Abstract
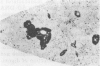
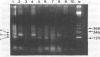
The prevalence of feline leukemia virus (FeLV) antigen and DNA was assessed in formalin-fixed, paraffin-embedded tumor tissues from 70 cats with lymphosarcoma (LSA). Tissue sections were tested for FeLV gp70 antigen using avidinbiotin complex (ABC) immunohistochemistry (IHC); DNA was extracted and purified from the same tissue blocks for polymerase chain reaction (PCR) amplification of a 166 base pair region of the FeLV long terminal repeat (LTR). Results were related to antemortem FeLV enzyme-linked immunosorbent assay (ELISA) for serum p27 antigen, anatomic site of LSA, and patient age. Viral DNA was detected by PCR in 80% of cases and viral antigen by IHC in 57% of cases. Seventeen cases were PCR-positive and IHC-negative; one case was PCR-negative and IHC-positive. Clinical records included FeLV ELISA results for 30 of 70 cats. All 19 ELISA-positive cats were positive by PCR and IHC; of the 11 ELISA-negative cats that were negative by IHC, seven were positive by PCR. When evaluated according to anatomic site, FeLV DNA and antigen were detected less frequently in intestinal LSAs than in multicentric and mediastinal tumors. Lymphosarcoma tissues from cats < 7 yr were several fold more likely to be positive for FeLV antigen by IHC than were tumors from cats > or = 7 yr. However, there was no significant difference in PCR detection of FeLV provirus between LSAs from cats < 7 yr and those > or = 7 yr.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Erlich H. Polymerase chain reaction strategy. Annu Rev Biochem. 1992;61:131–156. doi: 10.1146/annurev.bi.61.070192.001023. [DOI] [PubMed] [Google Scholar]
- Blue J. T., French T. W., Kranz J. S. Non-lymphoid hematopoietic neoplasia in cats: a retrospective study of 60 cases. Cornell Vet. 1988 Jan;78(1):21–42. [PubMed] [Google Scholar]
- Callanan J. J., McCandlish I. A., O'Neil B., Lawrence C. E., Rigby M., Pacitti A. M., Jarrett O. Lymphosarcoma in experimentally induced feline immunodeficiency virus infection [corrected]. Vet Rec. 1992 Apr 4;130(14):293–295. doi: 10.1136/vr.130.14.293. [DOI] [PubMed] [Google Scholar]
- Casey J. W., Roach A., Mullins J. I., Burck K. B., Nicolson M. O., Gardner M. B., Davidson N. The U3 portion of feline leukemia virus DNA identifies horizontally acquired proviruses in leukemic cats. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7778–7782. doi: 10.1073/pnas.78.12.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter S. M. Feline leukemia virus: pathophysiology, prevention, and treatment. Cancer Invest. 1992;10(2):173–181. doi: 10.3109/07357909209032778. [DOI] [PubMed] [Google Scholar]
- Cotter S. M., Hardy W. D., Jr, Essex M. Association of feline leukemia virus with lymphosarcoma and other disorders in the cat. J Am Vet Med Assoc. 1975 Mar 1;166(5):449–454. [PubMed] [Google Scholar]
- Defer C., Agut H., Garbarg-Chenon A., Moncany M., Morinet F., Vignon D., Mariotti M., Lefrère J. J. Multicentre quality control of polymerase chain reaction for detection of HIV DNA. AIDS. 1992 Jul;6(7):659–663. doi: 10.1097/00002030-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Donahue P. R., Hoover E. A., Beltz G. A., Riedel N., Hirsch V. M., Overbaugh J., Mullins J. I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988 Mar;62(3):722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. P., Cotter S. M., Hardy W. D., Jr, Essex M. Comparison of virus-positive and virus-negative cases of feline leukemia and lymphoma. Cancer Res. 1979 Oct;39(10):3866–3870. [PubMed] [Google Scholar]
- Francis D. P., Essex M., Cotter S. M., Gutensohn N., Jakowski R., Hardy W. D., Jr Epidemiologic association between virus-negative feline leukemia and the horizontally transmitted feline leukemia virus. Cancer Lett. 1981 Mar;12(1-2):37–42. doi: 10.1016/0304-3835(81)90035-5. [DOI] [PubMed] [Google Scholar]
