Abstract
Growth media and environmental conditions influence the expression of adhesion and invasion proteins in Listeria monocytogenes. Here, the expression of the 104-kDa Listeria adhesion protein (LAP) was studied in nutrient-rich media (Trypticase soy broth [TSB] and brain heart infusion [BHI]), minimal medium (Luria-Bertani [LB]), or nutrient-deficient medium (peptone water [PW]) by immunoblotting, enzyme-linked immunosorbent assay (ELISA), and immunoelectron microscopy. Also, the effect of incorporating different concentrations of glucose on LAP expression was studied. Immunoblotting showed that LAP expression was at least twofold higher in LB medium than in TSB or BHI, while PW supported very poor cell growth and LAP expression. ELISA and immunoblotting results showed that higher concentrations of glucose (>1.6 g/liter) lowered the culture pH and suppressed LAP expression by more than 75%; however, the addition of K2HPO4 reduced this effect. L. monocytogenes cells grown in LB media with lower concentrations of glucose showed higher adhesion to Caco-2 cells (3,716 and 4,186 cpm of attached bacteria for 0 and 0.2 g of glucose/liter, respectively), while L. monocytogenes cells grown in LB with higher glucose concentrations exhibited lower adhesion (2,126 and 2,221 cpm for 1.6 and 3.2 g of glucose/liter, respectively). A LAP-negative L. monocytogenes strain (A572) showed low adhesion profiles regardless of the amount of glucose added. Transmission electron microscopy revealed that LAP is localized mainly in the cytoplasm, with only a few molecules located on the cell surface. Growth in LB with high glucose (3.2 g/liter) showed the presence of only a few molecules in the cells, corroborating the results observed with ELISA or immunoblotting. In summary, nutrient-rich media and high concentrations of glucose suppressed LAP expression, which possibly is due to the changes in the pH of the media during growth from the accumulation of sugar fermentation by-products.
Listeria monocytogenes is an invasive food-borne pathogen that affects immunocompromised individuals, the elderly, and children, causing severe diseases ranging from gastroenteritis to meningitis or encephalitis. Infection may also lead to abortion or stillbirth in pregnant women. L. monocytogenes pathogenicity can be attributed to its ability to penetrate, survive, and replicate in professional and nonprofessional phagocytes. Attachment of L. monocytogenes to host intestinal cells is considered the first step in pathogenesis. After attachment, L. monocytogenes enters host cells either by phagocytosis or endocytosis, where it can infect adjacent cells, enter the bloodstream (7), and eventually infiltrate body organs such as the liver, spleen, and brain (2).
The establishment of successful listerial infection requires the presence and expression of virulence genes, demonstrated in various growth media (9, 21). The expression of these virulence genes is controlled by various environmental and host conditions, including temperature, osmolarity, and pH, and the presence of certain nutrients such as sugars or ions in the bacterial habitat (9, 27). Several virulence proteins that are involved in the process of adhesion and invasion have been characterized in L. monocytogenes (28). ActA, a surface protein of 90 kDa, is believed to be crucial in the movement of L. monocytogenes through the cytoplasm, by polymerizing the host actin filaments (8), and it has been shown to be involved in the attachment to host cells (1). InlA, a surface protein of 80 kDa, binds E-cadherin on epithelial cells and is essential for L. monocytogenes internalization, while InlB, a 71-kDa protein in the internalin family, is essential for entry into hepatocytes and other epithelial or fibroblast cells (7, 12). Other proteins, such as p60, also are reported to be involved in the adhesion and invasion of eukaryotic cells (17, 25). Recently, Milohanic et al. (23) identified a 102-kDa protein containing amidase activity, which is encoded by the ami gene, that plays a role in the adhesion of L. monocytogenes to Caco-2 cells via its cell wall anchoring domain. L. monocytogenes also contains a 24.6-kDa protein that binds human fibronectin (13). Furthermore, Pandiripally et al. (24) identified a 104-kDa Listeria adhesion protein (LAP) from L. monocytogenes and showed that it is involved in bacterial attachment to Caco-2 cells. This was confirmed by partially blocking the attachment with anti-LAP monoclonal antibodies (24).
Almost all known listerial virulence determinants are coordinately expressed under the positive control of the PrfA protein, a transcriptional factor that is structurally and functionally related to cyclic AMP receptor protein and is subjected to complex environmental control in L. monocytogenes (6, 28). For instance, L. monocytogenes expresses certain virulence factors at 37°C but not at 26°C or below, and it does not produce virulence factors in rich medium, such as brain heart infusion (BHI), at 37°C. However, a clear induction of virulence genes occurred when BHI was supplemented with charcoal or if the BHI medium was replaced with minimal essential medium (5, 32). Park and Kroll (27) reported that the presence of β-glucoside cellobiose in the growth medium strongly repressed the expression of the hly and plcA genes, while other common fermentable carbohydrates had no effect. In addition, Milenbachs et al. (22) found that several readily metabolized sugars, such as glucose, fructose, and cellobiose, caused down regulation of certain virulence genes.
