Abstract
Fourier-transform infrared (FT-IR) microspectroscopy was used in this study to identify yeasts. Cells were grown to microcolonies of 70 to 250 μm in diameter and transferred from the agar plate by replica stamping to an IR-transparent ZnSe carrier. IR spectra of the replicas on the carrier were recorded using an IR microscope coupled to an IR spectrometer, and identification was performed by comparison to reference spectra. The method was tested by using small model libraries comprising reference spectra of 45 strains from 9 genera and 13 species, recorded with both FT-IR microspectroscopy and FT-IR macrospectroscopy. The results show that identification by FT-IR microspectroscopy is equivalent to that achieved by FT-IR macrospectroscopy but the time-consuming isolation of the organisms prior to identification is not necessary. Therefore, this method also provides a rapid tool to analyze mixed populations. Furthermore, identification of 21 Debaryomyces hansenii and 9 Saccharomyces cerevisiae strains resulted in 92% correct identification at the strain level for S. cerevisiae and 91% for D. hansenii, which demonstrates that the resolution power of FT-IR microspectroscopy may also be used for yeast typing at the strain level.
Traditional identification of yeasts is achieved by applying physiological and morphological tests, which determine enzyme production profiles and growth characteristics (5, 16). Various rapid and in some cases automated identification systems for routine analyses of yeasts are commercially available and easy to use. However, identification results are questionable to a certain degree because these systems originally were developed for clinical application and databases therefore do not include an adequate number of common environmental yeast species (10, 27). In recent years many techniques that identify yeasts according to the fatty acid composition of the cell membrane (3, 6) or genotypic characteristics such as temperature gradient gel electrophoresis (14), electrophoretic karyotyping (28), restriction fragment length polymorphism (12, 25) and randomly amplified polymorphic DNA (1, 4, 25) restriction enzyme analysis of PCR-amplified rDNA (11) or mitochondrial DNA (24), RNA probes (15, 18, 23), and rDNA sequencing (7, 29) have been developed. However, their application in the routine analysis of yeasts in the food industry is limited by their high cost and the requirement for highly skilled personnel.
A rapid and very inexpensive method to identify microorganisms is Fourier-transform infrared (FT-IR) spectroscopy (13, 19). Absorption of infrared light by cellular compounds results in a fingerprint-like spectrum that can be identified by comparison to reference spectra. Once an extensive and well designed database of reference spectra is established, reliable identification results are obtained within 25 h of starting the identification from a single colony (17, 21, 22).
FT-IR microspectroscopy is a novel tool to characterize microorganisms (20). Microcolonies grown on a solid medium are transferred from the agar plate to a ZnSe carrier by means of a stamping device, resulting in spatially accurate replicas. Air-dried colonies on the ZnSe carrier are detected using a computer-driven xy stage and a light microscope, and spectra of single colonies are recorded by a mid-IR spectrometer coupled to the microscope. Isolation and purification of the organisms to be measured are not necessary in this case, provided that the microcolonies are well separated, which permits identification from food or an environmental sample within 1 day.
The practical applicability of this technique for the identification of microbes, however, has never been demonstrated. The aim of this work was to develop a standardized procedure for cultivation and sample preparation for the identification of food-borne yeasts and to compare this approach to FT-IR macrospectroscopy.
MATERIALS AND METHODS
Microorganisms.
A total of 63 yeast strains, 48 from international culture collections and 15 from the Weihenstephan yeast collection, covering 9 genera and 13 species were used as reference strains (Table 1).
TABLE 1.
