Abstract
Polystyrene petri dishes containing liquid medium were inoculated with single-cell suspensions of a fresh clinical isolate of Neisseria subflava and were incubated under conditions of low vibration. N. subflava colonies grew firmly attached to the surface of the dish, while the broth remained clear. Growing colonies released cells into the medium, resulting in the appearance of 102 to 104 small satellite colonies attached to the surface of the dish in an area adjacent to each mature colony after 24 h. Satellite colonies grew in patterns of streamers shaped like jets and flares emanating from mature colonies and pointing toward the center of the dish. This dispersal pattern evidently resulted from the surface translocation of detached biofilm cells by buoyancy-driven convection currents that were generated due to slight temperature gradients in the medium. Streamers of satellite colonies ranged from 2 to >40 mm in length. Satellite colonies in very long streamers were relatively uniform in size regardless of their distance from the mature colony, suggesting that mature colonies released single cells or small clusters of cells into the medium and that the detachment, surface translocation, and subsequent surface reattachment of released cells were a transitory process. Incubation of N. subflava single cells in a perfused biofilm fermentor resulted in a large spike of the number of CFU in the perfusate after 9.5 h of growth, consistent with a rapid release of cells into the medium. Biofilm colonies of several other phylogenetically diverse oral bacteria, including Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Streptococcus mitis, and a prevalent but previously uncultured oral Streptococcus sp., exhibited similar temperature-dependent dispersal patterns in broth culture. This in vitro spreading phenotype could be a useful tool for studying biofilm dispersal in these and other nonflagellated bacteria and may have physiological relevance to biofilm dispersal in the oral cavity.
Biofilms constitute a major portion of the bacterial biomass in most natural, industrial, and clinical environments (5). Like all sessile organisms, biofilm bacteria must be able to release and disperse cells into the environment in order to colonize new sites. Proposed mechanisms for the detachment of cells from biofilms include erosion (the continuous release of single cells or small clusters of cells) and sloughing (the rapid detachment of large portions of the biofilm) (24). Erosion and sloughing can result from biofilm-associated processes, such as enzyme production (3, 4, 15), chemical signal production (22), cell-cycle-mediated events (1, 7, 8), and global regulation (10) or from external factors such as shear forces (8, 18), abrasion (collision of solid particles with the biofilm), and predator grazing (23). Sloughing usually occurs in older biofilms (17) and is considered an important mechanism in the shedding of microcolonies from preformed biofilms on heart valves, which often results in infective emboli that can lead to stroke (6). Sloughing is also considered an important mechanism in the dissemination of Legionella from water-cooling systems (14). Abrasion may play a role in biofilm dispersal in environments such as the oral cavity, while predator grazing may play a role in environments such as streams and soil. Phagocytosis is a form of predator grazing that could result in the detachment and dispersal of cells from biofilms. Detached cells may be dispersed by passive mechanisms such as current flow or by active mechanisms such as swimming motility or surface translocation (20).
The commensal bacterium Neisseria subflava comprises part of the normal flora of the oral cavity and respiratory tract of humans (13). Infrequently, N. subflava can enter the submucosa and cause opportunistic infections, such as meningitis, septicemia, and endocarditis (19). In the present report we describe the temporal and spatial dispersal patterns of N. subflava biofilm colonies that were grown attached to polystyrene petri dishes containing liquid medium and were incubated under conditions of low vibration. We show that N. subflava biofilm colonies exhibit a novel dispersal phenotype characterized by streamers of dispersed biofilm colonies emanating from the mature colonies and pointing in the direction of higher temperature. We also show that four other phylogenetically diverse oral bacteria exhibit similar biofilm dispersal phenotypes. These include the gram-negative periodontal pathogen Actinobacillus actinomycetemcomitans (25); Haemophilus aphrophilus, a commensal species closely related to A. actinomycetemcomitans (12); the gram-positive species Streptococcus mitis, a numerically important member of the oral microbiota that has been implicated in the etiology of nursing bottle and root surface caries (9); and a prevalent but previously uncultured oral Streptococcus sp. that is closely related to S. mitis.
MATERIALS AND METHODS
Bacterial strains.