It is known that the catabolism of added sugars might change the pH of the medium, resulting in the suppression of some proteins and enhancement of others (9). Likewise, temperature and growth phase are important factors that influence the expression of many virulence factors, such as internalin, listeriolysin, phospholipases, and actin polymerization proteins (10, 20, 31, 34). Recently, Santiago et al. (33) demonstrated that LAP expression was dependent on both the temperature and the growth phase of the culture, showing a very high expression in stationary phase at 37 and 42°C.
Food as part of the bacterial growth environment may contain different levels and types of nutrients that might enhance or suppress virulence genes, and it is known that nutrient-limiting conditions often trigger the expression of different virulence genes (21). Glucose, for example, is a major carbon source for bacteria, yet it acts as a catabolic repressor for some virulence factors. Thus, the objectives of this study were to assess the effect of different nutrient concentrations on the expression of LAP and the subsequent attachment of L. monocytogenes to Caco-2 cells.
MATERIALS AND METHODS
Bacteria and growth conditions.
L. monocytogenes F4244 (serotype 4b), a wild-type (WT) strain, and an isogenic mutant strain, A572 (LAP deficient), were used in this study. The WT strain was stored in BHI (Difco Labs, Detroit, Mich.) agar slants containing erythromycin (10 μg/ml), while the mutant strain was stored in BHI slants containing tetracycline (10 μg/ml) (24). For experimental purposes, L. monocytogenes strains were cultured in nutrient-rich BHI broth, tryptic soy broth (TSB), nutrient-limiting Luria-Bertani (LB) broth (Difco Labs), or 1% peptone water (PW), with different concentrations of glucose (Sigma) (0, 0.2, 0.4, 0.8, 1.6, and 3.2 g/liter) at 37°C for 18 to 20 h. Cultures (10 ml) were grown in 15-ml sterile disposable centrifuge tubes without shaking. Some media contained 2.5 g of K2HPO4/liter (Fisher Scientific). The cell density and the final pH of each culture were recorded by measuring absorbance at 595 (DU-640; Beckman-Coulter, Fullerton, Calif.) and by using a pH meter.
Nutrient-rich or nutrient-limiting media were defined by the total amounts of proteins or other nutrients, including sugars, present in the media. Nutrient-rich BHI contained brain infusion (200 g/liter), heart infusion (250 g/liter), peptone (10 g/liter), and glucose (2 g/liter), while TSB contained tryptone (17 g/liter), Soytone (3 g/liter), and glucose (2.5 g/liter). Nutrient-limiting LB contained tryptone (10 g/liter) and yeast extract (5 g/liter), while PW contained only tryptone (10 g/liter) (Difco laboratory manual).
ELISA.
L. monocytogenes cells were harvested (6,000 × g, 10 min) and resuspended in 0.05 M carbonate buffer (pH 9.6), and cell concentrations were adjusted to 0.3 (A595). Microtiter plates (Immulon, Dynatech, Chantilly, Va.) were coated with 100 μl of cells and incubated overnight at 4°C. An enzyme-linked immunosorbent assay (ELISA) was done using anti-LAP monoclonal antibody (MAb-H7) diluted at 1:200 as described previously (4, 33).
SDS-PAGE and immunoblotting.
L. monocytogenes cultures (10 ml) were centrifuged (6,000 × g, 10 min) and resuspended in 5 ml of phosphate-buffered saline (PBS), and absorbance (A595) was determined. Cells were pelleted for the second time, resuspended in electrophoresis sample solvent buffer (18) at the ratio of 450 μl per U of absorbance (595 nm), and incubated at 37°C for at least 2 h. The mixtures were then centrifuged (16,000 × g for 10 min), and the supernatants (10 μl) were loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (7.5% acrylamide) gels. After electrophoresis, proteins were transferred to Immobilon-P membranes (Millipore, Bedford, Mass.) and immunoprobed with anti-LAP monoclonal antibody (MAb-H7) (4, 24). The antibody reaction intensity of 104-kDa bands was quantified using the Un-Scan-It software program from SilK Scientific (Orem, Utah).
Immunoelecton microscopy.
Electron microscopy was performed as described by van Tuinen and Riezman (35). L. monocytogenes cells from different growth media were harvested and washed three times with PBS. Cells were fixed in 3% paraformaldehyde containing 0.5% glutaraldehyde in PBS for 2 h at 4°C, washed three times in PBS, incubated in 1% sodium metaperiodate at room temperature for 1 h, and incubated in 50 mM ammonium chloride for 30 min. Pellets were embedded in 1.5% agarose and sliced into blocks that were dried in graded ethanol prior to embedding in LR White resin (EM Sciences, Fort Washington, Pa.). Cells were oven dried to polymerize the resins. The embedded bacteria were thin sectioned and placed on Formvar-coated nickel grids. The grids were first blocked with 20 mM Tris-buffered saline (pH 7.4) containing 0.3% Tween 20 (TBST) and 1% bovine serum albumin for 10 to 20 min at room temperature and then incubated with 10 μl of each LAP-specific purified rabbit polyclonal antibody (2.2 mg/ml) or monoclonal MAb-H7 antibody (2.26 mg/ml) overnight at 4°C. The grids were washed several times with TBST and then incubated with goat anti-rabbit or anti-mouse immunoglobulin G conjugated to 12-nm gold particles (1:10) (Jackson ImmunoResearch, West Grove, Pa.) for 1 h at room temperature. After washing with TBST, the grids were air dried and stained with 2% aqueous uranyl acetate (Sigma) for 3 min, washed again, and viewed under a transmission electron microscope (EM-400; Philips, Hillsboro, Oreg.).