Yeast species included in the database of FT-IR reference spectra
| Species | Straina | Origin |
|---|---|---|
| Candida intermedia | DSM 70753 | Pilsener beer |
| Candida intermedia | CBS 572 (T) | Feces |
| Candida intermedia | CBS 5310 | Grape |
| Candida parapsilosis | DSM 5784 (T) | Sprue |
| Candida parapsilosis | DSM 70125 | Sausage |
| Candida parapsilosis | CBS 1954 | Olives |
| Candida tropicalis | DSM 1346 | |
| Candida tropicalis | CBS 94 (T) | Bronchitic patient |
| Candida tropicalis | CBS 2310 | |
| Clavispora lusitaniae | CBS 1944 | Sputum |
| Clavispora lusitaniae | CBS 5299 | Milk of mastitic cow |
| Clavispora lusitaniae | CBS 6936 (T) | Citrus essence |
| Debaryomyces hansenii | CBS 941 | Rancid butter |
| Debaryomyces hansenii | CBS 767 (T) | Carlsberg laboratory |
| Debaryomyces hansenii | CBS 1099 | Cheese |
| Debaryomyces hansenii | CBS 6960 | Organ of cow |
| Debaryomyces hansenii | DSM 70238 | Sausage |
| Debaryomyces hansenii | DSM 70244 | Beer |
| Debaryomyces hansenii | CBS 789 | Interdigital mycotic lesion |
| Debaryomyces hansenii | WSYC 482 | Dairy |
| Debaryomyces hansenii | WSYC 483 | Dairy |
| Debaryomyces hansenii | WSYC 484 | Dairy |
| Debaryomyces hansenii | WSYC 485 | Dairy |
| Debaryomyces hansenii | WSYC 486 | Dairy |
| Debaryomyces hansenii | WSYC 487 | Dairy |
| Debaryomyces hansenii | WSYC 488 | Dairy |
| Debaryomyces hansenii | WSYC 489 | Dairy |
| Debaryomyces hansenii | WSYC 490 | Cheese starter |
| Debaryomyces hansenii | WSYC 491 | Yoghurt |
| Debaryomyces hansenii | WSYC 492 | Dairy |
| Debaryomyces hansenii | WSYC 493 | Dairy |
| Debaryomyces hansenii | WSYC 494 | Dairy |
| Debaryomyces hansenii | WSYC 495 | Dairy |
| Hanseniaspora uvarum | CBS 312 | Fermenting cacao |
| Hanseniaspora uvarum | CBS 314 (T) | Grapes |
| Hanseniaspora uvarum | CBS 5074 | Apple must |
| Kluyveromyces marxianus | CBS 397 | Yoghurt |
| Kluyveromyces marxianus | DSM 70344 | Yoghurt |
| Kluyveromyces marxianus | CBS 6432 | |
| Pichia anomala | CBS 5759 (T) | |
| Pichia anomala | DSM 70130 | Brewery |
| Pichia anomala | CBS 249 | Berries |
| Pichia guillermondii | DSM 6381 | Sputum |
| Pichia guillermondii | DSM 70052 | Butter |
| Pichia guillermondii | CBS 2030 (T) | Insect frass on Ulmus americana |
| Pichia membranaefaciens | CBS 1328 | Koumiss |
| Pichia membranaefaciens | DSM 70631 | Brewery |
| Pichia membranaefaciens | CBS 107 (T) | Elm exudate |
| Rhodotorula mucilaginosa | CBS 2395 | Butter starter |
| Rhodotorula mucilaginosa | CBS 316 (T) | |
| Rhodotorula mucilaginosa | CBS 2772 | Yeast infusion |
| Saccharomyces cerevisiae | DSM 1333 | Distillery yeast |
| Saccharomyces cerevisiae | DSM 70449 (T) | Beer |
| Saccharomyces cerevisiae | DSM 70470 | |
| Saccharomyces cerevisiae | DSM 70471 | |
| Saccharomyces cerevisiae | DSM 70478 | Palm wine |
| Saccharomyces cerevisiae | DSM 70509 | |
| Saccharomyces cerevisiae | DSM 70514 | Grape must |
| Saccharomyces cerevisiae | WSYC 220 | |
| Saccharomyces cerevisiae | ATCC 9763 | Distillery yeast |
| Torulaspora delbrueckii | CBS 1146 (T) | |
| Torulaspora delbrueckii | CBS 6795 | Juice |
| Torulaspora delbrueckii | DSM 70526 | Marzipan |
ATCC, American Type Culture Collection, Manassas, Va., CBS, Cenraalbureau voor Schimmelcultures, Delft, The Netherlands; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany; WSYC, Weihenstephan Yeast Collection, Institut für Mikrobiologie, FML Weihenstephan, Freising, Germany; T, Type strain.
Sample preparation.
Sample preparation and data acquisition for FT-IR macrospectroscopy were performed as described by Kümmerle et al. (17). For FT-IR microspectroscopy, one heaped platinum loop of yeast cells was suspended in 100 μl of water and a dilution series was done. Dilutions were plated on YGC agar (Merck, Darmstadt, Germany) and incubated for 24 ± 0.5 h at 25 ± 2°C. A few strains which had grown slowly were incubated until the colony diameter on the stamp reached 70 to 90 μm. The first cell layers of the microcolonies were transferred by replica stamping to an infrared transparent ZnSe carrier (25 mm in diameter) and air dried for approximately 10 min.
