Abstract
The success of Listeria monocytogenes as a food-borne pathogen owes much to its ability to survive a variety of stresses, both in the food environment and, after ingestion, within the animal host. Growth at high salt concentrations is attributed mainly to the accumulation of organic solutes such as glycine betaine and carnitine. We characterized L. monocytogenes LO28 strains with single, double, and triple deletions in the osmolyte transport systems BetL, Gbu, and OpuC. When single deletion mutants were tested, Gbu was found to have the most drastic effect on the rate of growth in brain heart infusion (BHI) broth with 6% added NaCl. The highest reduction in growth rate was found for the triple mutant LO28BCG (ΔbetL ΔopuC Δgbu), although the mutant was still capable of growth under these adverse conditions. In addition, we analyzed the growth and survival of this triple mutant in an animal (murine) model. LO28BCG showed a significant reduction in its ability to cause systemic infection following peroral coinoculation with the wild-type parent. Altering OpuC alone resulted in similar effects (R. D. Sleator, J. Wouters, C. G. M. Gahan, T. Abee, and C. Hill, Appl. Environ. Microbiol. 67:2692-2698, 2001), leading to the assumption that OpuC may play an important role in listerial pathogenesis. Analysis of the accumulation of osmolytes revealed that betaine is accumulated up to 300 μmol/g (dry weight) when grown in BHI broth plus 6% NaCl whereas no carnitine accumulation could be detected. Radiolabeled-betaine uptake studies revealed an inability of BGSOE (ΔbetL Δgbu) and LO28BCG to transport betaine. Indeed, for LO28BCG, no accumulated betaine was found, but carnitine was accumulated in this strain up to 600 μmol/g (dry weight) of cells, indicating the presence of a possible fourth osmolyte transporter.
Listeria monocytogenes is a gram-positive food-borne pathogen that is highly resistant to osmotic stress (NaCl concentrations of up to 10%) and can grow at refrigeration temperatures (31). This characteristic growth and survival under such adverse environmental conditions is attributed mainly to the accumulation of the organic compounds glycine betaine (N,N,N-trimethylglycine) (16, 18) and carnitine (β-hydroxy-γ-N-trimethylaminobutyrate) (3). Accumulated to high internal concentrations without adversely affecting vital cellular processes, these compounds are often referred to as compatible solutes (32). In general, compatible solutes are small, highly soluble molecules which carry no net charge at physiological pH and function to stabilize protein structure and function while also maintaining cell volume at elevated osmolarity (14, 27). As well as glycine betaine and carnitine, other compounds, such as sugars, amino acids, amino acid derivatives, sulfate esters, and small peptides, have been shown to function as effective compatible solutes in bacterial cells (14). For L. monocytogenes cells grown in complex media with 7.5% added NaCl, increases in glycine betaine and carnitine levels were accompanied by elevated concentrations of potassium, glutamate, glycine, alanine, and proline (18). However, while proline at extracellular concentrations of <10 mM is incapable of promoting growth at elevated osmolarity (1, 18), a recent study by Sleator et al. revealed that growth at high salt concentrations in complex media is significantly reduced in a strain lacking the proline biosynthesis pathway (25). In a defined medium with 4% added NaCl, betaine, carnitine, acetylcarnitine, γ-butyrobetaine, and proline betaine all act as osmoprotectants and resulted in significant growth stimulation (1). Of these, betaine and carnitine are the most effective osmoprotectants in L. monocytogenes (3, 18). Transport of glycine betaine into the cell is induced 200-fold upon salt stress (16). In another study, the duration of transport of betaine and carnitine was found to be directly related to the osmotic strength of the medium (30).
The uptake of glycine betaine and carnitine is thought to be mediated via three osmolyte transporters; BetL, Gbu, and OpuC (7, 15, 24, 28). The first, BetL of L. monocytogenes LO28, is a betaine transporter homologous to OpuD of Bacillus subtilis and BetP of Corynebacterium glutamicum (24). These systems belong to a family of secondary transporters transporting an ion in symport with the osmolyte (9). Deletion of the betL gene resulted in a lower specific growth rate at high osmolarities (26). However, no significant difference in virulence potential was observed in the absence of a functional BetL transporter (26). The second transport system identified, Gbu, encoded by the gbuABC operon, belongs to the binding-protein-dependent ATP binding cassette superfamily of transporters and is homologous to OpuA in B. subtilis (15). gbuA encodes an ATPase, gbuB encodes a permease, and gbuC encodes a substrate binding protein. Membrane translocation of an osmolyte via Gbu is dependent on ATP hydrolysis. Deletion of gbu in L. monocytogenes 10403S results in a decreased growth rate both at high salt concentrations and at reduced temperatures (15). Moreover, glycine betaine transport activity in a gbu mutant is about fourfold lower than in wild-type cells in modified Pine's medium with 8% NaCl (15). The most recently identified transporter, OpuC, also a member of the ATP binding cassette superfamily, is dedicated mainly to carnitine uptake (7, 28). The opuCABCD operon, encoding the transporter, is homologous to opuC and opuB of B. subtilis. As with the Gbu system, osmolyte uptake by OpuC is coupled to ATP hydrolysis. Carnitine transport is severely reduced in an opuC mutant of L. monocytogenes EGD (7). In addition, virulence studies revealed that a Listeria opuC deletion mutant exhibits a reduced ability to colonize the upper small intestine and cause subsequent systemic infection following peroral inoculation (28).