The polyclonal antibody to LAP, mentioned above, was developed by immunizing rabbits with 104-kDa LAP bands collected from SDS-PAGE (7.5%-acrylamide) gels following electrophoretic separation of L. monocytogenes WT surface protein preparations (15).
Radiolabeled adhesion assay.
Adhesion assays were performed using 3H-labeled L. monocytogenes cells according to the method of Hagman et al. (14), with some modifications. L. monocytogenes cells were grown overnight at 37°C in medium containing 20 μCi of methyl [3H]thymidine/ml (79 Ci/mmol; Amersham-Pharmacia Biotech, Inc., Piscataway, N.J.) (19), harvested by centrifugation (6,000 × g, 20 min), washed three times with PBS, and resuspended in the same buffer. The suspension was adjusted to an optical density at 595 nm of 0.3. For adhesion analysis, Caco-2 cell (HTB-37; American Type Culture Collection) monolayers were established in 24-well plates (33). The monolayers were washed once with Dulbecco's modified Eagle's medium, and 400 μl of fresh medium was added to each well. A 100-μl aliquot of radiolabeled bacteria (≈5 × 108 CFU/ml) was added to each well, and the plates were incubated for 30 min at 37°C and then washed five times with PBS to remove unbound bacteria. Adherent bacteria were recovered by incubating monolayers in 0.5 ml of 0.5% SDS for 30 min, collected, and mixed thoroughly with scintillation cocktail (CytoScint; ICN Biomedicals, Inc., Costa Mesa, Calif.), and the radioactivity was measured using a liquid scintillation counter (Beckman Coulter). All adhesion assays were performed at least three times with triplicate samples.
Statistical analysis.
Statistical analysis was performed using SAS software (Cary, N.C.), and differences in treatments were determined with Duncan's test at a P level of ≤0.05.
RESULTS
LAP expression in L. monocytogenes cells grown in different growth media.
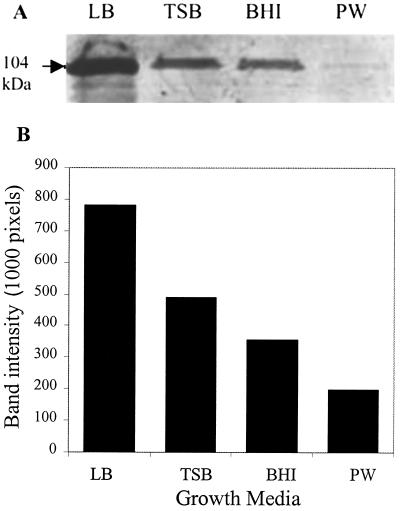
LAP expression was analyzed following growth of L. monocytogenes in BHI, TSB, LB, and PW by immunoblotting (Fig. 1). LAP expression in LB (139,223 pixels) as determined by band intensity was 1.44- to 1.62-fold higher than that in TSB (96,458 pixels) or BHI (85,551 pixels), while the PW medium yielded very poor LAP expression (30,288 pixels). Since LB medium supported the highest LAP expression, it was used to study the effect of various concentrations of glucose on LAP expression.
FIG. 1.
(A) Quantitative analysis of LAP expression by Western blotting from L. monocytogenes F4244 grown in nutrient-rich (TSB, BHI) and nutrient-limiting (LB, PW) growth media. (B) Densitometric quantification of LAP expression in the indicated growth media.
Cell density and final pH of cultures grown in LB with different concentrations of glucose.