FT-IR microspectroscopy.
Spectra from microcolonies 70 to 250 μm in diameter were recorded in transmission between wavenumbers of 4,000 to 500 cm−1 using the IRscope II coupled with the IFS 28B FT-IR spectrometer (both from Bruker, Karlsruhe, Germany). The following measurement parameters were used: 6-cm−1 resolution, 20-kHz scan speed, Blackman-Harris 3-Term apodization, zerofilling of 2. For each spectrum, 128 interferograms were averaged. To cover variations between different sample preparations, each strain was measured three times independently and three to six spectra from different colonies were recorded each time. Three representative spectra, one from each measurement, were then averaged. Data processing was performed using OPUS 3.1 software for windows NT (Bruker). Second derivatives of the spectra were found to yield the best identification results and were smoothed to diminish the effects of baseline shifts and to enhance the resolution of complex bands. Three spectral windows, from 3,030 to 2,830, 1,350 to 1,200, and 900 to 700 cm−1, were chosen for identification. Dissimilarity between spectra is expressed as the spectral distance value (SD) which reflects the nonoverlapping areas of the spectra.
Testing of the database.
To assess the accuracy of an identification, the database has to be tested. In our case, the average spectrum of one strain was excluded from the database and was then compared with the spectrum library containing the remaining spectra. If the first hit belonged to an average spectrum of the same species and had an SD of ≤1.0, the result was counted as a correct identification at the species level. If the first hit belonged to a spectrum of the same genus but to a different species, identification was only at the genus level. Identifications with an SD of >1.0 in the first hit were counted as not identified. Results with incorrect identification in the first hit were noted as misidentifications. A total of 45 strains were tested by this procedure. Furthermore, the improvement in the correctness of identification due to increasing numbers of strains per species included in the database was investigated by using Debaryomyces hansenii strains. Test sets comprising 3, 4, 6, 9, 12, 15, 18, and 21 strains of D. hansenii in 10 different, randomly chosen combinations were included in the library. One D. hansenii strain was then excluded and identified against the spectral library containing the remaining spectra. This was done for all strains of each test set. The results were averaged to obtain the mean correctness of identification for both the whole library and the test sets.
RESULTS AND DISCUSSION
Influence of colony shape and colony size.
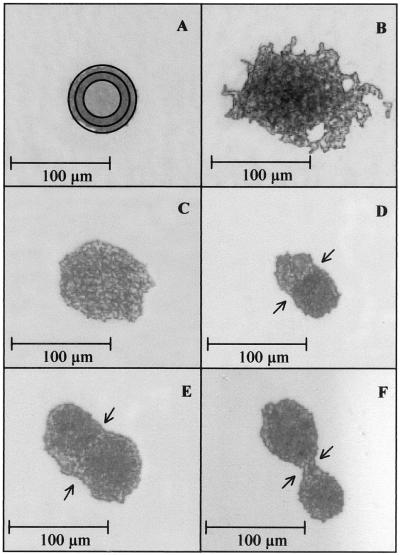
After replica stamping, different colony shapes occur on the ZnSe carrier. Figure 1 presents examples. The appearance of the colonies on the ZnSe window is strain dependent.
FIG. 1.
Shapes of microcolonies of different yeast species after replica stamping. (A) Torulaspora delbrueckii. Different apertures are indicated. While recording spectra, the field around the colony has to be covered by an aperture in order to measure only the sample. For this purpose, apertures of different sizes are implemented. The bright central area is left uncovered during measurement. (B) Candida intermedia. (C) Rhodotorula mucilaginosa. (D) R. mucilaginosa and T. delbrueckii growing into each other. (E) Two colonies of R. mucilaginosa growing into each other. (F) Two colonies of D. hansenii.
