Abstract
Deletions were made in Streptomyces lividans in either of two genes (zwf1 and zwf2) encoding isozymes of glucose-6-phosphate dehydrogenase, the first enzyme in the oxidative pentose phosphate pathway (PPP). Each mutation reduced the level of Zwf activity to approximately one-half that observed in the wild-type strain. When the mutants were transformed with multicopy plasmids carrying the pathway-specific transcriptional activator genes for either the actinorhodin (ACT) or undecylprodigiosin (RED) biosynthetic pathway, they produced higher levels of antibiotic than the corresponding wild-type control strains. The presumed lower flux of carbon through the PPP in each of the Δzwf mutants may allow more efficient glucose utilization via glycolysis, resulting in higher levels of antibiotic production. This appears to occur without lowering the concentration of NADPH (the major biochemical product of the oxidative PPP activity) to a level that would limit antibiotic biosynthesis. Consistent with this hypothesis, deletion of the gene (devB) encoding the enzyme that catalyzes the next step in the oxidative PPP (6-phosphogluconolactonase) also resulted in increased antibiotic production. However, deletion of both zwf genes from the devB mutant resulted in reduced levels of ACT and RED production, suggesting that some of the NADPH made by the PPP is utilized, directly or indirectly, for antibiotic biosynthesis. Although applied here to the model antibiotics ACT and RED, such mutations may prove to be useful for improving the yield of commercially important secondary metabolites.
The emergence of multiply antibiotic-resistant human pathogens has resulted in an urgent need for new antibiotics. Actinomycetes produce approximately two-thirds of all known antibiotics of microbial origin, including over 6,000 different chemical structures, and they continue to be an excellent source of novel compounds. Many of these natural products are commercially important medicinal compounds with a variety of therapeutic uses. Frequently, antibiotics are grouped on the basis of common or similar biosynthetic pathways (e.g., members of the polyketide, polyether, macrolide, and β-lactam families), and they are often produced from the same primary metabolic precursors.
One of the hurdles in the development of a newly discovered natural product as an antibiotic or in the development of a novel antibiotic made by combinatorial biosynthesis (16) is the ability to generate sufficient quantities of the compound for further study. Genetic manipulation of a producing organism can be used to improve the efficiency of production, which is classically achieved by empirical means but more recently has been conducted in a knowledge-based manner (8). One potentially important element in productivity is the availability of precursors and cofactors.
We chose to study Streptomyces lividans 66 strains containing multicopy plasmids carrying the pathway-specific transcriptional activator gene for either actinorhodin (ACT) (pIJ68) (30) or undecylprodigiosin (RED) (pIJ6014) (38). These strains each produced copious amounts of only one antibiotic (either ACT or RED), and they were presumed to contain such high levels of the pathway-specific activators that the antibiotic biosynthetic enzymes should not limit metabolic flux. Rather, it was assumed that the supply of metabolic precursors for antibiotic biosynthesis was limiting and might be manipulated to improve the antibiotic production capability.
Most antibiotic biosynthetic pathways involve some reductive steps in which NADPH is used as an essential cofactor. In most cells the majority of NADPH is produced by the activity of two enzymes of the pentose phosphate pathway (PPP), glucose-6-phosphate dehydrogenase (G6PDH) (Zwf) and 6-phosphogluconate dehydrogenase (6PGDH) (36). However, in S. lividans 6PGDH was reported to use NAD+ in preference to NADP+ as a cofactor (12). In Streptomyces antibioticus, glucose is catabolized by both the PPP and glycolysis, and the PPP is the dominant pathway during exponential growth (34). Of particular relevance to these studies is the fact that a positive correlation was observed between methylenomycin production and carbon flux through the PPP in Streptomyces coelicolor (26). This suggested that it might be possible to enhance antibiotic production by increasing the supply of NADPH by elevating the activity of Zwf. To address this possibility, deletions were made in the zwf genes of S. lividans in order to assess the contribution of the PPP to ACT and RED biosynthesis in derivatives of a strain that had been engineered to overproduce each of the antibiotics. Once the expected reduction in antibiotic biosynthesis had been observed in the