Abstract
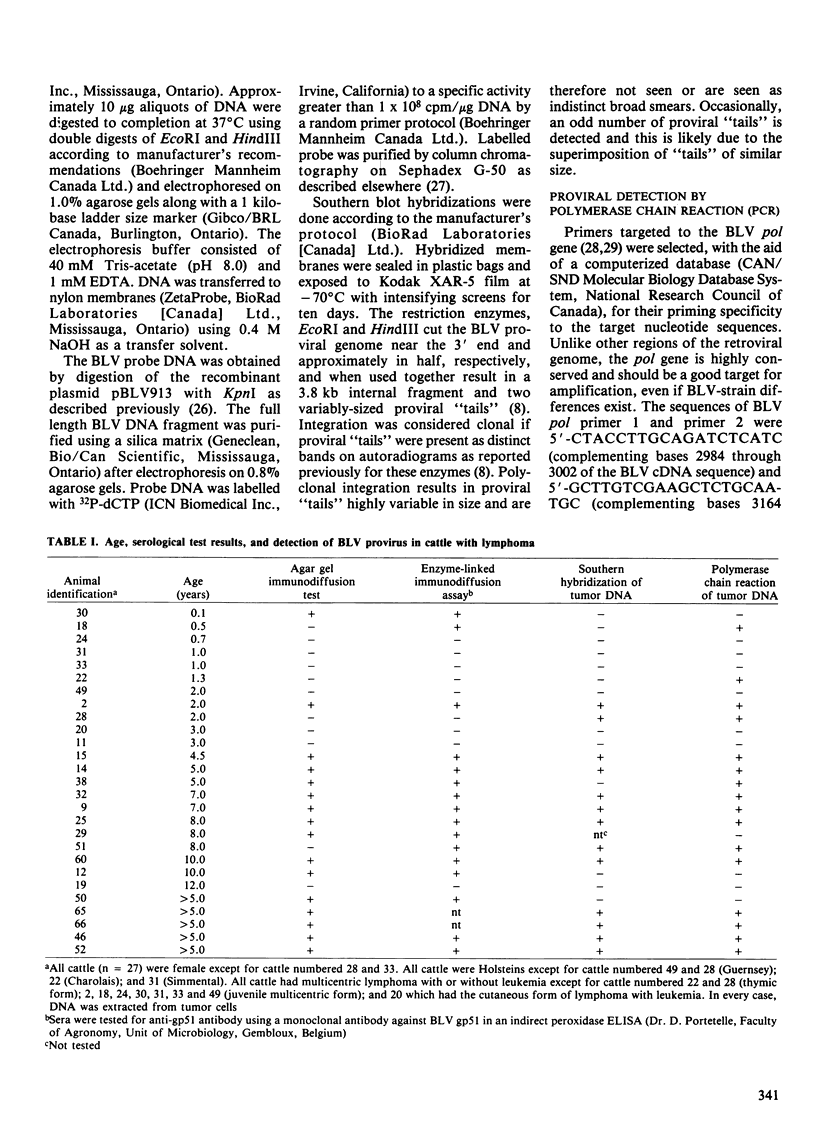
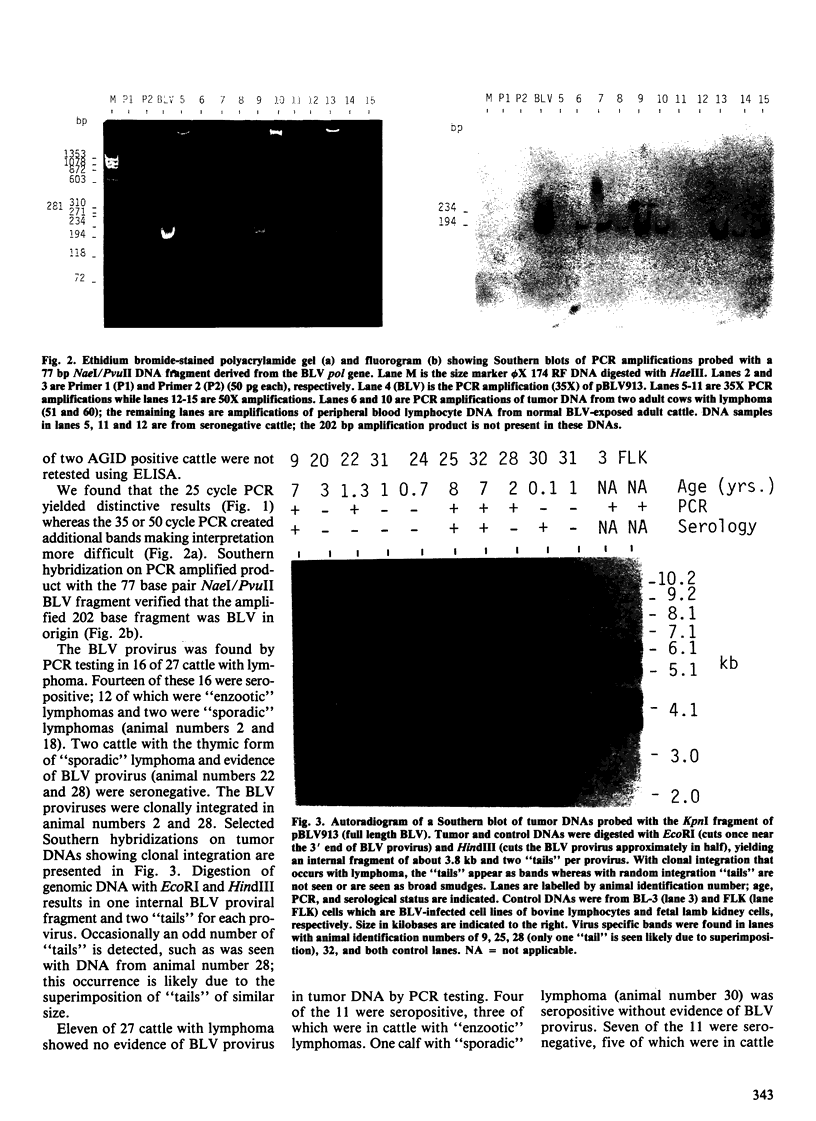
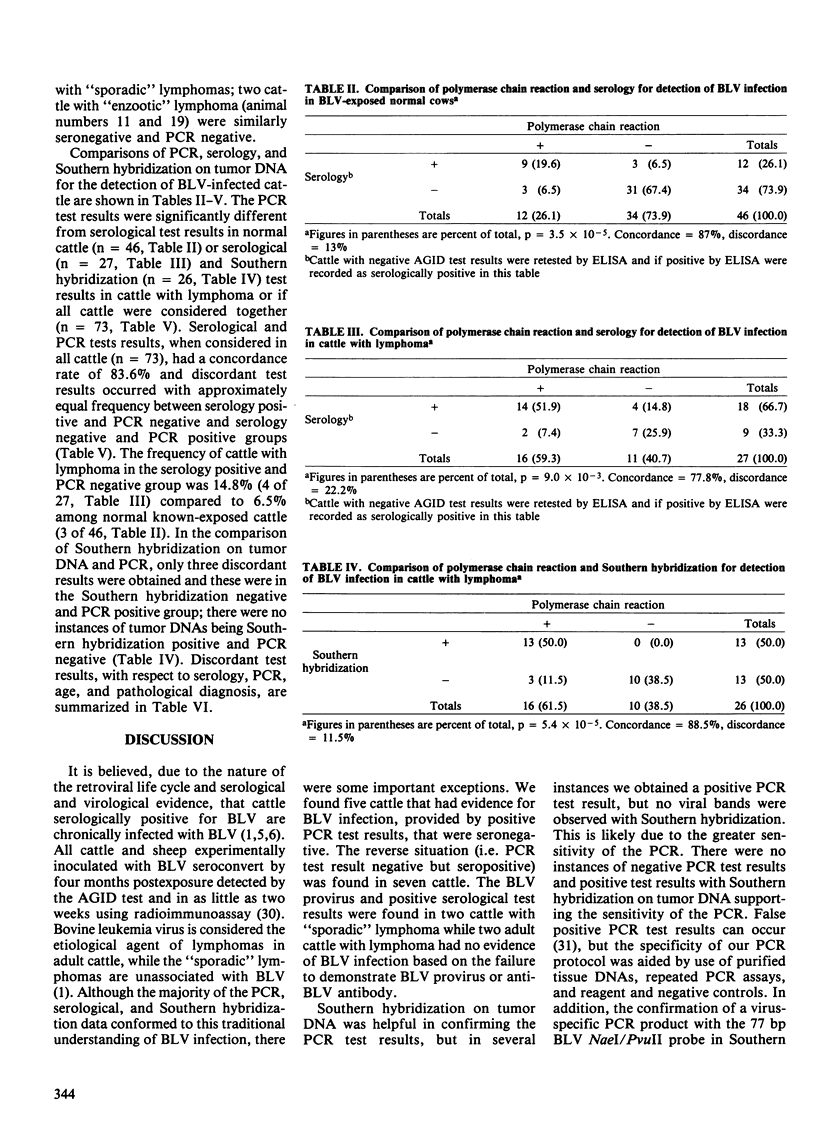
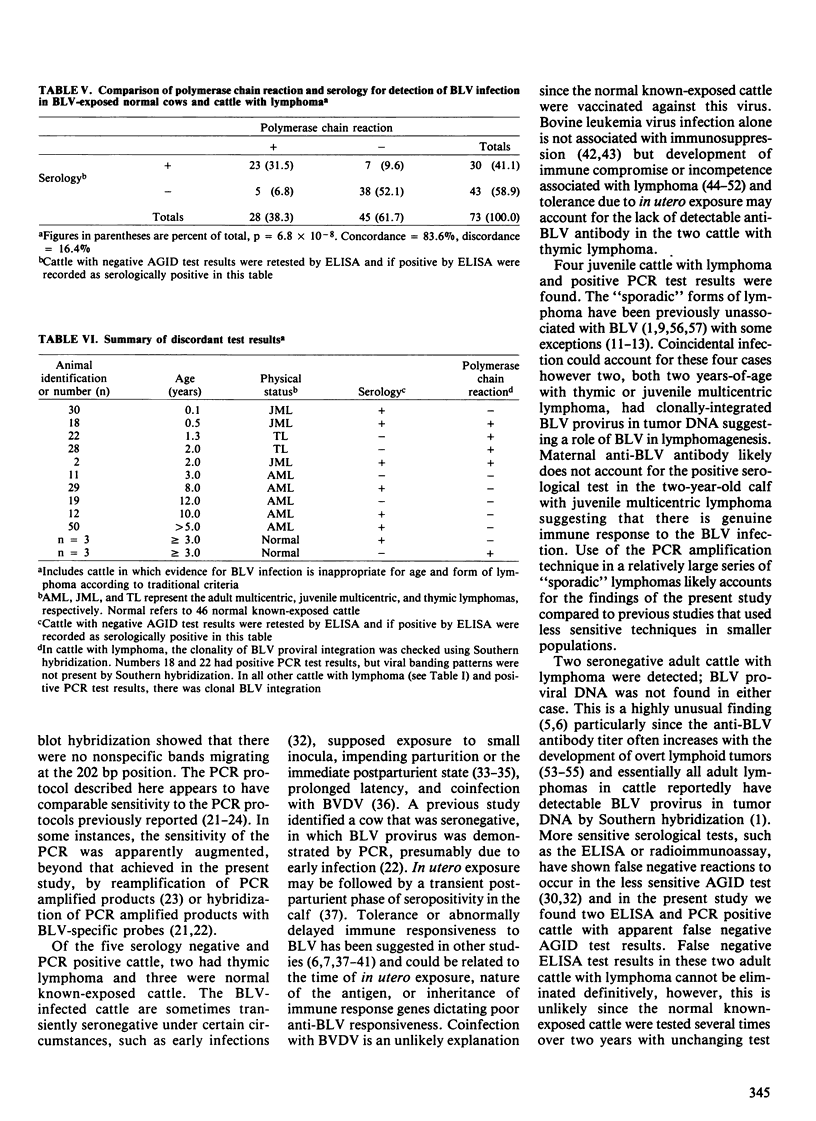
Twenty-seven cattle with lymphoma and 46 cows from a known bovine leukemia virus (BLV)-infected herd were tested for anti-BLV antibody by the agar gel immunodiffusion (AGID) test and an enzyme-linked immunosorbent assay (ELISA). The polymerase chain reaction (PCR) and Southern hybridization were used to detect BLV provirus in the tumor DNA of the 27 cattle with lymphoma. The PCR was used to detect BLV provirus in the peripheral blood mononuclear cell DNA of the 46 normal known-exposed cattle. Two presumed false negative AGID test results compared to ELISA were found. Of ten cattle three years of age or less with "sporadic" forms of lymphoma, four had BLV provirus in tumor DNA, detectable by PCR. In two of these four, BLV provirus was clonally integrated based on digestion of tumor DNA with restriction enzymes followed by Southern hybridization. The BLV provirus was not detected by PCR in 5 of 17 cattle with "enzootic" lymphoma and two of these five were seronegative. Among normal BLV-exposed cows, 6.5% (3 of 46) were serologically positive and PCR negative; serologically negative and PCR positive cows occurred with the same frequency. Serological and PCR test results, when considered in all cattle (n = 73), had a concordance rate of 83.6%. Discordant test results occurred with approximately equal frequency between serologically positive and PCR negative (7 of 73, 9.6%) and serologically negative and PCR positive (5 of 73, 6.8%) groups. These data suggest that the role of BLV in some "sporadic" bovine lymphomas, previously unassociated with BLV, should be reexamined. The BLV provirus was not demonstrable in the tumor DNA from five adult cattle with lymphoma, suggesting that BLV may not be the etiological agent in all adult bovine lymphomas. The findings of persistently seronegative PCR positive and seropositive PCR negative cattle indicate that further work is needed to more fully understand the host-virus interaction. Present serological screening methods may not have sufficient sensitivity for determining BLV status in some circumstances.