Abstract
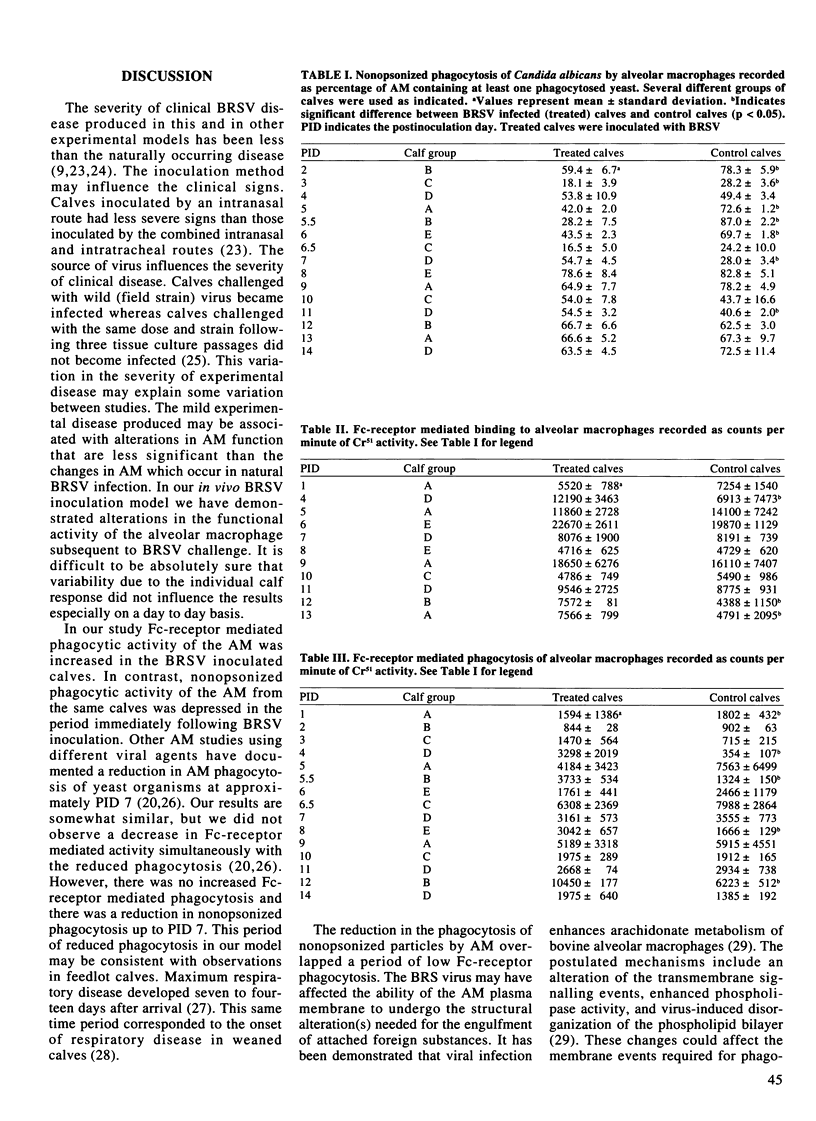
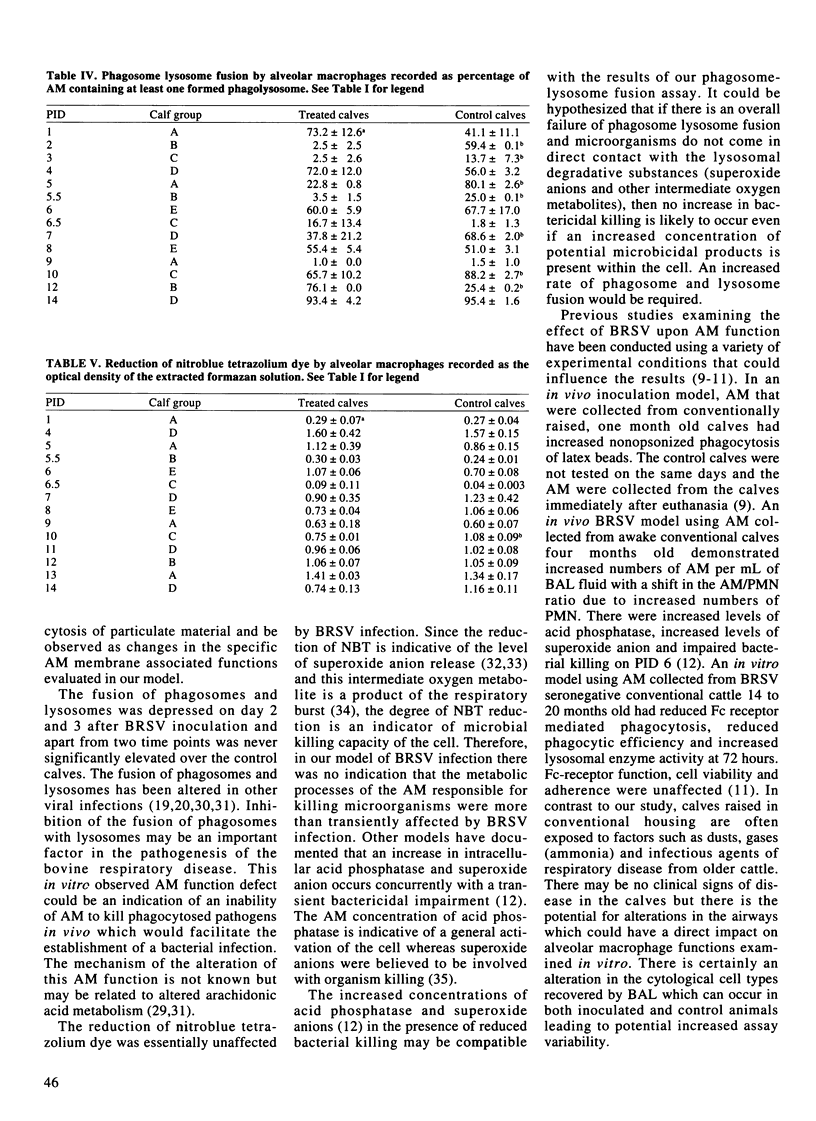
The effect of bovine respiratory syncytial virus (BRSV) upon alveolar macrophage (AM) function was investigated using an in vivo calf inoculation model. Alveolar macrophages were collected sequentially from live calves at multiple time points during the 14 day period following viral inoculation. Alveolar macrophages from bronchoalveolar lavage fluids were purified by density gradient centrifugation (> 95% AM) prior to in vitro evaluation of cell functions. There were significant but variable and inconsistent differences in the functions of AM from the BRSV inoculated calves compared to the control calves. Fc-receptor mediated phagocytosis was either increased or unchanged by BRSV inoculation. Nonopsonized phagocytosis was decreased during the early postinoculation period and later increased. There was a variable effect on AM phagosome lysosome fusion with increased fusion activity on postinoculation days 2 through 5, 7 and 12 but reduced activity on days 6 and 10. The AM respiratory burst, as measured by nitroblue tetrazolium dye reduction, was essentially unaffected with a reduction in activity on day 10 only. In this model, BRSV inoculation of calves primarily resulted in an alteration of the membrane associated phagocytic functions of the alveolar macrophages (p < 0.05).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Baker J. C., Ames T. R., Markham R. J. Seroepizootiologic study of bovine respiratory syncytial virus in a dairy herd. Am J Vet Res. 1986 Feb;47(2):240–245. [PubMed] [Google Scholar]
- Baker J. C., Ames T. R., Markham R. J. Serologic studies of bovine respiratory syncytial virus in Minnesota cattle. Am J Vet Res. 1985 Apr;46(4):891–892. [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Babiuk L. A. Alteration of alveolar macrophage functions after aerosol infection with bovine herpesvirus type 1. Infect Immun. 1986 Jan;51(1):344–347. doi: 10.1128/iai.51.1.344-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Babiuk L. A. Bovine alveolar macrophages: phenotypic and functional properties of subpopulations obtained by Percoll density gradient centrifugation. J Leukoc Biol. 1986 Feb;39(2):167–181. doi: 10.1002/jlb.39.2.167. [DOI] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Babiuk L. A. Viral-bacterial pneumonia in calves: effect of bovine herpesvirus-1 on immunologic functions. J Infect Dis. 1985 May;151(5):937–947. doi: 10.1093/infdis/151.5.937. