Abstract
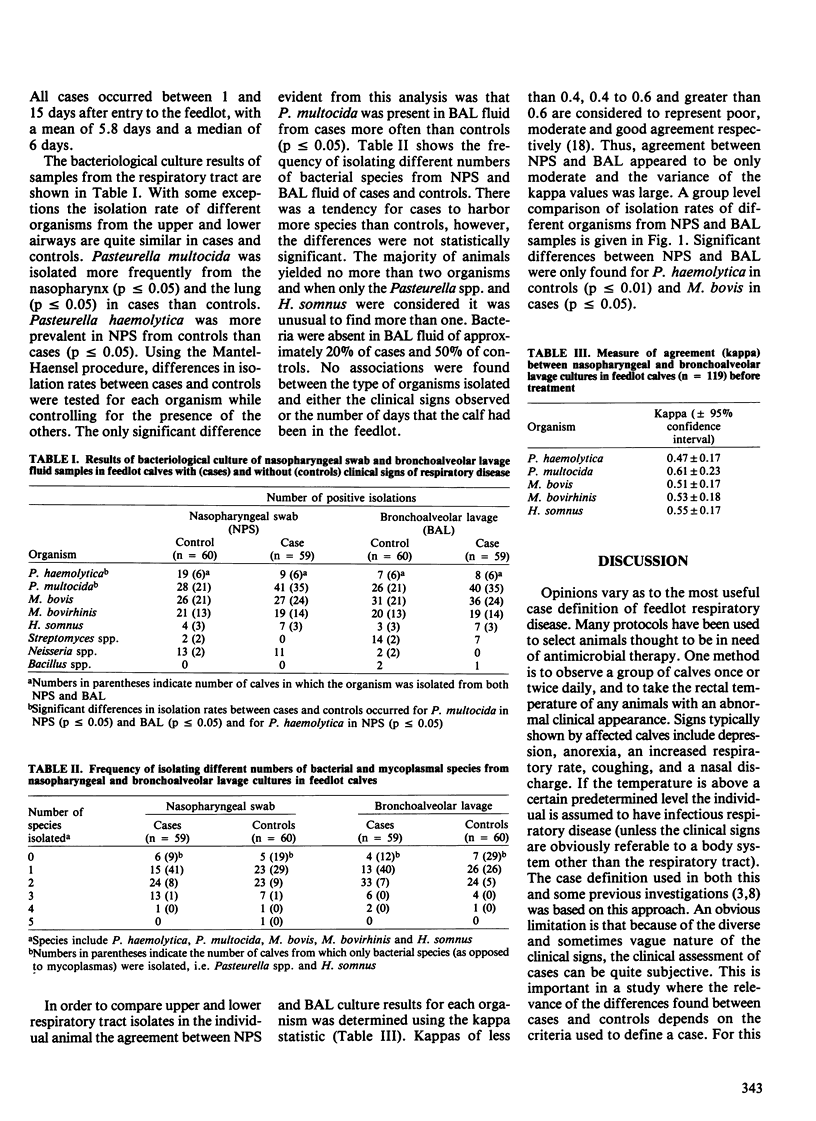
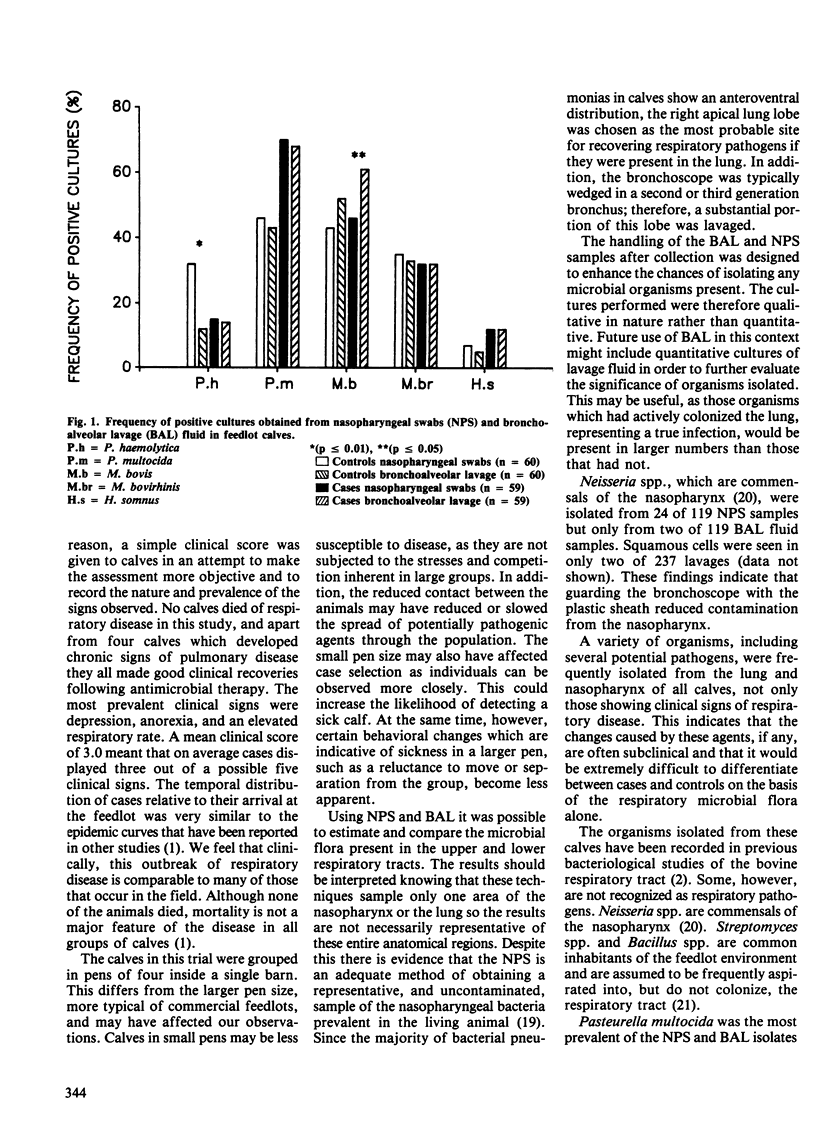
The upper and lower respiratory tracts of 59 feedlot calves with clinical signs of naturally occurring respiratory disease (cases) and 60 comparison (control) animals were cultured before treatment, using nasopharyngeal swabs (NPS) and bronchoalveolar lavage (BAL). The most prevalent organisms were Pasteurella multocida and Mycoplasma bovis. Isolations of P. multocida from NPS and BAL fluid were found to be significantly associated with morbidity (p less than or equal to 0.05), but the frequency with which other organisms were isolated from the nasopharynx and lungs was similar in cases and controls. There was evidence of moderate agreement between NPS and BAL isolates at the individual calf level using the kappa statistic, (range of kappa values = 0.47-0.61) but the variability of the kappa statistics was large. Therefore, in an individual calf NPS cultures did not accurately predict BAL cultures. The NPS and BAL culture results were quite similar at the group level, however.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett R. H., Jasper D. E. Immunosuppression of humoral and cell-mediated responses in calves associated wtih inoculation of Mycoplasma bovis. Am J Vet Res. 1977 Nov;38(11):1731–1738. [PubMed] [Google Scholar]
- Bryson D. G. Calf pneumonia. Vet Clin North Am Food Anim Pract. 1985 Jul;1(2):237–257. doi: 10.1016/S0749-0720(15)31326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis N. F. Selective isolation of slowly growing acidifying mycoplasmas from swine and cattle. Acta Vet Scand. 1979;20(4):607–609. doi: 10.1186/BF03546591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay R. N., Howard C. J., Thomas L. H., Wyld S. G. Pathogenicity of some Mycoplasma and Acholeplasma species in the lungs of gnotobiotic calves. Res Vet Sci. 1979 Sep;27(2):233–237. [PubMed] [Google Scholar]
- Jericho K. W., Carter G. R. Pneumonia in calves produced with aerosols of Pasteurella multocida alone and in combination with bovine herpesvirus 1. Can J Comp Med. 1985 Apr;49(2):138–144. [PMC free article] [PubMed] [Google Scholar]
- Kwiecien J. M., Little P. B. Failure of a selective medium to isolate Haemophilus somnus strains. Aust Vet J. 1989 May;66(5):159–160. doi: 10.1111/j.1751-0813.1989.tb09789.x. [DOI] [PubMed] [Google Scholar]
