Abstract
Five bacterial isolates enriched from fuel-contaminated Antarctic soils fixed nitrogen in the dark heterotrophically and nonsymbiotically. Two isolates utilized jet fuel vapors and volatile hydrocarbons for growth but not in N-deficient medium. Bacteria such as these may contribute to in situ biodegradation of hydrocarbons in Antarctic soils.
Phototrophic N2-fixing cyanobacteria have been detected in and isolated from Antarctic surface soils, snow, sea ice, and permanent lake ice (e.g., references 18 and 22), where they contribute to nutrient cycling. The presence of free-living heterotrophic diazotrophs in cyanobacterial mats on soils adjacent to McMurdo Dry Valley lakes has been inferred through measurement of nitrogenase activity in the dark and amplification of nifH genes having some sequence similarity to heterotrophic diazotrophs (17). However, axenic cultures of heterotrophic diazotrophs from these sources have not been described previously, nor have they been reported in soils distant from Dry Valley lakes or from subsurface soils, likely for lack of examination.
The presence of such organisms has environmental significance in Antarctic soils, which commonly have very low levels of fixed nitrogen (e.g., <0.01% [wt/wt] [8]). Spills of diesel and jet fuel on such soils result in extremely high soil carbon/nitrogen ratios (e.g., >250:1 [2]), which may limit microbial degradation of the hydrocarbons. In addition, spilled oils usually penetrate to a depth such that surficial photosynthetic nitrogen fixation would be of limited benefit in ameliorating the C/N ratio below the surface. Therefore, heterotrophic N2-fixing activity in contaminated soils may be important for natural attenuation of Antarctic fuel spills by providing combined nitrogen to hydrocarbon-degrading microbes, indirectly enhancing bioremediation at such sites. It is important to examine the potential biodegradative abilities of the indigenous microflora, as importation of microbes for bioremediation is banned by the Antarctic Treaty.
Enrichment and identification of heterotrophic diazotrophs.
Soils were collected aseptically from hydrocarbon-contaminated and uncontaminated sites in the Ross Sea region at Scott Base (Ross Island) and Marble Point (coastal continent) and in the Wright Valley (near Lake Vanda). Free-living heterotrophic N2-fixing bacteria were enriched by repeated transfer on nitrogen-deficient semisolid malate (NDSM) medium containing the following per liter of deionized water: 0.5 g of K2HPO4, 0.2 g of MgSO4 · 7H2O, 0.1 g of NaCl, 0.02 g of CaCl2 · 2H2O, 0.01 g of FeCl3, 0.002 g of Na2MoO4 · 2H2O, 0.002 g of sodium vanadate, 5.0 g of l-malic acid, 4.5 g of KOH, 0.01 g of MnSO4 · H2O, and 1.75 g of Noble agar (Difco), pH 6.8 to 7.0. Tubes of inoculated medium were incubated stationary in the dark at 22°C. From 19 soil enrichments, five isolates were purified on plate count agar (Difco) from Marble Point and the Wright Valley (Table 1). Diazotrophs were enriched from fuel-contaminated soils but not from nearby uncontaminated soils. Both surface and subsurface samples yielded N2 fixers; 35 to 40 cm was the greatest depth sampled.
TABLE 1.