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atluru D., Johnson D. W., Paul P. S., Muscoplat C. C. B-lymphocyte differentiation, using pokeweed mitogen stimulation: in vitro studies in leukemic and normal cattle. Am J Vet Res. 1979 Apr;40(4):515–520. [PubMed] [Google Scholar]
- Bause I., Maas-Inderwiesen F., Schmidt F. W. Results of an epidemiological survey of enzootic bovine leukosis in the northern part of Lower Saxony and a preliminary communication of an examination into relationship between BLV-antibody development and calving. Ann Rech Vet. 1978;9(4):765–769. [PubMed] [Google Scholar]
- Brandon R. B., Naif H., Daniel R. C., Lavin M. F. Early detection of bovine leukosis virus DNA in infected sheep using the polymerase chain reaction. Res Vet Sci. 1991 Jan;50(1):89–94. doi: 10.1016/0034-5288(91)90059-w. [DOI] [PubMed] [Google Scholar]
- Burridge M. J., Thurmond M. C., Miller J. M., Schmerr M. J., Van Der Maaten M. J. Fall in antibody titer to bovine leukemia virus in the periparturient period. Can J Comp Med. 1982 Jul;46(3):270–271. [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Lieber M. M., Todaro G. J., Graves D. C., Ferrer J. F. Bovine leukemia virus genes in the DNA of leukemic cattle. Science. 1976 Jun 4;192(4243):1005–1007. doi: 10.1126/science.179141. [DOI] [PubMed] [Google Scholar]
- Cockerell G. L., Parodi A. L., Levy D. Immunocompetence of sheep experimentally infected with bovine leukemia virus. Vet Immunol Immunopathol. 1986 Nov;13(3):189–202. doi: 10.1016/0165-2427(86)90072-3. [DOI] [PubMed] [Google Scholar]
- Cockerell G. L., Rovnak J. The correlation between the direct and indirect detection of bovine leukemia virus infection in cattle. Leuk Res. 1988;12(6):465–469. doi: 10.1016/0145-2126(88)90112-9. [DOI] [PubMed] [Google Scholar]
