Abstract
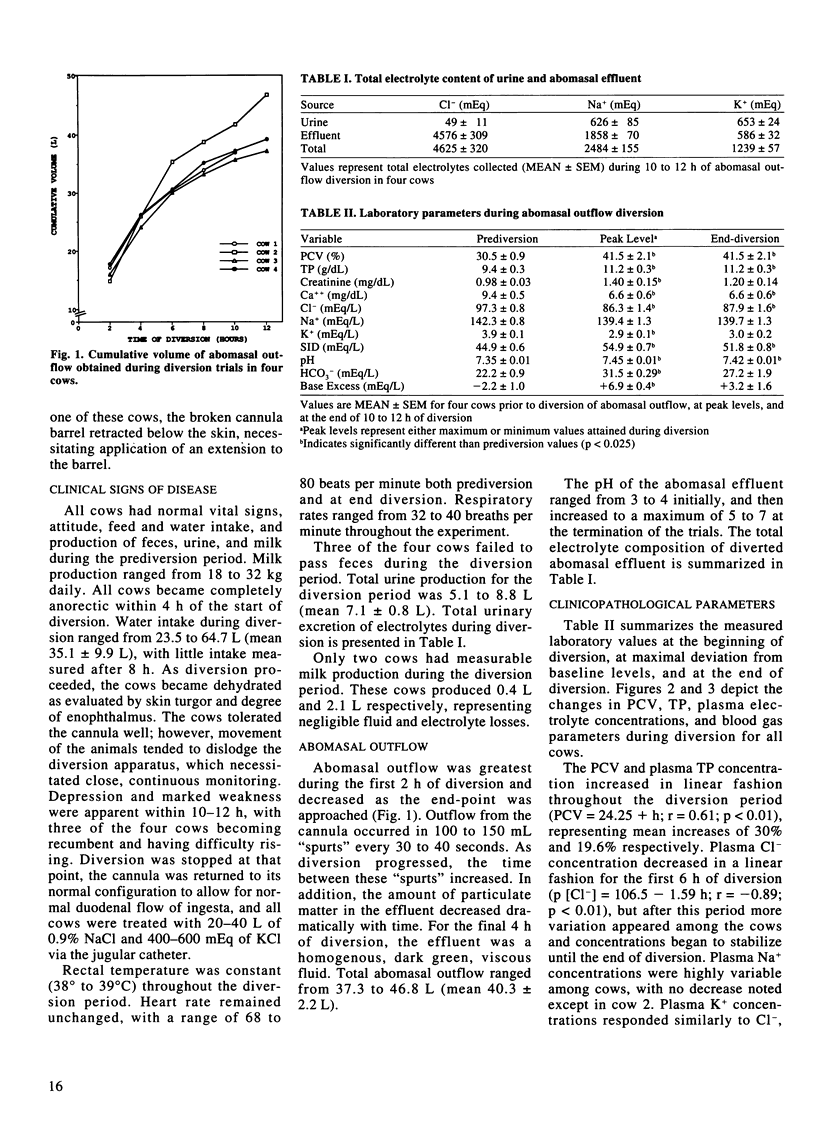
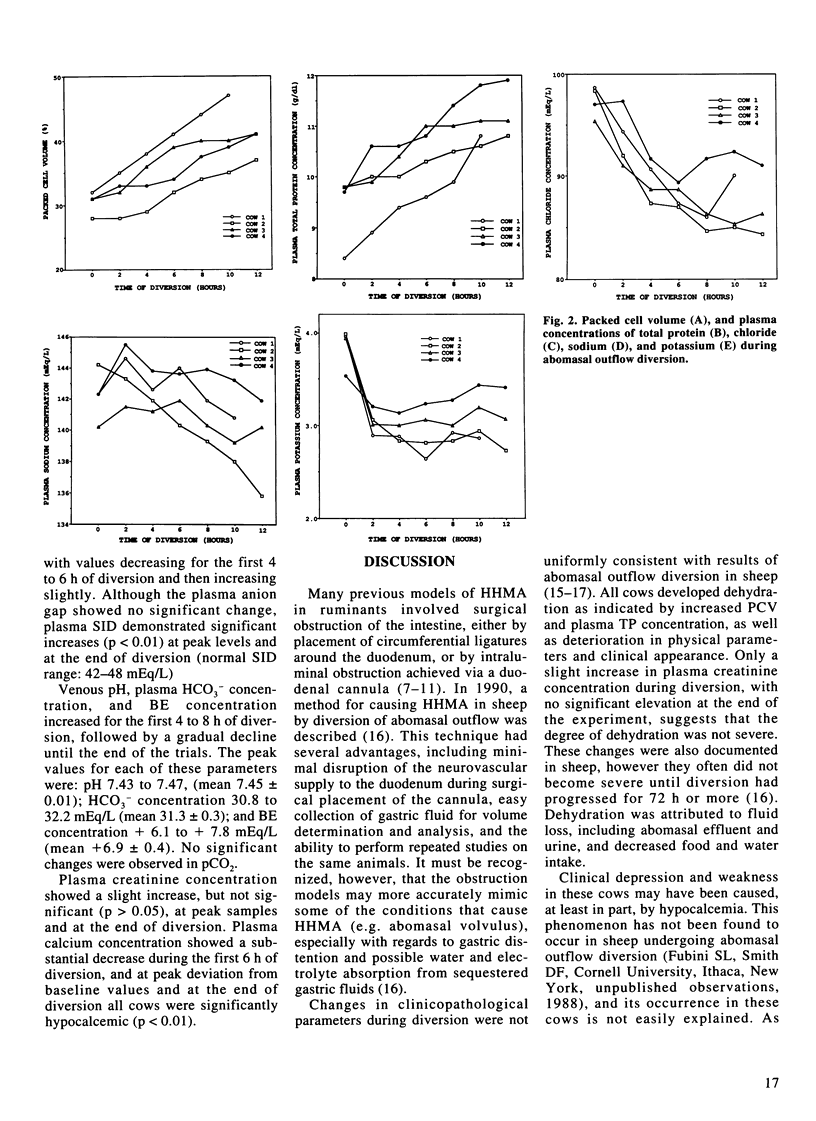
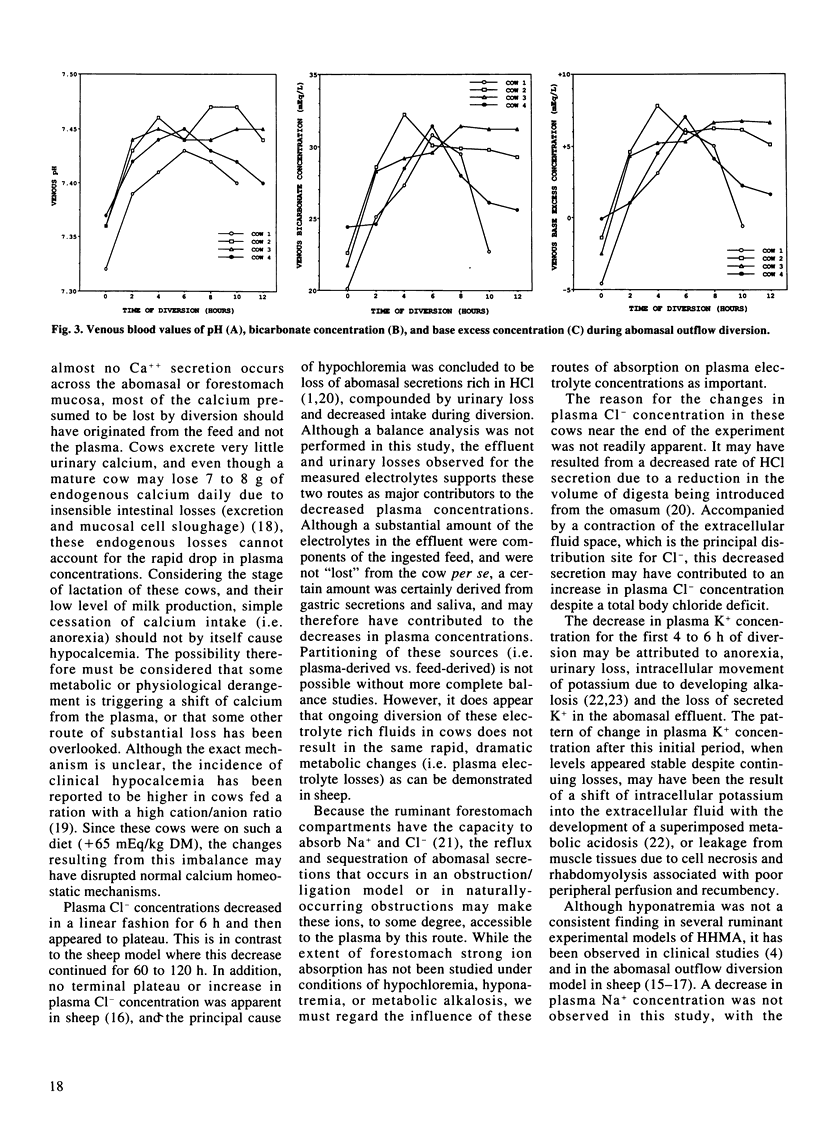
Four adult, lactating dairy cows were subjected to diversion (loss) of gastric contents through a T-shaped cannula placed in the cranial part of the duodenum just distal to the pylorus. Diversion was continued for 10 to 12 hours, at which point the cows were very weak and depressed. The volume of effluent during this period ranged from 37.3 to 46.8 L, with the largest volume being produced during the first four hours. All cows became dehydrated, with mean packed cell volume and total plasma protein concentration increasing 30% and 19.6%, respectively, but with only a slight increase in plasma creatinine concentration. Plasma Cl- concentrations decreased from a mean of 97.3 mEq/L at the beginning of diversion to a mean of 87.2 mEq/L at eight hours. This was followed by a plateau or slight increase in concentrations over the final hours of diversion. Plasma K+ concentration followed a similar pattern, decreasing from a mean of 3.9 mEq/L to a mean of 2.94 mEq/L at six hours, followed by increasing values until termination of diversion. No changes in plasma Na+ concentration were noted, except for a mild decrease in one cow. Plasma calcium concentrations decreased significantly, reaching 6.6 +/- 0.6 mEq/L at the end of diversion. Venous