Abstract
In vitro adherence of Lactobacillus strains to cell and tissue types along the chicken alimentary tract and to ileal mucus were determined. Fresh isolates from chickens adhered to the epithelium of crop and, in a strain-dependent manner, to follicle-associated epithelium and the apical surfaces of mature enterocytes of intestinal villi. No adherence to the apical surfaces of undifferentiated enterocytes, the mucus-producing goblet cells, or the ileal mucus was detected.
Species of Lactobacillus belong to the normal bacterial flora of gastrointestinal and genital tracts of humans and animals. Early studies on chicken microflora (2, 5-7) showed that the Lactobacillus flora lining the crop of the chicken gastrointestinal tract becomes established within a day after hatching and that the specific adherence of avian-associated lactobacilli onto the crop epithelium plays a role in the colonization. Overall, the tissue, cell, and molecular specificities of Lactobacillus adherence have remained poorly characterized, and the role of adherence in intestinal colonization by lactobacilli has remained a controversial topic (19). We initiated this study to analyze the cell and tissue tropism in the in vitro adhesion of commensal lactobacilli at different surfaces of the chicken intestinal tract, which offers a well-characterized tissue with structurally and functionally varied epithelial surfaces (9).
Isolation of lactobacilli from chicken intestine.
Lactobacillus crispatus strains ST1, A33, and 134mi, Lactobacillus reuteri CT7, and Lactobacillus gasseri CT5 were isolated from the crop of 2- to 12-day-old chickens by enrichment under anaerobic conditions on selection agar plates (LBS; Becton Dickinson Microbiology Systems, Cockeysville, Md.), and their identification was based on the 16S ribosomal DNA sequence determination (1). The strains L. crispatus ATCC 33820, L. reuteri ATCC 53609, L. gasseri ATCC 33323, and Lactobacillus casei ATCC 393 were obtained from the American Type Culture Collection (ATCC). L. reuteri 1063 was obtained from Stefan Roos, Uppsala, Sweden (16). For testing, the bacteria were cultivated in static MRS broth overnight at 37°C (20).
Bacterial adhesion to intestinal tissue.
Double staining of frozen tissue sections with bacteria and tissue markers has been successfully applied to determine tissue tropism in the adhesion of a variety of pathogenic bacteria and their adhesion proteins (reviewed in reference 11). Tissue samples of crop, duodenum, jejunum, ileum, cecum, and colon from four 12-day-old chickens, two males and two females, were excised and frozen as detailed in references 17 and 18. The fluorescein isothiocyanate (FITC)-labeled bacteria were tested at concentrations of 108 and 109/ml. Tetramethyl rhodamine isothiocyanate (TRITC)-conjugated wheat germ agglutinin lectin (20 μg/ml in phosphate-buffered saline [PBS]; Vector Laboratories, Inc., Burlingame, Calif.) was used as a general stain and a marker for chicken epithelium. Anti-chicken type III collagen immunoglobulin G (IgG) (0.02 μg/ml in PBS; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, Iowa) was used as a marker for extracellular tissue, anti-chicken T-cell receptor α/β Vβ1 (TCR2) IgG (10 μg/ml in PBS; Southern Biotechnology Associates, Inc., Birmingham, Ala.) was used for lymphoid organs, and anti-chicken β1-integrin IgG (50 μg/ml in PBS; Sigma, St. Louis, Mo.) was used for basolateral epithelia.
