Abstract
During light organ colonization of the squid Euprymna scolopes by Vibrio fischeri, host-derived mucus provides a surface upon which environmental V. fischeri forms a biofilm and aggregates prior to colonization. In this study we defined the temporal and spatial characteristics of this process. Although permanent colonization is specific to certain strains of V. fischeri, confocal microscopy analyses revealed that light organ crypt spaces took up nonspecific bacteria and particles that were less than 2 μm in diameter during the first hour after hatching. However, within 2 h after inoculation, these cells or particles were not detectable, and further entry by nonspecific bacteria or particles appeared to be blocked. Exposure to environmental gram-negative or -positive bacteria or bacterial peptidoglycan caused the cells of the organ's superficial ciliated epithelium to release dense mucin stores at 1 to 2 h after hatching that were used to form the substrate upon which V. fischeri formed a biofilm and aggregated. Whereas the uncolonized organ surface continued to shed mucus, within 48 h of symbiont colonization mucus shedding ceased and the formation of bacterial aggregations was no longer observed. Eliminating the symbiont from the crypts with antibiotics restored the ability of the ciliated fields to secrete mucus and aggregate bacteria. While colonization by V. fischeri inhibited mucus secretion by the surface epithelium, secretion of host-derived mucus was induced in the crypt spaces. Together, these data indicate that although initiation of mucus secretion from the superficial epithelium is nonspecific, the inhibition of mucus secretion in these cells and the concomitant induction of secretion in the crypt cells are specific to natural colonization by V. fischeri.
A recent study of the symbiosis between the Hawaiian squid Euprymna scolopes and the bioluminescent bacterial symbiont Vibrio fischeri (Fig. 1) demonstrated that during initiation of the symbiosis, the host squid uses ciliary currents and mucus secretions to concentrate V. fischeri near sites of colonization (35). The juvenile host hatches with complex superficial ciliated fields on the nascent light organ that potentiate the colonization process (Fig. 1B). Through the activity of the ciliated fields, V. fischeri cells aggregate in the mucus over a period of several hours (Fig. 1C) and subsequently migrate to and enter pores on either side of the organ (Fig. 1C and D). The symbionts then travel down ciliated ducts before colonizing epithelium-lined crypt spaces (Fig. 1D). The analyses further showed that the aggregation process is specific to gram-negative bacteria but that only V. fischeri is able to migrate through the ducts and colonize the host successfully. Indeed, the colonization of E. scolopes in this association has been shown to be very specific (30), and several symbiont genes have been identified as essential for the initiation, colonization, and persistence of V. fischeri in the host squid light organ (19, 20, 37, 45; K. Visick and E. G. Ruby, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., p. 310, 1997). After successful colonization, the ciliated fields undergo complete regression over the first 4 to 5 days of the symbiosis as part of the developmental program initiated by V. fischeri (13, 33). This description of bacterial aggregation (35) is the first in-depth description of a marine organism using ciliary mucus currents to assist in colonization by a specific bacterial symbiont, adding to the long list of the roles that mucus plays in animals.
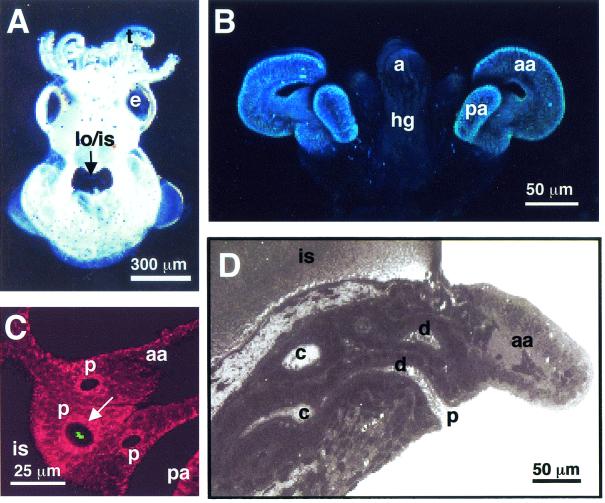
FIG. 1.
Path of symbiont cells into the juvenile host light organ. (A) Hatchling juvenile squid contain a bilobed light organ that is part of the ink sac complex located in the center of the mantle cavity (arrow). (B) Confocal image of the nascent light organ, revealing the ciliated epithelial fields on either side of the light organ, each with two appendages that direct seawater containing environmental bacteria towards sites of colonization. (C) Confocal image showing an aggregation of GFP-labeled V. fischeri (green, arrow) entering one of the three pores that occur on each side of the light organ. The cells of the light organ are stained with CellTracker (red), which is a general stain for eukaryotic cell cytoplasm. (D) Each pore leads to a ciliated duct that terminates in an epithelium-lined crypt space where permanent colonization by the symbiont population takes place. a, anus; aa, anterior appendage; c, crypt space; d, duct; e, eye; hg, hind gut; is, ink sac; lo, light organ; p, pore; pa, posterior appendage; t, tentacle.
In systems involving host mucus colonized by bacteria, such as in the squid-vibrio association (29, 46), a principal question is, to what extent and by what mechanism(s) do bacteria participate in the dynamics of mucus secretion? In pathogenic associations, bacteria and bacterial products, such as cell surface molecules and toxins, have been implicated in the control of mucus secretion at both the cellular and molecular levels (12). For example, in the pathogenesis of Vibrio cholerae, cholera toxin triggers mucin release from cultured human goblet cells (16, 24) and rat colonic tissue (7). In the lungs of patients suffering from cystic fibrosis, excess mucus secretion leads to creation of an environment that is conducive to persistent colonization by Pseudomonas aeruginosa (5, 27). Further studies of this association and others have shown that bacterial lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, is capable of upregulating genes involved in mucin synthesis (12, 25) and inducing mucus secretion (8).
In the present study we sought to (i) determine when and how mucus secretion is initiated in juvenile E. scolopes, (ii) define what host cells are responsible for secretion of the mucus that forms the matrix in which symbionts aggregate prior to colonization, and (iii) define the impact of the colonization of the light organ by V. fischeri on the secretory activity of mucus-producing host tissues.
MATERIALS AND METHODS
General procedures.
Adult E. scolopes squid were collected from shallow sand flats of Oahu, Hawaii (34), and breeding colonies were maintained as described previously (13, 18).
Unless otherwise indicated, bacterial strains were grown at 25°C in a seawater-based tryptone medium (3) to the mid-logarithmic phase, and cell density was estimated spectrophotometrically by determining optical density at 600 nm. Squid were placed individually in 5 ml of either filter-sterilized seawater (FSSW) or unfiltered natural seawater (USW), which contained around 106 bacteria/ml (14) but did not harbor V. fischeri in sufficient quantities to colonize the host. Juvenile E. scolopes placed in either FSSW or USW did not form a symbiotic association. Test bacterial strains were added to FSSW or USW to obtain a final density of approximately 103, 104, 105, or 106 test bacteria/ml of seawater. One of the following six bacterial strains was used for each inoculation experiment: the wild-type strain V. fischeri ES114; two nonmotile strains, NM200 and NF201, which are derivatives of ES114 (19); Vibrio parahaemolyticus KNH1 (35); Bacillus cereus 43-25 (grown in Luria-Bertani medium) (15), a marine Bacillus sp. strain; and heat-killed, tetramethyl rhodamine isocyanate-labeled Staphylococcus aureus (Molecular Probes, Eugene, Oreg.). The vibrio strains and B. cereus carried a plasmid containing a gene that encodes a green fluorescent protein (GFP) (35). Hatchling squid were also exposed to suspensions of various types of sterile particles, including 1-, 2-, or 10-μm-diameter fluorescent polystyrene beads (Molecular Probes) at a concentration of 106 beads/ml, silicon dioxide particles at a concentration of 1 mg/ml, or carmine particles at a concentration of 0.1 mg/ml, in FSSW.
