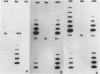
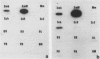
Abstract
Two regions of the primary structure of the small subunit rRNA of Sarcocystis muris bradyzoites were compared with nucleotide sequences of S. gigantea, Toxoplasma gondii, Plasmodium berghei and Mus musculus and used to design genus- and species-specific probes for the detection and identification of coccidia. Total cellular RNA of purified S. muris, S. cruzi, T. gondii and Eimeria nieschulzi and coccidia-infected tissues of mouse, ox, sheep and pig, were assayed using twenty-base oligomers labelled with 32P. Hybridization occurred at temperatures ranging from 21 degrees C to 41 degrees C or 51 degrees C. One probe detected only S. muris and another successfully hybridized to several members of coccidia, including S. muris, S. cruzi, T. gondii and E. nieschulzi. One ng of total cellular RNA was sufficient to yield detectable hybrids in slot blot assays. The excellent sensitivity suggests that rRNA-based probes are capable of detecting individual parasites, and can assay low levels of coccidial infections not detectable by other methods. The results of this study show that it is possible to customize the specificity of rRNA-based probes for diagnostic, epidemiological or taxonomic purposes.
Full text
PDF

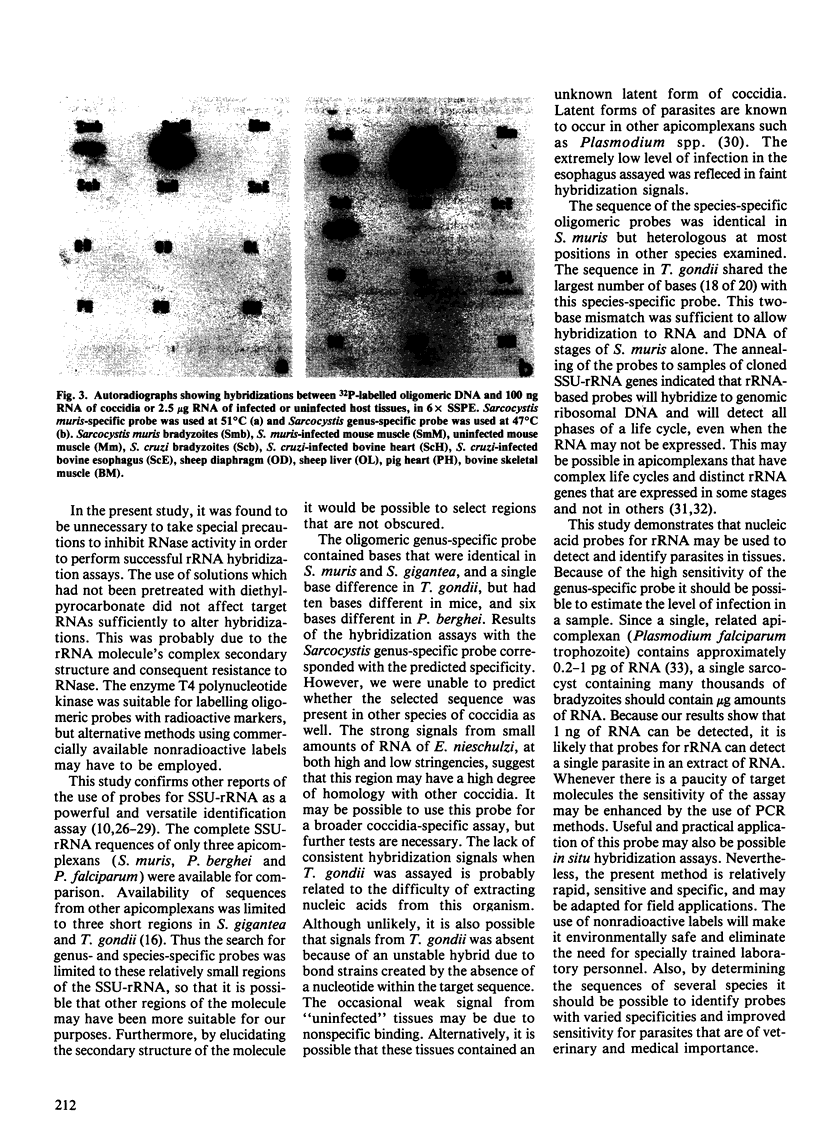

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr G. F. Quantitative cytochemistry of malaria infected erythrocytes (Plasmodium berghei, Plasmodium chabaudi and Plasmodium vinckei). Mil Med. 1969 Sep;134(10):1013–1025. [PubMed] [Google Scholar]
- Box E. D., Smith J. H. The intermediate host spectrum in a Sarcocystis species of birds. J Parasitol. 1982 Aug;68(4):668–673. [PubMed] [Google Scholar]
- Cazenave J., Cheyrou A., Blouin P., Johnson A. M., Begueret J. Use of polymerase chain reaction to detect Toxoplasma. J Clin Pathol. 1991 Dec;44(12):1037–1037. doi: 10.1136/jcp.44.12.1037-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeLong E. F., Wickham G. S., Pace N. R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989 Mar 10;243(4896):1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- Farooqui A. A., Adams D. D., Hanson W. L., Prestwood A. K. Studies on the enzymes of Sarcocystis suicanis: purification and characterization of an acid phosphatase. J Parasitol. 1987 Aug;73(4):681–688. [PubMed] [Google Scholar]
- Gajadhar A. A., Marquardt W. C., Hall R., Gunderson J., Ariztia-Carmona E. V., Sogin M. L. Ribosomal RNA sequences of Sarcocystis muris, Theileria annulata and Crypthecodinium cohnii reveal evolutionary relationships among apicomplexans, dinoflagellates, and ciliates. Mol Biochem Parasitol. 1991 Mar;45(1):147–154. doi: 10.1016/0166-6851(91)90036-6. [DOI] [PubMed] [Google Scholar]