pH, plasma HCO3- concentration, and plasma base excess concentration increased during the first four to eight hours of diversion, followed by a gradual decline. Although a mild hypochloremic metabolic alkalosis resulted from diversion of abomasal outflow in all cows, substantiated by a mild increase in plasma strong ion difference, the changes observed were not as great as expected.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrogué H. J., Madias N. E. Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med. 1981 Sep;71(3):456–467. doi: 10.1016/0002-9343(81)90182-0. [DOI] [PubMed] [Google Scholar]
- Avery T. B., Nagaraja T. G., Frey R. A. Blood, urine, and ruminal fluid changes associated with metabolic alkalosis induced by duodenal obstruction. Am J Vet Res. 1986 Apr;47(4):890–896. [PubMed] [Google Scholar]
- Block E. Manipulating dietary anions and cations for prepartum dairy cows to reduce incidence of milk fever. J Dairy Sci. 1984 Dec;67(12):2939–2948. doi: 10.3168/jds.S0022-0302(84)81657-4. [DOI] [PubMed] [Google Scholar]
- Corker E., Dziuk H. E. Obstructive ligation of digestive tract in sheep. Am J Vet Res. 1968 Jul;29(7):1429–1439. [PubMed] [Google Scholar]
- Eicker S. W. An introduction to strong ion difference. Vet Clin North Am Food Anim Pract. 1990 Mar;6(1):45–49. doi: 10.1016/s0749-0720(15)30893-8. [DOI] [PubMed] [Google Scholar]