- Fulton R., Plumb M., Shield L., Neil J. C. Structural diversity and nuclear protein binding sites in the long terminal repeats of feline leukemia virus. J Virol. 1990 Apr;64(4):1675–1682. doi: 10.1128/jvi.64.4.1675-1682.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines D. M., Chelack B. J. Technical considerations for developing enzyme immunohistochemical staining procedures on formalin-fixed paraffin-embedded tissues for diagnostic pathology. J Vet Diagn Invest. 1991 Jan;3(1):101–112. doi: 10.1177/104063879100300128. [DOI] [PubMed] [Google Scholar]
- Haines D. M., Clark E. G. Enzyme immunohistochemical staining of formalin-fixed tissues for diagnosis in veterinary pathology. Can Vet J. 1991 May;32(5):295–302. [PMC free article] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Hess P. W., MacEwen E. G., McClelland A. J., Zuckerman E. E., Essex M., Cotter S. M., Jarrett O. Biology of feline leukemia virus in the natural environment. Cancer Res. 1976 Feb;36(2 Pt 2):582–588. [PubMed] [Google Scholar]
- Hardy W. D., Jr, McClelland A. J., Zuckerman E. E., Snyder H. W., Jr, MacEwen E. G., Francis D., Essex M. Development of virus non-producer lymphosarcomas in pet cats exposed to FeLv. Nature. 1980 Nov 6;288(5786):90–92. doi: 10.1038/288090a0. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Zuckerman E. E., MacEwen E. G., Hayes A. A., Essex M. A feline leukaemia virus- and sarcoma virus-induced tumour-specific antigen. Nature. 1977 Nov 17;270(5634):249–251. doi: 10.1038/270249a0. [DOI] [PubMed] [Google Scholar]
- Harvey J. W., Shields R. P., Gaskin J. M. Feline myeloproliferative disease. Changing manifestations in the peripheral blood. Vet Pathol. 1978 Jul;15(4):437–448. doi: 10.1177/030098587801500401. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Mullins J. I. Feline leukemia virus infection and diseases. J Am Vet Med Assoc. 1991 Nov 15;199(10):1287–1297. [PubMed] [Google Scholar]
- Koshy R., Wong-Staal F., Gallo R. C., Hardy W., Essex M. Distribution of feline leukemia virus DNA sequences in tissues of normal and leukemic domestic cats. Virology. 1979 Nov;99(1):135–144. doi: 10.1016/0042-6822(79)90044-8. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lanzillo J. J. Preparation of digoxigenin-labeled probes by the polymerase chain reaction. Biotechniques. 1990 Jun;8(6):620–622. [PubMed] [Google Scholar]
- Lion T., Haas O. A. Nonradioactive labeling of probe with digoxigenin by polymerase chain reaction. Anal Biochem. 1990 Aug 1;188(2):335–337. doi: 10.1016/0003-2697(90)90616-h. [DOI] [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. False-positive results and the polymerase chain reaction. Lancet. 1988 Sep 17;2(8612):679–679. doi: 10.1016/s0140-6736(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Multiplying genes by leaps and bounds. Science. 1988 Jun 10;240(4858):1408–1410. doi: 10.1126/science.3375831. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Momoi Y., Watari T., Goitsuka R., Tsujimoto H., Hasegawa A. Detection of enhancer repeats in the long terminal repeats of feline leukemia viruses from cats with spontaneous neoplastic and nonneoplastic diseases. Virology. 1992 Aug;189(2):745–749. doi: 10.1016/0042-6822(92)90598-j. [DOI] [PubMed] [Google Scholar]