With the identification and characterization of these three osmolyte transporters, the mechanism of salt tolerance is becoming clearer. However, the role and impact of accumulated osmolytes, both betaine and carnitine, during high-osmolarity growth remains to be clarified. Using mutant strains with multiple deletion mutations in the known osmolyte transporters, the aim of this study was to describe the role of betaine and carnitine in growth at high osmolarity and in virulence.
MATERIALS AND METHODS
Strains, chemicals, and growth conditions.
The L. monocytogenes LO28 wild-type strain used throughout this study was a gift from P. Cossart, Institut Pasteur, Paris, France. Strains disrupted in one of the BetL (BSOE) (24, 26), Gbu (GSOE), or OpuC (LO28C) (28) transport systems were used, as well as double and triple mutants constructed against these single-mutant backgrounds. Since we encountered problems in growing the double mutant LO28BC (ΔbetL ΔopuC), this strain was excluded from the experiments. Strains were grown either in brain heart infusion (BHI) broth or in the defined medium (DM) described by Premaratne et al. (21). When required, the medium osmolarity was adjusted by the addition of NaCl to produce DMS (DM plus 3% NaCl). Where indicated, carnitine and glycine betaine (Sigma Chemical Co., St. Louis, Mo.) were added to DM(S) as filter-sterilized solutions to a final concentration of 1 mM. For growth of the single, double, and triple mutants LO28C (ΔopuC), LO28CG (ΔopuC Δgbu), and LO28BCG (ΔbetL ΔopuC Δgbu), erythromycin (10 μg/ml) was used as a selection marker. Experiments were performed at least twice, cell growth was monitored spectrophotometrically by measuring the optical density at 620 nm (OD620), and growth rates were calculated.
Generation of mutants.
The bank of single, double, and triple mutants used in this study was constructed using a combination of plasmid (pORI19)-mediated insertional mutagenesis and gene disruption based on the splicing by overlap extension (SOE) procedure. While the construction of BSOE (ΔbetL) and LO28C (ΔopuC) was described previously (24, 26, 28), mutants with deletions in gbuABC were obtained using the SOE technique to remove a 1-kb fragment from the center of the operon. Essentially two ≈325-bp PCR products (amplified by gbu SOEA [5′ GAATTCGTTAATTTTGAAAAAGACGG 3′] and gbu SOEB [5′ CCAGCATCAATTCCTGTGATTTCCAGAAAGTGCGGCCAG 3′] and gbu SOEC [5′ ATCACAGGAATTGATGCTGG 3′] and gbu SOED [5′ TCTAGAAGAAATTATCTAACACTTG 3′]) flanking the sequence to be deleted were spliced, giving a ≈650-bp hybrid which was subsequently cloned into the temperature-sensitive shuttle vector pKSV-7 and transformed into Escherichia coli DH5. The resulting plasmid, designated pCPL17, was subsequently transformed into the wild-type LO28 and mutant ΔbetL strains, and the resulting transformants were selected on BHI plates containing 10 μg of chloramphenicol per ml. Selection at 42°C of cells with chromosomal integration of pCPL17, followed by sequential passaging in BHI broth at 30°C in the absence of chloramphenicol, facilitated the recovery of cells in which allelic exchange between the intact gbuABC operon and the ≈650-bp insert on pCPL17 had occurred, thus giving rise to GSOE (Δgbu) and BGSOE (ΔbetL Δgbu). The double mutants LO28BC (ΔbetL ΔopuC) and LO28CG (ΔopuC Δgbu), together with the triple mutant LO28BCG (ΔbetL ΔopuC Δgbu), were constructed using pORI19-mediated insertional mutagenesis. Plasmid pCPL5 (pORI19 containing 1.1 kb of the listerial opuC operon [28]) was transformed into strains BSOE (ΔbetL), GSOE (Δgbu), and BGSOE (ΔbetL Δgbu) containing the RepA + temperature-sensitive helper plasmid pVE6007, and transformants were selected at 30°C on BHI plates containing 5 μg of erythromycin per ml. Temperature upshift from 30°C to the nonpermissive 42°C resulted in loss of pVE6007 and targeted chromosomal integration of pCPL5, thus creating LO28CG (ΔopuC Δgbu), LO28BC (ΔbetL ΔopuC), and LO28BCG (ΔbetL ΔopuC Δgbu).
