µM l-[3H]arginineo trans-stimulated by 2 mM leucine in the internal medium was calculated as in (A). Data (mean ± SEM) correspond to a representative experiment run in triplicate. In all panels, when not visible, error bars are smaller than the symbol.
Discussion
In this study we show system bo,+ transport activity in bo,+AT-reconstituted systems from HeLa and MDCK bo,+AT-transfected cells. This transport activity is dependent on (i) the expression of a functional bo,+AT protein in the liposomes, (ii) the presence of system bo,+ amino acid substrates on the trans-side and (iii) the membrane potential for electrogenic arginine/leucine exchange. System bo,+ transport activity in bo,+AT-reconstituted systems is independent of the expression of heterodimers of bo,+AT and rBAT. Thus, the bo,+AT subunit, in the absence of its heavy subunit rBAT, folds into an active form that shows full transport activity. In contrast, routing to the plasma membrane of rBAT and bo,+AT (Feliubadaló et al., 1999; this study) and expression of system bo,+ transport in the plasma membrane require the expression of both the heavy and the corresponding light subunit, as demonstrated earlier for this and other HATs (for reviews see Verrey et al., 2000; Chillarón et al., 2001). Indeed, processing of rBAT from the ER to the Golgi (i.e. maturation of the N-glycosylation of rBAT) depends on the presence of the light subunit bo,+AT. Therefore we have reconstituted functional bo,+AT from an intracellular location. Moreover, bo,+AT seems to protect rBAT against cellular degradation. In contrast, the level of expression of bo,+AT seems to be independent of the expression of rBAT. Therefore, bo,+AT might act as a specific chaperone for rBAT.
The biogenesis of the rBAT/bo,+AT holotransporter remains unknown, but the present study suggests a working model. First, bo,+AT folds into a functional conformation without the participation of rBAT. Then, bo,+AT recognizes rBAT and favours its folding, and finally the rBAT/bo,+AT heterodimers progress to the plasma membrane. Further research is needed to test this hypothesis. In any case, this is at odds with the biogenesis of the K+-transporting P-type ATPases in eukaryotes (for a review see Geering, 2001). These transporters also have an oligomeric structure with a polytopic catalytic α subunit, and a type II glycoprotein β subunit, which is needed for the enzyme function. In these ATPases the β subunit acts as a chaperone for the corresponding α subunit by facilitating the correct membrane integration and packing, acquisition of functional properties and routing to the cell surface of the α subunit. It is becoming more apparent that some transporters, besides HATs and K+-transporting ATPases, require ancillary glycoproteins for their proper plasma membrane expression and current function in eukaryotes (Bruce et al., 1994; Abumrad et al., 1998; Kirk et al., 2000). Thus, CD147 associates with the lactate transporter MCT1 or MCT4 for the expression of functional transporters at the cell surface (Kirk et al., 2000; Wilson et al., 2002). Reconstitution assays of MCT2 or MCT4 alone would indicate whether the ancillary subunit CD147 is necessary for the functional folding of these lactate transporters like the β subunit for the K+-transporting P-type ATPases, or whether it is only necessary for routing to the cell surface like the heavy subunit for HATs.
In addition to the chaperone function in K+-transporting P-type ATPases, β subunits also contribute to the intrinsic transport properties of the pump (i.e. they influence the apparent K+ affinity) (for a review see Geering, 2001). Interestingly, some cystinuria-specific rBAT mutations, in addition to having trafficking defects, also alter the apparent Km for some amino acids of the system bo,+ holotransporter (M.Pineda et al., in preparation). This suggests that mutations in the heavy subunit rBAT transmit conformational changes to the catalytic light subunit bo,+AT. Alternatively, rBAT per se might affect the kinetic properties of the holotransporter. Interestingly, the activity reconstituted in liposomes from bo,+AT-transfected cells is lower than that expressed by rBAT and bo,+AT at the cell surface. Moreover, arginine transport into HeLa cells expressing the holotransporter rBAT/bo,+AT has an apparent Km of ∼200 µM. This is at odds with the estimated apparent Km of 0.5 µM for arginine transport in bo,+AT-reconstituted systems. This difference could be due to the presence of an unstirred layer surrounding cells not present in liposomes that increases the apparent Km values. Indeed, this discrepancy in apparent Km values in cells and liposomes has been reported for other transporters (e.g. GABA, glutamate transporters; Pines et al., 1992). Alternatively, the holotransporter rBAT/bo,+AT might have a lower apparent affinity and a higher transport turnover for amino acids like arginine than the catalytic subunit bo,+AT alone. Reconstitution of the holotransporter is needed to solve this question.