- Fubini S. L., Smith D. F., Grohn Y. T., Levine S. A., Deuel D. M. Replacement of chloride deficit by use of 1.8% NaCl to correct experimentally induced hypochloremic metabolic alkalosis in sheep. Am J Vet Res. 1991 Nov;52(11):1898–1902. [PubMed] [Google Scholar]
- Gingerich D. A., Murdick P. W. Experimentally induced intestinal obstruction in sheep: paradoxical aciduria in metabolic alkalosis. Am J Vet Res. 1975 May;36(5):663–668. [PubMed] [Google Scholar]
- Gingerich D. A., Murdick P. W. Paradoxic aciduria in bovine metabolic alkalosis. J Am Vet Med Assoc. 1975 Feb 1;166(3):227–230. [PubMed] [Google Scholar]
- HAMMOND P. B., DZIUK H. E., USENIK E. A., STEVENS C. E. EXPERIMENTAL INTESTINAL OBSTRUCTION IN CALVES. J Comp Pathol. 1964 Apr;74:210–222. doi: 10.1016/s0368-1742(64)80027-8. [DOI] [PubMed] [Google Scholar]
- HILL K. J. Continuous gastric secretion in the ruminant. Q J Exp Physiol Cogn Med Sci. 1955 Jan;40(1):32–39. doi: 10.1113/expphysiol.1955.sp001095. [DOI] [PubMed] [Google Scholar]
- Ivan M., Johnston D. W. Reentrant cannulation of the small intestine in sheep: cannula and surgical method. J Anim Sci. 1981 Apr;52(4):849–856. doi: 10.2527/jas1981.524849x. [DOI] [PubMed] [Google Scholar]
- Lunn D. P., McGuirk S. M., Smith D. F., MacWilliams P. S. Renal net acid and electrolyte excretion in an experimental model of hypochloremic metabolic alkalosis in sheep. Am J Vet Res. 1990 Nov;51(11):1723–1731. [PubMed] [Google Scholar]