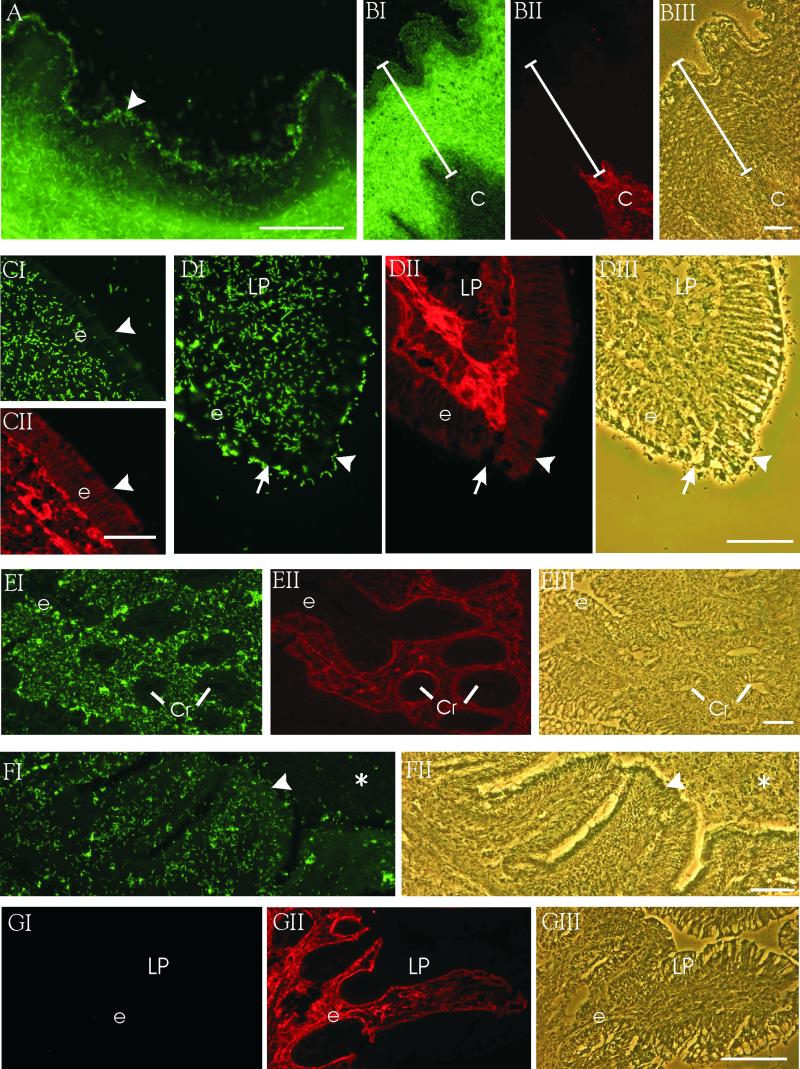
L. crispatus ST1 showed a strong adherence to the epithelial areas of the stratified squamous epithelium of the crop, to the simple columnar epithelium of intestine, and to the connective tissue areas along the lower intestinal tract (Fig. 1; Table 1). The pericellular tissue reactive with the anti-type III collagen monoclonal antibody (MAb) contained only a few adherent bacteria (Fig. 1B). The bacterium was observed to not adhere onto the endothelium. The epithelium-connective tissue bias of ST1 adhesion was different in the lower parts of the alimentary tract. In the small intestine (Fig. 1C through E), the adherence of L. crispatus ST1 was strong to the lamina propria of the villi and the basolateral poles of the villus enterocytes. Bacteria adhered strongly to the apical pole of the epithelium only at the areas of mature enterocytes at the villus tips (Fig. 1C and D). At the crypts containing undifferentiated columnar enterocytes, the bacteria adhered specifically to basolateral poles of the enterocytes but did not show any adherence to the apical poles (Fig. 1E). The adhesion of ST1 to the cecal epithelium and underlying tissues was less efficient and less distinctively localized than in the other parts of the digestive tract (Fig. 1F). The bacteria did not adhere to the indigenous debris remaining on the glass surface during preparation of the cecal sections (Fig. 1F). In the colon, the bacteria adhered to the lamina propria and less efficiently to the luminal poles of the villi. The adhesion patterns of the strains A33 and ST1 were highly similar (Table 1). The strain ATCC 33820 showed only a weak adhesion in the colon (Fig. 1G) as well as in the other parts of the intestine (data not shown). In contrast, L. crispatus strain 134mi adhered only to the crop and the duodenal epithelium (Table 1). The isolate CT7 of L. reuteri showed adherence only to the crop epithelium (Table 1). The adherence pattern shown by the isolate CT5 of L. gasseri was highly similar to those of L. crispatus strains ST1 and A33 (Table 1). The strains ATCC 33323 of L. gasseri, ATCC 53609 of L. reuteri, and ATCC 393 of L. casei showed only poor adhesiveness to the chicken tissue (data not shown).
FIG. 1.