In this study we were unable to reconstitute the holotransporter rBAT/bo,+AT, for two reasons. (i) Total integrity of the disulfide bound rBAT/bo,+AT heterodimers needs the presence of N-ethylmaleimide (NEM) in the homogenization buffer to prevent disulfide bond shuffling (Wang and Tate, 1995). Unfortunately, NEM dramatically inhibits human rBAT/bo,+AT-elicited system bo,+ transport activity, and reconstitution into liposomes of rBAT and bo,+AT in the absence of NEM diminishes the rBAT/bo,+AT heterodimers. (ii) Even in the presence of NEM, there is an excess of bo,+AT expression over that of rBAT in both transient and permanent transfections (data not shown), most probably due to the higher stability of bo,+AT in heterologous expression systems. One way to overcome this situation would be the expression of a bo,+AT–rBAT concatenamer, which expresses system bo,+ activity in Xenopus oocytes (Pfeiffer et al., 1999b). Reconstitution of this bo,+AT–rBAT concatenamer from HeLa transfected cells shows system bo,+ transport activity but the reconstituted activity is very low (i.e. 2-fold over background conditions) (data not shown). Purification before reconstitution will be needed to study the transport characteristics of the concatenamer. This may allow the study of the role of rBAT in the holotransporter function.
The reconstituted system bo,+ shows dramatic asymmetry: high-affinity (µM range) interaction of arginine and leucine from the outside and low-affinity (mM range) interaction of leucine from the inside. Very recently, similar asymmetry of substrate apparent affinities (extracellular apparent Km in the micromolar range and intracellular apparent Km in the millimolar range) has been reported for other heteromeric amino acid transporters (LAT-1 and LAT-2) expressed in oocytes (Meier et al., 2002). We do not know the orientation of bo,+AT protein in our reconstituted systems. Detection of the asymmetry for substrate affinity was possible due to the large difference in the substrate apparent affinity on either side of the transporter: since bo,+AT is an exchanger, at very low substrate concentration outside (µM), only the transporters oriented with the high-affinity site outside would be active and show low-affinity inside. Substrate affinity asymmetry was reported in system bo,+-like exchanger activity from intestinal chicken brush border vesicles (Torras-Llort et al., 2001): the estimated apparent Km for arginine was ∼8 µM outside and ∼180 µM inside. This suggests that the human bo,+AT may be oriented with the high-affinity side towards the renal tubule lumen and the low-affinity side inside the cell. This orientation has a physiological significance. System bo,+ is an exchanger for the influx of cystine and dibasic amino acids and the efflux of neutral amino acids (for a review see Chillarón et al., 2001; Palacín et al., 2001b). Immunoprecipitation studies in renal brush border preparations and clinical data on cystinuria strongly suggest that the rBAT/bo,+AT heterodimer is the main, if not the only, transporter responsible for the apical reabsorption of cystine along the renal proximal tubule (Fernández et al., 2002). More than 99% of the cystine and arginine filtered in the glomerulus is reabsorbed in the proximal tubule (Frimpter et al., 1962; Silbernagl, 1988). Then, a high-affinity system would ensure efficient reabsorption of the substrates even at the end of the proximal tubule. Moreover, low-affinity at the intracellular site of the bo,+ transporter would ensure efficient exchange with the high intracellular concentration of neutral amino acids in the epithelial cells of the proximal tubule.