N. subflava biovar perflava strains NJ9702 and NJ9703 and streptococcal strains NJ9704 and NJ9705 were isolated from the oral cavities of dental patients undergoing routine examination or treatment. Species were identified using cellular fatty acid analysis (Microbial ID, Newark, Del.), biochemical tests, and near-complete 16S rRNA sequence analysis. The 16S rRNA sequence of Streptococcus sp. strain NJ9704 was 99.9% identical (1 base change) to that of oral isolate H6 (GenBank accession no. AY005041), a prevalent but previously uncultured streptococcal phylotype isolated from human subgingival plaque that is closely related to Streptococcus oralis and S. mitis (16). A. actinomycetemcomitans strains CU1000 and DF2000 and H. aphrophilus strain NJ8700 were previously described (11).
Growth conditions.
Bacteria were cultured in standard 100-mm, tissue-culture-treated, polystyrene petri dishes (model no. 430167; Corning) in Trypticase soy broth (BD Biosystems) supplemented with 6 g of yeast extract and 8 g of glucose per liter in an atmosphere of 10% CO2. Inoculated petri dishes (containing 20 ml of medium) were incubated in a nonhumidified Forma model 3326 dual-chamber water-jacketed incubator equipped with an internal fan that circulated the air-CO2 mixture inside the chamber. Inoculated petri dishes were incubated directly on a 4- by 43- by 46-cm slab of marble (weight = 21 kg) supported by four 3- by 4- by 7-cm cellulose sponge feet placed on the bottom panel of the bottom chamber of the incubator, which greatly reduced the amount of vibration in the dishes.
Temperature gradients.
All experiments were performed with the incubator set at 37.0°C. Temperatures were measured using a dual-channel thermocouple thermometer equipped with bead-type microprobes (model 800006; Sper Scientific, Scottsdale, Ariz.) with a precision of ±0.1°C. Temperatures were measured in petri dishes containing medium with the probe suspended in the medium and in contact with the bottom of the dish. Stable temperatures were reached within 1 h. The temperature in a standard 100-mm-diameter petri dish was 0.4°C warmer in the center of the dish than at the edge (35.7 versus 35.3°C), resulting in a radial temperature gradient of 0.1°C cm−1 with a warm spot in the center (see Fig. 3, top). The temperature in a standard 100-mm-diameter petri dish incubated without the lid was 1.2°C cooler in the center than at the edge (31.1 versus 32.3°C), resulting in a radial temperature gradient of 0.3°C cm−1 with a cool spot in the center (see Fig. 3, middle). These two radial temperature gradients probably resulted from differences in the evaporation rate at the center and edge of the dish. Contamination of cultures in petri dishes without lids was prevented by means of a 20- by 20-cm sterile glass plate supported directly above the dish, 5 cm above the surface of the marble stabilizing slab. A linear temperature gradient was created by incubating 100-mm-diameter petri dishes (with lids) on a 2- by 13- by 29-cm slab of smooth-surfaced, high-density polyethylene containing two 1-cm-diameter, 29-cm-long holes passing lengthwise through the slab 1 cm from each edge. Water was passed through the two holes by means of rubber tubes connected to two circulating water baths located outside the incubator. The water baths were set to 34.0 and 40.0°C, which resulted in a linear temperature gradient of 0.1°C cm−1 from one side of the petri dish to the other (35.7 to 36.6°C; see Fig. 3, bottom).
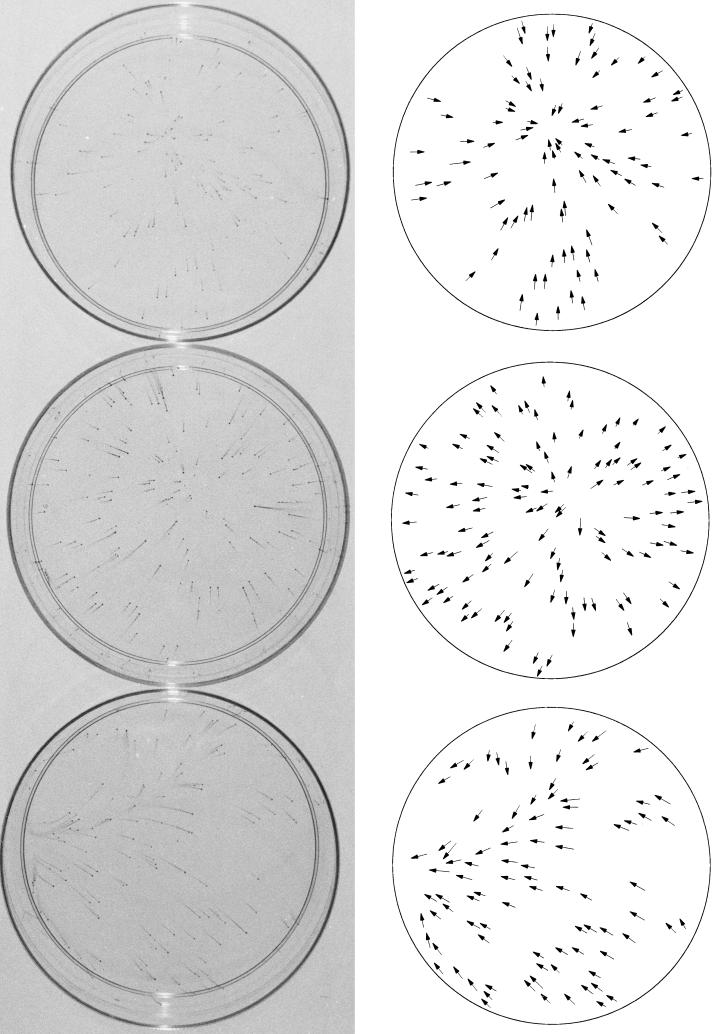
FIG. 3.