Virulence assays.
Bacterial virulence was determined by peroral coinoculation of 8- to 12-week-old BALB/c mice as described by Sleator et al. (28). Mutant and wild-type strains were suspended in buffered saline with gelatin (0.85% NaCl, 0.01% gelatin, 2.2 mM K 2HPO4, 4.2 mM Na 2HPO4). Each animal was infected with approximately 5 × 109 cells via a micropipette tip placed immediately behind the incisors. At 3 days postinfection, the mice were euthanized and the listerial numbers in the livers and spleens of infected animals were determined by spread plating homogenized samples onto BHI agar plates.
Uptake studies.
L. monocytogenes LO28 wild type and mutants were grown in DMS until they reached early exponential phase (OD620 ≈ 0.25). Osmolyte uptake studies were carried out essentially as described by Verheul et al. (30). The cells were concentrated to an OD620 of 20 in 50 mM potassium phosphate buffer (pH 6.8) containing 5 mM MgSO4, 3% NaCl, and chloramphenicol (100 μg/ml) to inhibit protein synthesis and were then stored on ice until use. Cells (OD620 of 1) were preenergized at 37°C with 1% glucose for 5 min prior to the addition of radiolabeled osmolyte N,N,N-[1-14C]trimethylglycine (final concentration, 19 μM), purchased from Campo Scientific (Veenendaal, The Netherlands). Samples were withdrawn after 0.5, 1, 2, 3, and 4 min, and uptake was stopped by the addition of 2 ml of 50 mM potassium phosphate buffer (pH 6.8) containing 5 mM MgSO4 and 3% NaCl. The cells were collected on 0.2-μm-pore-size cellulose nitrate filters (Schleicher & Schuell GmbH, Dassell, Germany) under vacuum; the filters were washed with another 2 ml of buffer, and the radioactivity trapped in the cells was measured using a scintillation counter (model 1600TR; Packard Instruments Co., Downers Grove, Ill.). Uptake of osmolytes by the cells was normalized to total cellular protein. The experiment was performed in triplicate, and the results of a typical experiment are presented.
Accumulation studies.
Cells were grown in BHI broth at 30°C with 3 or 6% added NaCl until they reached the midexponential growth-phase (OD620 ≈ 0.4). Then 100 ml of culture was pelleted and washed twice in 50 mM potassium phosphate buffer (pH 6.8) containing 5 mM MgSO4 with 3 or 6% added NaCl (isotonic with the growth medium). The cells were resuspended in 750 μl of water and freeze-dried for determination of dry weight. The cells were extracted by the procedure of Galinski and Herzog (8), using methanol-chloroform. Betaine and carnitine concentrations were determined as described by Verheul et al. (30). They were measured by obtaining the refractive index after high-performance liquid chromatography using a LiChrosphere 100-NH2, 5-μm column (Merck, Darmstadt, Germany) at a flow rate of 1 ml/min at 45°C with a mobile phase of 80:20 (vol/vol) acetonitrile-20 mM potassium phosphate (pH 7.0). The concentrations of betaine and carnitine were calculated from the area under the respective peaks using calibration curves. The experiment was performed in duplicate, and the results of a typical experiment are presented.
RESULTS
Growth of the osmolyte transporter mutants.
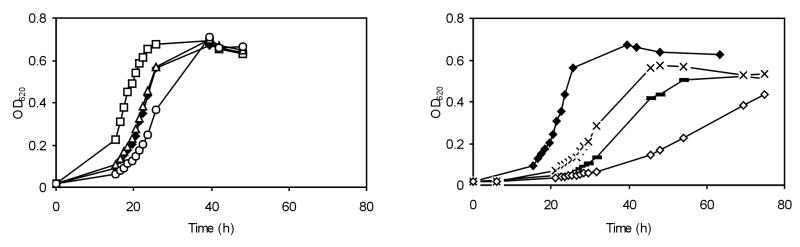
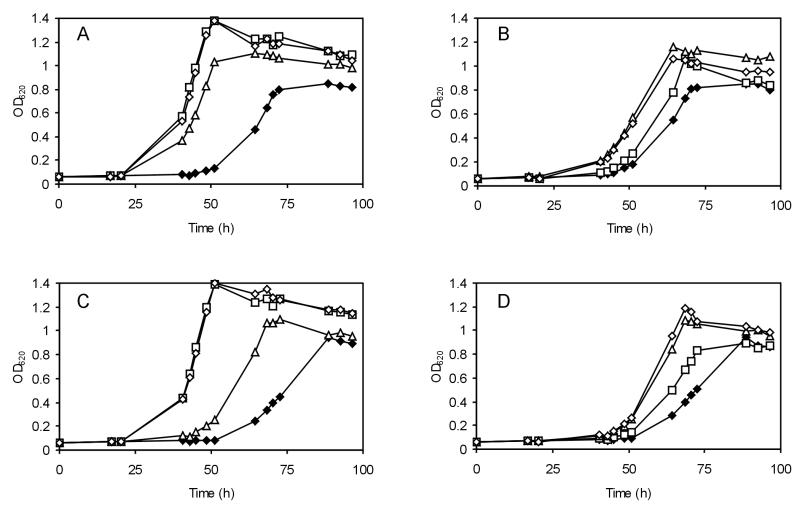