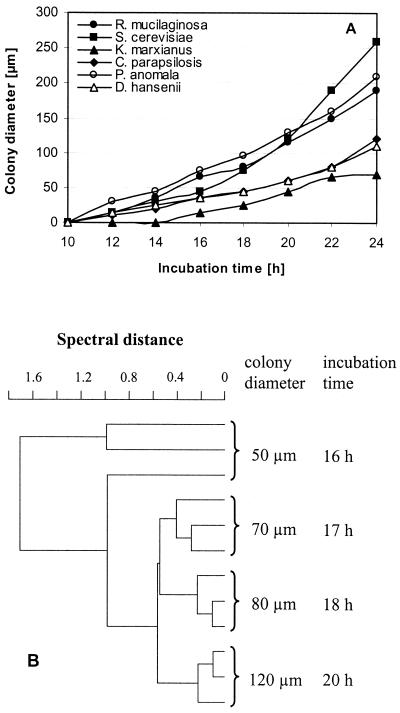
First, a standard protocol for sample preparation was developed. Cultivation parameters of the organisms strongly influence FT-IR spectra and, for that reason, must be strictly standardized. The age of the colonies, as reflected by the diameter of the microcolonies, was found to be an important parameter. As can be seen in Fig. 2A, the growth rates of six different yeast species are very different. Saccharomyces cerevisiae has a colony diameter on the stamp of more than 250 μm after 24 h of incubation, whereas Kluyveromyces marxianus reaches only 70 μm in the same time. Figure 2B displays the reproducibility of spectra depending on the colony size. The reproducibility of the spectra is enhanced by increasing diameter. There may be several reasons for this. First, cells on the surface of bigger colonies are entering stationary phase and may be more similar in their composition, which compares well with the findings of Choo-Smith et al. (8), who have shown that 24-h-old microcolonies of Escherichia coli can be divided into three layers from the bottom to the surface by Raman spectroscopy, with each layer most probably representing cells of different physiological state. Variance among spectra of the top layer is small compared to those in each of the deeper layers. Furthermore, slight differences between cells may be averaged over a larger number of cells. In addition, a larger aperture (Fig. 1A) can be chosen and therefore the signal intensity is increased. Assuming that an SD of <0.5 between spectra of the same colony diameter is required to yield a good reproducibility, a colony diameter of at least 70 μm is necessary. The K. marxianus strain tested in this experiment needed 24 h of growth at 25°C to reach this colony diameter, and slow-growing strains of other species may need the same time whereas fast-growing strains will exceed this size by far. Since the same standardized cultivation conditions for all yeast species are desirable, organisms were incubated for 24 h at 25°C and colonies were transferred by replica stamping and measured regardless of the diameter, provided that they had reached 70 μm. This ensures coverage of slow- as well as fast-growing yeast strains by the same procedure, which is of major importance for the analysis of mixed populations.
FIG. 2.
(A) Colony diameter of strains of six different yeast species after transfer to the ZnSe carrier, determined at different growth times. The organisms were incubated at 25°C. (B) Dendrogram of microcolonies of S. cerevisiae recorded at different growth times. Spectral ranges: 3,030 to 2,830 cm−1, 1,350 to 1,200 cm−1, and 900 to 700 cm−1. Average linkage, correlation with normalization to reprolevel (OPUS/Ident Handbook, Bruker Optik GmbH, Karlsruhe, Germany, 1995).
Testing of the database.
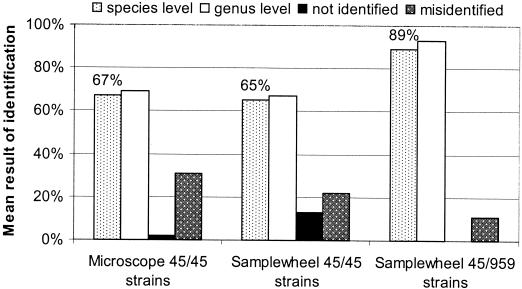
To investigate the potential of identification by FT-IR microspectroscopy, two model spectral libraries, one using FT-IR microspectroscopy and one using FT-IR macrospectroscopy were generated, comprising the average spectra of 45 yeast strains representing 9 genera and 13 species (Table 1). Both libraries were tested as described in Materials and Methods. FT-IR microspectroscopy identified 67% of the strains correctly at the species level, and FT-IR macrospectroscopy identified 65% (Fig. 3). Larger differences between the two methods were found in the number of false or unidentified spectra. Whereas the microscope misidentified 31% of the strains, the samplewheel method misidentified 22% of the spectra. Evaluation is highly dependent on the composition of the database. Except for S. cerevisiae, all species were represented by three strains only, and one of those, in addition, was excluded from the library to be identified. Especially for Clavispora lusitaniae and D. hansenii, identification by FT-IR microspectroscopy proved difficult. None of the three strains, could be identified correctly. Including more strains of the same species in the library will help to overcome these difficulties because new strains will close gaps between the strains included. This was demonstrated by increasing the number of D. hansenii strains in the database from 3 up to 21. The mean identification for 10 randomly chosen combinations of test sets comprising 3, 4, 6, 9, 12, 15, 18, and 21 strains each was calculated. The addition of more strains per species to the database led to an increased correctness of identification from 37% for three strains to 76% for 12 strains and 81% for 21 strains. Standard deviations were extremely high for identifications with few strains but decreased when the number of strains was increased. Similar findings were observed by Oberreuter et al. (22) for the identification of coryneform bacteria by FT-IR macrospectroscopy. Nevertheless, identification of D. hansenii was still difficult, since it is a relatively heterogenous species (Fig. 4) and there are some morphologically, physiologically and spectroscopically similar species, e.g., C. lusitaniae, Candida parapsilosis, and Candida intermedia.