Effect of different spatial temperature gradients on dispersal of N. subflava strain NJ9702. (Left) One-hundred-millimeter petri dishes inoculated with (from top to bottom) 116, 146, and 123 CFU and incubated at 32 to 36°C for 24 h. Top dish contained a warm spot in the center, middle dish contained a cool spot in the center, and bottom dish contained a warm spot on the left side. Temperature gradients ranged from 0.1 to 0.3°C cm−1. Dishes were stained with crystal violet as described in the legend of Fig. 1. (Right) Tracings of the petri dishes shown on the left, with arrows indicating the location and direction of dispersal of the mature biofilm colonies.
Preparation of single-cell suspensions.
Sixty milliliters of medium in a 150-mm-diameter, tissue-culture-treated, polystyrene petri dish (Corning) was inoculated with 2× 104 to 8 × 104 CFU of bacteria and was incubated at 37°C for 24 to 48 h. The culture medium was decanted, and the adherent cells were removed from the dish with a cell scraper. The cells were homogenized with five strokes of a Tenbroeck tissue grinder (Wheaton), transferred to a polypropylene tube, and sonicated for 30 s at 40% duty cycle and 70% capacity in a Branson model 200 sonicator equipped with a cup horn. The volume of the cell suspension was adjusted to 5 ml with fresh medium, and the cells were then passed by gravity through a 5-μm-pore-size polyvinylidene fluoride filter (Millipore). The filtrate (ca. 105 to 107 CFU ml−1) consisted of >99% single cells as determined by scanning electron microscopy.
Perfused biofilm fermentor.
Cells were grown in a perfused fermentor similar to the Swinnex biofilm fermentor described by Allison et al. (2) as follows: 10 μl of medium containing 20 to 100 CFU of a single-cell suspension of N. subflava strain NJ9702 was pipetted into the female Luer-Lok inlet port of a 25-mm-diameter, 0.2-μm-pore-size, cellulose acetate syringe filter (catalog no. 09-719A; Fisher) and was incubated at 37°C. Aliquots of fresh medium (40 μl each) were added after 10, 20, and 30 min to prevent the filter from drying. After 1 h, the filter was inverted and fresh medium (prewarmed to 37°C) was perfused through the filter at a rate of 5 ml h−1 using a peristaltic pump. Fractions (1 to 5 ml each) were collected directly into the wells of six-well polystyrene tissue culture plates (Corning) containing 2 ml of medium, serially diluted into the wells of additional plates, and incubated at 37°C. The mature colonies growing on the surface of each dish were counted after 12 to 24 h. The perfused fermentor experiment was carried out in an air incubator.
Nucleotide sequence accession numbers.
The results of the 16S rRNA analysis mentioned above have been deposited in GenBank under accession nos. AF479577 to AF479580.
RESULTS
Surface-associated growth and dispersal of N. subflava.