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. The pulmonary interstitial cell as immediate precursor of the alveolar macrophage. Am J Pathol. 1972 Sep;68(3):521–537. [PMC free article] [PubMed] [Google Scholar]
- Bryson D. G., Cush P. F., McNulty M. S., Platten M., Allan G. M. An immunoperoxidase method of detecting respiratory syncytial virus antigens in paraffin sections of pneumonic bovine lung. Am J Vet Res. 1988 Jul;49(7):1121–1126. [PubMed] [Google Scholar]
- Bryson D. G., McFerran J. B., Ball H. J., Neill S. D. Observations on outbreaks of respiratory disease in housed calves--(2) Pathological and microbiological findings. Vet Rec. 1978 Dec 2;103(23):503–509. doi: 10.1136/vr.103.23.503. [DOI] [PubMed] [Google Scholar]
- Bryson D. G., McNulty M. S., Logan E. F., Cush P. F. Respiratory syncytial virus pneumonia in young calves: clinical and pathologic findings. Am J Vet Res. 1983 Sep;44(9):1648–1655. [PubMed] [Google Scholar]
- Castleman W. L., Lay J. C., Dubovi E. J., Slauson D. O. Experimental bovine respiratory syncytial virus infection in conventional calves: light microscopic lesions, microbiology, and studies on lavaged lung cells. Am J Vet Res. 1985 Mar;46(3):547–553. [PubMed] [Google Scholar]
- Church T. L., Radostits O. M. A retrospective survey of diseases of feedlot cattle in Alberta. Can Vet J. 1981 Feb;22(2):27–30. [PMC free article] [PubMed] [Google Scholar]
- Eggers N. J., Cattle D. L. High-performance liquid chromatographic method for the determination of nitrate and nitrite in cured meat. J Chromatogr. 1986 Feb 28;354:490–494. doi: 10.1016/s0021-9673(01)87056-4. [DOI] [PubMed] [Google Scholar]
- Forman A. J., Babiuk L. A. Effect of infectious bovine rhinotracheitis virus infection on bovine alveolar macrophage function. Infect Immun. 1982 Mar;35(3):1041–1047. doi: 10.1128/iai.35.3.1041-1047.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Hesse R. A., Toth T. E. Effects of bovine parainfluenza-3 virus on phagocytosis and phagosome-lysosome fusion of cultured bovine alveolar macrophages. Am J Vet Res. 1983 Oct;44(10):1901–1907. [PubMed] [Google Scholar]