Adherence of L. crispatus strains ST1 (AI through FI) and ATCC 33820 (GI) to regions of the chicken alimentary tract. The adherence was visualized by double staining of frozen intestinal sections with FITC-conjugated bacteria (I panels) and with indirect immunofluorescence using a primary MAb against chicken type III collagen (BII, EII, and GII) or chicken β1-integrin (CII and DII). The tissue sections were from crop (A and B), ileum (C to E), cecum (F), and colon (G). Panels BIII, DIII, EIII, FII, and GIII show light microscopy images of the tissue areas. Arrowheads indicate outermost layer of the epithelium, and the arrow indicates sloughing enterocytes at the villus tip. e, epithelial layers; C, connective tissue; LP, lamina propria; Cr, crypts; asterisks, cecal mucus and unwashed digesta. The thickness of the crop epithelium is indicated in the B panels. Bars, 50 μm.
TABLE 1.
Adherence of freshly isolated lactobacilli to frozen tissue sections of chicken alimentary tract
| Tissue site | Bacterial adherencea
|
||||
|---|---|---|---|---|---|
|
L. crispatus strain
|
L. gasseri CT5 | L. reuteri CT7 | |||
| ST1 | A33 | 134mi | |||
| Crop | |||||
| Epithelium | ++ | ++ | + | ++ | + |
| Connective tissue | + | + | − | + | − |
| Endotheliumb | − | − | − | − | − |
| Duodenum, jejunum, ileum | |||||
| Apical epithelium | |||||
| Villus tip | ++ | ++ | −e | + | − |
| Villus crypt | − | − | − | − | − |
| Basolateral epithelium | ++ | + | −e | ++ | − |
| Lamina propria | ++ | ++ | −e | + | − |
| FAEc | + | + | − | − | − |
| Peyer's patchd | + | + | − | + | − |
| Goblet cells | − | − | − | − | − |
| Cecum | |||||
| Apical epithelium | |||||
| Villus tip | + | − | − | − | − |
| Villus crypt | − | − | − | − | − |
| Basolateral epithelium | + | − | − | ++ | − |
| Lamina propria | + | − | − | ++ | − |
| Goblet cells | − | − | − | − | − |
| Colon | |||||
| Apical epithelium | |||||
| Villus tip | + | + | − | + | − |
| Villus crypt | − | − | − | − | − |
| Basolateral epithelium | ++ | + | − | ++ | − |
| Lamina propria | ++ | ++ | − | ++ | − |
| Goblet cells | − | − | − | − | − |
Symbols: −, +, ++; undetectable, moderate, and strong adhesion, respectively. For examples, see Fig. 1 images GI (−), FI (+), and AI (++).
Endothelium of muscularis arteries.
FAE in ileum.
Lymphoid follicles in ileum.
The strain 134mi adhered weakly to the epithelium and lamina propria in the duodenum only.
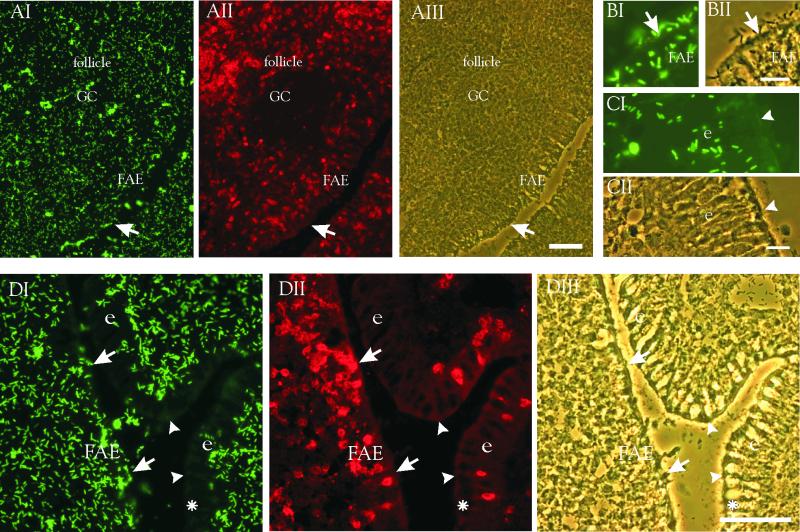
In the ileum, the follicle-associated epithelium (FAE) and the Peyer's patches of the mucous-associated lymphoid tissues are important sites for pathogen entry as well as for immune stimulation (8, 12). One application of orally administered lactobacilli has been to prevent or diminish intestinal infections. We therefore assessed the bacterial adherence to lymphatic follicles and FAE of the chicken ileum. The follicles and FAE were identified by their morphology, i.e., the