Source of isolates and presumptive identification or nearest phylogenetic neighbor
| Isolate | Source (depth in cm) | Presumptive identification | GenBank accession no. |
|---|---|---|---|
| 5.1 | Marble Point (0-2) | Pseudomonas stutzeria | AF411853 |
| 5A | Marble Point (5-10) | Pseudomonas stutzeria | AF411854 |
| 7C | Wright Valley (0-5) | Azospirillum brasilense | AF411852 |
| 5B | Marble Point (35-40) | Pseudomonas sp. (nearest phylogenetic neighbor) | AF411855 |
| 5C | Marble Point (0-2) | Aquaspirillum sp. (nearest phylogenetic neighbor) | AF411851b; AF413109c |
Presumptive identification (Table 1) was accomplished by standard biochemical tests (9), Gram staining, colony morphology determination, transmission electron microscopy of flagella, and use of API 20NE multitest strips (bioMerieux, Marcy l'Etoile, France), with appropriate control cultures. Full-length 16S ribosomal DNA sequences were determined as previously described (1). Preliminary comparison of sequences with the Ribosomal Database Project II (16) (June 2002) indicated affiliations with Azospirillum and Pseudomonas groups. However, two isolates gave poor matches. Isolate 5B had S_ab scores of ≤0.90 with named pseudomonads. Isolate 5C, which had two variant forms of its 16S rRNA gene differing by six substitutions and a single base insertion-deletion, had low similarity scores (S_ab, ≤0.70) with members of the Azospirillum group. Comparisons with a wider range of organisms indicated that isolate 5C shared the greatest sequence identity with Aquaspirillum peregrinum subsp. integrum (GenBank accession no. AB074521; S_ab = 0.915) and Aquaspirillum itersonii NCIMB 9070 (GenBank accession no. Z29620; S_ab = 0.912). Whether isolates 5B and 5C, with their low sequence identity to known species, are unique to Antarctica is not known at present.
A representative selection of Azospirillum spp. was chosen for more comprehensive phylogenetic analysis of isolate 7C with the portable version of PAUP∗ (25) with tasks distributed over 26 Pentium III computers running RedHat Linux 7.0. An initial phylogenetic tree was generated by using neighbor joining on a distance matrix corrected with the Hasegawa-Kishino-Yano model (HKY85) (10). This tree was refined by branch swapping with the tree bisection-reconnection algorithm using maximum likelihood with HKY+I+Γ. The gamma shape parameter, Ti/Tv (transition-to-transversion) ratio, proportion of invariant sites, and base frequencies were estimated from the starting tree. A single tree with −ln L (ln likelihood) of 4452 was found, with isolate 7C falling among the cluster of Azospirillum brasilense with good bootstrap support (data not shown).
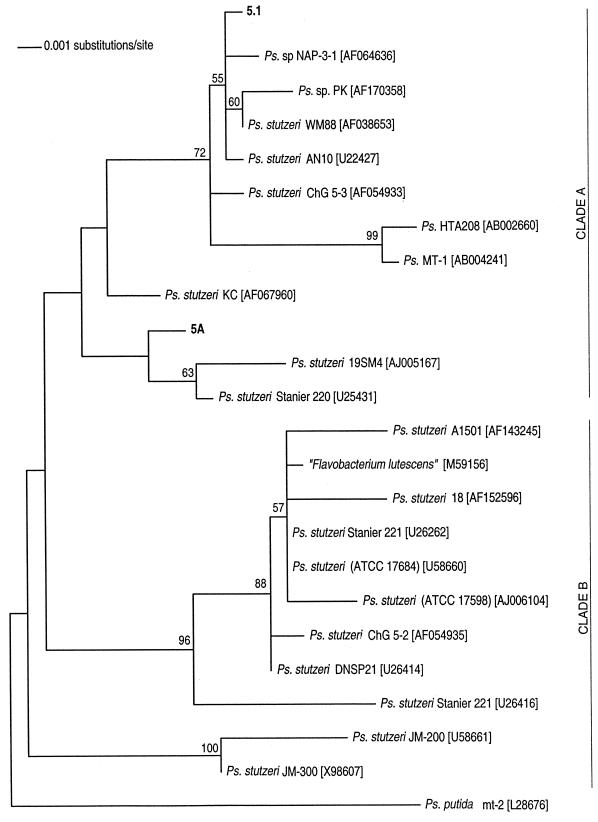
For isolates 5.1, 5A, and 5B, a primary phylogenetic analysis was performed with representatives of all the major Pseudomonas groups with Escherichia coli as the outgroup. Isolates 5.1 and 5A clearly fell within the Pseudomonas stutzeri clade. A second phylogenetic analysis was performed with these two isolates and P. stutzeri strains by using essentially the same approach as that for isolate 7C. The inferred tree is shown in Fig. 1. Bootstrap support for the placing of isolate 5A is low. Furthermore, a one-tailed Kishino-Hasegawa test (11) showed that this tree was not significantly better (P = 0.060) than a tree where 5A was constrained within the adjacent clade. Clearly then, although strain 5A optimally branches within clade A (Fig. 1), affiliation with P. stutzeri 19SM4 and Stanier 220 is not fully established. Isolate 5B, although clearly a member of the Pseudomonas genus, showed no well-supported affiliation with any of the major groups and branched deeply within the tree. For this reason, it is excluded from Fig. 1 and is identified only as Pseudomonas sp. (Table 1).