- Iglesias G., Pijoan C., Molitor T. Interactions of pseudorabies virus with swine alveolar macrophages: effects of virus infection on cell functions. J Leukoc Biol. 1989 May;45(5):410–415. doi: 10.1002/jlb.45.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Defect in intracellular killing of Staphylococcus aureus within alveolar macrophages in Sendai virus-infected murine lungs. J Clin Invest. 1976 Jun;57(6):1533–1539. doi: 10.1172/JCI108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A., Sannes P. L. Alveolar macrophage ingestion and phagosome-lysosome fusion defect associated with virus pneumonia. Infect Immun. 1980 Mar;27(3):960–968. doi: 10.1128/iai.27.3.960-968.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A., Sannes P. L. Alveolar macrophage ingestion and phagosome-lysosome fusion defect associated with virus pneumonia. Infect Immun. 1980 Mar;27(3):960–968. doi: 10.1128/iai.27.3.960-968.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimman T. G., Westenbrink F., Schreuder B. E., Straver P. J. Local and systemic antibody response to bovine respiratory syncytial virus infection and reinfection in calves with and without maternal antibodies. J Clin Microbiol. 1987 Jun;25(6):1097–1106. doi: 10.1128/jcm.25.6.1097-1106.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laegreid W. W., Liggitt H. D., Silflow R. M., Evermann J. R., Taylor S. M., Leid R. W. Reversal of virus-induced alveolar macrophage bactericidal dysfunction by cyclooxygenase inhibition in vitro. J Leukoc Biol. 1989 Apr;45(4):293–300. doi: 10.1002/jlb.45.4.293. [DOI] [PubMed] [Google Scholar]
- Laegreid W. W., Taylor S. M., Leid R. W., Silflow R. M., Evermann J. R., Breeze R. G., Liggitt H. D. Virus-induced enhancement of arachidonate metabolism by bovine alveolar macrophages in vitro. J Leukoc Biol. 1989 Apr;45(4):283–292. doi: 10.1002/jlb.45.4.283. [DOI] [PubMed] [Google Scholar]
- Lehmkuhl H. D., Gough P. M., Reed D. E. Characterization and identification of a bovine respiratory syncytial virus isolated from young calves. Am J Vet Res. 1979 Jan;40(1):124–126. [PubMed] [Google Scholar]
- Lehnert B. E., Valdez Y. E., Fillak D. A., Steinkamp J. A., Stewart C. C. Flow cytometric characterization of alveolar macrophages. J Leukoc Biol. 1986 Mar;39(3):285–298. doi: 10.1002/jlb.39.3.285. [DOI] [PubMed] [Google Scholar]