Mutation in either subunit of the rBAT/bo,+AT holotransporter produces loss of renal reabsorption of cystine and dibasic amino acid that results in cystinuria, and mutations in y+LAT-1 (4F2hc/y+LAT-1 holotransporter) impair renal reabsorption of dibasic amino acid in lysinuric protein intolerance (LPI) (for a review see Palacín et al., 2001b). Eighteen and seven missense mutations have been described for bo,+AT and y+LAT-1 in cystinuria and LPI, respectively (Font et al., 2001; Palacín et al., 2001b). These mutations may cause transport dysfunction by affecting trafficking to the plasma membrane or intrinsic transport activity (Mykkanen et al., 2000). Here we show that reconstitution of the A354T-mutated bo,+AT yields an almost inactive transporter, whereas A182T-mutated bo,+AT retains full reconstituted activity. This demonstrates that, in addition to possible trafficking defects not characterized here, mutation A354T inactivates the transporter, whereas mutation A182T may alter the routing of the holotransporter to the plasma membrane. The degree of the defect caused by these two mutations correlates with the levels of urine cystine and dibasic amino acids in their heterozygotes (i.e. higher hyperexcretion in A354T carriers than in A182T carriers) (Font et al., 2001). Residue A354 is located in putative transmembrane IX and corresponds to a residue with a short side chain (i.e. glycine, alanine or serine) in all known catalytic subunits of HATs. In contrast, residue A182, located in putative transmembrane V, is not conserved among the catalytic subunits of HATs (Font et al., 2001). Further studies are needed to characterize the defects that cause transport inactivation of A354T bo,+AT mutated proteins and the trafficking defect suspected for A182T bo,+AT. Reconstitution of mutated bo,+AT or y+LAT-1 proteins may help to dissect the defects associated with cystinuria and LPI mutations.
Materials and methods
Cell culture
MDCK and HeLa cells were grown in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% fetal calf serum (heat-inactivated), 100 U/ml penicillin (Gibco) and 0.1 mg/ml streptomycin (Gibco) (D10) at 37°C in a humidified atmosphere containing 5% CO2. Stably transfected MDCK-derived cell lines were grown in D10 supplemented with the appropriate selection agent (400 µg/ml geneticin and/or 100 µg/ml hygromycin B).
cDNAs and transfections in HeLa and MDCK cells
Transient transfections were performed in HeLa cells by standard calcium phosphate precipitation in 15 cm diameter plates with a mixture of DNA containing 4 µg of pEGFP (green fluorescence protein; Clontech), 18 µg of pCDNA3-rBAT and 18 µg of pCDNA3-bo,+AT as described (Font et al., 2001). When rBAT, bo,+AT or GAT-1 was transfected alone, the DNA transfection mixture contained 4 µg of pEGFP and 36 µg of pCDNA3-rBAT, pCDNA3-bo,+AT or pCDNA3-GAT-1 (wild-type rat GAT-1 cDNA cloned into the pCDNA3 vector (Invitrogen) between the HindIII and XbaI sites). The GFP-encoding plasmid was included to monitor transfection efficiencies, which ranged from 60 to 90% as assessed by FACS analysis. To obtain a bo,+AT MDCK cell line, parental MDCK-J cells were transfected with pCDNA3-bo,+AT following the standard protocol (Font et al., 2001). Forty-eight hours later, cells were plated at limiting dilution and grown in the presence of geneticin (400 µg/ml) for 2–3 weeks. Individual clones were isolated, expanded and analysed for expression of the bo,+AT protein by western blot. One clone, number 5, was selected for further experiments based on the high level and stable expression of the protein. Similarly, an rBAT/bo,+AT MDCK cell line was engineered starting with the selected bo,+AT-expressing cell line. Briefly, bo,+AT MDCK cells were transfected with pCDNA3Hygro-rBAT [rBAT cDNA cloned into the pCDNA3Hygro3.1 vector (Invitrogen) between the HindIII and XbaI sites] and individual colonies resistant to both geneticin (400 µg/ml) and hygromycin B (100 µg/ml) were isolated. Clones were screened by western blot analysis. In addition, a functional analysis was performed on preselected clones to identify clone 31B, which expresses a high l-cystine transport activity absent in parental MDCK cells.