- Miura T., Shibuya M., Tsujimoto H., Fukasawa M., Hayami M. Molecular cloning of a feline leukemia provirus integrated adjacent to the c-myc gene in a feline T-cell leukemia cell line and the unique structure of its long terminal repeat. Virology. 1989 Apr;169(2):458–461. doi: 10.1016/0042-6822(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Brody D. S., Binari R. C., Jr, Cotter S. M. Viral transduction of c-myc gene in naturally occurring feline leukaemias. 1984 Apr 26-May 2Nature. 308(5962):856–858. doi: 10.1038/308856a0. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Stephenson J. R., Gardner M. B., Roy-Burman P. RD-114 and feline leukaemia virus genome expression in natural lymphomas of domestic cats. Nature. 1977 Mar 24;266(5600):357–360. doi: 10.1038/266357a0. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Moore J. L., Schochetman G. Use of UV irradiation to reduce false positivity in polymerase chain reaction. Biotechniques. 1991 Apr;10(4):442–446. [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Riedel N., Hoover E. A., Mullins J. I. Transduction of endogenous envelope genes by feline leukaemia virus in vitro. Nature. 1988 Apr 21;332(6166):731–734. doi: 10.1038/332731a0. [DOI] [PubMed] [Google Scholar]
- Pacitti A. M., Jarrett O. Duration of the latent state in feline leukaemia virus infections. Vet Rec. 1985 Nov 2;117(18):472–474. doi: 10.1136/vr.117.18.472-a. [DOI] [PubMed] [Google Scholar]
- Persing D. H. Polymerase chain reaction: trenches to benches. J Clin Microbiol. 1991 Jul;29(7):1281–1285. doi: 10.1128/jcm.29.7.1281-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymerase chain reaction and direct DNA tests in detection of human papillomavirus (HPV) DNA in cytologically normal and abnormal cervical smears. Scandinavian Multicenter Study Group. Acta Obstet Gynecol Scand. 1992 Feb;71(2):98–103. doi: 10.3109/00016349209007964. [DOI] [PubMed] [Google Scholar]
- Reinacher M., Theilen G. Frequency and significance of feline leukemia virus infection in necropsied cats. Am J Vet Res. 1987 Jun;48(6):939–945. [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Gasper P. W., Nicolson M. O., Mullins J. I. Molecular analysis and pathogenesis of the feline aplastic anemia retrovirus, feline leukemia virus C-Sarma. J Virol. 1986 Oct;60(1):242–250. doi: 10.1128/jvi.60.1.242-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko J. L., Hoover E. A., Quackenbush S. L., Olsen R. G. Reactivation of latent feline leukaemia virus infection. Nature. 1982 Jul 22;298(5872):385–388. doi: 10.1038/298385a0. [DOI] [PubMed] [Google Scholar]
- Rojko J. L., Kociba G. J., Abkowitz J. L., Hamilton K. L., Hardy W. D., Jr, Ihle J. N., O'Brien S. J. Feline lymphomas: immunological and cytochemical characterization. Cancer Res. 1989 Jan 15;49(2):345–351. [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Parameters affecting susceptibility of PCR contamination to UV inactivation. Biotechniques. 1991 May;10(5):590–594. [PubMed] [Google Scholar]
- Shelton G. H., Grant C. K., Cotter S. M., Gardner M. B., Hardy W. D., Jr, DiGiacomo R. F. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: a retrospective study (1968-1988). J Acquir Immune Defic Syndr. 1990;3(6):623–630. [PubMed] [Google Scholar]
- Soe L. H., Devi B. G., Mullins J. I., Roy-Burman P. Molecular cloning and characterization of endogenous feline leukemia virus sequences from a cat genomic library. J Virol. 1983 Jun;46(3):829–840. doi: 10.1128/jvi.46.3.829-840.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. A., Warnock M., Wheeler A., Wilkie N., Mullins J. I., Onions D. E., Neil J. C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986 Jun;58(3):825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavaras T., Stewart M., McDougall A., Fulton R., Testa N., Onions D. E., Neil J. C. Molecular cloning and characterization of a defective recombinant feline leukaemia virus associated with myeloid leukaemia. J Gen Virol. 1990 Feb;71(Pt 2):343–354. doi: 10.1099/0022-1317-71-2-343. [DOI] [PubMed] [Google Scholar]
- Wright P. A., Wynford-Thomas D. The polymerase chain reaction: miracle or mirage? A critical review of its uses and limitations in diagnosis and research. J Pathol. 1990 Oct;162(2):99–117. doi: 10.1002/path.1711620203. [DOI] [PubMed] [Google Scholar]