Microscopy.
For light microscopic analyses of mucin histochemistry, juvenile host animals or light organs from adult squid were fixed in 20 ml of Carnoy’s fixative (50% ethanol, 40% glacial acetic acid, 10% chloroform) at 4°C for 2 h. Samples were then dehydrated by washing them two times (10 min each time) in 100% ethanol at 4°C and then two times (10 min each time) in xylene at room temperature. Samples were then embedded in paraffin, sectioned with a Microm HM340E microtome, mounted on glass slides, and stored at 4°C before histochemical analysis.
To stain for various mucin types, sections were deparaffinized by using two changes of xylene, followed by washes with 100 and 70% ethanol before rehydration in deionized water. Sections were stained with either (i) high-iron diamine (HID) to visualize sulfomucins and alcian blue (AB) (pH 2.5) to visualize sialomucins (43) or (ii) periodic acid-Schiff stain (PAS) to visualize neutral mucins and AB (pH 2.5) to visualize sialomucins (6). Briefly, for HID-AB staining, sections were incubated in a solution containing 0.24% dimethyl meta-phenylenediamine dihydrochloride, 0.04% dimethyl para-phenylenediamine dihydrochloride, and 1.7% FeCl3 for 16 h at 25°C with agitation during the first hour. The sections were then rinsed in running tap water (RTW) and stained with a 1% AB solution for 10 min before they were rinsed in RTW. Stained samples were dehydrated, cleared in xylene, and then mounted with Permount. For PAS-AB staining, sections were rinsed in 3% acetic acid for 3 min and then incubated for 30 min with a 1% AB solution. Samples were rinsed in RTW, placed in 0.5% periodic acid for 10 min, rinsed in RTW, placed in Schiff's reagent for 10 min, reduced in 0.5% sodium metabisulfite, rinsed in RTW for 10 min, dehydrated, cleared in xylene, mounted with Permount, and viewed with a compound microscope.
To prepare specimens for confocal microscopy following incubation with bacterial strains or particles, hatchling squid were either stained with a fluorochrome for laser-scanning confocal microscopy (LSM) or left unstained for differential interference contrast microscopy. For fluorescence staining, animals were placed for 30 min in FSSW containing 1 μM CellTracker Orange (Molecular Probes), a fluorochrome that labels the cytoplasm of viable eukaryotic cells. The animals were rinsed with several changes of FSSW and then anesthetized in either a 1:1 solution of 80 mM MgCl2 and FSSW or a 2% solution of ethanol in FSSW. The animals were placed ventral side up in a depression well slide, and the mantle and funnel of each animal were dissected away to expose the underlying light organ. Fluorescently labeled animals and bacteria were simultaneously viewed by LSM with a Zeiss LSM 510 confocal microscope. Bacterial aggregations in unstained animals were viewed by using LSM and differential interference contrast microscopy.
To visualize mucus secretions by LSM, animals were incubated with fluorescently labeled wheat germ agglutinin (WGA) (Oregon Green or tetramethyl rhodamine; Molecular Probes), a lectin that specifically binds to N-acetylneuraminic acid (sialic acid) residues, which occur in the mucus secretions of the E. scolopes light organ (35). The animals were incubated with WGA at a final concentration of 10 μg/ml of FSSW for 30 min, rinsed two times (1 min each time) in FSSW, dissected, and viewed by LSM. Fluorescein isothiocyanate-labeled Ulex europaeus agglutinin (Sigma-Aldrich, St. Louis, Mo.), a lectin that labels l-fucose residues, which do not occur in the mucus secretions of the juvenile light organ (35), was used as a control to assess the degree of nonspecific binding of lectins to host mucus. To determine whether sialated residues that might be associated with the surfaces of V. fischeri cells could be the source of sialic acid residues in the crypts, culture-grown V. fischeri cells and symbionts isolated from adult light organs (34) were stained with WGA.
Treatment with PGN and LPS.
Peptidoglycan (PGN) from either V. fischeri (17) or S. aureus (Fluka, Milwaukee, Wis.) was treated with a 1% (wt/wt) solution of lysozyme in 0.1 M sodium acetate buffer (pH 5) for 10 min to digest the PGN into smaller fragments. The lysozyme was then inactivated by boiling the solution for 5 min. A control containing lysozyme without PGN was prepared in a similar manner. The treated PGN at a final concentration between 100 and 300 μg/ml or a corresponding equal volume of the lysozyme control was then added to 1 ml of FSSW, and hatchling squid were added. The animals were incubated for 2 to 3 h in these solutions and then prepared for lectin staining for mucus and viewed by LSM.
In experiments in which animals were exposed to PGN-conjugated beads, a 2% aqueous suspension of 2-μm-diameter, fluorescent, carboxylate-modified polystyrene beads (Molecular Probes) was added (50:50) to a 1-mg/ml solution of lysozyme-treated PGN from S. aureus in 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 6.0). A set of negative control beads was similarly treated, but no PGN was added. After a 15-min incubation at room temperature, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (pH 6.5) was added to a concentration of 4 mg/ml to initiate covalent coupling of PGN to the beads. The 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide-treated suspensions were incubated overnight with shaking, and the coupling reaction was then quenched for 30 min by adding glycine to a final concentration of 100 mM. The beads were then washed 10 times in 50 mM sodium phosphate buffer with 0.1 M NaCl (pH 7.4) and then filtered through 10- and 3-μm-pore-size filters (Nuclepore, Pleasanton, Calif.) with repeated washing to remove coated beads larger than 3 μm. The beads were then washed again five times in FSSW before use. Both PGN-linked and control beads were stained with fluorescent WGA to confirm that PGN had been successfully bound to the beads. Both sets of beads were incubated for 3 h in FSSW and centrifuged, and the supernatants were collected and filtered through a 0.22-μm-pore-size filter (Millipore, Bedford, Mass.). The beads (106 beads/ml) with or without PGN or filtered supernatants were added to hatchling squid, and after a 3-h incubation the animals were stained for mucus detection as described above.
Hatchling squid were exposed to LPS from either V. fischeri (1), V. cholerae, or Escherichia coli (Sigma Chemical Co.) or to purified lipid A from V. fischeri (1) at concentrations of 10 and 100 ng/ml and 1 μg/ml in FSSW as previously described (18). These concentrations were chosen because they effectively promote mucus secretion in other animal systems (2, 25, 26, 49) and induce developmental changes in E. scolopes (18). The suspensions of both LPS and lipid A were sonicated for 30 s before they were added to the squid.
Experimental manipulation of colonization events.