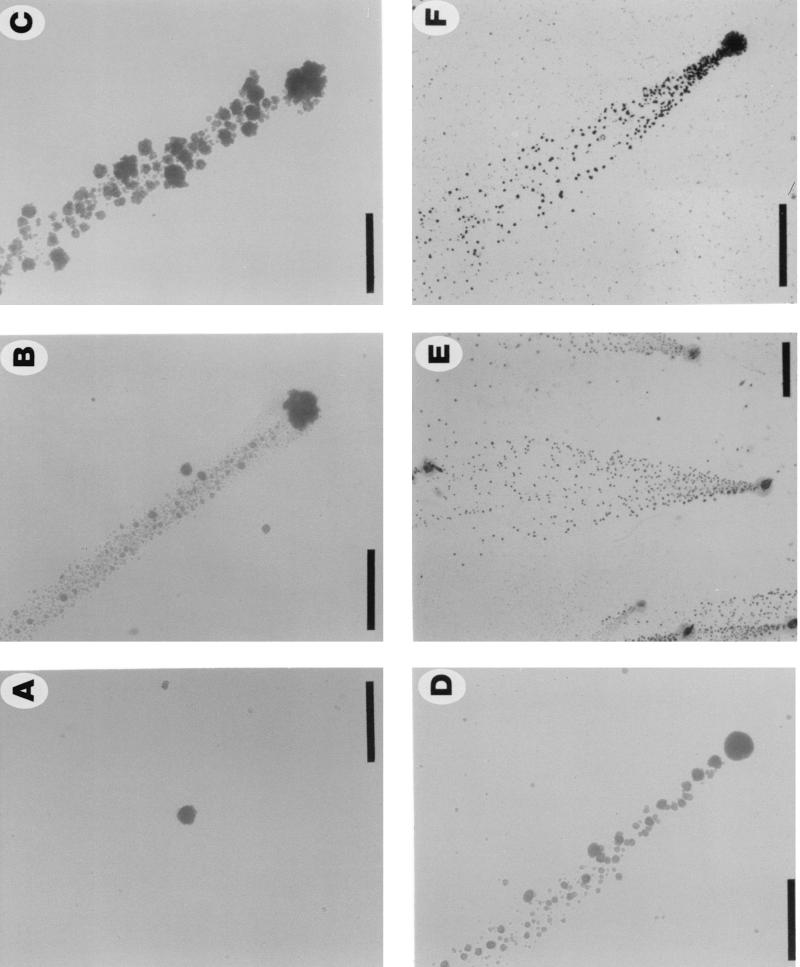
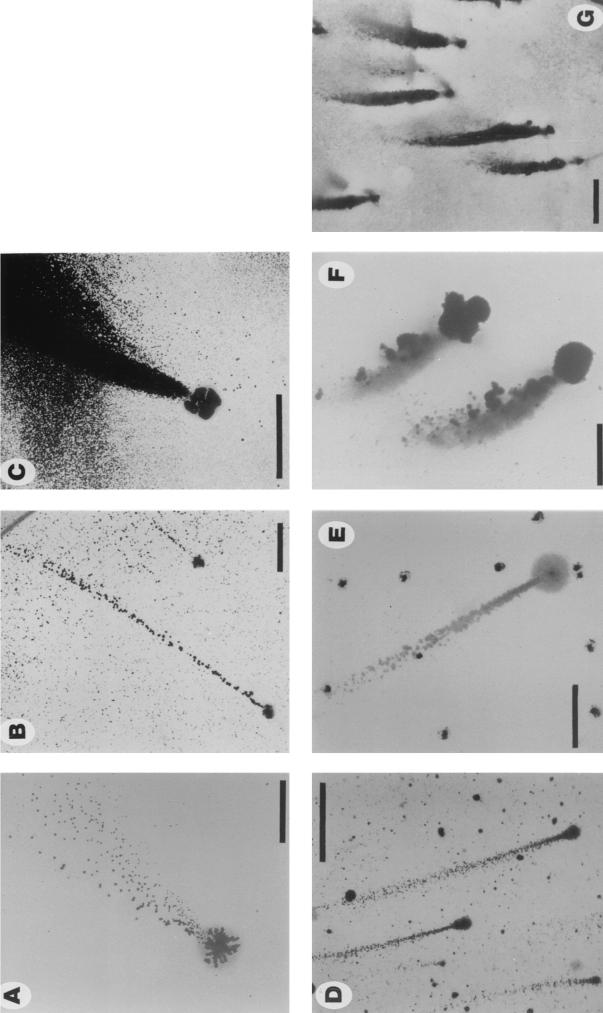
Single-cell suspensions of N. subflava strains NJ9702 and NJ9703 were inoculated into 100-mm petri dishes containing liquid medium and were incubated at 37°C for 24 h. Colonies grew firmly attached to the surface of the dish, while the broth remained clear. Colonies reached a diameter of 100 μm after 12 h (Fig. 1A) and 200 μm after 24 h (Fig. 1B to D). Colonies were smooth, translucent, and spheroidal and remained attached to the surface of the dish after gentle washing. After 24 h, numerous small satellite colonies were growing on the surface of the dish in areas adjacent to each mature colony (Fig. 1B to F). The number of mature colonies growing on the surface of each petri dish was the same as the number that grew on an agar plate inoculated with the same inoculum, indicating that satellite colonies arose from cells released by mature colonies into the medium and not from slow settlers from the planktonic phase. Satellite colonies grew in patterns shaped like narrow streamers (Fig. 1B to D) or wide flares (Fig. 1E and F) emanating from the mature colonies. In some streamers, satellite colonies varied in size (Fig. 1B and D), suggesting that growing colonies were capable of releasing a broad distribution of particle sizes into the medium (24) or that released cells exhibited different growth rates. The sizes of satellite colonies in long streamers were relatively uniform (Fig. 1E and F; see below). Figure 2 shows 100-mm petri dishes inoculated with various numbers of CFU of strains NJ9702 and NJ9703 and incubated at 37°C for 24 h. One hundred percent of mature colonies produced streamers of satellite colonies that reached lengths of >40 mm in petri dishes inoculated with low numbers of CFU (Fig. 2, top). Streamers contained 102 to 104 satellite colonies. All streamers pointed toward the center of the petri dish.
FIG. 1.
Surface-associated colonies of N. subflava strains NJ9702 and NJ9703 grown in polystyrene petri dishes in liquid medium. (A) Strain NJ9702 (12 h), (B) strain NJ9702 (24 h), (C and D) strain NJ9703 (24 h), and (E and F) strain NJ9702 (24 h). Panels A to D show light micrographs of live colonies taken with an Olympus IMT inverted microscope at a magnification of ×40. For panels E and F, colonies were stained for 2 min with Gram-staining reagent (2 g of crystal violet, 0.8 g of ammonium oxalate, and 20 ml of ethanol per 100 ml), washed extensively with tap water, air dried, and viewed under an Olympus SZ40 stereo microscope. The direction of dispersal was from bottom to top in panels B to F. Bar = 2 mm for panel E and 500 μm for others.