- Derse D. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J Virol. 1987 Aug;61(8):2462–2471. doi: 10.1128/jvi.61.8.2462-2471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes L., Levy D., Parodi A. L., Levy J. P. Spontaneous immune response of bovine leukemia-virus-infected cattle against five different viral proteins. Int J Cancer. 1980 Apr 15;25(4):503–508. doi: 10.1002/ijc.2910250412. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Stephenson J. R., Sarma P. S., Aaronson S. A., Charder S. Bovine lymphosarcoma: development of a radioimmunologic technique for detection of the etiologic agent. Science. 1976 Dec 24;194(4272):1428–1430. doi: 10.1126/science.188129. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F., Abt D. A., Bhatt D. M., Marshak R. R. Studies on the relationship between infection with bovine C-type virus, leukemia, and persistent lymphocytosis in cattle. Cancer Res. 1974 Apr;34(4):893–900. [PubMed] [Google Scholar]
- Grégoire D., Couez D., Deschamps J., Heuertz S., Hors-Cayla M. C., Szpirer J., Szpirer C., Burny A., Huez G., Kettmann R. Different bovine leukemia virus-induced tumors harbor the provirus in different chromosomes. J Virol. 1984 Apr;50(1):275–279. doi: 10.1128/jvi.50.1.275-279.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeney J. L., Valli V. E., Montesanti J. Alterations in humoral immune response to bovine leukaemia virus antigens in cattle with lymphoma. J Gen Virol. 1988 Mar;69(Pt 3):659–666. doi: 10.1099/0022-1317-69-3-659. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K., Yoshikura H. Frequent partial deletion of human adult T-cell leukemia virus type I proviruses in experimental transmission: pattern and possible implication. J Virol. 1986 May;58(2):508–512. doi: 10.1128/jvi.58.2.508-512.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. M., Valli V. E., Wilkie B. N. Inhibition of lymphocyte blastogenesis by sera from cows with lymphoma. Am J Vet Res. 1980 Mar;41(3):372–376. [PubMed] [Google Scholar]