- McGuirk S. M., Butler D. G. Metabolic alkalosis with paradoxic aciduria in cattle. J Am Vet Med Assoc. 1980 Sep 15;177(6):551–554. [PubMed] [Google Scholar]
- Papadopoulos P., Raptopoulos D., Dessiris A., Tsimopoulos G. Experimental intestinal obstruction in cattle. Part I: Changes in the clinical picture. Zentralbl Veterinarmed A. 1985 Apr;32(4):264–275. [PubMed] [Google Scholar]
- Papadopoulos P., Raptopoulos D., Dessiris A., Tsimopoulos G., Roumpies N. Experimental intestinal obstruction in cattle. Part II: Changes in blood, urine and rumen content chemistry. Zentralbl Veterinarmed A. 1985 Apr;32(4):276–288. [PubMed] [Google Scholar]
- Smith D. F., Lunn D. P., Robinson G. M., McGuirk S. M., Nordheim E. V., MacWilliams P. S. Experimental model of hypochloremic metabolic alkalosis caused by diversion of abomasal outflow in sheep. Am J Vet Res. 1990 Nov;51(11):1715–1722. [PubMed] [Google Scholar]
- Smith D. F. Right-side torsion of the abomasum in dairy cows: classification of severity and evaluation of outcome. J Am Vet Med Assoc. 1978 Jul 1;173(1):108–111. [PubMed] [Google Scholar]
- Whitlock R. H., Tasker J. B., Tenant B. C. Hypochloremic metabolic alkalosis and hypokalemia in cattle with upper-gastrointestinal obstruction. Am J Dig Dis. 1975 Jun;20(6):595–596. [PubMed] [Google Scholar]