zwf mutants, elevated levels of NADPH could then be engineered by overexpression of the appropriate genes, with the expectation that this would result in enhanced antibiotic production. S. coelicolor is closely related to S. lividans, and some primary metabolic genes show high levels of nucleotide sequence identity (e.g., glkA, encoding glucose kinase, shows 99.8% identity over 904 nucleotides [22]). Two clusters of genes encoding PPP enzymes were identified during the S. coelicolor genome sequencing project (http://www.sanger.ac.uk/Projects/S_coelicolor/; accession numbers AL031107 and AL096839). The genes appear to be organized into operons with many of the predicted translational initiation and termination sites either overlapping or lying extremely close together, suggesting that many of the genes are translationally coupled. Both clusters contain genes for transketolase (tkt) and transaldolase (tal) that lie 5′ of the zwf genes. The zwf genes (zwf1and zwf2) of each cluster appear to be translationally coupled with downstream opc genes that are essential for Zwf activity in both cyanobacteria (14, 37) and Corynebacterium glutamicum (25). The deduced amino acid sequences of Zwf1 and Zwf2 from S. coelicolor are very similar (85% identity), whereas the two Zwf proteins in Mycobacterium tuberculosis (10) show only 50% identity to each other. Both S. coelicolor Zwf proteins are markedly more similar to M. tuberculosis Zwf2 (75 and 77% identity for zwf1 and zwf2, respectively) than to Zwf1 (51 and 49% identity, respectively). The product of devB, which lies immediately downstream of opc in the zwf2 gene cluster, shows homology to an additional N-terminal domain found in a mammalian G6PDH. This domain is not directly involved in G6PDH catalytic activity but has recently been shown to encode the 6-phosphogluconolactonase (15). This enzyme catalyzes hydrolysis of the lactone (which undergoes slow spontaneous hydrolysis in the absence of the lactonase) to produce the substrate for the 6PGDH (Fig. 1).
FIG. 1.
Overview of central carbon metabolism, showing the early steps of the PPP. Genes encoding the enzymes relevant to this work are shown. 6PGA, 6-phosphogluconate; ksp, spontaneous hydrolysis of 6-phospho-δ-gluconolactone (6PGL); G6P, glucose-6-phosphate; TCA, tricarboxylic acid; CoA, coenzyme A.
MATERIALS AND METHODS
Bacterial strains, media, and plasmids.
Escherichia coli strains DH5α (35) and ET12567 (21) were used for routine subcloning and conjugation into S. lividans, respectively. Organisms were grown at 37°C in Luria-Bertani medium, and for transformation we used standard procedures (35). E. coli transformants were selected with carbenicillin (100 μg · ml−1), apramycin (50 μg · ml−1), or hygromycin (50 μg · ml−1). The cloning vectors used were pIJ2925, pIJ2920 (18), pKC1132 (3), and pGEM-T (Promega). S. lividans 66 (John Innes Centre strain 1326) and S. coelicolor M145 were grown and manipulated as described previously (18). For conjugation from E. coli we used helper plasmid pUZ8002 (27). S. lividans exconjugants were selected by adding 0.5 mg of nalidixic acid and 1 mg of apramycin or 1 mg of hygromycin to each agar plate. Strains were purified and maintained by using 20 μg of apramycin per ml or 20 μg of hygromycin per ml.
For shake flask fermentation experiments we used a 50-ml seed stage culture containing a mixture of 2 parts of yeast extract-malt extract medium and 1 part of Trypticase soy broth containing thiostrepton at a concentration of 50 μg · ml−1. Spores were inoculated directly into 250-ml conical spring flasks containing this medium and were incubated with shaking at 30°C for 40 to 44 h. Mycelium was collected by centrifugation and resuspended in a liquid minimal medium (6) containing 25 g of glucose per liter and 25 μg of thiostrepton per ml. The cultures were fermented for up to 7 days and monitored daily until antibiotic accumulation ceased.
For stirred-tank fermentation experiments, strains were stored as frozen mycelium at −80°C. The stock cultures were used to inoculate (1%, vol/vol) 100-ml portions of GG1 medium (15 g of glucose per liter, 15 g of glycerol per liter, 15 g of soy peptone per liter, 3 g of NaCl per liter, l g of CaCO3 per liter, 0.005 g of thiostrepton per liter) in 500-ml baffled Erlenmeyer flasks. Cultures were grown for 48 h at 28°C on a rotary shaker at 200 rpm. Then 8 ml of each culture was used to inoculate 100 ml of GYB medium (33 g of glucose per liter, 15 g of yeast extract per liter, 0.005 g of thiostrepton per liter) in a 500-ml baffled flask. The cultures were grown for 24 h at 28°C on a rotary shaker at 200 rpm, and 100-ml portions were used to inoculate 4-liter fermentors containing 2.5 liters of phosphate-limited Evans medium.