L. monocytogenes is known for its ability to grow under high-osmolarity conditions. To identify the role of osmolytes in salt stress, we monitored the growth of the osmolyte uptake mutants in BHI broth with 6% added NaCl (Fig. 1). Growth of wild-type LO28 and the mutants in BHI broth with no added NaCl at 30°C was similar (data not shown). On addition of 6% NaCl, the growth rate of wild type LO28 was 0.059 h−1 (Table 1). Similar growth rates were found for the single mutants BSOE (ΔbetL) and LO28C (ΔopuC), while the growth rate of GSOE (Δgbu) was reduced to 0.044 h−1. Surprisingly, the lag time for the single mutant BSOE (ΔbetL) was found to be shorter than that for the wild type. The growth rate of the double mutant BGSOE (ΔbetL Δgbu) was lower (0.022 h−1) than that of LO28CG (ΔopuC Δgbu) (0.029 h−1), indicating the importance of the betaine transporters BetL and Gbu. Deletion of all three transporters resulted in a strain severely affected in its growth (0.010 h−1). To identify the individual influence of betaine and carnitine, the strains were grown in DMS (3% NaCl) with added betaine and/or carnitine. The growth rate of L. monocytogenes in DMS was increased by the addition of betaine or carnitine (Table 1). Relief of osmotic pressure was only partly achieved by the addition of betaine to DMS for the double mutant BGSOE (ΔbetL Δgbu), while addition of carnitine resulted in significant growth stimulation (Fig. 2). While addition of betaine to LO28CG (ΔopuC Δgbu) resulted in similar growth to that observed for the wild type, the presence of carnitine also resulted in an increase in the growth of this strain. Surprisingly, the presence of carnitine resulted in a slight increase in the growth of the triple mutant too. Growth of this strain was promoted slightly by addition of betaine and even more so by addition of carnitine (Fig. 2), thus suggesting the presence of a fourth osmolyte transporter, transporting carnitine (and betaine).
FIG. 1.
Growth of wild-type L. monocytogenes and osmolyte transporter mutants in BHI with 6% NaCl at 30°C. Symbols: ⧫, LO28 (wild type); □, BSOE (ΔbetL); ○, GSOE (Δgbu); Δ, LO28C (ΔopuC); _, BGSOE (ΔbetL Δgbu); ×, LO28CG (ΔopuC Δgbu); ◊, LO28BCG (ΔbetL ΔopuC Δgbu).
TABLE 1.
Growth rates of L. monocytogenes LO28 and mutants in BHI with 6% added NaCl and DMS with or without additional compatible solutes at 30°C
| Medium | Growth rate (h−1) of straina:
|
|||
|---|---|---|---|---|
| LO28 | BGSOE | LO28CG | LO28BCG | |
| BHI + 6% NaCl | 0.059 ± 0.003 | 0.022 ± 0.001 | 0.029 ± 0.001 | 0.010 ± 0.001 |
| DMS | 0.057 ± 0.007 | 0.031 ± 0.001 | 0.028 ± 0.002 | 0.028 ± 0.004 |
| DMS + betaine | 0.086 ± 0.005 | 0.034 ± 0.004 | 0.095 ± 0.008 | 0.038 ± 0.005 |
| DMS + carnitine | 0.074 ± 0.008 | 0.051 ± 0.007 | 0.041 ± 0.002 | 0.050 ± 0.008 |
| DMS + betaine and carnitine | 0.110 ± 0.017 | 0.038 ± 0.001 | 0.091 ± 0.008 | 0.041 ± 0.008 |
Results are presented as mean ± standard deviation.
FIG. 2.
Growth at 30°C of L. monocytogenes LO28 (wild type) (A), BGSOE (ΔbetL Δgbu) (B), LO28CG (ΔopuC Δgbu) (C), and LO28BCG (ΔbetL ΔopuC Δgbu) (D) in DMS without added osmolytes (⧫), with 1 mM added betaine (□), with 1 mM added carnitine (Δ), and with 1 mM betaine and 1 mM carnitine (◊).
Virulence studies.