FIG. 2.
One-hundred-millimeter petri dishes inoculated with (from top to bottom) 14, 393, and 4 × 103 CFU of N. subflava strain NJ9702 (left) and 8, 290, and 3 × 103 CFU of strain NJ9703 (right). Dishes were incubated for 24 h and stained with crystal violet as described in the legend of Fig. 1.
Evidence for temperature-dependent dispersal.
Figure 3 shows 100-mm petri dishes inoculated with 1.2 × 102 to 1.4 × 102 CFU of N. subflava strain NJ9702 and incubated at 32 to 36°C in the presence of three different spatial temperature gradients. The top dish contained a warm spot in the center, the middle dish contained a cool spot in the center, and the bottom dish contained a warm spot on the left side of the dish. The temperature difference between the center and the edge in the top two dishes and between the left and right sides in the bottom dish was ca. 1°C. In each dish, the streamers of satellite colonies pointed in the direction of higher temperature. These data indicate that the directional component of the observed vectorial biofilm dispersal phenotype was determined by higher temperature.
Spatial and size distributions of satellite colonies.
The density of satellite colonies in the streamers was inversely proportional to the distance from the mature colony (Fig. 1E and F and Fig. 2, top). This relationship approximated a Gaussian curve, consistent with the hypothesis that satellite colonies arose from the diffusion or dispersion of cells released by mature colonies into the medium. In long streamers (12 to 42 mm), the sizes of satellite colonies were relatively uniform regardless of their distance from the mature colony. For example, in the streamer of satellite colonies shown in the center of Fig. 1E, satellite colonies that were located 2 to 4 mm from the mature colony were 0.052 ± 0.009 mm in diameter (n = 119), while colonies 10 to 12 mm from the mature colony were 0.051 ± 0.008 mm in diameter (n = 48). These data suggest that mature colonies released single cells or small clusters of cells into the medium and that the detachment, surface translocation, and subsequent surface reattachment of released cells occurred over a discrete period of time rather than as a continuous process.
Detachment of N. subflava cells grown in a perfused biofilm fermentor.