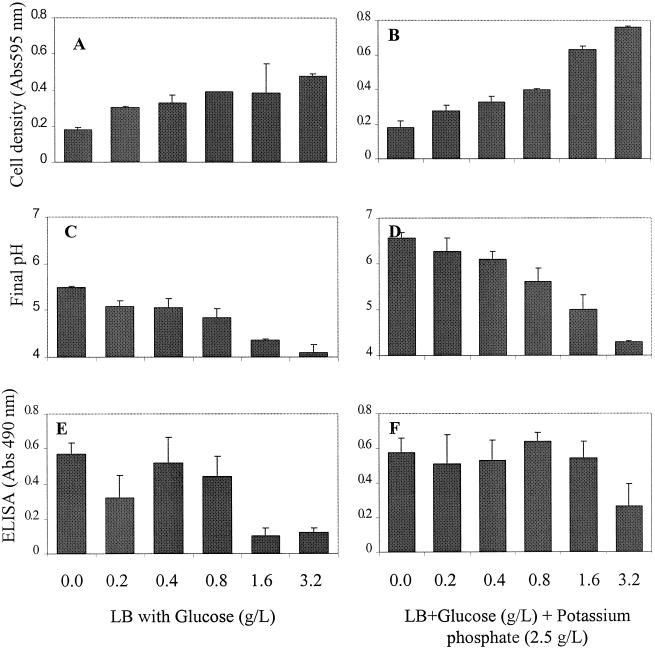
Cell density (A595) ranged from 0.33 to 0.45 for media containing 0.2 to 3.2 g of glucose/liter (Fig. 2A), and the final pH decreased gradually with increasing concentrations of glucose (Fig. 2C). The pH values were 5.5, 5.09, 5.05, 4.83, 4.37, and 4.1 for glucose concentrations of 0, 0.2, 0.4, 0.8, 1.6, and 3.2 g/liter, respectively. When K2HPO4 was added before inoculation, cell density (A595) increased dramatically with increased glucose concentrations, from 0.28 with 0.2 g of glucose/liter to 0.76 with 3.2 g of glucose/liter, which represents a threefold increase. The corresponding final pH of each culture was increased, with values of 6.57, 6.28, 6.1, 5.62, 4.99, and 4.3 for glucose concentrations of 0, 0.2, 0.4, 0.8, 1.6, and 3.2 g/liter, respectively (Fig. 2D). Overall, pH values were significantly higher with K2HPO4 (P < 0.05) than the media with glucose alone, whereas the differences in cell densities with or without K2HPO4 were not significant at a P level of <0.05.
FIG. 2.
Growth and expression of LAP in L. monocytogenes F4244 cultured in LB medium with different concentrations of dextrose and with or without K2HPO4 (2.5 g/liter). (A and B) Cell density of L. monocytogenes grown in LB with glucose only (A) or with both glucose and K2HPO4 (B). (C and D) Final pH after overnight growth of L. monocytogenes in LB with glucose only (C) or with both glucose and K2HPO4 (D). (E and F) ELISA results showing LAP expression in L. monocytogenes when grown in LB with glucose (E) or with both glucose and K2HPO4 (F).
Effect of glucose on LAP expression. (i) ELISA results.
ELISA data indicated that LAP expression without or with low amounts (0.2, 0.4, and 0.8 g/liter) of glucose was significantly higher (P < 0.05) than the expression in media containing 1.6 or 3.2 g of glucose/liter (Fig. 2E). When K2HPO4 was added to the media, LAP expression was generally increased for all treatments (Fig. 2F); however, expression in the presence of 0 to 1.6 g of glucose/liter was significantly higher than that with 3.2 g of glucose/liter. Overall, LAP expression was significantly (P < 0.05) higher for media with glucose and K2HPO4 than for media with glucose alone.
(ii) Immunoblotting.
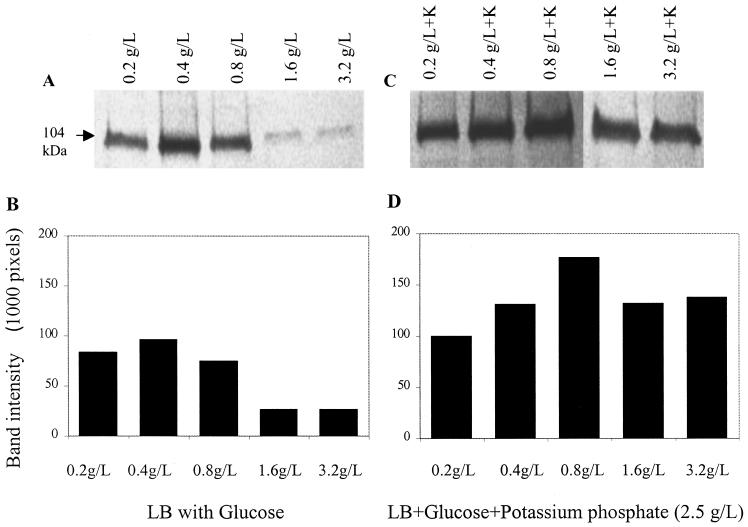
Similar to the ELISA results, Western blotting showed that LAP expression was highest when Listeria was grown in LB with 0.4 g of glucose/liter (96,501 pixels) (Fig. 3A and B). LAP expression decreased by 3.5-fold at glucose concentrations of 1.6 or 3.2 g/liter. In media containing K2HPO4, the effect of glucose on LAP expression was apparently low (Fig. 3C and D), although 0.8 g of glucose/liter appeared to support the highest expression (177,332 pixels), while 0.2 g of glucose/liter supported the lowest LAP expression (99,591 pixels).
FIG. 3.