Reconstitution
For liposome preparation, soybean phospholipids (Asolectin; Sigma) were partially purified as described (Kagawa and Racker, 1971) and crude pig brain lipids were extracted as described previously (Folch et al., 1957). As detergent, cholic acid (Sigma) was used, recrystallized (Kagawa and Racker, 1971) and neutralized with NaOH to pH 7.4. Reconstitution of the transporter into liposomes was performed as described by Radian and Kanner (1985) with minor changes. Briefly, cells from a 15 cm diameter plate were washed twice in phosphate-buffered saline (PBS) and then harvested in 100 µl of PBS. A 25 µl aliquot of the cell suspension was mixed with liposomes and cholate up to a final volume of 200 µl. The mixture was passed through a Sephadex G-50 (Sigma) column pre-equilibrated with the reconstitution buffer (120 mM KPi pH 7.4, 5 mM Tris–SO4, 0.5 mM EDTA, 1 mM MgSO4, 1% glycerol and, where indicated, with the desired concentration of amino acids). Liposomes were passed through a second spin column of identical composition but lacking the amino acid. Control liposomes (without entrapped amino acid) were also passed through a second spin column. The protein concentration in the cell suspension and in the liposomes was determined using a protein assay kit (Bio-Rad).
Uptake measurements
l-[35S]cystine (Amersham) uptake measurements (20 µM) on whole cells were made as described (Font et al., 2001). l-[3H]arginine (American Radiolabelled Chemicals) uptake measurements were performed following the same protocol, using 50 µM l-arginine (40 Ci/mmol, 0.5 µCi/well) and without l-glutamate in the uptake solution described (Font et al., 2001). For l-[3H]arginine transport studies in the reconstituted system, 10 µl of liposomes was mixed with 180 µl of uptake solution [150 mM choline chloride, 10 mM Tris–HEPES, 1 mM MgCl2, 1 mM CaCl2, 0.5 µCi l-[3H]arginine, 2.8 µM valinomycin (Sigma) and l-arginine to the final concentration] equilibrated at 37°C, and incubated at 37°C for different periods. Reactions were terminated by the addition of 1 ml of ice-cold stop solution (150 mM choline chloride, 10 mM Tris–HEPES and 5 mM l-arginine) and filtration through membrane filters (Sartorius, 0.45 µm pore size). Filters were then washed three times in 2 ml of stop solution and dried, and the trapped radioactivity was counted. All experimental values were corrected by subtracting zero-time values obtained by adding the stop solution before the liposomes. Unless otherwise indicated only l-isomers of amino acids were used in this study.
Western blot analysis
Western blot analyses using anti-human bo,+AT antibody (P6-870 Ab) and anti-human rBAT antibody (hrBAT-251 Ab) were carried out as described (Font et al., 2001; Fernández et al., 2002). For both total cell extracts and liposomes, 100 µg of protein in Laemmli sample buffer containing or not 100 mM dithiothreitol (DTT) was loaded in each lane for SDS–PAGE (10% polyacrylamide). As a control, brush border membrane preparations from human kidney were prepared by the Ca2+ precipitation method (Malathi et al., 1979). For brush border membrane preparations, NEM at 5 mM was present in all buffers used (except the resuspension buffer) following Tate and colleagues (Wang and Tate, 1995), in order to prevent artifactual reduction/shuffling of disulfides. The reconstituted systems used in this study were prepared from cell extracts in the absence of NEM because it inactivates system bo,+ in transfected HeLa cells (data not shown). Cell extracts, liposomes and membranes were stored at –80°C until used.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank W.J.Nelson for the MDCK-J cells. We are grateful to A.Rosales and F.Rousaud, from the Fundació Puigvert-IUNA (Barcelona, Spain), for help in kidney biopsies. We thank R.Rycroft for editorial help. This research was supported in part by BIOMED2 CT98-BMH4-3514 EC grant to M.P. and B.K., by DGICYT research grant PM99/0172 from Spain to M.P., by Fundació La Marató-TV3 research grant 981930 to M.P., and by Generalitat de Catalunya Grant 2001 SGR 00118 and support of the Comissionat per a Universitats i Recerca de la Generalitat de Catalunya (Catalonia, Spain) to A.Z. and M.P. N.R. is a recipient of a fellowship from CIRIT (Catalonia, Spain). P.B. and E.F. are recipients of a fellowship from the Ministerio de Educación, Cultura y Deporte (Spain).