FIG. 1.
Maximum likelihood tree of P. stutzeri strains. GenBank accession numbers of the sequences are shown in square brackets. Bootstrap values were obtained from 284 maximum likelihood replicates. Each bootstrap replicate was a full heuristic search with 10 random additions of sequences. Pseudomonas putida was used as an outgroup.
Diazotrophy.
Nitrogenase activity was detected indirectly by gas chromatographic (GC) measurement of acetylene reduction to ethylene (27), by using a 2-m stainless steel Poropak R (80/100 mesh) column at 60°C in a Hewlett-Packard 5890A GC connected to a Hewlett-Packard 3392 integrator. Acid-washed screw-cap glass vials with TFE-silicon septa (Fisher) containing NDSM medium were incubated stationary in the dark at 22°C. Growth was observed as subsurface (microaerophilic) bands at variable depths. After incubation, acetylene was injected to ca. 10% of the headspace volume. After 24 h, 300-μl headspace samples were analyzed by GC with standard mixtures of acetylene and ethylene and parallel uninoculated controls. All five cultures demonstrated acetylene reduction that was completely repressed in control cultures amended with 1 g of NH4Cl liter−1.
DNA-DNA hybridization analyses with probes derived from classical molybdenum-dependent (nifD) and alternate Mo-independent nitrogenase genes (vnfD and anfD) (15) were conducted by P. Bishop (North Carolina State University). nifD genes were detected in all isolates, but none hybridized to the alternate nitrogenase gene probes. PCR amplification of the alternate nitrogenase genes vnfG and anfG (14) was unsuccessful, and the isolates were unable to grow in N-deficient, Mo-deficient media (P. Bishop, personal communication), suggesting that they harbor only the classical Mo-dependent nitrogenase.
Temperature effects.
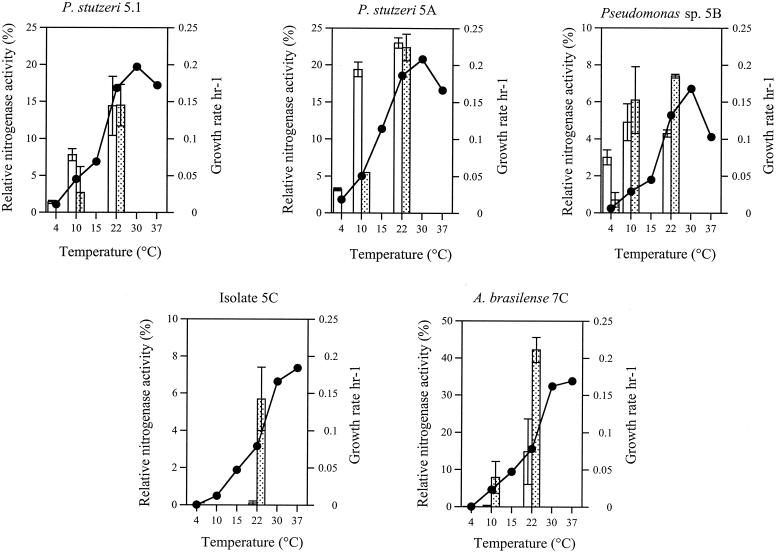
The effect of temperature on growth rate was determined in replicate flasks containing 25 ml of quarter-strength Trypticase soy broth (Difco) inoculated with cells suspended in phosphate buffer. Duplicate flasks having an initial optical density at 600 nm (OD600) of 0.03 to 0.05 were incubated at various temperatures at 150 rpm. Growth was determined spectrophotometrically, with duplicates typically varying by <10%. The maximum exponential growth rate was calculated graphically for each temperature from the mean OD600, as described previously (1). Isolates 5C and 7C had optima of ≥37°C (Fig. 2, line graph) and minima of ≥4°C, whereas the psychrotolerant pseudomonads had optima of 30°C and minima of <4°C. Significantly, all isolates could grow at 10°C, well within the upper summer temperatures of the Antarctic source soils (≥18°C [3]).