PCR conditions.
Appropriate oligonucleotide primers were used with the ExpandHF PCR system (Boehringer). After an initial denaturation step (3 min at 96°C), 20 cycles of amplification (1 min at 94°C, 1 min at 50°C, 1 min per kb of DNA at 68°C) was followed by a final extension period of 15 min at 72°C. Taq polymerase (Boehringer) was used for other PCRs when the presence or absence of a particular fragment needed to be determined.
Construction of plasmids and mutant strains.
The mutant strains made in this work are listed in Table 1.
TABLE 1.
S. lividans mutant strains used in this work
| Strain | Genotype | Deletion plasmid | Brief description of deletion |
|---|---|---|---|
| M704 | Δzwf1 | pIJ8702 | In-frame deletion in zwf1 |
| M706 | Δzwf1 Δzwf2-opc2-devB | pIJ8706 | In-frame deletion in zwf1; deletion of majority of zwf2 and all of opc2 and devB at the 3′ end of zwf2 transcription unit |
| M714 | ΔdevB | pIJ8718 | Deletion of devB at the 3′ end of zwf2 transcription unit; hygromycin resistance gene inserted in place of devB |
| M715 | Δzwf2 | pIJ8717 | In-frame deletion in zwf2 |
(i) S. lividans M704 (Δzwf1).
To make an in-frame deletion in zwf1, a DNA fragment that contained the 3′ end of zwf1 and flanking DNA (length, 1.7 kb) was first produced by PCR. Cosmid SC5A7 was used as the DNA template with primers g6pdh1 and g6pdh2 (Table 2), and the amplified fragment was cloned into pGEM-T, which introduced a HindIII site downstream of the 3′ end of the zwf1 sequence. The resulting plasmid was designated pIJ8719. The nucleotide sequences of the ends of the cloned DNA were confirmed by using universal and reverse primers. The internal sequence of the DNA was confirmed by using sequencing primers g6pdh.seq1 to g6pdh.seq4. A second DNA fragment containing the 5′ end of zwf1 and flanking DNA was purified as a 2.8-kb BamHI fragment from the same cosmid and cloned into the BamHI site of pIJ2925, yielding pIJ8720. Insertion of the 1.7-kb HindIII-MluI fragment from pIJ8719 into pIJ8720 that had been digested with the same enzymes led to replacement of the 3′ end of zwf1, generating the required in-frame deletion. The overall result was deletion of a 1,113-bp MluI fragment (within the zwf1 coding region) which was now flanked by 1.7- and 1.8-kb DNA fragments. This fragment was excised by using HindIII and EcoRI sites flanking the BamHI site in pIJ2925 and cloned between the HindIII and EcoRI sites of pKC1132 (which carries an apramycin resistance gene) to produce pIJ8722. The desired deletion was confirmed by nucleotide sequencing, and the plasmid was introduced into S. lividans 1326 by conjugation. Apramycin-resistant exconjugants were grown nonselectively to allow excision of the integrated DNA. Apramycin-sensitive colonies were examined by Southern hybridization to assess whether they segregated to yield the wild-type or zwf1-deleted genotype. When the labeled 2.8-kb BamHI DNA fragment (Fig. 2a) was used to probe BamHI-digested chromosomal DNAs, the wild-type DNA showed a strong signal at 2.8 kb, whereas the two mutant DNA samples produced the larger band expected for deletion of the MluI fragment, which contained a BamHI site. These zwf1 mutant strains were designated M704-1 and M704-2.
TABLE 2.