FIG. 3.
External validation of databases containing 45 or 959 average reference spectra recorded with FT-IR microspectroscopy (microscope) and FT-IR macrospectroscopy (samplewheel). If the first hit belonged to an average spectrum of the same species and had an SD of ≤1.0, the result was counted as a correct identification at the species level. If the first hit belonged to a spectrum of the same genus but to a different species, identification was correct at the genus level. Identifications with SD of >1.0 in the first hit were counted as not identified. Results with incorrect identification in the first hit were noted as misidentifications. Abbreviations: 45/45, database consists of 45 average spectra and all 45 spectra were subjected to the external validation; 45/959, database consists of 959 average spectra and 45 spectra were identified against this database.
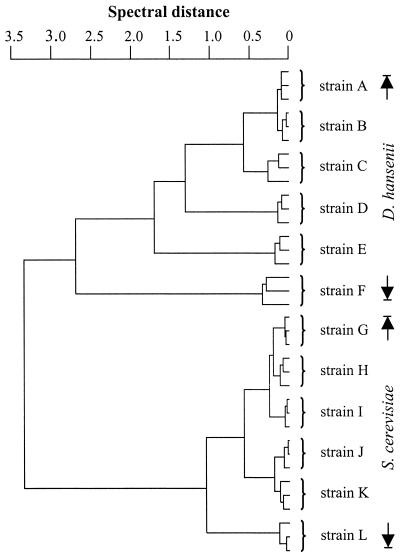
FIG. 4.
Dendrogram of six different D. hansenii (A to F) and six S. cerevisiae (G to L) strains, each measured three times independently by FT-IR microspectroscopy.
An extended database consisting of 959 yeast reference spectra has been established over the years in our laboratory for FT-IR macrospectroscopy. The 45 strains of the original model library were identified against this database and yielded 89% correct identifications at the species level (Fig. 3). Considering that the microscope obtained similar results to FT-IR macrospectroscopy with the small libraries, this is what we expect microspectroscopy to be capable of at least. Other systems for routine yeast identification using physiological methods obtain the following percentages of correct identifications: less than 20% with the API YEAST IDENT (27), 12 to 35% with the API 20C (2, 9, 27), 53 to 76% with the API ATB 32C system (9, 26), 40% with MicroScan (10), ∼70 to 91% with the SIM (9, 27), 74% by applying keys and descriptions by Barnett (2), and ∼80% applying the identification scheme of Kreger-van Rij (27). On the basis of these data, FT-IR microspectroscopy is most probably going to become a very good alternative to automated test systems.
Typing of yeast strains using FT-IR microspectroscopy.
Differentiation among various strains is particularly important for the analysis of contamination routes and the control of production organisms. Traditional identification methods based on morphological and physiological tests, however, are not suitable for strain identification. The strains of two yeast species, D. hansenii and S. cerevisiae, were subjected to typing by FT-IR microspectroscopy. Figure 4 demonstrates that multiple measurements of individual D. hansenii and S. cerevisiae strains cluster together. In each case, repeated measurements of the same strain form a subcluster which is clearly separated from subclusters of other strains.
Since spectra of D. hansenii strains show less similarity than those of S. cerevisiae strains (Fig. 4), we expected them to give better identification results than S. cerevisiae at the strain level. However, the mean strain identification rate was 92% for S. cerevisiae and 91% for D. hansenii. These data demonstrate that FT-IR microspectroscopy is a promising tool for the identification of yeasts even to the level of individual strains.
Time cost of FT-IR macro- and microspectroscopy.