(A and B) Quantitative analysis of LAP expression by Western blotting from L. monocytogenes F4244 grown in LB with different concentrations of glucose (A) and densitometric quantification of LAP bands (B). (C and D) Quantitative analysis (C) and densitometric quantification (D) of LAP from L. monocytogenes F4244 when grown in LB with different concentrations of glucose and K2HPO4 (2.5 g/liter).
(iii) TEM results.
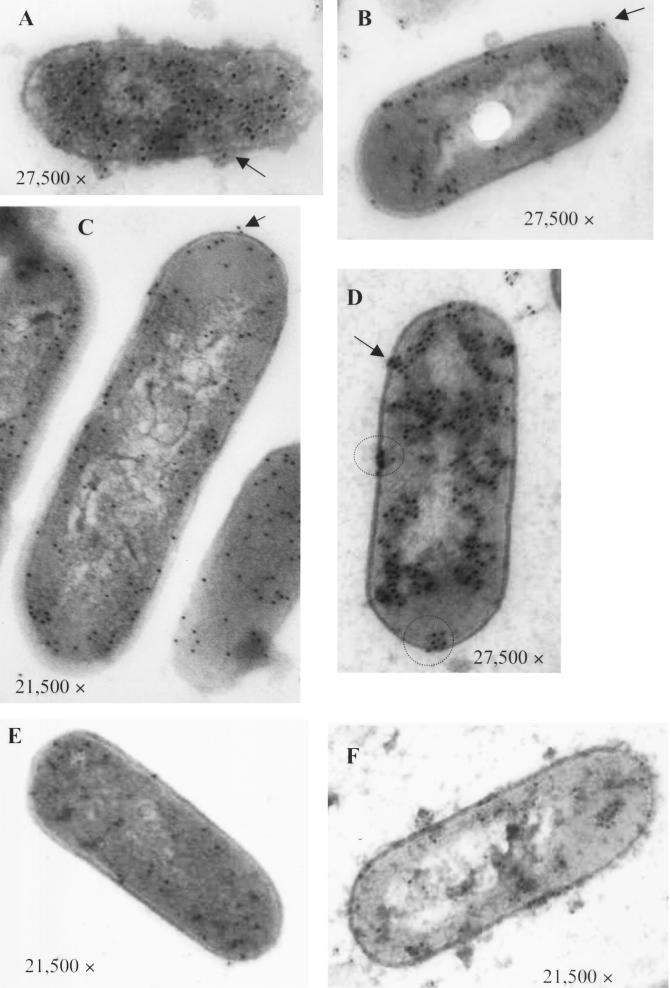
Transmission electron microscopy (TEM) was done to visualize LAP distribution and location in bacterial cell components and to compare results from ELISA and immunoblotting (Fig. 4). The blots shown in Fig. 4A, C, and E were immunoprobed with anti-LAP monoclonal antibody (MAb-H7), while the blots in panels B, D, and F were probed with anti-LAP polyclonal antibody. Upon counting gold particles on the surface of the cells, we noticed that LAP expression was higher in LB with 0.0 g of glucose/liter (≈90 particles/cell) (Fig. 4A) or 0.8 g of glucose/liter (≈95 particles/cell) (Fig. 4C) than in LB with 3.2 g of glucose/liter (≈20 particles/cell) (Fig. 4E).
FIG. 4.
TEM images of L. monocytogenes F4244 cells grown in LB with 0.0 (A and B), 0.8 (C and D), or 3.2 g of glucose/liter (E and F) and probed with immunogold-labeled LAP-specific monoclonal antibody (A, C, and E) or with polyclonal antibody (B, D, and F). Arrows indicate the cell surface location of LAP, and the circle indicates patchy distribution.
When L. monocytogenes cells were probed with polyclonal antibody, the results were similar to those with the monoclonal antibody; however, a different distribution profile of the gold particles was observed. The gold particles were clustered in patches, with the highest numbers present on L. monocytogenes grown in LB with 0.8 g of glucose/liter (Fig. 4D). Several of these patches were distributed in the cytoplasm, cytoplasmic membrane, and the cell wall. LAP was also observed when L. monocytogenes was grown in LB containing 3.2 g of glucose/liter (Fig. 4F), although much less than what was expressed during growth with 0.8 g of glucose/liter.
Adhesion assay.
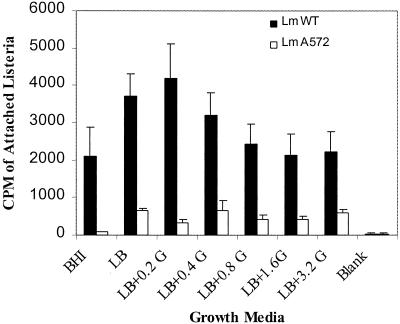
In order to determine if variable LAP expression would affect the adhesion of L. monocytogenes cells to Caco-2 cells, bacteria were grown in LB with or without glucose and analyzed for adhesion. L. monocytogenes cells grown in LB with 0.2 g of glucose/liter or LB without glucose exhibited the highest adhesion to Caco-2 cells (3,716 to 4,155 cpm), which was significantly (P < 0.05) higher than that for LB with 0.8, 1.6, or 3.2 g of glucose/liter (Fig. 5). When adhesion was compared for L. monocytogenes cells grown in nutrient-limiting LB and nutrient-rich BHI broth, the adhesion was significantly (P < 0.05) lower for the latter medium (Fig. 5). However, when the LAP-deficient L. monocytogenes mutant (A572) was grown in LB with different glucose concentrations and tested for its adhesion to Caco-2 cells, attachment was minimal (≈500 cpm) regardless of the amount of glucose added. In general, the number of L. monocytogenes cells that adhered to Caco-2 cells decreased with increasing concentrations of glucose.