Oligonucleotides used in this study
| Designation | Nucleotide sequence | Cosmid location |
|---|---|---|
| g6pdh1 | AAAAAAGCTTCCGAGGCCGTCCAGCAGCCACGAG | SC5A7:9,801-9,820 |
| g6pdh2 | AGGAGTACTGGGCCTCGCACGACAA | SC5A7:11,535-11,511 |
| g6pdh.seq1 | GTTCTCGCCGTCGACCTCGATC | SC5A7:10,151-10,172 |
| g6pdh.seq2 | GGTGTCAGACATGGACAGGGGT | SC5A7:10,487-10,508 |
| g6pdh.seq3 | TTGTCCGCCTCGGCCTCCACGA | SC5A7:10,801-10,822 |
| g6pdh.seq4 | GTTCCGGCCTCGGAGCCGACCC | SC5A7:11,149-11,170 |
| zwf2.1 | GAATTCAGTCACGGTCGAAGAGGCCAT | StC22:16,387-16,407 |
| zwf2.2 | GGATCCTGACGCCGAAGATGACCAGA | StC22:17,387-17,368 |
| zwf2.3 | GGATCCACCACGTACGGAGAACGCCGGGTT | StC22:20,659-20,682 |
| zwf2.4 | AAGCTTTACGCGCCCGGCCCGCACCACC | StC22:21,682-21,661 |
| zwf2.seq1 | ACCTCTCGCAGATCCACTCCGT | StC22:16,752-16,773 |
| zwf2.seq2 | TGTGAAGGACAAGGCGTACAGC | StC22:16,987-17010 |
| zwf2.seq3 | ACGAAGGAGCGGTACTTCATGT | StC22:20,919-20,940 |
| zwf2.seq4 | GGGCGTCGTCCAGGGAAGCGTA | StC22:21,340-21,361 |
| zwf2.seq6 | TCGGTTACCTCGAAGAGGAC | StC22:18,254-18,264 |
| opc2.3 | GAATTCATATGGTGCTCACCCTGGTCATCGTCA | StC22:18,910-18,934 |
| opc2.4 | GGATCCTCATTTCGCCGCCGCCTTCCTCACC | StC22:19,975-19,851 |
| zwf2bgl2 | AGATCTCCATGGCCGAGGACAT | StC22:17,994-18,015 |
| zwf2opc2h3 | AAGCTTGATGACGACGAGGGTG | StC22:19,021-19,002 |
| zwf2.6 | TTCATGGCCGGCGCCAGCTCCGTCCGT | StC22:18,834-18,798 |
FIG. 2.
(a) Map of cosmid SC5A7 containing a cluster of PPP-related genes. The open arrows indicate the directions and extents of the predicted protein-encoding regions. Genes encoding predicted proteins showing strong homology to proteins having known metabolic functions are indicated by boldface italic type. Genes encoding predicted proteins having unknown functions are designated hyp. Segments of DNA that were used to make the in-frame zwf1 deletion are indicated by lines terminating in solid circles. The extent and location of the in-frame deletion in the deletion plasmid pIJ8722 and the Δzwf1 deletion strain M704 are indicated by a dotted line. The fragments (from pIJ8707 and pIJ8719) used to produce pIJ8715 are indicated by shaded boxes. The region of the zwf1 cluster cloned in pIJ8715 and used to complement the Δzwf1 mutation is indicated by a line terminating in solid diamonds. Naturally occurring restriction enzyme sites are indicated by boldface type, while sites introduced by PCR or from vector polylinkers are indicated by lightface type in parentheses. (b) Map of cosmid StC22 containing a second cluster of PPP-related genes. The restriction enzyme sites used to join the various fragments were added by using PCR primers. The box labeled hygR shows the position of the insertion of the hygromycin resistance gene in the ΔdevB mutant strain. The extents of deleted DNA in the deletion plasmids and strains are indicated by dotted lines.
(ii) S. lividans M715 (Δzwf2).
An in-frame deletion mutant for the zwf2 locus was made by using a strategy similar to that described above for the Δzwf1 mutant. The 3′ end of zwf2 was amplified by PCR by using cosmid StC22 DNA and zwf2bgl2 and zwf2opc2h3 as the primers. The amplified DNA added BglII and HindIII sites at the 5′ and 3′ ends, respectively, of the zwf2 fragment. The DNA fragment was cloned in pGEM-T, and the nucleotide sequence was confirmed by using zwf2.seq6 and the universal and reverse sequencing primers. The DNA fragment was excised with BglII and HindIII and ligated with pIJ8706 that had been digested with BamHI and HindIII. Construction of the in-frame deletion was confirmed by nucleotide sequence analysis by using the universal, reverse, and zwf2.seq2 primers. The deletion construct (pIJ8717) (Fig. 2b) was introduced into S. lividans 1326 by conjugation, with selection for apramycin-resistant exconjugants. Purification in the presence of nalidixic acid and apramycin, followed by three rounds of nonselective growth, yielded a population of spores from which apramycin-sensitive colonies were identified at a frequency of ca. 1 in 2,000. PCR analysis of chromosomal DNA derived from the apramycin-sensitive segregants by using zwf2.6 and zwf2.seq2 produced 1.8- and 1.2-kb amplification products for zwf2+ strains and zwf2 deletion mutants, respectively. Two strains were shown to produce the smaller mutant PCR product, and the Δzwf2 strains were designated S. lividans M715-1 and M715-2, respectively.
