Abstract
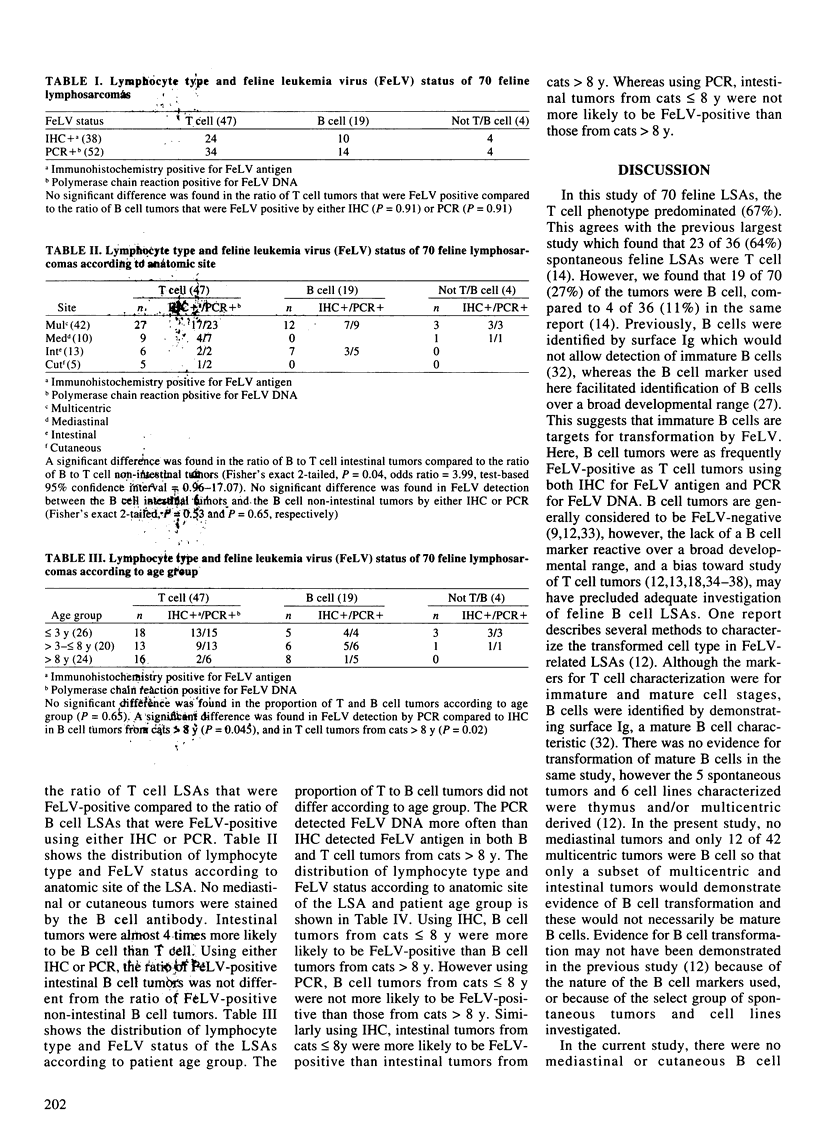
The lymphocyte phenotype of 70 formalin-fixed, paraffin-embedded feline lymphosarcomas (LSAs) was determined immunohistochemically using a T cell polyclonal antibody, and a B cell monoclonal antibody. Forty-seven of 70 (67%) tumors were T cell, 19/70 (27%) were B cell, and 4/70 (6%) did not stain with either marker. Thirty-eight of 70 (54%) tumors were positive for feline leukemia virus (FeLV) antigen by immunohistochemistry (IHC), and 52/70 (74%) tumors were positive for FeLV DNA using the polymerase chain reaction (PCR). B cell tumors were as frequently FeLV-positive as T cell tumors using either IHC or PCR. Intestinal tumors were more likely to be B cell than T. The incidence of B and T cell tumors was not different among young (< or = 3 y), middle-aged (> 3 y to < or = 8 y), and old (> 8 y) cats. Both B and T cell tumors from old cats were FeLV-positive more often by PCR than by IHC. Feline leukemia virus DNA but not antigen, was detected in B cell tumors and intestinal tumors from cats > 8 y as often as it was detected in B cell tumors and intestinal tumors from cats < or = 8 y. Previously, most B cell and intestinal tumors from old cats were considered to be negative for FeLV. Here, the results suggest involvement of latent or replication-defective forms of the virus in such tumors from old cats. This study supports a role for FeLV in feline B cell as well as T cell tumorigenesis.
