With recent advances in molecular genetics, there has been a surge in interest in using stored blood samples for genetic research, even though informed consent at the time of blood sampling did not include this possibility. One of the cornerstones of the World Medical Association's Declaration of Helsinki on ethical principles for medical research is the need for informed consent and the right of any participant in a research project to withdraw at any time.1 We report here our experiences of seeking informed consent for academic and commercial genetic research on blood samples collected more than a decade earlier.
Methods and results
A total of 1583 out of 2000 (79.2%) randomly selected men and women in the age group 25-64 years participated in the 1990 risk factor survey in the World Health Organization's MONICA project.2,3 Participants were given written information and asked to donate blood for “future research on cardiovascular disorders and diabetes.” A total of 1494 blood samples were taken (in 89 participants, consent was not given or technical problems arose in obtaining a blood sample). The blood samples were fractionated and stored at –80° C.
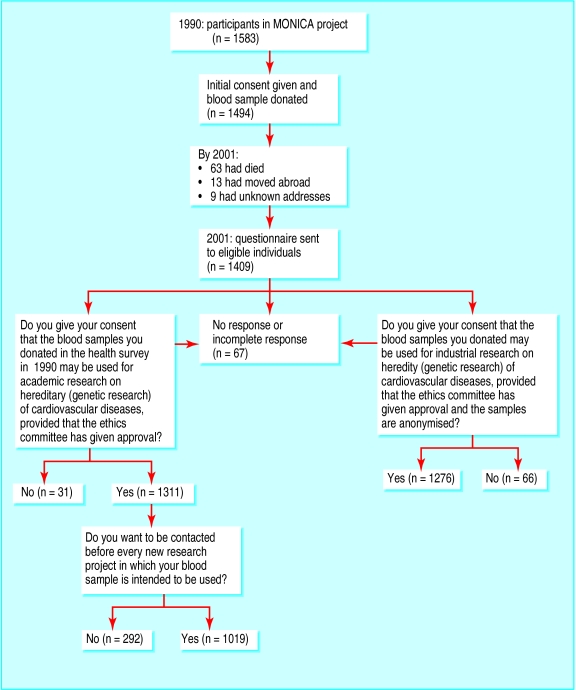
In 2001, 11 years later, 85 of the 1494 individuals had died, moved abroad, or had an unknown address (figure). We sent a letter to the remaining 1409 participants with information about ongoing genetic studies and seeking consent at three different levels (figure). Of the 1409 subjects, 1342 (95.2%) responded.
A total of 1311 out of 1409 (93.0%) eligible participants gave their consent for their blood samples to be used for academic genetic research, provided that the ethics committee had approved the research. Thirty one (2.2%) participants did not give their consent; 64 participants did not reply and three provided incomplete answers (4.8% together).
Of the 1311 participants who gave their consent, 292 (22.3%) wanted to be informed about, and give new consent for, each new genetic project (figure). The rest gave general consent to genetic research, as long as an ethics committee had approved the research. However, a further 35 (2.5%) participants did not give consent for their blood to be used for industrial genetic research (figure).
Comment
Eleven years after donation of samples, only a very small proportion of participants did not give consent for their blood to be used in academic or industrial genetic research.
To our knowledge, this report is the first to provide empirical data from a “real life” situation—that is, people's willingness to give consent to genetic research on their own blood donated more than a decade previously, when genetic research was not an issue. Participation in risk factor screening is, in itself, an expression of willingness to contribute to research. The participants in the original survey in 1990 were randomly selected, with a participation rate as high as 79%, thus suggesting that our results are representative. The MONICA project is well known and well regarded among the people in this part of Sweden, where it has been running since 1985, and the great willingness to give consent to genetic research emphasises the importance of close researcher-participant interaction.
We conclude that (a) it is feasible to obtain individual consent for genetic research many years after blood was donated; (b) people's readiness to contribute to genetic research is high, at least in the framework of a carefully conducted study that is well known to the population; and (c) consent is given nearly as often for industrial genetic research as for academic genetic research, provided that the blood samples are anonymised and an ethics committee has approved the research.
Figure.
Follow up in 2001 of participants in 1990 MONICA survey, showing numbers of participants lost to follow up and responses to questionnaire about consent for blood to be used in genetic research
Eductation and debate p 648
Footnotes
Funding: Swedish Foundation for Strategic Research, the Swedish Research Council, the Swedish Heart and Lung Foundation, and the Swedish Stroke Foundation.
Competing interests: None declared.
References
- 1.World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000;284:3043–3045. [PubMed] [Google Scholar]
- 2.Huhtasaari F, Asplund K, Stegmayr B, Lundberg V, Wester PO. Trends in cardiovascular risk factors in the northern Sweden MONICA study. Who are the winners? Cardiovasc Risk Factors. 1993;3:215–221. [Google Scholar]
- 3.Tunstall-Pedoe H. The World Health Organization MONICA project (monitoring of trends and determinants in cardiovascular diseases): a major international collaboration. J Clin Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]