Given that Listeria is a food-borne pathogen, the most common route of infection is the oral route. Also, since the osmolarity of the gastrointestinal tract (equivalent to 0.3 M NaCl) is approximately twice that of the bloodstream, the ability of the mutants to reach and proliferate within the target organs (liver and spleen) was investigated following peroral coinoculation with the wild-type parent. Consistent with the results of previous investigations, only mutants carrying deletions in the opuC operon appeared significantly affected in their ability to reach, and multiply within, the livers and spleens of infected animals (Fig. 3). These results suggest that OpuC is the most important osmolyte uptake system, at least during infection of the animal (murine) model. This is not altogether surprising, given that OpuC represents the major carnitine transporter in Listeria (7, 28) and carnitine is most probably the predominant osmolyte in animal tissues (4). Further evidence to suggest that OpuC may represent an important virulence factor in Listeria is the existence of two PrfA boxes. PrfA, the global regulator of virulence potential in L. monocytogenes, may thus control the transcription of opuC, or at least a component thereof. Located at positions 1458048 and 1457691 on the genome, the putative PrfA boxes carry only a single and double mismatch, respectively (10). The existence of such highly conserved regulatory domains further supports our finding that carnitine uptake, at least via OpuC, plays an important role in listerial pathogenesis.
FIG. 3.
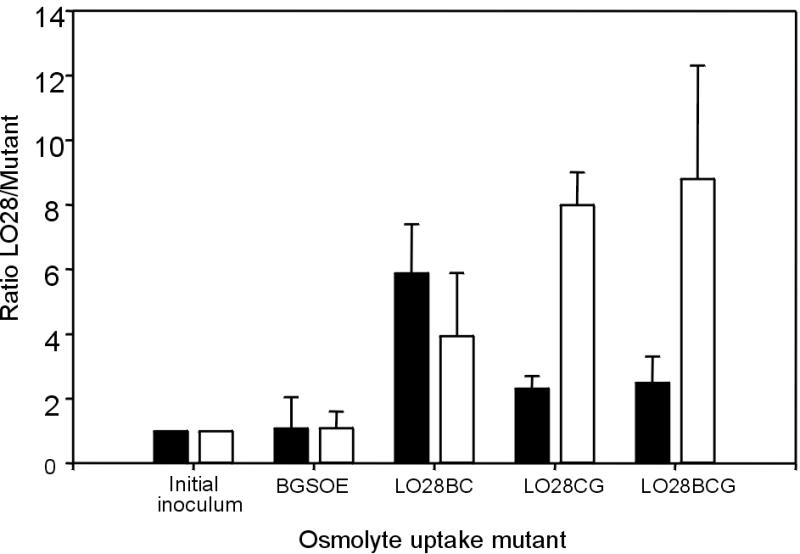
Virulence potential of the double and triple mutants following peroral coinoculation of BALB/c mice. The ratio of the strains was determined in the inoculum and in the livers (black bars) and spleens (white bars) of infected animals 3 days postinfection (n = 4).
Effect of osmolyte transporter deletions on betaine uptake.
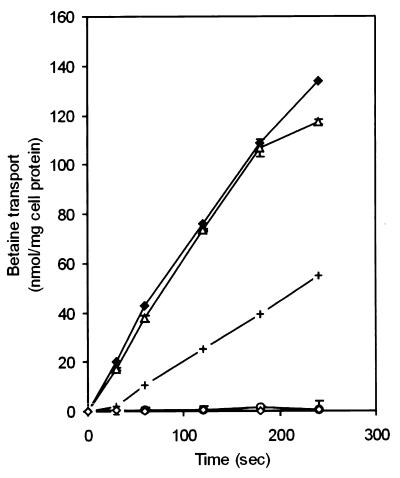
Radiolabeled-osmolyte uptake studies were performed to assess the ability of the various osmolyte uptake mutants to transport betaine (Fig. 4). Betaine uptake was 43 nmol mg of cell protein−1 min−1 in wild-type LO28. Betaine transport was reduced only slightly in LO28C (ΔopuC) (37 nmol mg of cell protein−1 min−1), indicating that transport of betaine occurs mainly via Gbu and BetL. This is supported by the finding that in strain BGSOE (ΔbetL Δgbu), no residual betaine transport could be detected. In strain LO28CG (ΔopuC Δgbu), betaine transport was severely reduced (12 nmol mg of cell protein−1 min−1); however, its growth in DMS with added betaine was comparable to the growth of wild-type LO28. Consistent with the results obtained for the ΔbetL Δgbu mutant, we were unable to detect any betaine uptake in the triple mutant. However, growth of these strains was stimulated slightly upon addition of betaine to DMS. We were able to detect only very low levels of carnitine uptake for the wild-type LO28 and the mutants, as was also described by Sleator et al. (28).
FIG. 4.
Glycine betaine transport. Strain LO28 (wild type) (⧫), LO28C (ΔopuC) (Δ), LO28CG (ΔopuC Δgbu) (+), BGSOE (ΔbetL Δgbu) (○), and LO28BCG (ΔbetL ΔopuC Δgbu) (◊) were assayed for N,N,N,-[1-14C]trimethylglycine uptake in the presence of 3% NaCl.
Accumulation of betaine and carnitine.