References
- Abumrad N., Harmon,C. and Ibrahimi,A. (1998) Membrane transport of long-chain fatty acids: evidence for a facilitated process. J. Lipid Res., 39, 2309–2318. [PubMed] [Google Scholar]
- Bassi M.T., Gasol,E., Manzoni,M., Pineda,M., Riboni,M., Martín,R., Zorzano,A., Borsani,G. and Palacín,M. (2001) Identification and characterisation of human xCT that coexpresses, with 4F2hc heavy chain, the amino acid transport system xc–. Pflügers Arch., 442, 286–296. [DOI] [PubMed] [Google Scholar]
- Bertran J., Werner,A., Moore,M.L., Stange,G., Markovich,D., Biber,J., Testar,X., Zorzano,A., Palacín,M. and Murer,H. (1992) Expression-cloning of a cDNA from rabbit kidney cortex that induces a single transport system for cystine, dibasic and neutral amino acids. Proc. Natl Acad. Sci. USA, 89, 5601–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce L.J., Groves,J.D., Okubo,Y., Thilaganathan,B. and Tanner,M.J. (1994) Altered band 3 structure and function in glycophorin A- and B-deficient (MkMk) red blood cells. Blood, 84, 916–922. [PubMed] [Google Scholar]
- Busch A.E., Herzer,T., Waldegger,S., Schmidt,F., Palacín,M., Biber,J., Markovich,D., Murer,H. and Lang,F. (1994) Opposite directed currents induced by the transport of dibasic and neutral amino acids in Xenopus oocytes expressing the protein rBAT. J. Biol. Chem., 269, 25581–25586. [PubMed] [Google Scholar]
- Calonge M.J. et al. (1994) Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nature Genet., 6, 420–425. [DOI] [PubMed] [Google Scholar]
- Chairoungdua A. et al. (1999) Identification of an amino acid transporter associated with the cystinuria-related Type II membrane glycoprotein. J. Biol. Chem., 274, 28845–28848. [DOI] [PubMed] [Google Scholar]
- Chillarón J. et al. (1996) Obligatory amino acid exchange via systems bo,+-like and y+L-like. A tertiary active transport mechanism for renal reabsorption of cystine and dibasic amino acids. J. Biol. Chem., 271, 17761–17770. [DOI] [PubMed] [Google Scholar]
- Chillarón J., Estévez,R., Samarzija,I., Waldegger,S., Testar,X., Lang,F., Zorzano,A., Busch,A. and Palacín,M. (1997) An intracellular trafficking defect in type I cystinuria rBAT mutants M467T and M467K. J. Biol. Chem., 272, 9543–9549. [DOI] [PubMed] [Google Scholar]
- Chillarón J., Roca,R., Valencia,A., Zorzano,A. and Palacín,M. (2001) Heteromeric amino acid transporters: biochemistry, genetics and physiology. Am. J. Physiol. Renal. Physiol., 281, F995–F1018. [DOI] [PubMed] [Google Scholar]
- Coady M.J., Jalal,F., Chen,X., Lemay,G., Berteloot,A. and Lapointe,J.Y. (1994) Electrogenic amino acid exchange via the rBAT transporter. FEBS Lett., 356, 174–178. [DOI] [PubMed] [Google Scholar]
- Coady M.J., Chen,X.Z. and Lapointe,J.Y. (1996) rBAT is an amino acid exchanger with variable stoichiometry. J. Membr. Biol., 149, 1–8. [DOI] [PubMed] [Google Scholar]
- Devés R. and Boyd,C.A. (2000) Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J. Membr. Biol., 173, 165–177. [DOI] [PubMed] [Google Scholar]
- Feliubadaló L. et al. (International Cystinuria Consortium) (1999) Non-non-Type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo,+AT) of rBAT. Nature Genet., 23, 52–57. [DOI] [PubMed] [Google Scholar]
- Fernández E., Carrascal,M., Rousaud,F., Abián,J., Zorzano,A., Palacín, M. and Chillarón,J. (2002) The rBAT-bo,+AT heterodimer is the main apical reabsorption system for cystine in kidney. Am. J. Physiol. Renal Physiol., 283, F540–F548. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees,M. and Sloane Stanley,G.H. (1957) A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem., 226, 497–509. [PubMed] [Google Scholar]