To characterize the early events leading to mucus secretion and aggregation formation, the interaction of host tissues with bacteria or bacterium-sized particles was analyzed during the first 2 h after hatching. Freshly hatched squid were exposed to bacterial strains or 1-, 2-, or 10-μm-diameter fluorescent polystyrene beads (Molecular Probes), all at a concentration of 106 cells or beads per ml of FSSW. The animals were viewed by LSM between time zero and 1 h at 5-min intervals following exposure and then at 1.5 and 2 h, and the presence of any bacteria or beads attached to the ciliated surfaces of the light organ or within the ducts or crypt spaces was noted.
A series of experiments was conducted to determine whether colonization of the light organ affected mucus shedding and aggregation formation. To determine whether the ability to secrete mucus and form aggregations persists following early colonization of the crypts by V. fischeri, hatchling squid were continually exposed to wild-type V. fischeri cells for the first 24 h, and the ability of V. fischeri to form aggregations was observed by LSM at 2-h intervals. Then, to determine whether the host continued to aggregate newly available bacteria from the environment after prolonged colonization of the crypts (i.e., after 24 h), hatchling squid were first infected with unlabeled wild-type V. fischeri cells, and successful colonization was detected by monitoring luminescence with a TD-20/20 luminometer (Turner, Sunnyvale, Calif.). Animals that were symbiotic for 24, 48, 72, and 96 h were then exposed to GFP-labeled V. fischeri cells and viewed by LSM after 3 to 4 h of incubation to determine whether they remained capable of aggregating symbionts outside the organ after colonization of the crypts for specific times. The ability of animals maintained as aposymbiotic organisms (i.e., animals exposed only to USW) to form aggregations with added V. fischeri cells was also assessed at each of these time points.
In experiments in which light organs were cured of their symbionts following colonization, animals were continuously exposed to chloramphenicol at a final concentration of 10 μg/ml, and the decline in the symbiont population was monitored by monitoring luminescence as previously described (13). To determine whether cured E. scolopes juveniles are capable of forming bacterial aggregations, the chloramphenicol-treated animals were then inoculated with GFP-labeled V. fischeri and viewed by LSM after 3 to 4 h of incubation to determine whether aggregations had formed. In all experiments, aposymbiotic, symbiotic, and cured animals were stained with WGA to detect host mucus.
RESULTS
Early light organ permissiveness.
Whereas permanent colonization of the E. scolopes light organ is restricted to motile strains of V. fischeri (19, 35), the results of the present study indicate that this specificity develops over the first few hours following hatching of the juvenile hosts. Viewing the animals with a dissection microscope during hatching revealed that the cilia on the surface of the light organ are not beating at hatching but begin to beat within 10 to 20 s after hatching in either USW or FSSW. In experiments in which the animals were then exposed to fluorescently labeled, ∼1- or 5-μm-diameter, living or dead, gram-negative or -positive bacteria or to 1-, 2-, or 10-μm-diameter particles, neither bacteria nor particles were observed in the crypt spaces during the first 20 to 25 min after hatching. Between approximately 30 and 60 min following exposure, up to 90% of the animals exposed to 1-μm-diameter gram-negative and -positive bacteria or to 1-μm-diameter particles had between one and three bacterial cells or particles in the crypt spaces, as determined by LSM (Table 1). The 5-μm-diameter bacteria and 2- and 10-μm-diameter beads were never observed in the crypt spaces. These data indicate that passive early entry into host crypt spaces is not limited by particle type or viability but by size.
TABLE 1.
Permissiveness of the host light organ
| Bacteria or particle type | Avg size (μm) | % of squid with bacteria or particles (n = 20)a |
|---|---|---|
| Gram-negative bacteria | ||
| V. fischeri ES114 (wild type) | 1 | 90 |
| V. fischeri NM200 (nonmotile) | 1 | 88 |
| V. parahaemolyticus | 1 | 73 |
| V. parahaemolyticus at 3.5 to 4.0 h | 1 | 55b |
| Gram-positive bacteria | ||
| S. aureus (heat killed) | 1 | 70 |
| B. cereus | 5 | 0 |
| Polystyrene beads | 1 | 10 |
| 2 | 0 | |
| 10 | 0 |
Percentage of squid having one to three bacterial cells or particles in their crypt spaces between 30 and 60 min after hatching. Unless otherwise noted, animals were exposed to cells or beads immediately following hatching.
Percentage of squid having one to three bacterial cells in their crypt spaces between 30 and 60 min after they were maintained in FSSW for 3 h.
Within the permissive period (from 30 to 60 min), bacteria and particles were observed either outside the light organ or in deep portions of the ducts or crypt spaces, unlike the eventual migrations of aggregated symbionts, which progress slowly through the ducts and into the crypts between 2 and 5 h after inoculation (35). These data suggest that the mechanism responsible for the initial entry into the crypt spaces provides a relatively rapid means of transport for these nonspecific particles.
Unlike the V. fischeri cells that later colonize the host, the motile strains of both wild-type V. fischeri and V. parahaemolyticus were no longer visibly motile after they entered the crypts during this early permissive period. When the light organs were plated, no CFU resulted, although culture-grown strains of bacteria exposed to the animals have a plating efficiency of 100% (38), suggesting that either the transport process itself or the conditions of the hatchling crypt space compromised the viability of the cells. Furthermore, the ability of the bacteria or particles to enter the crypts was restricted to a limited time frame. Although bacteria and 1-μm-diameter beads entered the crypts during the first hour after exposure, neither bacteria nor beads were observed in the crypt spaces of squid 2 h after inoculation (n = 20). These data indicate that the bacteria or particles that entered during the initial phase were cleared or degraded by the host in some manner.
Taken together, these data demonstrate that while colonization eventually occurs as the result of migration of motile strains of aggregated V. fischeri into the crypts between 2 and 5 h after inoculation (35), the light organ crypts are temporarily permissive to entry by other bacteria or particles that are 1 μm in diameter. However, these early recruits are cleared, and V. fischeri cells that may initially enter do not appear to contribute to the population of cells that eventually colonizes the host.
Depletion of mucus stores from the ciliated epithelial appendages of the hatchling host light organ.
Mucus secretion for aggregation formation, which normally commences 1 to 2 h following hatching, occurred principally from the cells of the ciliated epithelial appendages on the surface of the juvenile light organ. Although the entire ciliated epithelial fields of the juvenile light organ were coated with sialomucin (Fig. 2A and B), only the cells of the appendages of newly hatched squid had the histological appearance suggestive of mucus secretion, i.e., vivid staining of mucins localized to the apical regions of the cells (Fig. 2C). Distinct blue staining, indicative of sialomucins, and pink staining, indicative of neutral mucins, were observed in these intracellular stores (Fig. 2C). Sialomucin appeared to be the dominant mucin type found embedded in the densely ciliated cell surfaces, and neutral mucin was detected at relatively low levels in these extracellular environments (Fig. 2B and C). In animals maintained in FSSW, a coat of sialomucin covered the appendages, but little shedding of the mucus was observed (Fig. 2A and B). HID-AB staining showed that few sulfated mucins occurred anywhere except in the reflector tissue of the light organ, and this staining also confirmed that sialomucin covered the ciliated fields (data not shown).