The cultivation and sample preparation procedures of FT-IR macro- and microspectroscopy are different. Starting with an unknown sample, organisms have to be isolated prior to measurement by FT-IR macrospectroscopy which takes 2 days and requires an additional purification streak to ensure that a pure culture is present. A single colony from the pure culture is then subjected to the sample preparation procedure described by Kümmerle et al. (17). These three steps are combined in one step applying FT-IR microspectroscopy. After dilution of the sample, organisms are grown to microcolonies and directly transferred from the agar plate to the IR-transparent ZnSe carrier. While marking measurement positions using the light microscope, colonies that are positioned very close together and would probably appear as one colony after 2 days of incubation can be detected (Fig. 1D to F) and excluded. For this reason, further purification of the colonies is not necessary, enabling microbiologists to obtain identification results after 24 h only, compared to 5 days when using FT-IR macrospectroscopy.
Acknowledgments
This work was supported by the FEI (Forschungskreis der Ernährungsindustrie e.V., Bonn, Germany), the AiF (Arbeitskreis für industrielle Forschung), and the Ministry of Economics and Technology (project 12634N).
REFERENCES
- 1.Andrighetto, C., E. Psomas, N. Tzanetakis, G. Suzzi, and A. Lombardi. 2000. Randomly amplified polymorphic DNA (RAPD) PCR for the identification of yeasts isolated from dairy products. Lett. Appl. Microbiol. 30:5-9. [DOI] [PubMed] [Google Scholar]
- 2.Arias, C. R., J. K. Burns, L. M. Friedrich, R. M. Goodrich, and M. E. Parish. 2002. Yeast species associated with orange juice: evaluation of different identification methods. Appl. Environ. Microbiol. 68:1955-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustyn, O. P. H., J. L. F. Kock, and D. Ferreira. 1992. Differentiation between yeast species and strains within a species by cellular fatty acid analysis. 5. A feasible technique? Syst. Appl. Microbiol. 15:105-115. [Google Scholar]
- 4.Baleiras Couto, M. M., J. M. van der Vossen, H. Hofstra, and J. H. Huis in't Veld. 1994. RAPD analysis: a rapid technique for differentiation of spoilage yeasts. Int. J. Food Microbiol. 24:249-260. [DOI] [PubMed] [Google Scholar]
- 5.Barnett, J. A., R. W. Payne, and D. Yarrow. 2000. Yeasts: characteristics and identification. Cambridge University Press, Cambridge, United Kingdom.
- 6.Botha, A., and J. L. Kock. 1993. Application of fatty acid profiles in the identification of yeasts. Int. J. Food Microbiol. 19:39-51. [DOI] [PubMed] [Google Scholar]
- 7.Cappa, F., and P. S. Cocconcelli. 2001. Identification of fungi from dairy products by means of 18S rRNA analysis. Int. J. Food Microbiol. 69:157-160. [DOI] [PubMed] [Google Scholar]
- 8.Choo-Smith, L. P., K. Maquelin, T. van Vreeswijk, H. A. Bruining, G. J. Puppels, N. A. Ngo Thi, C. Kirschner, D. Naumann, D. Ami, A. M. Villa, F. Orsini, S. M. Doglia, H. Lamfarraj, G. D. Sockalingum, M. Manfait, P. Allouch, and H. P. Endtz. 2001. Investigating microbial (micro)colony heterogeneity by vibrational spectroscopy. Appl. Environ. Microbiol. 67:1461-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deak, T., and L. R. Beuchat. 1993. Comparison of the SIM, API 20C, and ID 32C systems for identification of yeasts isolated from fruit juice concentrates and beverages. J. Food Prot. 56:585-592. [DOI] [PubMed] [Google Scholar]
- 10.Deak, T., and L. R. Beuchat. 1995. Evaluation of the MicroScan enzyme-based system for the identification of foodborne yeasts. J. Appl. Bacteriol. 79:439-446. [DOI] [PubMed] [Google Scholar]
- 11.Dlauchy, D., J. Tornai Lehoczki, and G. Peter. 1999. Restriction enzyme analysis of PCR amplified rDNA as a taxonomic tool in yeast identification. Syst. Appl. Microbiol. 22:445-453. [DOI] [PubMed] [Google Scholar]