- Jacobs R. M., Valli V. E., Wilkie B. N. Response of cows with lymphoma to the intradermal injection of tumor cell antigens and phytohemagglutinin. Can J Comp Med. 1981 Jan;45(1):43–50. [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. M., Valli V. E., Wilkie B. N. Serum electrophoresis and immunoglobulin concentrations in cows with lymphoma. Am J Vet Res. 1980 Dec;41(12):1942–1946. [PubMed] [Google Scholar]
- Jacobsen K. L., Kaneene J. B., Miller J. M., Bull R. W. Comparison of the commercial agar-gel immunodiffusion test and radioimmunoprecipitation assay for detection of antibodies to bovine leukemia virus. Am J Vet Res. 1985 Jul;46(7):1430–1433. [PubMed] [Google Scholar]
- Kaaden O. R., Lange S., Romanowski W., Marré H., Pfeilsticker J., Roselius R. Transient viraemia with bovine leukaemia virus in bulls. Zentralbl Veterinarmed B. 1982 May;29(4):269–274. doi: 10.1111/j.1439-0450.1982.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Kaaden O. R., Neth R., Frenzel B. Sequential studies of bovine leukemia virus antibody development in dairy cattle over a four-year period. Ann Rech Vet. 1978;9(4):771–776. [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Couez D., Claustriaux J. J., Palm R., Burny A. Chromosome integration domain for bovine leukemia provirus in tumors. J Virol. 1983 Jul;47(1):146–150. doi: 10.1128/jvi.47.1.146-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Marbaix G., Cleuter Y., Portetelle D., Mammerickx M., Burny A. Genomic integration of bovine leukemia provirus and lack of viral RNA expression in the target cells of cattle with different responses to BLV infection. Leuk Res. 1980;4(6):509–519. doi: 10.1016/0145-2126(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Kettmann R., Meunier-Rotival M., Cortadas J., Cuny G., Ghysdael J., Mammerickx M., Burny A., Bernardi G. Integration of bovine leukemia virus DNA in the bovine genome. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4822–4826. doi: 10.1073/pnas.76.10.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Portetelle D., Mammerickx M., Cleuter Y., Dekegel D., Galoux M., Ghysdael J., Burny A., Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Sentsui H., Arai K., Fujigaki A., Enomoto C., Iwasaki H., Ishida H. Serological methods to detect calves infected in utero with bovine leukemia virus. Nihon Juigaku Zasshi. 1983 Aug;45(4):453–461. doi: 10.1292/jvms1939.45.453. [DOI] [PubMed] [Google Scholar]