presence of germinal centers and lack of tall columnar enterocytes, as well as the high number of cells recognized by the anti-T-cell receptor MAb. L. crispatus ST1 adhered to the follicles and the germinal center regions of the follicles as well as to the FAE located immediately above the follicles (Fig. 2A). Adherent bacteria could be located at the luminal FAE surfaces (Fig. 2B) but were absent at the luminal surfaces of enterocytes at the same ileal region (Fig. 2C and D). The adhesion of L. crispatus A33 was also efficient on the ileal Peyer's patches and the overlying FAE; L. gasseri strain CT5 showed moderate adhesion to the lymphoid follicles, whereas the adhesiveness to the FAE was poor (Table 1). The strain L. reuteri ATCC 53609 exhibited selective adherence to the tissue domains of ileal Peyer's patches, whereas poor adherence to the intestinal mucous-associated lymphoid areas was observed with other Lactobacillus strains (data not shown).
FIG. 2.
Adherence of L. crispatus ST1 to the ileal lymphoid follicles, its overlying FAE, and to columnar epithelial cells in the ileum. The tissue sections were double stained with FITC-labeled bacteria as well as MAbs against chicken T-cell receptor 2 (TCR α/β Vβ1) and TRITC-labeled secondary antibodies. (A) Adherence to the lymphoid follicle, with the characteristic germinal center (GC) rich in B cells and surrounded by the T-cell zone strongly positive for the tissue marker. (B and C) Bacterial adherence to the FAE (B) and to the corresponding ileal epithelium (e) not associated with lymphoid follicles (C). (D) Adherence to FAE and enterocytes in the same microscopic tissue section. Arrows indicate FAE surface rich in intraepithelial T cells reactive with the tissue marker; arrowheads indicate the apical surfaces of tall columnar enterocytes with only a few intracellular T cells. The asterisk indicates a mucus-producing goblet cell. Bars, 50 μm.
Mucus adherence.
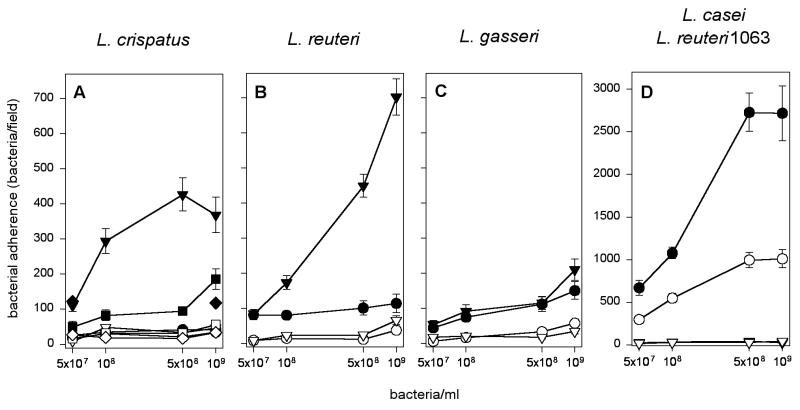
The procedure to prepare the tissue sections used in this study removes the protective mucus layer covering epithelial surfaces (4). Isolates of Lactobacillus have been reported to bind to intestinal mucus or fecal glycoproteins (16, 21); we therefore compared the strains for adhesiveness onto immobilized mucus scraped from the chicken ileum (18). For comparison, we also tested adhesion to an unrelated protein, type IV collagen, and the study also included strain 1063 of L. reuteri, which was previously found to adhere to mucus (16). Strains A33 of L. crispatus and ATCC 53609 of L. reuteri showed adhesiveness to mucus which, however, was low compared to that seen with the adhesive strain 1063. The other test strains showed nondetectable levels of adhesion (Fig. 3). The poor adhesion by chicken-associated lactobacilli is in line with the finding that the crop epithelium does not contain mucus-producing cells (9).