FIG. 5.
Adhesion analysis of L. monocytogenes F4244 (LmWT) and LAP-deficient L. monocytogenes A572 (LmA572) to secondary intestinal cell line Caco-2 (colon) using radioisotope [3H]thymidine-labeled bacteria. Listeria was grown in BHI or LB with different concentrations of glucose. Data represent mean values of nine replicates from three different experiments. The average background reading was about 35 cpm.
DISCUSSION
The expression of virulence genes often is triggered by changes in the growth environment, such as the transition from free to parasitic life (32). Along with pH, osmolarity, growth phase, and temperature, sugars in their simple or complex forms are believed to play a key role in virulence gene regulation (3, 6, 9, 11, 16, 21, 26, 27, 30). In this communication, we describe the effects of different growth media (nutrient-limiting to nutrient-rich media) and different concentrations of glucose on the expression of a 104-kDa LAP in L. monocytogenes. This protein was shown to play a role in adhesion to Caco-2 cells, indicating that it is a virulence determinant (24). To examine LAP expression, cells were grown in nutrient-rich (BHI and TSB) and nutrient-limiting (LB and PW) media for 18 to 20 h and probed by Western blotting; cell concentrations were adjusted for each culture before Western blotting. Nutrient-rich BHI and TSB contained large quantities of proteins and sugars (for BHI, 460 g of proteins/liter and 2 g of dextrose/liter; for TSB, 23 g of proteins/liter and 2.5 g of dextrose/liter). Nutrient-limiting LB contained protein (10 g/liter) and yeast extract (5 g/liter) without any glucose, while PW contained only protein (10 g/liter). Both BHI and TSB supported high cell densities yet low LAP expression, while LB did not support high cell density yet yielded high LAP expression. In contrast, PW did not support good cell growth; therefore, only a little LAP expression was observed. The apparent low expression in PW medium could be because this medium is nutritionally extremely poor and, therefore, may not support the expression of not only LAP but also other proteins. A reduced or relative lack of protein expression in PW medium was visualized in Coomassie blue-stained SDS-PAGE gels (data not shown). In a similar study, Milenbachs et al. (22) reported that the expression of hly and plcA were strongly suppressed when L. monocytogenes was grown in a rich medium, TSB. Bohne et al. (5) reported that L. monocytogenes does not express some of the virulence genes (hly and actA) in rich medium such as BHI. However, when charcoal (a nonspecific chelating agent) was added to the medium or the BHI was replaced with minimal essential medium, these virulence genes were highly expressed (32). Similarly, in this study when L. monocytogenes was grown in LB medium, which is nutritionally poor, LAP was expressed in higher amounts than that expressed in rich media (TSB or BHI). This behavior indicates that under nutrient-limiting stress conditions, L. monocytogenes might produce more of its virulence factors to adapt to the new environment.
To further understand the regulation of LAP, we studied the effect of glucose on its expression. Glucose is used as a carbon source for L. monocytogenes and is possibly used by the organism to sense the transition from the mucosal surfaces to the bloodstream (21). Furthermore, different carbon sources can affect the expression of virulence factors in many systems by altering growth rate, metabolic pools, or pH (21). ELISA data indicated that LAP expression was not affected or even increased at low concentrations of glucose. However, LAP was suppressed when L. monocytogenes cells were grown in LB with higher levels of glucose (1.6 and 3.2 g/liter). Concomitant with this decrease in LAP expression, there was a decrease in the final pH. The pH was decreased gradually as the glucose amount was increased, until it dropped below 4.5 with 3.2 g of glucose/liter. Milenbachs et al. (22) found that cellobiose (4-O-β-d-glucopyranosyl-d-glucopyranose) completely repressed hly expression at 25 mM or more, and it was found that the final pH was 4 to 4.5; however, the decrease in hly expression was not due to the pH decrease, as the repression was not abolished in pH-maintained cultures. Similar to cellobiose, glucose appeared to decrease the pH when metabolized. However, this decrease in pH is the possible reason for LAP repression, as the addition of K2HPO4 possibly maintained pH homeostasis and thus restored LAP expression. Datta and Kothary (9) studied the effect of glucose on hly expression and found that the repression was due to the decrease in pH, which presumably resulted from the accumulation of sugar fermentation by-products, rather than it being a catabolite repressor. Similarly, Staphylococcus aureus alpha toxin and enterotoxin B production were greatly suppressed in the presence of glucose, possibly due to the accumulation of glycolytic end products (11).