- Kono Y., Sentsui H., Arai K., Irishio W., Fujigaki A. Studies of foetuses from cows clinically affected with bovine leucosis. Vet Microbiol. 1983 Oct;8(5):505–509. doi: 10.1016/0378-1135(83)90044-5. [DOI] [PubMed] [Google Scholar]
- Koyama H., Hohdatsu T., Nagai T., Tsubaki S. Determination of lymphocyte count necessary for isolating bovine leukaemia virus (BLV) from BLV-infected cattle and correlation between lymphocyte count and antibody titre. Zentralbl Veterinarmed B. 1988 Nov;35(9):648–653. doi: 10.1111/j.1439-0450.1988.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Kwok S., Ehrlich G., Poiesz B., Kalish R., Sninsky J. J. Enzymatic amplification of HTLV-I viral sequences from peripheral blood mononuclear cells and infected tissues. Blood. 1988 Oct;72(4):1117–1123. [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. False-positive results and the polymerase chain reaction. Lancet. 1988 Sep 17;2(8612):679–679. doi: 10.1016/s0140-6736(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Mammerickx M., Burny A., Kettmann R., Portetelle D. A bovine thymic lymphosarcoma case showing a negative serological response to bovine leukemia virus antigens, in a herd with high incidence of enzootic bovine leukosis. Zentralbl Veterinarmed B. 1981;28(9-10):733–742. doi: 10.1111/j.1439-0450.1981.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Miller J. M., Schmerr M. J., Van Der Maaten M. J. Comparison of four serologic tests for the detection of antibodies to bovine leukemia virus. Am J Vet Res. 1981 Jan;42(1):5–8. [PubMed] [Google Scholar]
- Murtaugh M. P., Lin G. F., Haggard D. L., Weber A. F., Meiske J. C. Detection of bovine leukemia virus in cattle by the polymerase chain reaction. J Virol Methods. 1991 Jun;33(1-2):73–85. doi: 10.1016/0166-0934(91)90009-o. [DOI] [PubMed] [Google Scholar]