Full text
PDF

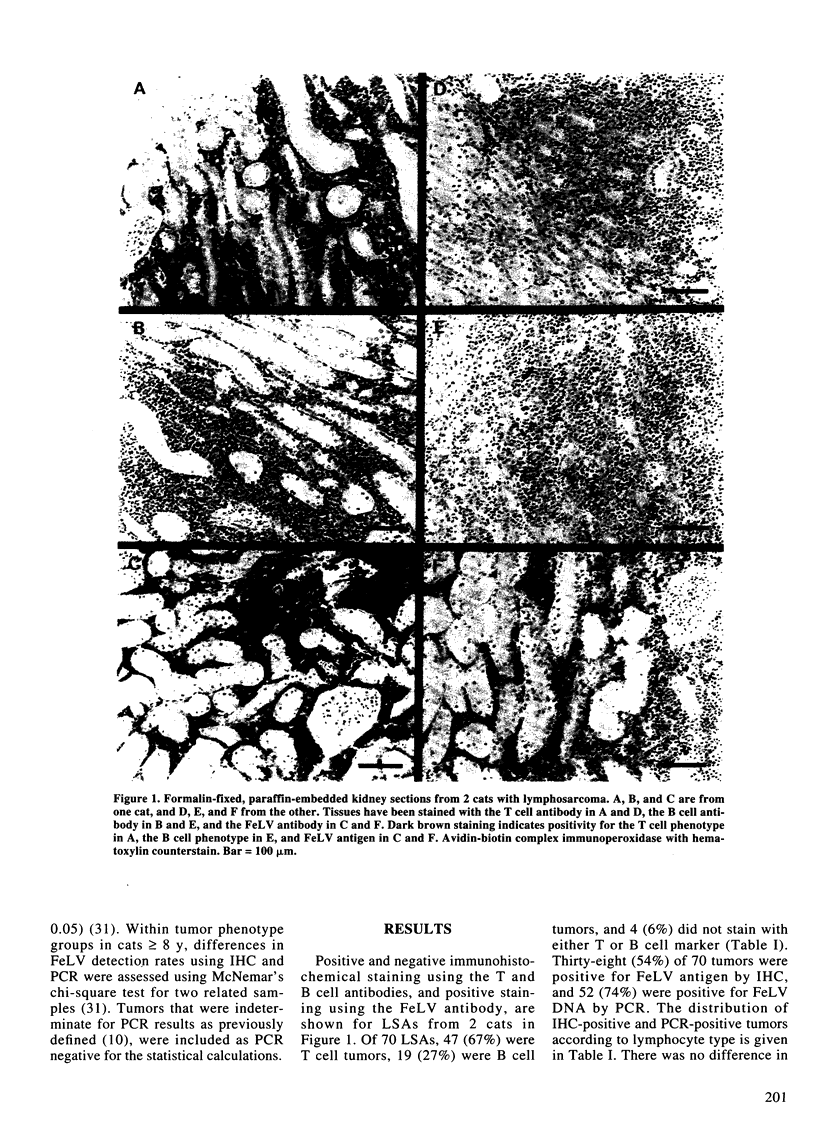


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackley C. D., Hoover E. A., Cooper M. D. Identification of a CD4 homologue in the cat. Tissue Antigens. 1990 Feb;35(2):92–98. doi: 10.1111/j.1399-0039.1990.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Beebe A. M., Dua N., Faith T. G., Moore P. F., Pedersen N. C., Dandekar S. Primary stage of feline immunodeficiency virus infection: viral dissemination and cellular targets. J Virol. 1994 May;68(5):3080–3091. doi: 10.1128/jvi.68.5.3080-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerell G. L., Krakowka S., Hoover E. A., Olsen R. G., Yohn D. S. Characterization of feline T-and B-lymphocytes and identification of an experimentally induced T-cell neoplasm in the cat. J Natl Cancer Inst. 1976 Oct;57(4):907–913. doi: 10.1093/jnci/57.4.907. [DOI] [PubMed] [Google Scholar]
- Cotter S. M. Feline leukemia virus: pathophysiology, prevention, and treatment. Cancer Invest. 1992;10(2):173–181. doi: 10.3109/07357909209032778. [DOI] [PubMed] [Google Scholar]
- Crighton G. W. Lymphosarcoma in the cat. Vet Rec. 1969 Mar 29;84(13):329–331. doi: 10.1136/vr.84.13.329. [DOI] [PubMed] [Google Scholar]
- Dean G. A., Quackenbush S. L., Ackley C. D., Cooper M. D., Hoover E. A. Flow cytometric analysis of T-lymphocyte subsets in cats. Vet Immunol Immunopathol. 1991 Jul;28(3-4):327–335. doi: 10.1016/0165-2427(91)90124-u. [DOI] [PubMed] [Google Scholar]
- Dorn C. R., Taylor D. O., Hibbard H. H. Epizootiologic characteristics of canine and feline leukemia and lymphoma. Am J Vet Res. 1967 Jul;28(125):993–1001. [PubMed] [Google Scholar]
- Dorn C. R., Taylor D. O., Schneider R., Hibbard H. H., Klauber M. R. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968 Feb;40(2):307–318. [PubMed] [Google Scholar]
- English R. V., Johnson C. M., Gebhard D. H., Tompkins M. B. In vivo lymphocyte tropism of feline immunodeficiency virus. J Virol. 1993 Sep;67(9):5175–5186. doi: 10.1128/jvi.67.9.5175-5186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foon K. A., Todd R. F., 3rd Immunologic classification of leukemia and lymphoma. Blood. 1986 Jul;68(1):1–31. [PubMed] [Google Scholar]
- Forrest D., Onions D., Lees G., Neil J. C. Altered structure and expression of c-myc in feline T-cell tumours. Virology. 1987 May;158(1):194–205. doi: 10.1016/0042-6822(87)90253-4. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Cotter S. M., Hardy W. D., Jr, Essex M. Comparison of virus-positive and virus-negative cases of feline leukemia and lymphoma. Cancer Res. 1979 Oct;39(10):3866–3870. [PubMed] [Google Scholar]
- Francis D. P., Essex M., Cotter S. M., Gutensohn N., Jakowski R., Hardy W. D., Jr Epidemiologic association between virus-negative feline leukemia and the horizontally transmitted feline leukemia virus. Cancer Lett. 1981 Mar;12(1-2):37–42. doi: 10.1016/0304-3835(81)90035-5. [DOI] [PubMed] [Google Scholar]
- Fulton R., Forrest D., McFarlane R., Onions D., Neil J. C. Retroviral transduction of T-cell antigen receptor beta-chain and myc genes. Nature. 1987 Mar 12;326(6109):190–194. doi: 10.1038/326190a0. [DOI] [PubMed] [Google Scholar]