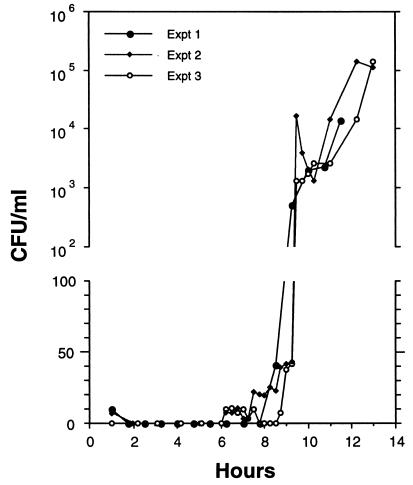
In a perfused biofilm fermentor (2), cells attached to a filter or other substrate are perfused with fresh medium and fractions of the perfusate can be collected and plated to measure the number of CFU detached from the growing biofilm. We used cellulose acetate syringe filters (inoculated with 20 to 100 CFU of a single-cell suspension and perfused with fresh medium at a rate of 5 ml h−1) to quantify detachment of N. subflava strain NJ9702 biofilm cells. Figure 4 shows the results from three independent experiments. Loosely bound cells (corresponding to 0 to 50% of the original inoculum) were removed within the first hour of elution. No further cells were released within the first 6 h. Fractions that eluted between 6 and 9.5 h contained 0 to 45 CFU ml−1, indicating that a small amount of biofilm erosion occurred during this period. At 9.5 h a spike of CFU (103 to 104 CFU ml−1) appeared in the perfusate, indicating that cells were rapidly released into the medium at this time. The perfusate contained high CFU counts (103 to 105 CFU ml−1) from 9.5 to 13 h, suggesting that continuous erosion of the growing biofilm occurred once dispersal had begun. Cells that were released in the perfusate produced colonies that were relatively uniform in size (data not shown), consistent with the hypothesis that mature colonies released primarily single cells or small clusters of cells into the medium.
FIG. 4.
Elution of N. subflava strain NJ9702 cells from a 0.22-μm-pore-size cellulose acetate syringe filter perfused with fresh medium at a constant flow rate of 5 ml h−1. The graphs represent three individual experiments performed on three different days. The ordinate scale is linear from 0 to 100 and logarithmic from 102 to 106. Filters were inoculated with 100 CFU in experiments 1 and 2 and 20 CFU in experiment 3.
Biofilm dispersal of A. actinomycetemcomitans and H. aphrophilus.
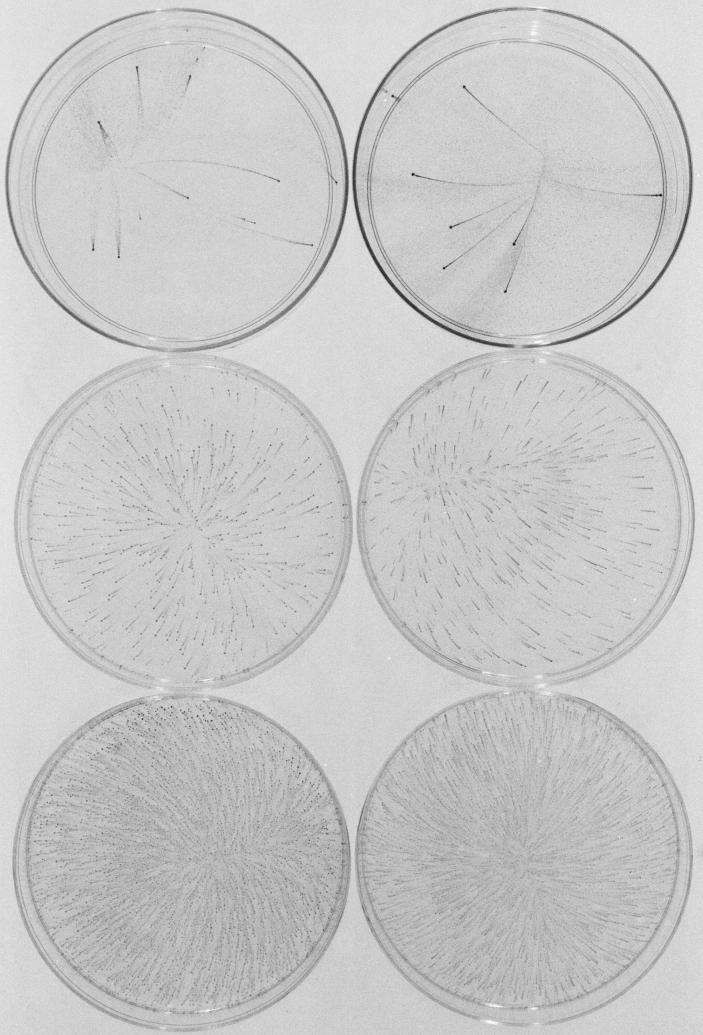
A. actinomycetemcomitans strain CU1000 (serotype f) displayed a biofilm dispersal phenotype that was very similar to that observed in N. subflava (Fig. 5A and B). Mature A. actinomycetemcomitans CU1000 colonies displayed a lobate morphology (Fig. 5A) and grew firmly attached to the surface of the petri dish. One hundred percent of mature colonies produced streamers of satellite colonies that displayed the same spatial and size distributions and temperature-dependent dispersal patterns as those produced by N. subflava. A. actinomycetemcomitans CU1000 produced more satellite colonies than N. subflava (up to 106 per mature colony), and satellite colonies took 3 days to appear. Directional dispersal was not always evident with strain CU1000 because large numbers of satellite colonies often covered the entire surface of the dish. Dispersal of A. actinomycetemcomitans strain DF2000 (serotype c) was similar to that of strain CU1000, except that even larger numbers of satellite colonies were produced, resulting in large areas of densely packed satellite colonies (Fig. 5C) and that dispersal was directional but not always toward higher temperature. The dispersal phenotype displayed by H. aphrophilus strain NJ8700 was nearly identical to that of A. actinomycetemcomitans strain CU1000, except that only 1 to 10% of mature biofilm colonies dispersed (Fig. 5D and E).
FIG. 5.