- Naif H. M., Brandon R. B., Daniel R. C., Lavin M. F. Bovine leukaemia proviral DNA detection in cattle using the polymerase chain reaction. Vet Microbiol. 1990 Nov;25(2-3):117–129. doi: 10.1016/0378-1135(90)90071-3. [DOI] [PubMed] [Google Scholar]
- Odawara T., Onuma M., Yoshikawa H., Yoshikawa T., Izawa H. Circulating immune complex levels in cows with enzootic bovine leukosis. Nihon Juigaku Zasshi. 1987 Aug;49(4):657–661. doi: 10.1292/jvms1939.49.657. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Sagata N., Tsuzuku-Kawamura J., Onuma M., Izawa H., Ikawa Y. No involvement of bovine leukemia virus in sporadic bovine lymphosarcoma. Microbiol Immunol. 1986;30(7):697–701. doi: 10.1111/j.1348-0421.1986.tb02995.x. [DOI] [PubMed] [Google Scholar]
- Olson C., Miller L. D., Miller J. M., Hoss H. E. Transmission of lymphosarcoma from cattle to sheep. J Natl Cancer Inst. 1972 Nov;49(5):1463–1467. [PubMed] [Google Scholar]
- Olson C., Miller L. D., Miller J. M. Role of C-type virus in bovine lymphosarcoma. Bibl Haematol. 1973;39:198–205. doi: 10.1159/000427842. [DOI] [PubMed] [Google Scholar]
- Onuma M., Okada K., Yamazaki Y., Fujinaga K., Fujimoto Y., Mikami T. Induction of C-type virus in cell lines derived from calf form bovine lymphosarcoma. Microbiol Immunol. 1978;22(11):683–691. doi: 10.1111/j.1348-0421.1978.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Portetelle D., Bruck C., Mammerickx M., Burny A. In animals infected by bovine leukemia virus (BLV) antibodies to envelope glycoprotein gp51 are directed against the carbohydrate moiety. Virology. 1980 Aug;105(1):223–233. doi: 10.1016/0042-6822(80)90169-5. [DOI] [PubMed] [Google Scholar]
- Portetelle D., Mammerickx M., Burny A. Use of two monoclonal antibodies in an ELISA test for the detection of antibodies to bovine leukaemia virus envelope protein gp51. J Virol Methods. 1989 Feb;23(2):211–222. doi: 10.1016/0166-0934(89)90135-3. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Burny A., Gilden R. V. The gag and pol genes of bovine leukemia virus: nucleotide sequence and analysis. Virology. 