The concentrations of betaine and carnitine in cells growing at elevated osmolarity in BHI broth were analyzed to determine the effects of mutating the different osmolyte transporters and to detect differences in the preferences of the respective transporters for the two osmolytes. Growth of the wild type and the mutants in BHI broth at 30°C was similar (data not shown), and the concentration of accumulated betaine was low, about 70 μmol/g (dry weight) of cells, while carnitine was accumulated to a nondetectable level (below 40 μmol/g [dry weight] of cells) (Fig. 5). The level of betaine accumulation increased with increasing osmolarity; addition of 3 or 6% NaCl resulted in 200 and 300 μmol of betaine/g (dry weight) of cells, respectively. For the mutants BSOE (ΔbetL) and LO28C (ΔopuC), the concentrations of accumulated betaine were similar to that in the wild type; also, no carnitine accumulation was observed in these mutants (Fig. 5). In GSOE (Δgbu), less betaine accumulated, and this was compensated by increased accumulation of carnitine. Growing this strain in BHI broth with 6% added NaCl resulted in betaine levels of 125 μmol/g (dry weight) of cells, while carnitine accumulated to 300 μmol/g (dry weight). For BGSOE (ΔbetL Δgbu), which lacks the two principal betaine transporters, no betaine accumulation from BHI broth was observed at low or elevated osmolarity. However, carnitine levels reached almost 600 μmol/g (dry weight) when this strain was cultured in BHI with 6% added NaCl. For the double mutant LO28CG (ΔopuC Δgbu), betaine accumulation is possible via BetL; however, since this strain was found to be severely reduced in its ability to transport betaine (Fig. 4), betaine accumulation levels were also found to be threefold lower than in the wild-type strain. Moreover, carnitine accumulation was observed in this mutant, even though uptake of betaine by BetL is known not to be inhibited by carnitine (9). Also, the triple mutant was able to accumulate carnitine to a concentration of about 600 μmol/g (dry weight) of cells, although all the osmolyte transporters characterized to date are deleted in this strain. Furthermore, its growth was promoted by the addition of carnitine to DMS. In combination, these results seem to indicate the presence of a fourth functional uptake system transporting carnitine from BHI broth at elevated osmolarity. Although the concentration of accumulated carnitine was very high in the triple mutant, its growth was severely impaired, indicating that carnitine might be a less effective osmoprotectant than betaine.
FIG. 5.
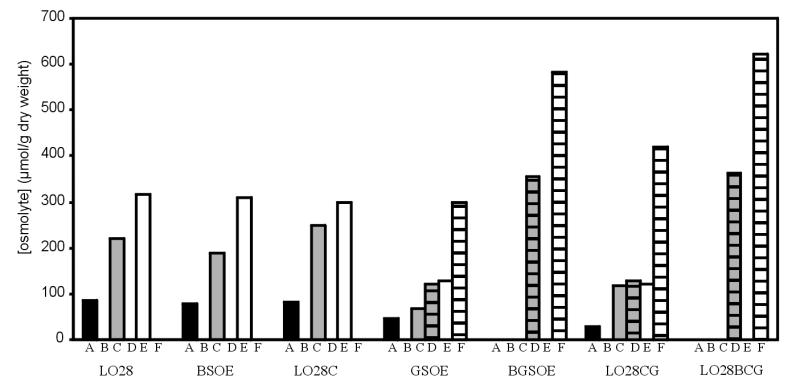
Accumulated betaine and carnitine from BHI by L. monocytogenes LO28 and osmolyte transporter mutants. A, betaine accumulated from BHI with 0% NaCl; B, carnitine accumulated from BHI with 0% NaCl; C, betaine accumulated from BHI with 3% NaCl; D, carnitine accumulated from BHI with 3% NaCl; E, betaine accumulated from BHI with 6% NaCl; F, carnitine accumulated from BHI with 6% NaCl.
DISCUSSION
L. monocytogenes is a food-borne pathogen capable of growing at high osmolarity (NaCl concentrations of up to 10%). This characteristic growth and survival at elevated osmolarities result mainly from the accumulation of betaine and carnitine, the most effective osmoprotectants in L. monocytogenes. Molecular characterization of the salt tolerance of L. monocytogenes has been the focus of much attention in recent years (see, e.g., references 7, 15, 24, 26, 27, and 28). Combined with previous physiological investigations, genetic analysis has provided new insights into the mechanisms of listerial osmotolerance. By using multiple deletion mutants with mutations in the known osmolyte transporters, we were able to describe the role of betaine and carnitine is growth at high osmolarity and in virulence.