- Fulton R., Plumb M., Shield L., Neil J. C. Structural diversity and nuclear protein binding sites in the long terminal repeats of feline leukemia virus. J Virol. 1990 Apr;64(4):1675–1682. doi: 10.1128/jvi.64.4.1675-1682.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines D. M., Chelack B. J. Technical considerations for developing enzyme immunohistochemical staining procedures on formalin-fixed paraffin-embedded tissues for diagnostic pathology. J Vet Diagn Invest. 1991 Jan;3(1):101–112. doi: 10.1177/104063879100300128. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, McClelland A. J., Zuckerman E. E., Snyder H. W., Jr, MacEwen E. G., Francis D., Essex M. Development of virus non-producer lymphosarcomas in pet cats exposed to FeLv. Nature. 1980 Nov 6;288(5786):90–92. doi: 10.1038/288090a0. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Zuckerman E. E., MacEwen E. G., Hayes A. A., Essex M. A feline leukaemia virus- and sarcoma virus-induced tumour-specific antigen. Nature. 1977 Nov 17;270(5634):249–251. doi: 10.1038/270249a0. [DOI] [PubMed] [Google Scholar]
- Holmberg C. A., Manning J. S., Osburn B. I. Feline malignant lymphomas: comparison of morphologic and immunologic characteristics. Am J Vet Res. 1976 Dec;37(12):1455–1460. [PubMed] [Google Scholar]
- Jackson M. L., Haines D. M., Meric S. M., Misra V. Feline leukemia virus detection by immunohistochemistry and polymerase chain reaction in formalin-fixed, paraffin-embedded tumor tissue from cats with lymphosarcoma. Can J Vet Res. 1993 Oct;57(4):269–276. [PMC free article] [PubMed] [Google Scholar]
- Klotz F. W., Cooper M. D. A feline thymocyte antigen defined by a monoclonal antibody (FT2) identifies a subpopulation of non-helper cells capable of specific cytotoxicity. J Immunol. 1986 Apr 1;136(7):2510–2514. [PubMed] [Google Scholar]
- Lippman S. M., Miller T. P., Spier C. M., Slymen D. J., Grogan T. M. The prognostic significance of the immunotype in diffuse large-cell lymphoma: a comparative study of the T-cell and B-cell phenotype. Blood. 1988 Aug;72(2):436–441. [PubMed] [Google Scholar]
- Mackey L. J., Jarrett W. F. Pathogenesis of lymphoid neoplasia in cats and its relationship to immunologic cell pathways. I. Morphologic aspects. J Natl Cancer Inst. 1972 Sep;49(3):853–865. [PubMed] [Google Scholar]
- Mackey L. J., Jarrett W. F. Two populations of lymphocytes in a cat. Vet Rec. 1975 Jan 11;96(2):41–41. doi: 10.1136/vr.96.2.41-a. [DOI] [PubMed] [Google Scholar]
- Mackey L., Jarrett W., Jarrett O., Wilson L. B and T cells in a cat with thymic lymphosarcoma. J Natl Cancer Inst. 1975 Jun;54(6):1483–1487. doi: 10.1093/jnci/54.6.1483. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Momoi Y., Watari T., Goitsuka R., Tsujimoto H., Hasegawa A. Detection of enhancer repeats in the long terminal repeats of feline leukemia viruses from cats with spontaneous neoplastic and nonneoplastic diseases. Virology. 1992 Aug;189(2):745–749. doi: 10.1016/0042-6822(92)90598-j. [DOI] [PubMed] [Google Scholar]
- Miura T., Tsujimoto H., Fukasawa M., Kodama T., Shibuya M., Hasegawa A., Hayami M. Structural abnormality and over-expression of the myc gene in feline leukemias. Int J Cancer. 1987 Oct 15;40(4):564–569. doi: 10.1002/ijc.2910400422. [DOI] [PubMed] [Google Scholar]