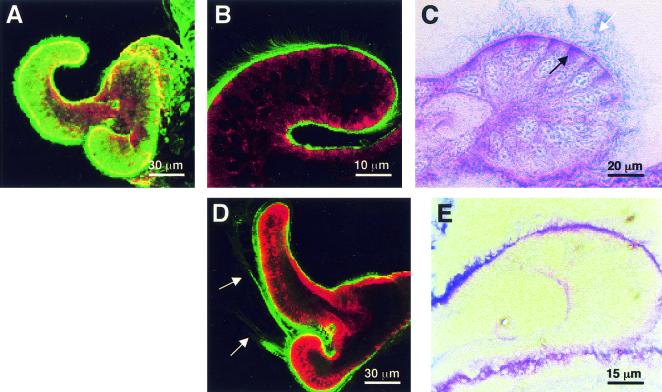
FIG. 2.
Mucin storage and secretion in the superficial ciliated epithelium of the E. scolopes light organ. (A) Confocal image of the light organ surface of a representative live hatchling squid, showing uniform staining with WGA (green) for N-acetylneuraminic acid (sialic acid) residues characteristic of sialomucins. (B) WGA staining of a confocal section through the ciliated fields of a live specimen, confirming the presence of a coat of sialomucin (green) on the surfaces. (C) PAS-AB histological staining of a section of hatchling squid, demonstrating that the cytoplasm of the apical regions of the cells comprising the ciliated epithelial appendages contains dense neutral mucin and sialomucin stores (purple, black arrow), while the extracellular surfaces are coated with sialomucin (blue, white arrow). (D) Representative confocal micrograph showing the typical sialomucin shedding event (arrows) that occurred in the surface epithelium within 1 h of exposure to USW, gram-negative or -positive bacteria, or bacterial PGN. (E) After several hours of exposure to environmental bacteria, the abundance of mucins within the appendages decreased compared with the abundance in hatchling animals (panel C). In the confocal images, the host cells are counterstained with CellTracker (red).
Shedding of sialomucin from the appendages of animals occurred by 2 h after exposure to USW or FSSW that contained gram-negative bacterial strains (V. fischeri, V. parahaemolyticus, and E. coli) or gram-positive bacterial strains (B. cereus, a marine Bacillus sp., and S. aureus) (Fig. 2D and Table 2). Concomitantly, the intensity of PAS-AB staining decreased in the appendages between hatching and 2 h after the animals were exposed to USW or V. fischeri (Fig. 2E). The decreased intensity of PAS-AB staining in the apical regions of the appendages was observed in both aposymbiotic and symbiotic animals maintained in USW for at least 96 h. Mucus secretion was not detected in animals maintained in FSSW alone (Table 2). A 30-min exposure to V. fischeri or B. cereus, followed by rinsing in FSSW and lectin staining, resulted in staining patterns similar to those of animals that were incubated with bacteria for up to 6 h (data not shown), suggesting that only a transient exposure to bacteria or bacterial products is required to trigger continuous mucus secretion. None of the particle suspensions tested (polystyrene beads, silicon dioxide, or carmine) induced mucus shedding (Table 2). These data demonstrate that the induction of host mucus secretion is not symbiont specific but does require interaction with some bacterial component.
TABLE 2.
Inducers of host mucus secretion
| Inducer | n | Relative level of mucus secretiona |
|---|---|---|
| Gram-negative bacteria | ||
| V. fischeri | 24 | + |
| V. fischeri (nonmotile) | 12 | + |
| V. fischeri (heat or azide killed) | 6 | + |
| V. parahaemolyticus | 24 | + |
| E. coli (heat killed) | 10 | + |
| Gram-positive bacteria | ||
| B. cereus | 12 | ++ |
| Bacillus sp. (marine) | 12 | ++ |
| S. aureus (heat killed) | 12 | + |
| Bacterial products | ||
| Bacterial LPS (V. fischeri, E. coli, or V. cholerae) | 9 | − |
| Lipid A (V. fischeri) | 9 | − |
| PGN (V. fischeri) | 12 | + |
| PGN (S. aureus) | 12 | ++ |
| Filtered supernatant of either Bacillus culture | 12 | ++ |
| Nonbacterial particles | ||
| PGN-conjugated 2-μm-diameter beads | 6 | ++ |
| Unlinked beads | 6 | − |
| Filtered supernatant of PGN-linked beads | 6 | − |
| Filtered supernatant of unlinked beads | 6 | − |
| Carmine particles | 6 | − |
| Silicon dioxide particles | 6 | − |
| USW | 10 | + |
| FSSW | 80 | − |
−, not detectable; +, moderate; ++, abundant.
An additional set of experiments was performed to determine whether exposure to intact bacteria is required for the induction of host mucus shedding. Squid were exposed to supernatants of either B. cereus cells or cells of a marine Bacillus sp. To obtain these supernatants, the bacteria were incubated in FSSW for 3 to 4 h at a concentration of 106 bacteria/ml, and this was followed by centrifugation and filtration of the supernatants with a syringe filter (pore size, 0.22 μm). Supernatants from either Bacillus species were capable of inducing mucus secretion (Table 2), suggesting that a diffusible or shed bacterial component and not necessarily direct contact with intact bacterial cells is required to induce host mucus secretion.
Because exposure to both gram-positive and gram-negative bacteria and their products led to mucus secretion, the ability of PGN, a common cellular component of both bacterial types, to induce host mucus secretion was tested. In addition, the abilities of the gram-negative bacterial cell wall molecule LPS and lipid A, the endotoxic component of LPS, to induce host mucus secretion were also tested; these molecules are known inducers of animal cell mucus secretion (12, 25). Animals exposed to V. fischeri or S. aureus PGN that had been lysozyme treated and heat inactivated exhibited mucus secretion from the ciliated appendages (Fig. 2D and Table 2). Heat-inactivated lysozyme by itself did not cause detectable mucus secretion. Neither LPS or lipid A from V. fischeri nor LPS from V. cholerae or E. coli caused detectable mucus secretion from host ciliated cells (Table 2). These data indicate that bacterial PGN, but not LPS or lipid A, is capable of inducing the release of host mucus from intracellular stores, although they do not prove that PGN is the only bacterial product that causes mucus secretion from these host cells.
Link between early permissiveness and mucus shedding.
A series of experiments was performed to determine whether early permissiveness of the light organ is required for mucus shedding and whether the eventual loss of permissiveness is due to the mucus shedding. Because the size of PGN-conjugated, 2-μm-diameter beads precluded their entrance into the crypt spaces (Table 1), these beads were used to determine their ability to cause host mucus shedding from outside the crypts. Exposure of the animals to these beads caused mucus shedding from the light organ surface (Table 2), while supernatants derived from incubation of the PGN-conjugated beads in FSSW for the same period (i.e., 4 h) did not cause mucus shedding in five of six animals tested. Since it is difficult to render the animals free of all contaminating material in FSSW, we feel that a response in a low number of animals (one of six animals in this instance) is not unexpected. These findings confirmed that the PGN on the beads was stably linked, providing few small molecules that might be shed from the beads that would interact with the crypt cells. Beads exposed to linkage agents in the absence of PGN caused mucus shedding in only one of six animals tested, confirming that the linkage treatment of the beads is relatively ineffective at causing this effect in the host. These data suggest that (i) host receptors for the induction of mucus secretion occur on the outside of the light organ (i.e., outside the crypts) and (ii) the early permissiveness of the organ to bacterial cells is not essential for the induction of mucus secretion.