It was established previously that LAP is an adhesion protein (24), and here we have investigated the effect of glucose on LAP expression and cellular adhesion properties by using a Caco-2 cell line. Interestingly, adhesion of L. monocytogenes to Caco-2 was decreased as increased amounts of glucose were added to the media. This correlates LAP expression to adhesion; however, it is well known that there are other adhesion determinants that are also responsible for L. monocytogenes adhesion to intestinal cells, and the increased glucose levels may not affect the expression of these adhesion factors. This may explain the decrease in adhesion at high glucose levels to only half of that at low or no glucose. Furthermore, a LAP-negative mutant, L. monocytogenes A572, was grown in LB with different glucose concentrations and then used in the adhesion assay to determine the effect of LAP on L. monocytogenes adhesion properties to Caco-2 cells. Interestingly, A572 bound equally to Caco-2 cells regardless of the concentration of glucose, indicating that LAP is involved in the adhesion process.
TEM results were similar to ELISA and Western blotting results. There was very little expression of LAP when L. monocytogenes cells were grown in LB with high glucose (3.2 g/liter), while the expression in LB without glucose or with a low amount (0.8 g/liter) was high. It appeared that LAP is largely located in the cytoplasm, with only some LAP on the surface of the cell in patchy patterns. It is possible that most of the LAP remained intracellular; however, during infection some LAP is found on the surface to initiate attachment, similar to the listeriolysin O production observed by Quinn et al. (29). In addition, LAP is not the only adhesion protein involved in the attachment of L. monocytogenes to Caco-2 cells; multiple other proteins are also involved. This explains why the adhesion to Caco-2 cells was not abolished when LAP expression was strongly repressed in LB with 1.6 or 3.2 g of glucose/liter. Furthermore, it appears that only a few molecules of LAP are sufficient to assist in binding of L. monocytogenes to Caco-2 cells. The polyclonal antibody, compared to the monoclonal antibody, recognized higher numbers of LAP molecules; this could be due to the multiple epitopes recognized by a polyclonal antibody.
In conclusion, it appears that nutrient-limiting medium (LB) supports higher LAP expression than nutrient-rich media (BHI and TSB). In contrast, nutrient-deficient medium (PW) supported neither good cell growth nor LAP expression. Glucose appears to enhance cell growth by providing energy; however, it affects LAP expression. This effect may be due to the decrease in pH to levels that are incompatible with LAP production. This behavior indicates that, under nutrient-limiting stress conditions, L. monocytogenes might produce more of its virulence factors to adapt in the new environment for survival and pathogenesis. This study further confirms the role of LAP in L. monocytogenes adhesion to mammalian cells. Based on this study, it could be speculated that the growth environment, including food, may influence the overall expression of LAP and thereby affect the attachment of L. monocytogenes to intestinal epithelium in vivo and the onset or severity of disease.
Acknowledgments
This study was funded by a grant from the Showalter Trust Fund.
We sincerely thank Debby Sherman for her assistance with the electron microscopy, Jennifer Wampler for statistical analysis, and Brad Reuhs and Maribeth Cousin for critical review of the manuscript.
References
- 1.Alvarez-Dominguez, C., J. A. Vasquez-Boland, E. Carrsaco-Marin, P. Lopez-Mato, and F. Leyva-Cobian. 1997. Host cell heparin sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparin sulfate receptor recognition. Infect. Immun. 65:78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour, A. H., A. Rampling, and C. E. Hormaeche. 2001. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infect. Immun. 69:4657-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behari, J., and P. Youngman. 1998. Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA. Infect. Immun. 66:3635-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhunia, A. K., P. H. Ball, A. T. Fuad, B. Kurz, J. W. Emerson, and M. G. Johnson. 1991. Development and characterization of a monoclonal antibody specific for Listeria monocytogenes and Listeria innocua. Infect. Immun. 59:3176-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohne, J., Z. Sokolovic, and W. Goebel. 1994. Transcriptional regulation of prfA and PrfA-regulated virulence genes in Listeria monocytogenes. Mol. Microbiol. 11:1141-1150. [DOI] [PubMed] [Google Scholar]
- 6.Brehm, K., M.-T. Ripo., J. Kreft, and J.-A. Vasquez-Boland. 1999. The bvr locus of Listeria monocytogenes mediates virulence gene repression by β-glucosides. J. Bacteriol. 181:5024-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraborty, T. 1999. Molecular and cell biological aspects of infection by Listeria monocytogenes. Immunobiology 201:155-163. [DOI] [PubMed] [Google Scholar]