- Monteith C. E., Chelack B. J., Davis W. C., Haines D. M. Identification of monoclonal antibodies for immunohistochemical staining of feline B lymphocytes in frozen and formalin-fixed paraffin-embedded tissues. Can J Vet Res. 1996 Jul;60(3):193–198. [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Brody D. S., Binari R. C., Jr, Cotter S. M. Viral transduction of c-myc gene in naturally occurring feline leukaemias. 1984 Apr 26-May 2Nature. 308(5962):856–858. doi: 10.1038/308856a0. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Hughes D., McFarlane R., Wilkie N. M., Onions D. E., Lees G., Jarrett O. Transduction and rearrangement of the myc gene by feline leukaemia virus in naturally occurring T-cell leukaemias. 1984 Apr 26-May 2Nature. 308(5962):814–820. doi: 10.1038/308814a0. [DOI] [PubMed] [Google Scholar]
- Onions D., Lees G., Forrest D., Neil J. Recombinant feline viruses containing the myc gene rapidly produce clonal tumours expressing T-cell antigen receptor gene transcripts. Int J Cancer. 1987 Jul 15;40(1):40–45. doi: 10.1002/ijc.2910400108. [DOI] [PubMed] [Google Scholar]
- Quackenbush S. L., Donahue P. R., Dean G. A., Myles M. H., Ackley C. D., Cooper M. D., Mullins J. I., Hoover E. A. Lymphocyte subset alterations and viral determinants of immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J Virol. 1990 Nov;64(11):5465–5474. doi: 10.1128/jvi.64.11.5465-5474.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinacher M. Diseases associated with spontaneous feline leukemia virus (FeLV) infection in cats. Vet Immunol Immunopathol. 1989 May;21(1):85–95. doi: 10.1016/0165-2427(89)90132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinacher M., Theilen G. Frequency and significance of feline leukemia virus infection in necropsied cats. Am J Vet Res. 1987 Jun;48(6):939–945. [PubMed] [Google Scholar]
- Rezanka L. J., Rojko J. L., Neil J. C. Feline leukemia virus: pathogenesis of neoplastic disease. Cancer Invest. 1992;10(5):371–389. doi: 10.3109/07357909209024796. [DOI] [PubMed] [Google Scholar]
- Rojko J. L., Kociba G. J., Abkowitz J. L., Hamilton K. L., Hardy W. D., Jr, Ihle J. N., O'Brien S. J. Feline lymphomas: immunological and cytochemical characterization. Cancer Res. 1989 Jan 15;49(2):345–351. [PubMed] [Google Scholar]
- Rojko J. L., Olsen R. G. The immunobiology of the feline leukemia virus. Vet Immunol Immunopathol. 1984 May;6(1-2):107–165. doi: 10.1016/0165-2427(84)90050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton G. H., Grant C. K., Cotter S. M., Gardner M. B., Hardy W. D., Jr, DiGiacomo R. F. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: a retrospective study (1968-1988). J Acquir Immune Defic Syndr. 1990;3(6):623–630. [PubMed] [Google Scholar]
- Tompkins M. B., Gebhard D. H., Bingham H. R., Hamilton M. J., Davis W. C., Tompkins W. A. Characterization of monoclonal antibodies to feline T lymphocytes and their use in the analysis of lymphocyte tissue distribution in the cat. Vet Immunol Immunopathol. 1990 Dec;26(4):305–317. doi: 10.1016/0165-2427(90)90115-9. [DOI] [PubMed] [Google Scholar]
- Tompkins M. B., Nelson P. D., English R. V., Novotney C. Early events in the immunopathogenesis of feline retrovirus infections. J Am Vet Med Assoc. 1991 Nov 15;199(10):1311–1315. [PubMed] [Google Scholar]
- Wellman M. L., Kociba G. J., Rojko J. L. Guinea pig erythrocyte rosette formation as a nonspecific cell surface receptor assay in the cat. Am J Vet Res. 1986 Feb;47(2):433–437. [PubMed] [Google Scholar]