FIG. 3.
Adherence of chicken-associated lactobacilli isolates to immobilized ileal mucus. Adherence to mucus preparations is shown by filled symbols, and the adherence to type IV collagen is shown by open symbols. (A) Adherence of L. crispatus strains ST1 (•), A33 (▾), 134mi (▪), and ATCC 33820 (⧫). (B) Adherence of L. reuteri strains CT7 (•) and ATCC 53609 (▾). (C) Adherence of L. gasseri strains CT5 (•) and ATCC 33323 (▾). (D) For comparison, the adhesion by strains 1063 of L. reuteri (•) and ATCC 393 of L. casei (▾) are shown.
Our results on the in vitro tissue and cell specificity of lactobacillar adherence are in agreement with the findings of Fuller and Turvey (7) and others (13). We studied the tissue distribution of the in vivo colonization by lactobacilli in the chicken intestine. Our data also give support for the observation (5) that adherence to crop epithelial cells is a major determinant of lactobacillar colonization in the chicken intestine. The importance of the crop epithelium as an adhesion target for lactobacilli was stressed by the behavior of L. reuteri CT7, which was isolated from the chicken intestine in this study and adhered efficiently to the crop epithelium but very poorly to the other regions of the chicken intestinal tract. We observed that the isolates ST1 and A33 of L. crispatus as well as CT5 of L. gasseri adhered to the apical aspects of mature enterocytes at the tips of villi. Fuller and Turvey (7) observed massive bacterial colonization in the chicken cecum, where a thick layer of bacteria covered the epithelium. We found that the lactobacilli adhered to the cecal epithelium only poorly. On the other hand, it has been suggested that the bacterial adherence plays a less important role in cecal colonization due to the slow peristalsis of cecal loops, which gives a longer time for bacteria to multiply (6).
The M cells located in the FAE are important for pathogen invasion into the circulation and for presentation of antigens to the immune system (14). The adherence of lactobacilli to FAE observed in this study may denote presentation of lactobacilli to the immune cells and is in line with the recent report of association of certain lactobacilli with Peyer's patches of the mouse (15). The light microscopy techniques employed here do not allow identification of individual M cells in the chicken ileum (3, 10), and the possible interaction of Lactobacillus with M cells requires a more detailed study. Our ongoing research (18) has shown that avian pathogenic Escherichia coli adhere to the crop epithelium and the FAE by using their mannose-binding type 1 fimbriae and that the strain ST1 efficiently inhibits such binding in vitro.
In summary, our results show that Lactobacillus isolates exhibit considerable cell and tissue tropism in their adherence to the tissue domains of the chicken intestine. Isolates from the chicken shared adhesiveness to the crop epithelium but showed differences in their adherence to enterocytes at the villi and to the FAE in the ileum. With the lack of knowledge on the adhesin molecules included in lactobacillar adhesion, we have no explanation at the present for the low adhesiveness of the lactobacilli obtained from the culture collection. Our results support the hypothesis (7) that bacterial adherence to the crop is important for colonization in the chicken and suggest, on the other hand, that some lactobacillar isolates colonize the crop and simply pass through the other parts of the chicken intestine.
Acknowledgments
This study was supported by the Academy of Finland (grant numbers 44168, 44600, and 50725).
We thank Brita Mäki and Raili Lameranta for skilled technical assistance.