Experiments were performed to determine whether the loss of the early nonspecific permissiveness of the light organ, which normally occurs 1 to 2 h following hatching, is a hard-wired, time-dependent, developmental change or the result of induced mucus secretion. To determine whether the block involves a developmental change, freshly hatched animals were maintained in FSSW for 3 h and then were exposed to GFP-labeled V. parahaemolyticus cells. Within 30 to 60 min after inoculation (3.5 to 4.0 h after hatching), these nonspecific bacterial cells were still able to enter the crypt spaces (Table 1), and they were cleared from the host crypts by 2 h after exposure (5 h after hatching). These data suggest that the block of permissiveness is not merely a time-dependent developmental process.
To determine whether the induced mucus secretion, which normally occurs concomitant with the block of permissiveness at 1 to 2 h following hatching, is responsible for the block, fresh hatchling squid were exposed to PGN from S. aureus for 3 h and then inoculated with GFP-labeled V. parahaemolyticus. Copious mucus secretion was observed, and 30% of the animals viewed (n = 10) contained between one and three nonspecific cells, levels which are lower than the levels of permissiveness observed before mucus secretion (Table 1). These data suggest that mucus secretion is, at most, only partly responsible for the block of early light organ permissiveness and that other factors or activities are required to achieve a complete block.
Taken together, these studies of the relatedness of early permissiveness and induction of mucus secretion suggest that although these events occur in close sequence, they are relatively independent. However, mucus secretion likely contributes to the cessation of nonspecific entry.
Developmental changes in mucus secretion and symbiont aggregation.
A series of experiments were conducted to determine whether host mucus secretion and bacterial aggregation patterns change after colonization of the light organ. The appendages of 72-h aposymbiotic juveniles maintained in USW and stained with fluorescently labeled WGA contained a markedly thicker covering of sialated mucus than the appendages of symbiotic juveniles after 48 h of colonization (Fig. 3A and B). Aposymbiotic juveniles that were maintained in V. fischeri-free seawater were able to secrete mucus and aggregate bacteria when they were exposed for 3 to 4 h to V. fischeri at any time over the first 96 h (Fig. 3C). In symbiotic animals, the ability to secrete mucus and aggregate V. fischeri persisted up to 24 h after colonization (Fig. 3D). However, by 48 h after successful colonization with V. fischeri, little host mucus shedding was detected (Fig. 3B), and GFP-labeled V. fischeri that was in the environment of the host was no longer detected in aggregations outside the light organ (Fig. 3E). When a colonized host light organ was cured with the antibiotic chloramphenicol, mucus shedding from the ciliated fields was again detected (Fig. 3F). Concomitantly, the host's ability to aggregate V. fischeri was restored (Fig. 3G), although the number of cells amassed was lower (∼10%) than the number of cells detected in animals that had been maintained as aposymbiotic organisms over same time frame and then exposed to V. fischeri (Fig. 3C and G). The lower numbers of symbiont cells in the aggregations were most likely due to the fact that because morphogenesis had been induced by colonization (33), fewer cells were present in the ciliated epithelium that secreted mucus. These results indicate that the presence of the symbiont in the crypts inhibits the ability of the host to aggregate bacteria further after the association is established, although this host response can be reinitiated by eliminating the symbiont. Thus, symbiont suppression of host mucus secretion from the ciliated appendages and bacterial aggregation requires persistent interaction with intact V. fischeri cells in the crypt spaces, which are several tissue layers away from the ciliated fields of the juvenile light organ.
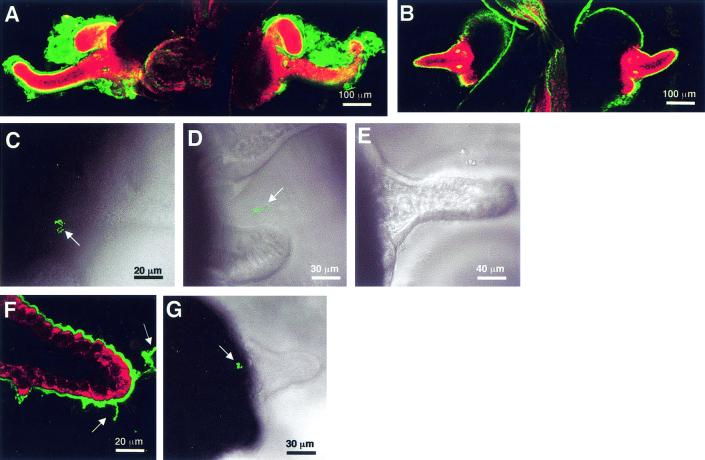
FIG. 3.
Influence of colonization on mucus shedding and bacterial aggregation. (A) Representative confocal micrograph of a 72-h aposymbiotic animal, showing the characteristic thick layer of sialated mucus (green) covering the superficial ciliated epithelial fields. (B) Although a thin coat of sialomucin-containing mucus (green) was present on the outside of the ciliated epithelium, no secretion of mucus was detected by 48 h after successful colonization by V. fischeri. (C) Aposymbiotic animals were capable of aggregating GFP-labeled V. fischeri (arrow) up to 96 h after hatching. (D) Wild-type V. fischeri cells formed loose aggregations (arrow) outside 24-h symbiotic animals. (E) By 48 h, no aggregations of V. fischeri cells were observed outside symbiotic light organs. (F) After the symbionts were removed from the light organ with the antibiotic chloramphenicol, mucus shedding was again detected (green, arrows), although not at the levels seen in aposymbiotic animals that were the same age. (G) The ability to form bacterial aggregations (arrow) was restored in 48-h symbiotic animals that had been cured with antibiotics for 24 h. In the confocal images, the sialomucin is labeled with WGA and the host cells are counterstained with CellTracker (red).
Colonization with V. fischeri also affected the amount of mucus in the crypts, having an effect opposite that observed in the mucus in the ciliated appendages. Neutral mucins were detected within the crypt spaces of both aposymbiotic and symbiotic animals (Fig. 4A and C). HID-AB staining revealed that while aposymbiotic animals had a thin coat of sialomucin along the brush border of the crypt epithelium (Fig. 4B), sialomucin was found throughout the crypt spaces of symbiotic animals (Fig. 4D). An abundance of sialomucin was also observed in adult crypt spaces (Fig. 4E). Because many gram-negative bacteria are capable of secreting exopolysaccharides composed of polysialic acid (44), which would stain positively in our assay, we tested V. fischeri cells for evidence of the presence of sialic acid residues. Staining of V. fischeri cells with the sialomucin label WGA was not observed in culture-grown cells (data not shown) or in cells obtained from adult light organs (Fig. 4F), although the crypt matrix material that surrounded these cells did stain with the lectin (Fig. 4F). These data suggest that interaction of V. fischeri with crypt cells induces host mucus secretion into the crypt spaces.