In wild-type LO28, betaine is the preferred osmolyte when during growth in BHI broth at elevated osmolarity, whereas carnitine was accumulated only by the mutant strains impaired in their betaine uptake. The ability of glycine betaine to suppress the accumulation of other osmolytes, both exogenously and endogenously produced, is typical in bacterial species that can accumulate glycine betaine along with other osmolytes (13, 20, 22). For the single mutants, only deletion of Gbu reduced betaine accumulation, indicating that Gbu is the main betaine transporter for L. monocytogenes LO28. The double mutant BGSOE (ΔbetL Δgbu) is dependent on OpuC for its osmolyte uptake. No accumulated betaine could be detected, and the growth of this mutant at high osmolarity was severely impaired. However, a high concentration of carnitine was present in the cells. These data indicate that carnitine is less effective than betaine in promoting listerial osmotolerance, a result which is in accordance with the lower growth rate observed for wild-type cells grown in DMS supplemented with carnitine than in DMS with the same concentration of betaine (29). It was also found that betaine is a better osmoprotectant than carnitine in E. coli, and it was suggested that the longer carbon chain of carnitine decreases its osmoprotective function (19).
In virulence assays, a significant reduction in virulence potential was observed following peroral coinoculation of the wild type and the triple mutant. In a previous study, LO28C (ΔopuC) exhibited similar effects (28), leading to the assumption that OpuC, and hence carnitine uptake, is an important virulence factor in L. monocytogenes. The relative abundance of carnitine in mammalian tissues (4) makes it the most readily available and thus possibly the most important osmolyte contributing to the survival of L. monocytogenes during infection (28). Growth experiments in DMS with carnitine as the only available osmolyte show an increase in the growth rate of wild-type LO28 and the BGSOE (ΔbetL Δgbu) mutant. Carnitine only slightly promotes the growth of LO28CG (ΔopuC Δgbu) and the triple mutant, indicating that these strains are not as well protected as the wild type in high-osmolarity environments, a factor which may play a role during virulence, particularly given the elevated osmolarity of the bloodstream (equivalent to 0.15 M NaCl) and gastrointestinal tract (equivalent to 0.3 M NaCl). Analysis of the genome of L. monocytogenes EGD-e (available at http://genolist.pasteur.fr/listilist) revealed a putative fourth osmolyte transporter with homology to opuB of B. subtilis (12) and exhibiting a functional organization similar to that of BusA (OpuA) of Lactococcus lactis (17). The operon consists of two genes, opuBA and opuBB, located in the vicinity of and oppositely orientated with respect to the opuC operon on the L. monocytogenes EGD-e genome. In B. subtilis, OpuB transports choline, which is converted into betaine by two intracellular enzymes (GbsA and GbsB) (6). The transport of choline and its subsequent conversion to betaine affords considerable osmoprotection to the cells (5). We propose that the listerial OpuB transporter is also capable of transporting carnitine. Transport studies using the double mutant BGSOE (ΔbetL Δgbu) and the triple mutant fail to show any betaine transport, indicating that the remaining transporter, OpuB, may not have a high affinity for betaine.
The fact that at least three, and probably four, osmolyte transporters are present in L. monocytogenes emphasizes the physiological significance of osmoregulation for this strain, similar to that for B. subtilis and C. glutamicum. The need for several osmoregulatory systems may lie in the need to mediate the uptake of structurally diverse compatible solutes available in diverse ecological niches, the involvement of different transporters in response to environments with different osmolalities, and the associated need for differential regulation of transporter expression. Betaine, for example, is abundant in plant tissue (23), while carnitine is a major component of muscle tissue (29). BetL is relatively effective only when the osmotic stress is provided by or accompanied by sodium ions, since transport of betaine via this transporter is known to be coupled to the influx of Na+ ions (9). Hence, this transport system cannot explain the osmotically activated transport observed at low Na+ concentrations. In such an environment, L. monocytogenes must possess an active ATP-dependent osmolyte transporter. Another benefit of the ATP-dependent transporter, Gbu, is that it is able to concentrate osmolytes to a much higher level than ion/metabolite symporters. From RNA slot blot analyses and transport studies, it is clear that, as is also observed for OpuD in B. subtilis (11), both the expression of gbu and betL and the activity of the encoded enzymes are subject to regulation by the external osmolarity (16, 26). The stress-inducible sigma factor, σB, may play a role in regulating the expression of both betaine and carnitine transporters. An L. monocytogenes mutant lacking σB is defective for growth in a defined medium with elevated osmolarity in the presence of either betaine or carnitine, whereas no significant difference in growth is observed between the mutant and wild type in defined medium at normal osmolarity (2).
Currently, the OpuB transporter is being analyzed and a mutant with a mutation in this transporter is being generated in our laboratory. The generation of a quadruple mutant would make it possible to investigate the role of osmolytes under several stress conditions in more detail, since it is predicted that this strain will be totally impaired in the uptake of betaine and carnitine.