Surface-associated colonies of various oral bacteria grown in polystyrene petri dishes in liquid medium. (A and B) A. actinomycetemcomitans strain CU1000 (3 days); (C) A. actinomycetemcomitans strain DF2000 (3 days); (D and E) H. aphrophilus strain NJ8700 (3 days); (F) S. mitis strain NJ9705 (24 h); and (G) Streptococcus sp. strain NJ9704 (24 h). Panel F shows a micrograph of live colonies. Others show colonies stained with crystal violet as described in the legend of Fig. 1. The direction of dispersal in all panels was from bottom to top. Bars = 500 μm for panels A and F and 2 mm for others.
Biofilm dispersal of oral streptococci.
S. mitis strain NJ9705 displayed a biofilm dispersal phenotype that was similar to that seen in N. subflava (Fig. 5F). Biofilm colonies of S. mitis were irregularly shaped and not as tightly adherent to the polystyrene surface as those of N. subflava, A. actinomycetemcomitans, and H. aphrophilus. One hundred percent of S. mitis biofilm colonies produced streamers of satellite colonies after 1 day, and dispersal was consistently in the direction of higher temperature. Streamers of S. mitis satellite colonies were short (<10 mm), even in petri dishes containing small inocula, and were densely packed, similar to those produced by A. actinomycetemcomitans strain DF2000 (Fig. 5C). S. mitis satellite colonies were also highly variable in size and shape (Fig. 5F). The biofilm dispersal phenotype of Streptococcus sp. strain NJ9704 was nearly identical to that of S. mitis NJ9705 (Fig. 5G).
DISCUSSION
In this report we describe a novel biofilm dispersal phenotype displayed by several phylogenetically diverse species of oral bacteria. This phenotype is characterized by long streamers of satellite biofilm colonies emanating from the mature biofilm colony and pointing in the direction of higher temperature. The most likely explanation for this dispersal phenotype is the surface translocation of detached biofilm cells by buoyancy-driven convection currents that are generated due to temperature gradients in the medium. Another possible mechanism is the thermotactic migration of detached cells along the surface of the dish by a flagellum-independent mode of surface translocation, such as twitching, sliding, or gliding motility. Simple diffusion probably cannot account for the surface translocation of nonflagellated bacterial cells over long distances within the time frame of our experiments (21).
In working with various species of oral bacteria, we found that fresh clinical isolates often exhibit biofilm-related phenotypes that are not exhibited by laboratory strains. For example, we found that four of four fresh clinical isolates of N. subflava but only one of four N. subflava strains that we obtained from the American Type Culture Collection (Manassas, Va.) exhibited the biofilm formation and dispersal phenotypes described in the present work. Also, nearly all of the cultures of A. actinomycetemcomitans that we obtained from the American Type Culture Collection had completely lost their ability to form biofilms in vitro, whereas 100% of fresh clinical isolates formed tenacious biofilms. We observed similar results with laboratory versus clinical strains of H. aphrophilus. These observations suggest that it may be important to utilize fresh clinical isolates when studying biofilm-related phenotypes in certain oral bacteria.