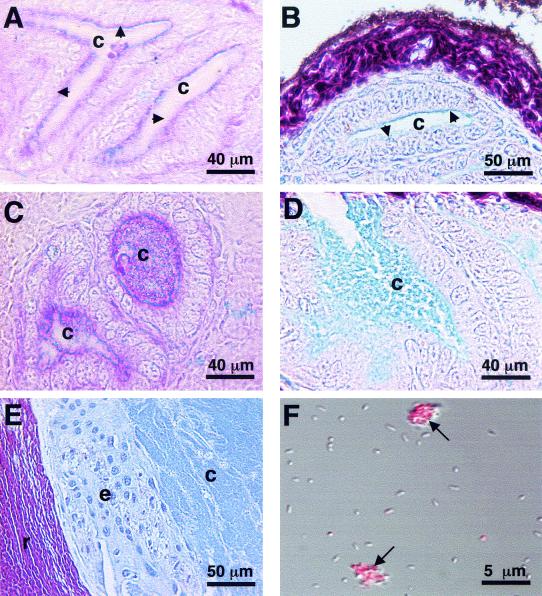
FIG. 4.
Symbiont-induced mucus secretion into the crypt spaces. (A) After 24 h of incubation, PAS-AB staining revealed a thin coat of sialomucin (blue, arrows) along the brush border of the crypt epithelium in aposymbiotic animals. (B) The presence of sialomucin (blue, arrows) was confirmed in aposymbiotic animals stained with HID-AB. Sulfomucins (purple-brown) were confined to areas outside the tissues in direct contact with the symbionts, such as the reflective tissue. (C and D) PAS-AB (C) and HID-AB (D) staining of 24-h symbiotic juveniles revealed dense sialomucin (blue) within the light organ crypt spaces. (E) HID-AB staining of the crypt spaces of adult light organs resulted in a staining pattern similar to that observed in the symbiotic juveniles. (F) WGA staining of symbionts isolated from adult light organs revealed no staining of the bacterial cells, although the matrix material stained bright for sialomucins (red, arrows). c, crypt space; e, crypt epithelium; r, reflector.
DISCUSSION
The results of this study provide evidence that (i) the light organ crypts are open to nonspecific bacteria and particles that are less than 2 μm in diameter for a brief period after hatching, and these initial bacteria or particles are not detectable in the crypts by 2 h following this first exposure; (ii) exposure to gram-positive or -negative bacteria or to PGN induces the cells of the ciliated epithelial appendages on the light organ surface to secrete sialated mucus; (iii) the early permissiveness of the light organ and the initiation of subsequent mucus shedding do not appear to be interdependent processes; (iv) by 48 h after colonization, V. fischeri inhibits further host mucus shedding by the ciliated appendages and bacterial aggregate formation, and the inhibition of these processes is reversible by removal of the symbionts from the light organ with antibiotics; and (v) host colonization by V. fischeri leads to an increase in mucus within the crypt spaces.
In the host's natural environment, the light organ contains only strains of V. fischeri (30). However, the finding that hatchling crypts are transiently open to 1-μm-diameter bacteria and particles indicates that the factors regulating exclusive entry of V. fischeri do not fully function during early stages of the symbiosis. Because the early entrants, including V. fischeri, were cleared from the crypt spaces, the crypts might be considered hyperspecific during this period (i.e., they allow entry of symbiotic and nonsymbiotic bacteria and particles but inhibit subsequent persistence of these bacteria and particles). The process of removal remains to be described but may involve a harsh chemical environment in the crypt spaces (42, 47; Visick and Ruby, Abstr. 97th Gen. Meet. Am. Soc. Microbiol.) or the activity of the crypt cells. A previous study of the E. scolopes light organ showed that host blood cells or hemocytes, which have activities similar to those of vertebrate macrophage cells, are found in the crypt space environment (34). To what extent these macrophages remove early bacterial invaders from the light organ crypts remains to be determined. Whatever the mechanism(s), the results of this study and previous studies (30) suggest that once the early entrants into the organ are cleared, V. fischeri is the sole occupant of the crypt space throughout the life of the host animal.
Production of the mucus in which symbionts form a biofilm.
The present results suggest that during embryogenesis the host develops a nascent light organ that is biochemically and biophysically ready to interact with the environment to harvest symbionts. Upon exposure of a hatchling animal to the natural seawater environment, the ciliary activity of the superficial light organ epithelium commences and mucus is shed by the cells. In the context of the squid-vibrio symbiosis, these findings reveal another role for the ciliated epithelial fields in the promotion of colonization of host tissues by the symbionts. Thus, these tissues are responsible not only for currents and entrainment of V. fischeri-containing seawater and mucus during aggregate formation (35) but also for production and delivery of the mucus.
Under the experimental conditions of the present study, the induction of mucus secretion was relatively nonspecific; i.e., mucus secretion was induced by both gram-negative and gram-positive bacteria, as well as by PGN, although it was not induced by nonbiological particles that were the same size as the bacteria (Table 2). Previous studies of the colonization process have indicated that only gram-negative bacteria aggregate in the mucus (35) and that only V. fischeri sets up permanent residence in the crypt spaces (24). Taken together, these data indicate that the colonization of the E. scolopes light organ is a process of ever-increasing specificity.
PGN was the molecule found in both gram-negative and gram-positive bacteria that caused host mucus secretion in these experiments. Although the PGN of gram-positive bacteria is exposed, PGN is buried beneath the outer membrane of gram-negative bacteria. Thus, for the PGN of gram-negative bacteria to be active in the induction of mucus secretion, cells must either lyse or bleb their surfaces to expose PGN. Studies of V. fischeri have shown that it actively blebs its cell surface (Apicella, unpublished data), and similar activity has been noted in other environmental and pathogenic gram-negative bacteria (9, 21, 28, 36). Studies of the activity of PGN in other systems have demonstrated that it is a potent activator of the eukaryotic immune system and leads to the production of numerous inflammatory mediators (31), although we are not aware of any other studies implicating PGN in the induction of mucus secretion.
The induction of mucus secretion by PGN-conjugated beads suggests that the receptors for PGN are outside the light organ. The nature of these receptors remains to be determined, but several recent studies of animal receptors of bacterial products provide some intriguing candidate molecules. PGN is a known ligand for certain Toll receptors, where its binding induces the activity of the evolutionarily conserved NF-κB pathway (39, 48). In addition, recently, a new class of PGN receptors has been described, whose pathway of activation has not yet been elucidated (22, 26). Similar PGN recognition proteins in E. scolopes may be involved in the binding of PGN that could promote or induce, either directly or indirectly, the shedding of mucus.
The data obtained from histological and lectin staining demonstrate that sialated mucus is shed from the ciliated epithelial appendages in response to bacteria. The apparent decrease in these intracellular mucus stores, along with the continued mucus secretion in animals that were maintained in USW but were not exposed to V. fischeri, indicates that there is a high rate of turnover of mucus within the ciliated appendages. The ability to dispense and alter internal mucus stores quickly is not unprecedented in E. scolopes. A previous study of mucus secretory cells found in the mantle epidermis of E. scolopes demonstrated that this squid has the ability to secrete and chemically modify its mucus secretions from this tissue almost instantaneously (40).