REFERENCES
- 1.Apajalahti, J. H. A., H. Kettunen, M. Bedford, and W. E. Holben. 2001. Percent G+C profiling accurately reveals diet-related differences in the gastrointestinal microbial community of broiler chickens. Appl. Environ. Microbiol. 67:5656-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooker, B. E., and R. Fuller. 1975. Adhesion of lactobacilli to the chicken crop epithelium. J. Ultrastruct. Res. 52:21-31. [DOI] [PubMed] [Google Scholar]
- 3.Clark, M. A., M. A. Jepson, N. L. Simmons, T. A. Booth, and B. H. Hirst. 1993. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J. Histochem. Cytochem. 41:1679-1687. [DOI] [PubMed] [Google Scholar]
- 4.Deplancke, B., and H. R. Gasskins. 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 73:1131S-1141S. [DOI] [PubMed] [Google Scholar]
- 5.Fuller, R. 1973. Ecological studies on the lactobacillus flora associated with the crop epithelium of the fowl. J. Appl. Bacteriol. 36:131-139. [Google Scholar]
- 6.Fuller, R. 1975. Nature of the determinant responsible for the adhesion of lactobacilli to chicken crop epithelial cells. J. Gen. Microbiol. 87:245-250. [DOI] [PubMed] [Google Scholar]
- 7.Fuller, R., and A. Turvey. 1971. Bacteria associated with the intestinal wall of the fowl (Gallus domesticus). J. Appl. Bact. 34:617-622. [DOI] [PubMed] [Google Scholar]
- 8.Hathaway, L. J., and J.-P. Kraehenbuhl. 2000. The role of M cells in mucosal immunity. Cell. Mol. Life Sci. 57:323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodges, R. D. 1974. The histology of the fowl, p. 35-88. London Academic Press, London, England.
- 10.Kitagawa, H., S. Shiraishi, T. Imagawa, and M. Uehara. 2000. Ultrastructural characteristics and lectin-binding properties of M cells in the follicle-associated epithelium of chicken caecal tonsils. J. Anat. 197:607-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korhonen, T. K., R. Virkola, B. Westerlund, H. Holthöfer, and J. Parkkinen. 1990. Tissue tropism of Escherichia coli adhesins in human extraintestinal infections. Curr. Top. Microbiol. Immunol. 151:115-127. [DOI] [PubMed] [Google Scholar]
- 12.McCracken, V. J., and R. G. Lorenz. 2001. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell. Microbiol. 3:1-11. [DOI] [PubMed] [Google Scholar]
- 13.Morishita, Y., T. Mitsuoka, C. Kaneuchi, S. Yamamoto, and M. Ogata. 1971. Specific establishment of lactobacilli in the digestive tract of germ-free chickens. Jpn. J. Microbiol. 15:531-538. [DOI] [PubMed] [Google Scholar]
- 14.Neutra, M. R., N. J. Mantis, A. Frey, and P. J. Giannasca. 1999. The composition and function of M cell apical membranes: implications for microbial pathogenesis. Immunology 11:171-181. [DOI] [PubMed] [Google Scholar]
- 15.Plant, L., and P. Conway. 2001. Association of Lactobacillus spp. with Peyer's patches in mice. Clin. Diagn. Lab. Immunol. 8:320-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos, S., F. Karner, L. Axelsson, and H. Jonsson. 2000. Lactobacillus mucosae sp. nov., a new species with in vitro mucus-binding activity isolated from pig intestine. Int. J. Syst. Evol. Microbiol. 50:251-258. [DOI] [PubMed] [Google Scholar]
- 17.Sillanpää, J., B. Martinez, J. Antikainen, T. Takahiro, N. Kalkkinen, S. Tankka, K. Lounatmaa, J. Keränen, M. Höök, B. Westerlund-Wikström, P. H. Pouwels, and T. K. Korhonen. 2000. Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. J. Bacteriol. 182:6440-6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tankka, S., S. Leskelä, E. Ron, J. Apajalahti, and T. K. Korhonen. In-vitro adhesion of an avian pathogenic Escherichia coli 078 strain to the surfaces of the chicken intestinal tract and to ileal mucus. Vet. Microbiol., in press. [DOI] [PubMed]
- 19.Tannock, G. W. 1999. Analysis of the intestinal microflora: a renaissance. Antonie Leeuwenhoek 76:265-278. [PubMed] [Google Scholar]
- 20.Toba, T., R. Virkola, B. Westerlund, Y. Björkman, J. Sillanpää, T. Vartio, N. Kalkkinen, and T. K. Korhonen. 1995. A collagen-binding S-layer protein in Lactobacillus crispatus. Appl. Environ. Microbiol. 61:2467-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuomola, E. M., A. C. Ouwenhand, and S. J. Salminen. 1999. Human ileostomy glycoproteins as a model for small intestinal mucus to investigate adhesion of probiotics. Lett. Appl. Microbiol. 28:159-163. [DOI] [PubMed] [Google Scholar]