REFERENCES
- 1.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 2.Becker, L. A., M. S. Çetin, R. W. Hutkins, and A. K. Benson. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beumer, R. R., M. C. Te Giffel, L. J. Cox, F. M. Rombouts, and T. Abee. 1994. Effect of exogenous proline, betaine, and carnitine on growth of Listeria monocytogenes in a minimal medium. Appl. Environ. Microbiol. 60:1359-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bieber, L. L. 1988. Carnitine. Annu. Rev. Biochem. 57:261-283. [DOI] [PubMed] [Google Scholar]
- 5.Boch, J., B. Kempf, and E. Bremer. 1994. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 176:5364-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boch, J., B. Kempf, R. Schmid, and E. Bremer. 1996. Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: characterization of the gbsAB genes. J. Bacteriol. 178:5121-5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser, K. R., D. Harvie, P. J. Coote, and C. P. O'Byrne. 2000. Identification and characterization of an ATP binding cassette L-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galinski, E. A., and R. M. Herzog. 1990. The role of trehalose as a substitute for nitrogen-containing compatible solutes. Arch. Microbiol. 153:607-613. [Google Scholar]
- 9.Gerhardt, P. N. M., L. Tombras Smith, and G. M. Smith. 1996. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J. Bacteriol. 178:6105-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Domínguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K.-D. Entian, H. Fsihi, F. Garcia-Del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gómez-López, T. Hain, J. Hauf, D. Jackson, L.-M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueño, A. Maitournam, J. Mata Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J.-C. Pérez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vázquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 11.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 178:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kappes, R. M., B. Kempf, S. Kneip, J. Boch, J. Gade, J. Meier-Wagner, and E. Bremer. 1999. Two evolutionarily closely related ABC transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol. Microbiol. 32:203-216. [DOI] [PubMed] [Google Scholar]
- 13.Kempf, B., and E. Bremer. 1998. Stress responses of Bacillus subtilis to high osmolarity environments: uptake and synthesis of osmoprotectants. J. Biosci. 23:447-455. [Google Scholar]
- 14.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 15.Ko, R., and L. Tombras Smith. 1999. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl. Environ. Microbiol. 65:4040-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko, R., L. Tombras Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obis, D., A. Guillot, J.-C. Gripon, P. Renault, A. Bolotin, and M.-Y. Mistou. 1999. Genetic and biochemical characterization of a high-affinity betaine uptake system (BusA) in Lactococcus lactis reveals a new functional organization within bacterial ABC transporters. J. Bacteriol. 181:6238-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patchett, R. A., A. F. Kelly, and R. G. Kroll. 1992. Effect of sodium chloride on the intracellular solute pools of Listeria monocytogenes. Appl. Environ. Microbiol. 58:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peddie, B. A., M. Lever, C. M. Hayman, K. Randall, and S. T. Chambers. 1994. Relationship between osmoprotection and the structure and intracellular accumulation of betaines by Escherichia coli. FEMS Microbiol. Lett. 120:125-132. [DOI] [PubMed] [Google Scholar]
- 20.Peter, H., B. Weil, A. Burkovski, R. Kramer, and S. Morbach. 1998. Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing, and characterization of the proline/ectoine uptake system, ProP, and the ectoine/proline/glycine betaine carrier, EctP. J. Bacteriol. 180:6005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Premaratne, R. J., W. Lin, and E. A. Johnson. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randall, K., M. Lever, B. A. Peddie, and S. T. Chambers. 1995. Competitive accumulation of betaines by Escherichia coli K-12 and derivative strains lacking betaine porters. Biochim. Biophys. Acta 1245:116-120. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes, D., and A. D. Hanson. 1993. Quarternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. 44:357-384. [Google Scholar]
- 24.Sleator, R. D., C. G. M. Gahan, T. Abee, and C. Hill. 1999. Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl. Environ. Microbiol. 65:2078-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sleator, R. D., C. G. M. Gahan, and C. Hill. 2001. Identification and disruption of the proBA locus in Listeria monocytogenes: role of proline biosynthesis in salt tolerance and murine infection. Appl. Environ. Microbiol. 67:2571-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sleator, R. D., C. G. M. Gahan, B. O'Driscoll, and C. Hill. 2000. Analysis of the role of betL in contributing to the growth and survival of Listeria monocytogenes LO28. Int. J. Food Microbiol. 60:261-268. [DOI] [PubMed] [Google Scholar]
- 27.Sleator, R. D., and C. Hill. 2002. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26:49-71. [DOI] [PubMed] [Google Scholar]
- 28.Sleator, R. D., J. Wouters, C. G. M. Gahan, T. Abee, and C. Hill. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl. Environ. Microbiol. 67:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, L. 1996. Role of osmolytes in adaptation of osmotically stressed and chill-stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl. Environ. Microbiol. 62:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verheul, A., E. Glaasker, B. Poolman, and T. Abee. 1997. Betaine and l-carnitine transport by Listeria monocytogenes ScottA in response to osmotic signals. J. Bacteriol. 179:16979-16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker, S. J., P. Archer, and J. G. Banks. 1990. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 68:157-162. [DOI] [PubMed] [Google Scholar]
- 32.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]