Because symbiotic animals retain the ability to shed mucus and aggregate bacteria over the first 24 to 48 h, the host may be continually sampling the environment using mucus secretions and the ciliated fields until it is successfully colonized by V. fischeri. By 48 h after colonization, host mucus shedding decreases, and further aggregation formation is inhibited. These data suggest that V. fischeri is able to inhibit the ability of the host to aggregate bacteria by suppressing mucus secretion after colonization, because curing of the light organ resulted in renewed mucus shedding and subsequent bacterial aggregation, although at lower levels than the levels in aposymbiotic animals of the same age (Fig. 3). A previous study showed that 12 h of exposure to V. fischeri cells leads to irreversible apoptosis and regression of the ciliated epithelial fields (13). Therefore, the inability of cured animals to aggregate bacteria and secrete mucus at the same levels as aposymbiotic animals may be due to fewer secretory cells being present.
Whereas colonization of the crypt spaces by V. fischeri inhibits mucus secretion by the cells of the superficial epithelium, an increase in the sialated mucin level was observed in the crypts in response to colonization. Previous studies of symbiont-induced changes in the host have shown that direct interactions with V. fischeri cause the crypt epithelial cells to swell (33) and induce increases in the microvillar densities of these cells (23). Thus, the present study adds to our knowledge concerning the effects that the symbionts have on the host cells with which they directly interact. The function of the mucus in the crypts (e.g., as a nutrient source for the bacteria or as part of a type of host immune response) and whether mucus secretion is coupled to these developmental events remain to be determined.
The mechanism by which V. fischeri changes the secretory activity in the two epithelia of the symbiotic organ, the remote superficial ciliated epithelium and the directly associated crypt epithelium, is also unresolved. One possibility is that the symbionts remotely change the density or activity of host receptors for bacterial signaling molecules. Once the PGN receptors are identified in E. scolopes, whether mucus secretion changes due to alterations in the density of these receptors can be investigated. Another possibility is that the symbionts influence the expression of mucin-encoding genes directly in either or both fields of cells. Bacterium-induced changes in mucus production have been reported previously for both cooperative and pathogenic associations, but most often the bacteria have a direct effect on the host cells (5, 10). A previous study of LPS from the human stomach commensal bacterium Helicobacter pylori showed that while exposure to the LPS of this bacterium initially induced mucus secretion, after prolonged exposure it actually led to concentration-dependent inhibition of secretion (41). Intact, viable H. pylori cells cause similar effects and have also been shown to suppress expression of the mucin-encoding genes in cultured human gastric cells (4, 32). Although V. fischeri LPS does not appear to induce mucus secretion in E. scolopes, it may have an indirect suppressive effect on host cells of the superficial epithelium once the symbiont colonizes the light organ. Other pathogenic bacteria, such as P. aeruginosa, have been reported to cause increases in mucus production by epithelia in the lungs of patients suffering from cystic fibrosis (11), similar to the observed mucus induction of the squid crypt epithelia after colonization by V. fischeri.
The present study demonstrated that a bacterium in a beneficial association is able to alter host mucus production in a variety of ways. An initial nonspecific response of superficial tissues to environmental bacteria and/or their products sets the stage for harvesting of the specific partner. Once colonization by this partner has occurred, the activity of the partner changes mucus production in both remote tissues and the tissues with which they directly interact. Thus, the squid-vibrio symbiosis may be a useful model for studying the role of microbially derived factors in altering mucin synthesis and secretion during the interactions of bacteria with their animal hosts.
Acknowledgments
We thank W. Crookes, M. Goodson, J. Kimbell, T. Koropatnick, E. Ruby, and J. Stewart for helpful discussions and comments on the manuscript. We thank J. Handelsman for donating B. cereus 43-25 and M. Hadfield and C. Unabia for donating a marine Bacillus sp.
This research was funded by a grant from the W. M. Keck Foundation (to M.M.-N. and M. A. A.), by NIH grant RR12294 (to E. G. Ruby and M.M.-N.), by NSF grant IBN 9904601 (to M.M.-N. and E. G. Ruby), and by USDA NRI grant 98-35206-6429 (to H.R.G.).
REFERENCES
- 1.Apicella, M. A., J. M. Griffiss, and H. Schneider. 1994. Isolation and characterization of lipopolysaccharides, lipooligosaccharides and lipid A. Methods Enzymol. 235:242-252. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman, D. D., M. Sathyamoorthy, and S. E. Goldblum. 1998. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J. Biol. Chem. 273:35371-35380. [DOI] [PubMed] [Google Scholar]
- 3.Boettcher, K. J., and E. G. Ruby. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 172:3701-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd, J. C., C. K. Yunker, Q. S. Xu, L. R. Sternberg, and R. S. Bresalier. 2000. Inhibition of gastric mucin synthesis by Helicobacter pylori. Gastroenterology 118:1072-1079. [DOI] [PubMed] [Google Scholar]
- 5.Carnoy, C., R. Ramphal, A. Scharfman, J. Lo-Guidice, N. Houdret, A. Klein, C. Galabert, G. Lamblin, and P. Roussel. 1993. Altered carbohydrate composition of salivary mucins from patients with cystic fibrosis and the adhesion of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 9:323-334. [DOI] [PubMed] [Google Scholar]
- 6.Carson, F. 1990. Histotechnology: a self-instructional text. American Society of Clinical Pathologists Press, Chicago, Ill.
- 7.Chadee, K., K. Keller, J. Forstner, D. J. Innes, and J. I. Ravdin. 1991. Mucin and nonmucin secretagogue activity of Entamoeba histolytica and cholera toxin in rat colon. Gastroenterology 100:986-997. [DOI] [PubMed] [Google Scholar]
- 8.Choi, J., J. Klinkspoor, T. Yoshida, and S. Lee. 1999. Lipopolysaccharide from Escherichia coli stimulates mucin secretion by cultured dog gallbladder epithelial cells. Hepatology 29:1352-1357. [DOI] [PubMed] [Google Scholar]
- 9.Decho, A. 1990. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr. Mar. Biol. Annu. Rev. 28:73-153. [Google Scholar]
- 10.Deplancke, B., and H. R. Gaskins. 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 73:1131S-1141S. [DOI] [PubMed] [Google Scholar]
- 11.Dinwiddie, R. 2000. Pathogenesis of lung disease in cystic fibrosis. Respiration 67:3-8. [DOI] [PubMed] [Google Scholar]
- 12.Dohrman, A., S. Miyata, M. Gallup, J. Li, C. Chapelin, A. Coste, E. Escudier, J. Nadel, and C. Basbaum. 1998. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim. Biophys. Acta 1406:251-259. [DOI] [PubMed] [Google Scholar]
- 13.Doino, J. A., and M. J. McFall-Ngai. 1995. Transient exposure to competent bacteria initiates symbiosis-specific squid light organ morphogenesis. Biol. Bull. 189:347-355. [DOI] [PubMed] [Google Scholar]
- 14.Ducklow, H. 2000. Bacterial production and biomass in the oceans., p. 85-120. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, New York, N.Y.