- 8.Cossart, P., and M. Lecuit. 1998. Interaction of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 17:3797-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta, A. R., and M. H. Kothary. 1993. Effects of glucose, growth temperature, and pH on listeriolysin O production in Listeria monocytogenes. Appl. Environ. Microbiol. 59:3495-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dramsi, S., C. Kocks, C. Forestier, and P. Cossart. 1993. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature and the pleiotropic activator prfA. Mol. Microbiol. 9:931-941. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, J. L., and G. J. Cho. 1972. Production of staphylococcal alpha toxin. II. Glucose repression of toxin formation. Infect. Immun. 6:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard, J. L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 13.Gilot, P., Y. Jossin, and J. Content. 2000. Cloning, sequencing and characterization of a Listeria monocytogenes gene encoding a fibronectin-binding protein. J. Med. Microbiol. 49:887-896. [DOI] [PubMed] [Google Scholar]
- 14.Hagman, M. M., J. B. Dale, and D. L. Stevens. 1999. Comparison of adherence to and penetration of human laryngeal epithelial cell line by group A streptococci of various M protein types. FEMS Immunol. Med. Microbiol. 23:194-204. [DOI] [PubMed] [Google Scholar]
- 15.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 16.Iandolo, J. J., and W. M. Shafer. 1977. Regulation of staphylococcal enterotoxin B. Infect. Immun. 16:610-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn, M., and W. Goebel. 1989. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect. Immun. 57:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Laux, D. C., F. F. McSweegan, and P. S. Cohen. 1984. Adhesion of enterotoxigenic Escherichia coli to immobilized intestinal mucosal preparations: a model for adhesion to mucosal surface compartments. J. Microbiol. Methods 2:27-39. [Google Scholar]
- 20.Leimeister-Wachter, M., E. Domann, and T. Chakraborty. 1992. The expression of virulence genes in Listeria monocytogenes is thermoregulated. J. Bacteriol. 174:947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milenbachs, A. A., D. P. Brown, M. Moors, and P. Youngman. 1997. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 23:1075-1085. [DOI] [PubMed] [Google Scholar]
- 23.Milohanic, E., R. Jonquieres, P. Cossart, P. Berche, and J.-L. Gaillard. 2001. The autolysin ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol. Microbiol. 39:1212-1224. [DOI] [PubMed] [Google Scholar]
- 24.Pandiripally, V. K., D. G. Westbrook, G. R. Sunki, and A. K. Bhunia. 1999. Surface protein p104 is involved in adhesion of Listeria monocytogenes to human intestinal cell line, Caco-2. J. Med. Microbiol. 48:117-124. [DOI] [PubMed] [Google Scholar]
- 25.Park, J. H., Y. S. Lee, Y. K. Lim, S. H. Kwon, C. V. Lee, and B. S. Yoon. 2000. Specific binding of recombinant Listeria monocytogenes p60 protein to Caco-2 cells. FEMS Microbiol. Lett. 186:35-40. [DOI] [PubMed] [Google Scholar]
- 26.Park, S. F. 1994. The repression of listeriolysin O expression in Listeria monocytogenes by the phenolic β-d-glucoside, arbutin. Lett. Appl. Microbiol. 19:258-260. [DOI] [PubMed] [Google Scholar]
- 27.Park, S. F., and R. G. Kroll. 1993. Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant derived molecule cellobiose in Listeria monocytogenes. Mol. Microbiol. 8:653-661. [DOI] [PubMed] [Google Scholar]
- 28.Portnoy, D. A., T. Chakraborty, W. Goebel, and P. Cossart. 1992. Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun. 60:1263-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn, F., L. Pine, E. White, V. George, K. Gutekunst, and B. Swaminathan. 1993. Immunogold labeling of Listeria monocytogenes virulence-related factors within Caco-2 cells. Res. Microbiol. 144:597-608. [DOI] [PubMed] [Google Scholar]
- 30.Regassa, L. B., R. P. Novick, and M. J. Betley. 1992. Glucose and nonmaintained pH decrease expression of the accessory gene regulator (agr) in Staphylococcus aureus. Infect. Immun. 60:3381-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renzoni, A., A. Klarsfeld, S. Dramsi, and P. Cossart. 1997. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infect. Immun. 65:1515-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ripio, M. T., G. Dominguez-Bernal, M. Suarez, K. Brehm, P. Berche, and J.-A. Vazquez-Boland. 1996. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res. Microbiol. 147:371-384. [DOI] [PubMed] [Google Scholar]
- 33.Santiago, N. I., A. Zipf, and A. K. Bhunia. 1999. Influence of temperature and growth phase on expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl. Environ. Microbiol. 65:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokolovic, Z., J. Riedel, M. Weenscher, and W. Goebel. 1993. Surface-associated, PrfA-regulated proteins of Listeria monocytogenes synthesized under stress conditions. Mol. Microbiol. 8:219-227. [DOI] [PubMed] [Google Scholar]
- 35.van Tuinen, E., and H. Riezman. 1987. Immunolocalization of glyceraldehyde-3-phosphate dehydrogenase, hexokinase, and carboxypeptidase Y in yeast cells at the ultrastructural level. J. Histochem. Cytochem. 35:327-333. [DOI] [PubMed] [Google Scholar]