- Gajadhar A. A., Marquardt W. C. Ultrastructural and transmission evidence of Sarcocystis cruzi associated with eosinophilic myositis in cattle. Can J Vet Res. 1992 Jan;56(1):41–46. [PMC free article] [PubMed] [Google Scholar]
- Gajadhar A. A., Yates W. D., Allen J. R. Association of eosinophilic myositis with an unusual species of Sarcocystis in a beef cow. Can J Vet Res. 1987 Jul;51(3):373–378. [PMC free article] [PubMed] [Google Scholar]
- Gunderson J. H., McCutchan T. F., Sogin M. L. Sequence of the small subunit ribosomal RNA gene expressed in the bloodstream stages of Plasmodium berghei: evolutionary implications. J Protozool. 1986 Nov;33(4):525–529. doi: 10.1111/j.1550-7408.1986.tb05656.x. [DOI] [PubMed] [Google Scholar]
- Gunderson J. H., Sogin M. L., Wollett G., Hollingdale M., de la Cruz V. F., Waters A. P., McCutchan T. F. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987 Nov 13;238(4829):933–937. doi: 10.1126/science.3672135. [DOI] [PubMed] [Google Scholar]
- Göbel U. B., Geiser A., Stanbridge E. J. Oligonucleotide probes complementary to variable regions of ribosomal RNA discriminate between Mycoplasma species. J Gen Microbiol. 1987 Jul;133(7):1969–1974. doi: 10.1099/00221287-133-7-1969. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Goman M., Hall R., Osland A., Hope I. A., Langsley G., Zolg J. W., Scaife J. G. Characterisation and translation studies of messenger RNA from the human malaria parasite Plasmodium falciparum and construction of a cDNA library. Mol Biochem Parasitol. 1984 Mar;10(3):269–285. doi: 10.1016/0166-6851(84)90026-4. [DOI] [PubMed] [Google Scholar]
- Johnson A. M., McDonald P. J., Illana S. Characterization and in vitro translation of Toxoplasma gondii ribonucleic acid. Mol Biochem Parasitol. 1986 Mar;18(3):313–320. doi: 10.1016/0166-6851(86)90088-5. [DOI] [PubMed] [Google Scholar]
- Johnson A. M., Murray P. J., Illana S., Baverstock P. J. Rapid nucleotide sequence analysis of the small subunit ribosomal RNA of Toxoplasma gondii: evolutionary implications for the Apicomplexa. Mol Biochem Parasitol. 1987 Oct;25(3):239–246. doi: 10.1016/0166-6851(87)90087-9. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. E., Dubey J. P., Goldschmidt M. H., Saik J. E., Schmitz J. A. Sarcocystis sp. in muscles of domestic cats. Vet Pathol. 1986 Jan;23(1):88–90. doi: 10.1177/030098588602300119. [DOI] [PubMed] [Google Scholar]