FIG. 2.
Effect of temperature on exponential growth rate (solid circles) and on relative nitrogenase activity (bars). Growth rate was determined graphically from the mean OD600 of duplicate liquid cultures (1). Relative nitrogenase activity was determined for cultures pregrown at 10°C (open bars) or 22°C (stippled bars) and subsequently incubated with acetylene at 4, 10, or 22°C. Acetylene reduction activity was expressed as the percentage of total GC peak area corresponding to the ethylene peak for each injection. Values are the means of duplicate cultures; error bars represent 1 standard deviation.
The effect of temperature on nitrogenase activity, detected as acetylene reduction, was measured by pregrowing the isolates in duplicate vials of NDSM medium at 10°C (the lowest temperature tested at which all isolates could grow) or 22°C (the upper end of environmental relevance in situ). After growth was established (4 days at 22°C or 3 weeks at 10°C), the vials were sealed and equilibrated at 4, 10, or 22°C for 2 h, and acetylene was injected to 10% by volume of headspace. Headspace samples were removed at 24-h intervals for GC analysis up to 48 h at 22°C and to 72 h at 4 and 10°C. Relative nitrogenase activity was expressed as the percentage of the total GC peak area representing the ethylene peak for each injection (Fig. 2, bars), without correction for biomass. The Pseudomonas spp. exhibited greater detectable nitrogenase activity at 4 and 10°C when pregrown at 10°C than when pregrown at 22°C. In contrast, pregrowth of isolate 5C and A. brasilense 7C at 10°C did not enhance nitrogenase activity at lower temperatures, and neither strain showed measurable acetylene reduction at 4°C even after 72 h. Diazotrophic pseudomonads from Canadian High Arctic soils also showed variability in their tolerance to low temperatures, with some unable to grow or reduce nitrogen at 9°C (13). Our data suggest that the three Pseudomonas spp. can grow and fix nitrogen at environmentally relevant soil temperatures of ≤10°C and therefore may be active seasonally in situ. However, the slow growth and poor nitrogenase activity of A. brasilense 7C and isolate 5C at low temperatures raise questions about their activity in situ.
Carbon source utilization.
To determine whether the isolates could support diazotrophy by utilizing hydrocarbon vapors, they were inoculated into N-deficient semisolid (NDS) medium lacking a carbon source. Serum bottles, containing NDS prepared with or without 1 g of NH4Cl liter−1, were fitted with stoppers and suspended plastic cupules (Kontes, Vineland, N.J.) containing sterile filter paper strips soaked with filter-sterilized JP-8 jet fuel; parallel controls had no jet fuel. After incubation in the dark, stationary, at 22°C for 10 days, Pseudomonas sp. isolates 5A and 5B grew in distinct subsurface bands with jet fuel vapors but only when provided with NH4Cl. Under the conditions used, no isolate grew or demonstrated acetylene reduction with jet fuel as the sole carbon source in NDS medium; however, it is possible that alkane vapors from the jet fuel interfered with the acetylene reduction assay (7). Furthermore, P. stutzeri 5A grew on plates of Bushnell-Haas agar (Difco) incubated individually with vapors of benzene, toluene, and m-xylene, and Pseudomonas sp. isolate 5B grew on hexane vapors. The remaining three strains did not grow on JP-8 or any of the pure hydrocarbons tested.
Authenticated cases of axenic N2-fixing hydrocarbon-degrading microbes are rare and biased toward methane oxidizers (e.g., reference 6). Infrequent reports spanning 4 decades (e.g., references 5, 7, 12, 21, 23, and 24) have described pure isolates that can both fix N2 and utilize gaseous or liquid hydrocarbons, but not necessarily simultaneously.
Significance.
Our success in isolating heterotrophic diazotrophs from contaminated Antarctic soils may have resulted from in situ selection imposed by the presence of hydrocarbons in these soils. Pristine Antarctic soils are limited in organic carbon for heterotrophic diazotrophy, whereas fuel-contaminated soils have adequate carbon but limited nitrogen. This combination of circumstances may be the key to in situ enrichment of consortia capable of hydrocarbon degradation under N2-limited conditions, and/or to selection of diazotrophs with the uncommon ability to utilize hydrocarbons.