- 12.Esteve-Zarzoso, B., C. Belloch, F. Uruburu, and A. Querol. 1999. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 49:329-337. [DOI] [PubMed] [Google Scholar]
- 13.Helm, D., H. Labischinski, G. Schallehn, and D. Naumann. 1991. Classification and identification of bacteria by Fourier-transform infrared spectroscopy. J. Gen. Microbiol. 137:69-79. [DOI] [PubMed] [Google Scholar]
- 14.Hernan-Gomez, S., J. C. Espinosa, and J. F. Ubeda. 2000. Characterization of wine yeasts by temperature gradient gel electrophoresis (TGGE). FEMS Microbiol. Lett. 193:45-50. [DOI] [PubMed] [Google Scholar]
- 15.Kosse, D., H. Seiler, R. Amann, W. Ludwig, and S. Scherer. 1997. Identification of yoghurt-spoiling yeasts with 18S rRNA-targeted oligonucleotide probes. Syst. Appl. Microbiol. 20:468-480. [Google Scholar]
- 16.Kreger-van Rij, N. J. W. 1984. The yeasts—a taxonomic study. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 17.Kümmerle, M., S. Scherer, and H. Seiler. 1998. Rapid and reliable identification of food-borne yeasts by Fourier-transform infrared spectroscopy. Appl. Environ. Microbiol. 64:2207-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lischewski, A., R. I. Amann, D. Harmsen, H. Merkert, J. Hacker, and J. Morschhauser. 1996. Specific detection of Candida albicans and Candida tropicalis by fluorescent in situ hybridization with an 18S rRNA-targeted oligonucleotide probe. Microbiology 142:2731-2740. [DOI] [PubMed] [Google Scholar]
- 19.Naumann, D., D. Helm, and H. Labischinski. 1991. Microbiological characterizations by FT-IR spectroscopy. Nature 351:81-82. [DOI] [PubMed] [Google Scholar]
- 20.Ngo Thi, N. A., C. Kirschner, and D. Naumann. 2000. FT-IR microspectrometry: a new tool for characterizing micro-organisms, p. 36-44. In G. J. Puppels (ed.), Biomedical spectroscopy: vibrational spectroscopy and other novel techniques. Proceedings of SPIE, vol. 3918. Society of Photo-Optical Engineers, Bellingham, Wash.
- 21.Oberreuter, H., J. Charzinski, and S. Scherer. 2002. Intraspecific diversity of Brevibacterium linens, Corynebacterium glutamicum and Rhodococcus erythropolis based on partial 16S rDNA sequence analysis and Fourier-transform infrared (FT-IR) spectroscopy. Microbiology 148:1523-1532. [DOI] [PubMed] [Google Scholar]
- 22.Oberreuter, H., H. Seiler, and S. Scherer. 2002. Identification of coryneform bacteria and related taxa by Fourier-transform infrared (FT-IR) spectroscopy. Int. J. Syst. Evol. Microbiol. 52:91-100. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira, K., G. Haase, C. Kurtzman, J. J. Hyldig-Nielsen, and H. Stender. 2001. Differentiation of Candida albicans and Candida dubliniensis by fluorescent in situ hybridization with peptide nucleic acid probes. J. Clin. Microbiol. 39:4138-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Querol, A., E. Barrio, and D. Ramon. 1992. A comparative study of different methods of yeast strain characterization. Syst. Appl. Microbiol. 15:439-446. [Google Scholar]
- 25.Romano, A., S. Casaregola, P. Torre, and C. Gaillardin. 1996. Use of RAPD and mitochondrial DNA RFLP for typing of Candida zeylanoides and Debaryomyces hansenii yeast strains isolated from cheese. Syst. Appl. Microbiol. 19:255-264. [Google Scholar]
- 26.Tornadijo, M. E., J. M. Fresno, R. M. Sarmiento, and J. Carballo. 1997. Comparison of the classical methods and the API ATB 32C system in the identification of yeasts isolated from goat cheese. Food Res. Int. 30:653-658. [Google Scholar]
- 27.Török, T., and A. D. King, Jr. 1991. Comparative study on the identification of food-borne yeasts. Appl. Environ. Microbiol. 57:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Török, T., D. Rockhold, and A. D. King, Jr. 1993. Use of electrophoretic karyotyping and DNA-DNA hybridization in yeast identification. Int. J. Food Microbiol. 19:63-80. [DOI] [PubMed] [Google Scholar]
- 29.Valente, P., J. P. Ramos, and O. Leoncini. 1999. Sequencing as a tool in yeast molecular taxonomy. Can. J. Microbiol. 45:949-958. [DOI] [PubMed] [Google Scholar]