- 15.Dunn, A. K., and J. Handelsman. 1999. A vector for promoter trapping in Bacillus cereus. Gene 226:297-305. [DOI] [PubMed] [Google Scholar]
- 16.Epple, H. J., K. M. Kreusel, C. Hanski, J. D. Schulzke, E. O. Riecken, and M. Fromm. 1997. Differential stimulation of intestinal mucin secretion by cholera toxin and carbachol. Pflugers Arch. Eur. J. Physiol. 433:638-647. [DOI] [PubMed] [Google Scholar]
- 17.Fleming, T. J., D. E. Wallsmith, and R. S. Rosenthal. 1986. Arthropathic properties of gonococcal peptidoglycan fragments: implications for the pathogenesis of disseminated gonococcal disease. Infect. Immun. 52:600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster, J. S., M. A. Apicella, and M. J. McFall-Ngai. 2000. Vibrio fischeri lipopolysaccharide induces developmental apoptosis, but not complete morphogenesis, of the Euprymna scolopes symbiotic light organ. Dev. Biol. 226:242-254. [DOI] [PubMed] [Google Scholar]
- 19.Graf, J., P. V. Dunlap, and E. G. Ruby. 1994. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol. 176:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graf, J., and E. G. Ruby. 2000. Novel effects of a transposon insertion in the Vibrio fischeri glnD gene: defects in iron uptake and symbiotic persistence, as well as nitrogen utilization. Mol. Microbiol. 37:168-179. [DOI] [PubMed] [Google Scholar]
- 21.Hellman, J., P. Loiselle, M. Tehan, J. Allaire, L. Boyle, J. Kurnick, D. Andrews, K. Kim, and H. Warren. 2000. Outer membrane protein A, peptidoglycan-associated lipoprotein, and murein lipoprotein are released by Escherichia coli bacteria into serum. Infect. Immun. 68:2566-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang, D., G. Liu, A. Lundstrom, E. Gelius, and H. Steiner. 1998. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl. Acad. Sci. USA 95:10078-10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamarcq, L. H., and M. J. McFall-Ngai. 1998. Induction of a gradual, reversible morphogenesis of its host's epithelial brush border by Vibrio fischeri. Infect. Immun. 66:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lencer, W. I., F. D. Reinhart, and M. R. Neutra. 1990. Interaction of cholera toxin with cloned human goblet cells in monolayer culture. Am. J. Physiol. 258:G96-G102. [DOI] [PubMed] [Google Scholar]
- 25.Li, J. D., A. F. Dohrman, M. Gallup, S. Miyata, J. R. Gum, Y. S. Kim, J. A. Nadel, A. Prince, and C. B. Basbaum. 1997. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc. Natl. Acad. Sci. USA 94:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, C., Z. Xu, D. Gupta, and R. Dziarski. 2001. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J. Biol. Chem. 276:34686-34694. [DOI] [PubMed] [Google Scholar]
- 27.Marshall, B., and K. Carroll. 1991. Interaction between Pseudomonas aeruginosa and host defenses in cystic fibrosis. Semin. Respir. Infect. 6:11-18. [PubMed] [Google Scholar]
- 28.McCarthy, M., J. Hedges, and R. Benner. 1998. Major bacterial contribution to dissolved organic nitrogen. Science 281:231-234. [DOI] [PubMed] [Google Scholar]
- 29.McFall-Ngai, M. J. 1999. Consequences of evolving with bacterial symbionts: insights from the squid-vibrio associations. Annu. Rev. Ecol. Syst. 30:235-256. [Google Scholar]
- 30.McFall-Ngai, M. J., and E. G. Ruby. 1991. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial symbiosis. Science 254:1491-1494. [DOI] [PubMed] [Google Scholar]
- 31.Michelsen, K., A. Aichers, M. Mohaupt, T. Hartung, S. Dimmeler, C. Kirschning, and R. Schumann. 2001. The role of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCs). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Biol. Chem. 276:25680-25686. [DOI] [PubMed] [Google Scholar]
- 32.Micots, I., C. Augeron, C. L. Laboisse, F. Muzeau, and F. Megraud. 1993. Mucin exocytosis: a major target for Helicobacter pylori. J. Clin. Pathol. 46:241-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery, M. K., and M. J. McFall-Ngai. 1994. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development 120:1719-1729. [DOI] [PubMed] [Google Scholar]
- 34.Nyholm, S. V., and M. J. McFall-Ngai. 1998. Sampling the light organ microenvironment of Euprymna scolopes: description of a population of host cells in association with the bacterial symbiont Vibrio fischeri. Biol. Bull. 195:89-97. [DOI] [PubMed] [Google Scholar]
- 35.Nyholm, S. V., E. V. Stabb, E. G. Ruby, and M. J. McFall-Ngai. 2000. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. USA 97:10231-10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa, H., Y. Amagai, I. Koike, K. Kaiser, and R. Benner. 2001. Production of refractory dissolved organic matter by bacteria. Science 292:917-920. [DOI] [PubMed] [Google Scholar]
- 37.Ruby, E. G. 1996. Lessons from a cooperative bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu. Rev. Microbiol. 50:591-624. [DOI] [PubMed] [Google Scholar]
- 38.Ruby, E. G., and L. M. Asato. 1993. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 159:160-167. [DOI] [PubMed] [Google Scholar]
- 39.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 40.Singley, C. T. 1982. Histochemistry and fine structure of the ectodermal epithelium of the sepiolid squid Euprymna scolopes. Malacologia 23:177-192. [Google Scholar]
- 41.Slomiany, B. L., Y. H. Liau, R. A. Lopez, J. Piotrowski, A. Czajkowski, and A. Slomiany. 1992. Effect of Helicobacter pylori lipopolysaccharide on the synthesis of sulfated gastric mucin. Biochem. Int. 27:687-697. [PubMed] [Google Scholar]
- 42.Small, A. L., and M. J. McFall-Ngai. 1999. A myeloperoxidase-like protein occurs in both cooperative and pathogenic associations of a single host animal. J. Cell. Biochem. 72:445-457. [PubMed] [Google Scholar]
- 43.Spicer, S., T. Leppi, and P. Stoward. 1965. Suggestions for a histochemical terminology of carbohydrate-rich tissue components. J. Histochem. Cytochem. 13:599-603. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland, I. W. 1989. Microbial polysaccharides—a comparison with eukaryotic polymers, p. 389-402. In E. Chantler and N. A. Ratcliffe (ed.), Mucus and related topics. The Company of Biologists, Ltd., Cambridge, United Kingdom. [PubMed]
- 45.Visick, K. L., J. Foster, J. Doino, M. J. McFall-Ngai, and E. G. Ruby. 2000. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 182:4578-4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visick, K. L., and M. J. McFall-Ngai. 2000. An exclusive contract: specificity in the Vibrio fischeri-Euprymna scolopes partnership. J. Bacteriol. 182:1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weis, V. M., A. L. Small, and M. J. McFall-Ngai. 1996. A peroxidase related to the mammalian antimicrobial protein myeloperoxidase in the Euprymna-Vibrio mutualism. Proc. Natl. Acad. Sci. USA 93:13683-13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshimura, A., E. Lien, R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 49.Zychlinsky, A., K. Thirumalai, J. Arondel, J. R. Cantey, A. O. Aliprantis, and P. J. Sansonetti. 1996. In vivo apoptosis in Shigella flexneri infections. Infect. Immun. 64:5357-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]