1985 Apr 30;142(2):357–377. doi: 10.1016/0042-6822(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Roberts D. H., Lucas M. H., Wibberley G., Westcott D. Response of cattle persistently infected with bovine virus diarrhoea virus to bovine leukosis virus. Vet Rec. 1988 Mar 26;122(13):293–296. doi: 10.1136/vr.122.13.293. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Ehrlich G. D., Ferrer J. F., Sninsky J. J., Zandomeni R., Dock N. L., Poiesz B. Amplification and analysis of specific DNA and RNA sequences of bovine leukemia virus from infected cows by polymerase chain reaction. J Clin Microbiol. 1992 Jan;30(1):185–191. doi: 10.1128/jcm.30.1.185-191.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu H., Inumaru S., Nakajima H. Inhibition of in vitro immunocyte function by sera from cattle with bovine leukosis. Vet Immunol Immunopathol. 1988 Jun;18(4):349–359. doi: 10.1016/0165-2427(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Takashima I., Olson C., Driscoll D. M., Baumgartener L. E. B-lymphocytes and T-lymphocytes in three types of bovine lymphosarcoma. J Natl Cancer Inst. 1977 Oct;59(4):1205–1209. doi: 10.1093/jnci/59.4.1205. [DOI] [PubMed] [Google Scholar]
- Trainin Z., Ungar-Waron H., Meirom R., Barnea A., Sela M. IgG and IgM antibodies in normal and leukaemic cattle. J Comp Pathol. 1976 Oct;86(4):571–580. doi: 10.1016/0021-9975(76)90066-9. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. Human T-lymphotropic retroviruses. Nature. 1985 Oct 3;317(6036):395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]
- Wyatt C. R., Wingett D., White J. S., Buck C. D., Knowles D., Reeves R., Magnuson N. S. Persistent infection of rabbits with bovine leukemia virus associated with development of immune dysfunction. J Virol. 1989 Nov;63(11):4498–4506. doi: 10.1128/jvi.63.11.4498-4506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Hattori S., Seiki M. Molecular biology of human T-cell leukemia virus associated with adult T-cell leukemia. Curr Top Microbiol Immunol. 1985;115:157–175. doi: 10.1007/978-3-642-70113-9_11. [DOI] [PubMed] [Google Scholar]