- Font M. et al. (International Cystinuria Consortium) (2001) Functional analysis of mutations in SLC7A9 and genotype/phenotype correlation in non-Type I cystinuria. Hum. Mol. Genet., 10, 305–316. [DOI] [PubMed] [Google Scholar]
- Frimpter G.W., Horwith,M., Furth,E., Fellows,R.E. and Thompson,D.D. (1962) Inulin and endogenous amino acid renal clearances in cystinuria: evidence for tubular secretion. J. Clin. Invest., 41, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa Y., Segawa,H., Kim,J.Y., Chairoungdua,A., Kim,D.K., Matsuo,H., Cha,S.H., Endou,H. and Kanai,Y. (2000) Identification and characterization of a Na+-independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral d- and l-amino acids. J. Biol. Chem., 275, 9690–9698. [DOI] [PubMed] [Google Scholar]
- Geering K. (2001) The functional role of beta subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr., 33, 425–438. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. and Racker,E. (1971) Partial resolution of the enzymes catalyzing oxidative phosphorylation. J. Biol. Chem., 246, 5477–5487. [PubMed] [Google Scholar]
- Kanai Y., Segawa,H., Miyamoto,K., Uchino,H., Takeda,E. and Endou,H. (1998) Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem., 273, 23629–23632. [DOI] [PubMed] [Google Scholar]
- Kanai Y. et al. (2000) Transport properties of a system y+L neutral and basic amino acid transporter. Insights into the mechanisms of substrate recognition. J. Biol. Chem., 275, 20787–20793. [DOI] [PubMed] [Google Scholar]
- Kirk P., Wilson,M.C., Heddle,C., Brown,M.H., Barclay,A.N. and Halestrap,A.P. (2000) CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J., 19, 3896–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi P., Preiser,H., Fairclough,P., Malett,P. and Crane,R.K. (1979) A rapid method for the isolation of kidney brush border membranes. Biochim. Biophys. Acta, 554, 259–263. [DOI] [PubMed] [Google Scholar]
- Mastroberardino L., Spindler,B., Pfeiffer,R., Skelly,P.J., Loffing,J., Shoemaker,C.B. and Verrey,F. (1998) Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature, 395, 288–291. [DOI] [PubMed] [Google Scholar]
- Meier C., Ristic,Z., Klauser,S. and Verrey,F. (2002) Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J., 21, 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K. et al. (2001) Human cystinuria-related transporter: Localization and functional characterization. Kidney Int., 59, 1821–1833. [DOI] [PubMed] [Google Scholar]
- Mykkanen J. et al. (2000) Functional analysis of novel mutations of y+LAT-1 amino acid transporter gene causing lysinuric protein intolerance (LPI). Hum. Mol. Genet., 9, 431–438. [DOI] [PubMed] [Google Scholar]
- Nakauchi J. et al. (2000) Cloning and characterization of a human brain Na+-independent transporter for small neutral amino acids that transport d-serine with high affinity. Neurosci. Lett., 287, 231–235. [DOI] [PubMed] [Google Scholar]
- Palacín M., Borsani,G. and Sebastio,G. (2001a) The molecular bases of cystinuria and lysinuric protein intolerance. Curr. Opin. Genet. Dev., 11, 328–335. [DOI] [PubMed] [Google Scholar]
- Palacín M., Goodyer,P., Nunes,V. and Gasparini,P. (2001b) Cystinuria. In Scriver,C.R, Beaudet,A.L., Sly,S.W. and Valle,D. (eds), Metabolic and Molecular Bases of Inherited Diseases. 8th edn. McGraw-Hill, New York, pp. 4909–4932.