Currently we have no evidence that these diazotrophs fix nitrogen or degrade hydrocarbons in situ. If so, it is possible that they alternate between hydrocarbon utilization and diazotrophy. Another attractive hypothesis is that they exist in association with nondiazotrophic hydrocarbon-degrading bacteria that provide reduced oxygen levels and hydrocarbon metabolites as a carbon source: for example, acetate (a common metabolite of alkane biodegradation) supported N2 fixation by all five Antarctic isolates. The diazotrophs in return may provide fixed nitrogen to the hydrocarbon degraders. Microbial consortia with N2-fixing and hydrocarbon-utilizing activities have been reported recently (e.g., references 19, 20, and 26). Such consortia in microsites could maintain a lower, more favorable pH than the bulk Antarctic soils, which were typically pH 8 to 9 (2). A third possibility is that the diazotrophs indirectly contribute to soil nitrogen by releasing nitrogenous biomass components after their death (4). The intriguing possibility that free-living Antarctic heterotrophic diazotrophs contribute to fuel spill bioremediation in situ deserves further examination.
Acknowledgments
We gratefully acknowledge funding from NSERC and the Petro-Canada Young Innovators Award to J.F., from Sigma Xi The Research Society to R.E., and from PGSF to J.A.
Logistical field support was provided by Antarctica New Zealand. We thank Sara Ebert, Kathy Semple (Canada), and Lisa Harris (New Zealand) for technical assistance; P. Bishop (North Carolina State University) for nif gene analysis; and W. J. Page (University of Alberta) for helpful discussions.
REFERENCES
- 1.Aislabie, J., J. Foght, and D. Saul. 2000. Aromatic hydrocarbon-degrading bacteria from soil near Scott Base, Antarctica. Polar Biol. 23:183-188. [Google Scholar]
- 2.Aislabie, J., M. McLeod, and R. Fraser. 1998. Potential for biodegradation of hydrocarbons in soil from the Ross Dependency, Antarctica. Appl. Microbiol. Biotechnol. 49:210-214. [Google Scholar]
- 3.Balks, M., J. Kimble, R. Paetzold, J. Aislabie, and I. Campbell. 2001. Effects of hydrocarbon contaminants on the temperature and moisture regimes of cryosols of the Ross Sea region, Antarctica, p. 33-39. In Collected proceedings, 2nd International Conference on Contaminants in Freezing Soil. Scott Polar Research Institute, Cambridge University, Cambridge, United Kingdom, in association with Geotechnical Science Laboratories, Carleton University, Ottawa, Canada.
- 4.Chan, Y.-K., W. L. Barraquio, and R. Knowles. 1994. N2-fixing pseudomonads and related soil bacteria. FEMS Microbiol. Rev. 13:95-118. [Google Scholar]
- 5.Coty, V. F. 1967. Atmospheric nitrogen fixation by hydrocarbon-oxidizing bacteria. Biotechnol. Bioeng. 9:25-32. [Google Scholar]
- 6.Davis, J. B., V. F. Coty, and J. P. Stanley. 1964. Atmospheric nitrogen fixation by methane-oxidizing bacteria. J. Bacteriol. 88:468-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bont, J. A. M., and E. G. Mulder. 1976. Invalidity of the acetylene reduction assay in alkane-utilizing, nitrogen-fixing bacteria. Appl. Environ. Microbiol. 31:640-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritsen, C. H., A. M. Grue, and J. C. Priscu. 2000. Distribution of organic carbon and nitrogen in surface soils in the McMurdo Dry Valleys, Antarctica. Polar Biol. 23:121-128. [Google Scholar]
- 9.Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg. 1994. Methods for general and molecular microbiology. American Society for Microbiology, Washington, D.C.