- Magwood S. E., Barnum D. A., Thomson R. G. Nasal bacterial flora of calves in healthy and in pneumonia-prone herds. Can J Comp Med. 1969 Oct;33(4):237–243. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W., Bateman K. G., Shewen P. E., Rosendal S., Bohac J. E. The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can J Vet Res. 1989 Jul;53(3):355–362. [PMC free article] [PubMed] [Google Scholar]
- Pass D. A., Thompson R. G. Wide distribution of Pasteurella haemolytica type 1 over the nasal mucosa of cattle. Can J Comp Med. 1971 Jul;35(3):181–186. [PMC free article] [PubMed] [Google Scholar]
- Pringle J. K., Viel L., Shewen P. E., Willoughby R. A., Martin S. W., Valli V. E. Bronchoalveolar lavage of cranial and caudal lung regions in selected normal calves: cellular, microbiological, immunoglobulin, serological and histological variables. Can J Vet Res. 1988 Apr;52(2):239–248. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Black F. T. Direct and indirect immunofluorescence of unfixed and fixed Mycoplasma colonies. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):615–622. doi: 10.1111/j.1699-0463.1972.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Martin S. W. The association between serological evidence of mycoplasma infection and respiratory disease in feedlot calves. Can J Vet Res. 1986 Apr;50(2):179–183. [PMC free article] [PubMed] [Google Scholar]
- Schiefer B., Ward G. E., Moffatt R. E. Correlation of microbiological and histological findings in bovine fibrinous pneumonia. Vet Pathol. 1978 May;15(3):313–321. doi: 10.1177/030098587801500305. [DOI] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Antibody titers to Pasteurella haemolytica A1 in Ontario beef cattle. Can J Comp Med. 1982 Oct;46(4):354–356. [PMC free article] [PubMed] [Google Scholar]
- Thomas L. H., Howard C. J., Parsons K. R., Anger H. S. Growth of Mycoplasma bovis in organ cultures of bovine foetal trachea and comparison with Mycoplasma dispar. Vet Microbiol. 1987 Feb;13(2):189–200. doi: 10.1016/0378-1135(87)90044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson R. G., Benson M. L., Savan M. Pneumonic pasteurellosis of cattle: microbiology and immunology. Can J Comp Med. 1969 Jul;33(3):194–206. [PMC free article] [PubMed] [Google Scholar]
- Thomson R. G., Chander S., Savan M., Fox M. L. Investigation of factors of probable significance in the pathogenesis of pneumonic pasteurellosis in cattle. Can J Comp Med. 1975 Apr;39(2):194–207. [PMC free article] [PubMed] [Google Scholar]
- Yates W. D., Kingscote B. F., Bradley J. A., Mitchell D. The relationship of serology and nasal microbiology to pulmonary lesions in feedlot cattle. Can J Comp Med. 1983 Jul;47(3):375–378. [PMC free article] [PubMed] [Google Scholar]