- Pfeiffer R., Rossier,G., Spindler,B., Meier,C., Kuhn,L. and Verrey,F. (1999a) Amino acid transport of y(+)L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J., 18, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer R., Loffing,J., Rossier,G., Bauch,C., Meier,C., Eggermann,T., Loffing-Cueni,D., Kuhn,L.C. and Verrey,F. (1999b) Luminal heterodimeric amino acid transporter defective in cystinuria. Mol. Biol. Cell, 10, 4135–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda M., Fernández,E., Torrents,D., Estévez,R., Lopez,C., Camps,M., Lloberas,J., Zorzano,A. and Palacín,M. (1999) Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J. Biol. Chem., 274, 19738–19744. [DOI] [PubMed] [Google Scholar]
- Pines G., Danbolt,N.C., Bjoras,M., Zhang,Y., Bendahan,A., Eide,L., Koepsell,H., Storm-Mathisen,J., Seeberg,E. and Kanner,B.I. (1992) Cloning and expression of a rat brain l-glutamate transporter. Nature, 360, 464–467. [DOI] [PubMed] [Google Scholar]
- Radian R. and Kanner,B.I. (1985) Reconstitution and purification of the sodium- and chloride-coupled γ-aminobutyric acid transporter from rat brain. J. Biol. Chem., 260, 11859–11865. [PubMed] [Google Scholar]
- Rossier G., Meier,C., Bauch,C., Summa,V., Sordat,B., Verrey,F. and Kuhn,L.C. (1999) LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J. Biol. Chem., 274, 34948–34954. [DOI] [PubMed] [Google Scholar]
- Sato H., Tamba,M., Ishii,T. and Bannai,S. (1999) Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem., 274, 11455–11448. [DOI] [PubMed] [Google Scholar]
- Sato H., Tamba,M., Kuriyama-Matsumura,K., Okuno,S. and Bannai,S. (2000) Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc–. Antioxid. Redox Signal., 2, 665–671. [DOI] [PubMed] [Google Scholar]
- Segawa H., Fukasawa,Y., Miyamoto,K., Takeda,E., Endou,H. and Kanai,Y. (1999) Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J. Biol. Chem., 274, 19745–19751. [DOI] [PubMed] [Google Scholar]
- Silbernagl S. (1988) The renal handling of amino acids and oligopeptides. Physiol. Rev., 68, 911–1007. [DOI] [PubMed] [Google Scholar]
- Torras-Llort M., Torrents,D., Soriano-Garcia,J.F., Gelpi,J.L., Estévez,R., Ferrer,R., Palacín,M. and Moreto,M. (2001) Sequential amino acid exchange across b0,+-like system in chicken brush border jejunum. J. Membr. Biol., 180, 213–220. [DOI] [PubMed] [Google Scholar]
- Torrents D., Estévez,R., Pineda,M., Fernández,E., Lloberas,J., Shi,Y.B., Zorzano,A. and Palacín,M. (1998) Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J. Biol. Chem., 273, 32437–32445. [DOI] [PubMed] [Google Scholar]
- Verrey F., Jack,D.L., Paulsen,I.T., Saier,M.H.,Jr and Pfeiffer,R. (1999) New glycoprotein-associated amino acid transporters. J. Membr. Biol., 172, 181–192. [DOI] [PubMed] [Google Scholar]
- Verrey F., Meier,C., Rossier,G. and Kuhn,L.C. (2000) Glycoprotein-associated amino acid exchangers: broadening the range of transport specificity. Pflügers Arch., 440, 503–512. [DOI] [PubMed] [Google Scholar]
- Wagner C.A., Lang,F. and Bröer,S. (2001) Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. Cell Physiol., 281, C1077–C1093. [DOI] [PubMed] [Google Scholar]
- Wang Y. and Tate,S.S. (1995) Oligomeric structure of a renal cystine transporter: implications in cystinuria. FEBS Lett., 368, 389–392. [DOI] [PubMed] [Google Scholar]
- Wilson M.C., Meredith,D. and Halestrap,A.P. (2002) Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J. Biol. Chem., 277, 3666–3672. [DOI] [PubMed] [Google Scholar]