- 10.Hasegawa, M., H. Kishino, and T. Yano. 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 21:160-174. [DOI] [PubMed] [Google Scholar]
- 11.Kishino, H., and M. Hasegawa. 1989. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from sequence data and the branching order in the Hominoidea. J. Mol. Evol. 29:170-179. [DOI] [PubMed] [Google Scholar]
- 12.Laguerre, G., B. Bossand, and R. Bardin. 1987. Free-living dinitrogen-fixing bacteria isolated from petroleum refinery oily sludge. Appl. Environ. Microbiol. 53:1674-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lifshitz, R., J. W. Kloepper, F. M. Scher, E. M. Tipping, and M. Laliberté. 1986. Nitrogen-fixing pseudomonads isolated from roots of plants grown in the Canadian High Arctic. Appl. Environ. Microbiol. 51:251-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loveless, T. M., and P. E. Bishop. 1999. Identification of genes unique to Mo-independent nitrogenase systems in diverse diazotrophs. Can. J. Microbiol. 45:312-317. [PubMed] [Google Scholar]
- 15.Loveless, T. M., J. R. Saah, and P. E. Bishop. 1999. Isolation of nitrogen-fixing bacteria containing molybdenum-independent nitrogenases from natural environments. Appl. Environ. Microbiol. 65:4223-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson, J. B., T. F. Steppe, R. W. Litaker, and H. W. Paerl. 1998. N2-fixing microbial consortia associated with the ice cover of Lake Bonney, Antarctica. Microb. Ecol. 36:231-238. [DOI] [PubMed] [Google Scholar]
- 18.Paerl, H. W., and J. C. Priscu. 1998. Microbial phototrophic, heterotrophic, and diazotrophic activities associated with aggregates in the permanent ice cover of Lake Bonney, Antarctica. Microb. Ecol. 36:221-230. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Vargas, J., H. M. Poggi-Varaldo, G. Calva-Calva, E. Rios-Leal, R. Rodriguez-Varquez, R. Ferrera-Cerralto, and F. Esparza-Garcia. 2000. Nitrogen-fixing bacteria capable of utilizing kerosene hydrocarbons as a sole carbon source. Water Sci. Technol. 42:407-410. [Google Scholar]
- 20.Piehler, M. F., J. G. Swistak, J. L. Pinckney, and H. W. Paerl. 1999. Stimulation of diesel fuel biodegradation by indigenous nitrogen fixing bacterial consortia. Microb. Ecol. 38:69-78. [DOI] [PubMed] [Google Scholar]
- 21.Prantera, M. T., A. Drozdowicz, S. G. Letie, and A. S. Rosado. 2002. Degradation of gasoline aromatic hydrocarbons by two N2-fixing soil bacteria. Biotechnol. Lett. 24:85-89. [Google Scholar]
- 22.Priscu, J. D., C. H. Fritsen, E. E. Adams, S. J. Giovannoni, H. W. Paerl, C. P. McKay, P. T. Doran, D. A. Gordon, B. D. Lanoil, and J. L. Pinckney. 1999. Perennial Antarctic lake ice: an oasis for life in a polar desert. Science 280:2095-2098. [DOI] [PubMed] [Google Scholar]
- 23.Riviére, J., J. Oudot, J. Jonquères, and G. Gatellier. 1974. Fixation d'azote atmosphérique par des bactéries utilisant l'hexadécane comme source de carbone et d'énergie. Ann. Agron. 25:633-644. [Google Scholar]
- 24.Roy, I., S. K. Shukla, and A. K. Mishra. 1988. n-Dodecane as a substrate for nitrogen fixation by an alkane-utilizing Azospirillum sp. Curr. Microbiol. 16:303-309. [Google Scholar]
- 25.Swofford, D. L. 2001. PAUP. Phylogenetic analysis using parsimony, version 4. Sinauer Associates, Sunderland, Mass.
- 26.Toccalino, P. L., R. L. Johnson, and D. R. Boone. 1993. Nitrogen limitation and nitrogen fixation during alkane biodegradation in a sandy soil. Appl. Environ. Microbiol. 59:2977-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner, G. L., and A. J. Gibson. 1980. Measurement of nitrogen fixation by indirect means, p. 111-138. In F. J. Bergerson (ed.), Methods for evaluating biological nitrogen fixation. John Wiley and Sons, Ltd., Chichester, United Kingdom.