Acknowledgments
We thank Aseel Toni, Markus Meyenhofer, and Rama Sood for technical assistance; Aseel Toni and Enrico Tinoco for providing clinical samples; and Mrinal Bhattacharjee, David Figurski, Peter Fredrikse, David Furgang, Paul Goncharoff, Scott Kachlany, Markus Meyenhofer, Paul Planet, Helen Schreiner, and Corey Weiss for helpful discussions.
REFERENCES
- 1.Allison, D. G., D. J. Evans, M. R. W. Brown, and P. Gilbert. 1990. Possible involvement of the division cycle in dispersal of Escherichia coli from biofilms. J. Bacteriol. 172:1667-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, D. G., T. Maira-Litran, and P. Gilbert. 1999. Perfused biofilm fermenters. Methods Enzymol. 310:232-248. [DOI] [PubMed] [Google Scholar]
- 3.Allison, D. G., B. Ruiz, C. SanJose, A. Jaspe, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179-184. [DOI] [PubMed] [Google Scholar]
- 4.Boyd, A., and A. M. Chakrabarty. 1994. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 60:2355-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 6.Donlan, R. M., and W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert, P., D. G. Allison, D. J. Evans, P. S. Handley, and M. R. W. Brown. 1989. Growth rate control of adherent bacterial populations. Appl. Environ. Microbiol. 55:1308-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert, P., D. J. Evans, and M. R. W. Brown. 1993. Formation and dispersal of bacterial biofilms in vivo and in situ. J. Appl. Bacteriol. 74:67S-78S. [DOI] [PubMed] [Google Scholar]
- 9.Hohwy, J., J. Reinholdt, and M. Kilian. 2001. Population dynamics of Streptococcus mitis in its natural habitat. Infect. Immun. 69:6055-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan, J. B., H. C. Schreiner, D. Furgang, and D. H. Fine. 2002. Population structure and genetic diversity of Actinobacillus actinomycetemcomitans strains isolated from localized juvenile periodontitis patients. J. Clin. Microbiol. 40:1181-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King, E. O., and H. W. Tatum. 1962. Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. J. Infect. Dis. 111:85-94. [DOI] [PubMed] [Google Scholar]
- 13.Knapp, J. S., and R. J. Rice. 1995. Neisseria and Branhamella, p. 324-340. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 14.Lee, J. V., and A. A. West. 1991. Survival and growth of Legionella species in the environment. J. Appl. Bacteriol. 70:121S-130S. [PubMed] [Google Scholar]
- 15.Lee, S. F., Y. H. Li, and G. W. Bowden. 1996. Detachment of Streptococcus mutans biofilm cells by an endogenous enzymatic activity. Infect. Immun. 64:1035-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyton, B. M., and W. G. Characklis. 1993. A statistical analysis of the effect of substrate utilization and shear stress on the kinetics of biofilm detachment. Biotechnol. Bioeng. 41:728-735. [DOI] [PubMed] [Google Scholar]
- 18.Picioreanu, C., M. C. M. van Loosdrecht, and J. J. Heijnen. 2001. Two-dimensional model of biofilm detachment caused by internal stress from liquid flow. Biotechnol. Bioeng. 72:205-218. [PubMed] [Google Scholar]
- 19.Pollack, S., A. Mogtader, and M. Lange. 1984. Neisseria subflava endocarditis. Case report and review of the literature. Am. J. Med. 76:752-758. [DOI] [PubMed] [Google Scholar]
- 20.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 21.Purcell, E. M. 1977. Life at low Reynolds number. Am. J. Phys. 45:3-11. [Google Scholar]
- 22.Puskas, A., E. P. Greenberg, S. Kaplan, and A. L. Schaefer. 1997. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J. Bacteriol. 179:7530-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart, P. S. 1993. A model of biofilm detachment. Biotechnol. Bioeng. 41:111-117. [DOI] [PubMed] [Google Scholar]
- 24.Stoodley, P., S. Wilson, L. Hall-Stoodley, J. D. Boyle, H. M. Lappin-Scott, and J. W. Costerton. 2001. Growth and detachment of cell clusters from mature mixed-species biofilms. Appl. Environ. Microbiol. 67:5608-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]