- Lal A. A., Changkasiri S., Hollingdale M. R., McCutchan T. F. Ribosomal RNA-based diagnosis of Plasmodium falciparum malaria. Mol Biochem Parasitol. 1989 Aug;36(1):67–71. doi: 10.1016/0166-6851(89)90201-6. [DOI] [PubMed] [Google Scholar]
- Levine N. D. The taxonomy of Sarcocystis (Protozoa, Apicomplexa) species. J Parasitol. 1986 Jun;72(3):372–382. [PubMed] [Google Scholar]
- Markus M. B. Possible support for the sporozoite hypothesis of relapse and latency in malaria. Trans R Soc Trop Med Hyg. 1976;70(5-6):535–535. doi: 10.1016/0035-9203(76)90153-x. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H., Heydorn A. O. The sarcosporidia (Protozoa, Sporozoa): life cycle and fine structure. Adv Parasitol. 1978;16:43–91. doi: 10.1016/s0065-308x(08)60572-2. [DOI] [PubMed] [Google Scholar]
- Munday B. L., Obendorf D. L. Morphology of Sarcocystis gigantea in experimentally-infected sheep. Vet Parasitol. 1984 Nov;16(3-4):193–199. doi: 10.1016/0304-4017(84)90036-0. [DOI] [PubMed] [Google Scholar]
- Raynal F., Michot B., Bachellerie J. P. Complete nucleotide sequence of mouse 18 S rRNA gene: comparison with other available homologs. FEBS Lett. 1984 Feb 27;167(2):263–268. doi: 10.1016/0014-5793(84)80139-8. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H. Structural diversity of eukaryotic small subunit ribosomal RNAs. Evolutionary implications. Ann N Y Acad Sci. 1987;503:125–139. doi: 10.1111/j.1749-6632.1987.tb40603.x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Berger S. L., Kimmel A. R. Molecular hybridization of immobilized nucleic acids: theoretical concepts and practical considerations. Methods Enzymol. 1987;152:399–407. doi: 10.1016/0076-6879(87)52046-8. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Miyada C. G. Oligonucleotide probes for the screening of recombinant DNA libraries. Methods Enzymol. 1987;152:432–442. doi: 10.1016/0076-6879(87)52050-x. [DOI] [PubMed] [Google Scholar]
- Waters A. P., McCutchan T. F. Rapid, sensitive diagnosis of malaria based on ribosomal RNA. Lancet. 1989 Jun 17;1(8651):1343–1346. doi: 10.1016/s0140-6736(89)92800-6. [DOI] [PubMed] [Google Scholar]
- Waters A. P., Syin C., McCutchan T. F. Developmental regulation of stage-specific ribosome populations in Plasmodium. Nature. 1989 Nov 23;342(6248):438–440. doi: 10.1038/342438a0. [DOI] [PubMed] [Google Scholar]
- da Silveira J. F., Mercereau-Puijalon O. Plasmodium chabaudi antigens synthesized in an mRNA-dependent cell-free translation system. Mol Biochem Parasitol. 1983 Feb;7(2):159–172. doi: 10.1016/0166-6851(83)90042-7. [DOI] [PubMed] [Google Scholar]