- Lepoivre M., Roche A. C., Tenu J. P., Petit J. F., Nolibe D., Monsigny M. Identification of two macrophage populations by flow cytometry monitoring of oxidative burst and phagocytic functions. Biol Cell. 1986;57(2):143–146. doi: 10.1111/j.1768-322x.1986.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Liggitt D., Huston L., Silflow R., Evermann J., Trigo E. Impaired function of bovine alveolar macrophages infected with parainfluenza-3 virus. Am J Vet Res. 1985 Aug;46(8):1740–1744. [PubMed] [Google Scholar]
- McNulty M. S., Bryson D. G., Allan G. M. Experimental respiratory syncytial virus pneumonia in young calves: microbiologic and immunofluorescent findings. Am J Vet Res. 1983 Sep;44(9):1656–1659. [PubMed] [Google Scholar]
- Muscoplat C. C., Chen A. W., Johnson D. W., Alhaji I. In vitro stimulation of bovine peripheral blood lymphocytes: standardization and kinetics of the response. Am J Vet Res. 1974 Dec;35(12):1557–1561. [PubMed] [Google Scholar]
- Nagahata H., Yatsu A., Noda H. The evaluation of a quantitative assay for estimating the bacterial activity of bovine neutrophils by nitroblue tetrazolium reduction. Br Vet J. 1986 Nov-Dec;142(6):578–584. doi: 10.1016/0007-1935(86)90117-x. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Pringle J. K., Viel L., Shewen P. E., Willoughby R. A., Martin S. W., Valli V. E. Bronchoalveolar lavage of cranial and caudal lung regions in selected normal calves: cellular, microbiological, immunoglobulin, serological and histological variables. Can J Vet Res. 1988 Apr;52(2):239–248. [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Taylor G. Respiratory syncytial virus. Brief review. Arch Virol. 1985;84(1-2):1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- Trigo E., Liggitt H. D., Evermann J. F., Breeze R. G., Huston L. Y., Silflow R. Effect of in vitro inoculation of bovine respiratory syncytial virus on bovine pulmonary alveolar macrophage function. Am J Vet Res. 1985 May;46(5):1098–1103. [PubMed] [Google Scholar]
- Zembala M., Lemmel E. M., Uracz W. Activation of human monocytes for nitroblue tetrazolium reduction and the suppression of lymphocyte response to mitogens. Clin Exp Immunol. 1980 Aug;41(2):309–316. [PMC free article] [PubMed] [Google Scholar]


