Abstract
During the summer and fall of 1990 hundreds of striped dolphins (Stenella coeruleoalba) died in the Spanish Mediterranean as a result of morbillivirus infection. A pathological investigation was carried out on dolphins from Valencia and Murcia which were among the first to die in the epizootic. The dolphins were in poor body condition and pneumonia was the main necropsy finding. Microscopic lung lesions characterized by necrosis of bronchial and bronchiolar epithelium and infiltration of alveoli with macrophages, lymphocytes, neutrophils and multinucleated syncytia were seen in most dolphins. Cytoplasmic and nuclear eosinophilic viral inclusions were present in bronchial and bronchiolar epithelium and in syncytia. Focal granulomatous inflammation associated with nematodes was also present. Brain lesions included diffuse degeneration and necrosis of neurons, microgliosis, perivascular cuffing, formation of syncytia and focal demyelination. Cytoplasmic and nuclear eosinophilic inclusions were present in neurons and glial cells. There was severe lymphoid necrosis and depletion of spleen and lymph nodes and syncytia also occurred in lymph nodes. Biliary and transitional epithelium contained nuclear and cytoplasmic eosinophilic inclusions. Immunoperoxidase staining using monoclonal antibodies to phocine distemper virus confirmed the presence of morbillivirus antigens in lung and brain. The distribution and severity of lesions in striped dolphins are similar to those of distemper in seals, harbor porpoises and terrestrial mammals. The formation of syncytia in the lung and brain may be a useful pathological indicator of morbillivirus infection and may be used in the investigation of pinniped and cetacean strandings in North America.
Full text
PDF





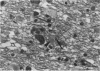
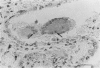
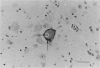
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON P. J., COHEN S., BARKA T. Hepatic injury. A histochemical study of intracytoplasmic globules occurring in liver injury. Arch Pathol. 1961 Jan;71:89–95. [PubMed] [Google Scholar]
- Appel M. J. Pathogenesis of canine distemper. Am J Vet Res. 1969 Jul;30(7):1167–1182. [PubMed] [Google Scholar]
- Brown C. C., Mariner J. C., Olander H. J. An immunohistochemical study of the pneumonia caused by peste des petits ruminants virus. Vet Pathol. 1991 Mar;28(2):166–170. doi: 10.1177/030098589102800209. [DOI] [PubMed] [Google Scholar]
- Dietz R., Ansen C. T., Have P., Heide-Jørgensen M. P. Clue to seal epizootic? Nature. 1989 Apr 20;338(6217):627–627. doi: 10.1038/338627a0. [DOI] [PubMed] [Google Scholar]
- Distemper virus in Baikal seals. Nature. 1989 Mar 16;338(6212):209–210. doi: 10.1038/338209a0. [DOI] [PubMed] [Google Scholar]
- Domingo M., Ferrer L., Pumarola M., Marco A., Plana J., Kennedy S., McAliskey M., Rima B. K. Morbillivirus in dolphins. Nature. 1990 Nov 1;348(6296):21–21. doi: 10.1038/348021a0. [DOI] [PubMed] [Google Scholar]
- Geraci J. R., St Aubin D. J., Barker I. K., Webster R. G., Hinshaw V. S., Bean W. J., Ruhnke H. L., Prescott J. H., Early G., Baker A. S. Mass mortality of harbor seals: pneumonia associated with influenza A virus. Science. 1982 Feb 26;215(4536):1129–1131. doi: 10.1126/science.7063847. [DOI] [PubMed] [Google Scholar]
- Holmes D. D., Smith P. D. Inclusion bodies in hepatic cytoplasm of dogs and rats after administering endotoxin. Am J Vet Res. 1969 May;30(5):811–815. [PubMed] [Google Scholar]
- Kennedy S., Smyth J. A., Cush P. F., Duignan P., Platten M., McCullough S. J., Allan G. M. Histopathologic and immunocytochemical studies of distemper in seals. Vet Pathol. 1989 Mar;26(2):97–103. doi: 10.1177/030098588902600201. [DOI] [PubMed] [Google Scholar]
- Kennedy S., Smyth J. A., Cush P. F., McAliskey M., McCullough S. J., Rima B. K. Histopathologic and immunocytochemical studies of distemper in harbor porpoises. Vet Pathol. 1991 Jan;28(1):1–7. doi: 10.1177/030098589102800101. [DOI] [PubMed] [Google Scholar]
- Kennedy S., Smyth J. A., Cush P. F., McCullough S. J., Allan G. M., McQuaid S. Viral distemper now found in porpoises. Nature. 1988 Nov 3;336(6194):21–21. doi: 10.1038/336021a0. [DOI] [PubMed] [Google Scholar]
- Kennedy S., Smyth J. A., McCullough S. J., Allan G. M., McNeilly F., McQuaid S. Confirmation of cause of recent seal deaths. Nature. 1988 Sep 29;335(6189):404–404. doi: 10.1038/335404a0. [DOI] [PubMed] [Google Scholar]
- Koller L. D. A note on eosinophilic cytoplasmic bodies in the liver of a rabbit. Vet Pathol. 1973;10(4):295–298. doi: 10.1177/030098587301000402. [DOI] [PubMed] [Google Scholar]
- Ladds P. W., Strafuss A. C. Eosinophilic cytoplasmic bodies in a bovine liver. Cornell Vet. 1971 Jul;61(3):486–489. [PubMed] [Google Scholar]
- Osterhaus A. D., Vedder E. J. Identification of virus causing recent seal deaths. Nature. 1988 Sep 1;335(6185):20–20. doi: 10.1038/335020a0. [DOI] [PubMed] [Google Scholar]
- POPPER H., PARONETTO F., BARKA T. PAS-positive structures of nonglycogenic character in normal and abnormal liver. Arch Pathol. 1960 Sep;70:300–313. [PubMed] [Google Scholar]
- Summers B. A., Appel M. J. Syncytia formation: an aid in the diagnosis of canine distemper encephalomyelitis. J Comp Pathol. 1985 Jul;95(3):425–435. doi: 10.1016/0021-9975(85)90047-7. [DOI] [PubMed] [Google Scholar]