(iii) S. lividans M706 (Δzwf1 Δzwf2-opc2-devB).
DNA fragments flanking the 5′ end of zwf2 and the 3′ end of devB were amplified by PCR by using cosmid StC22 DNA. Primers zwf2.1 and zwf2.2 (3′ end of tal2) were used to add EcoRI and BamHI sites at opposite ends of the fragment containing the 5′ end of zwf2. Similarly, primers zwf2.3 and zwf2.4 (3′ end of the gene encoding a hypothetical protein adjacent to and divergent from devB) added BamHI and HindIII sites at the fragment ends. The amplified DNA fragments were cloned into pGEM-T. Nucleotide sequence analysis (performed by using primers zwf2.seq3 and zwf2.seq4, as well as universal and reverse sequencing primers) confirmed that the expected DNA fragment had been isolated for the product of the zwf2.3 and zwf2.4 primers. However, two different PCR errors were observed in DNAs from different colonies (when zwf2.seq1 and zwf2.seq2 as well as universal and reverse primers were used) for the product of amplification with zwf2.1 and zwf2.2. The wild-type sequence of the amplified fragment was obtained by recombining appropriate segments of each clone.
The Δzwf2-opc2-devB deletion plasmid (pIJ8706) was assembled in a three-fragment ligation mixture containing the EcoRI-BamHI and BamHI-HindIII fragments and pKC1132 that had been digested with EcoRI and HindIII. pIJ8706 was introduced into S. lividans M704-1 by conjugation. Apramycin-resistant colonies were selected and purified. After three rounds of sporulation on nonselective agar, the progeny were screened for sensitivity to apramycin. Southern hybridization with the cloned EcoRI-BamHI fragment containing tal2 (Fig. 2b) as the probe revealed patterns corresponding to genotypes for either the wild type (a 6.37-kb MluI fragment) or Δzwf2-opc2-devB (a 5.28-kb MluI fragment), consistent with loss of the 3.2-kb fragment which included a single MluI site. Two Δzwf2-opc2-devB colonies were identified (from a population of approximately 4,000 colonies) and were designated M706-1 and M706-2.
(iv) S. lividans M714 (ΔdevB).
PCR amplification of cosmid StC22 DNA with the opc2.3 and opc2.4 primers produced a fragment encoding opc2 with an EcoRI site at the 5′ end and a BamHI site at the 3′ end. After cloning and nucleotide sequence confirmation, the fragment was purified and ligated into pIJ8706, which had been digested with the same enzymes. The resulting plasmid was cleaved at its unique BamHI site and treated with calf intestinal alkaline phosphatase before ligation with a BglII fragment purified from pIJ963 (18), which contained a hygromycin resistance gene. The ligation mixture was used to transform competent cells of E. coli DH5α with selection for hygromycin resistance. Digestion of the resulting plasmid (pIJ8718) (Fig. 2b) with EcoRI showed that in all of the transformants the hygromycin resistance gene had replaced devB and was inserted in the same orientation as the zwf2 operon. pIJ8718 was introduced into S. lividans 1326 by conjugation, which produced strains that were resistant to both hygromycin and apramycin. After nonselective growth, one apramycin-sensitive, hygromycin-resistant colony was isolated after ca. 2,500 colonies were screened. The ΔdevB strain was designated S. lividans M714.
Complementation of Δzwf1.
A 768-nucleotide HindIII-SacI DNA fragment was isolated from pIJ8719 (Fig. 2a), and a 6.52-kb SacI-XhoI DNA fragment was isolated from cosmid SC5A7. These two fragments were ligated with pIJ2925 that had been digested with HindIII and SalI. The resulting plasmid (pIJ8707) contained the majority of the putative zwf1 operon with the 3′ end truncated within pgi (downstream of opc) and the 5′ end truncated within a putative divergent transcriptional regulator, the role of which is not known yet (Fig. 2a). A 4.5-kb PstI fragment from pIJ8707 (extending from the polylinker PstI site in pIJ2925 [adjacent to the HindIII site] to the PstI site at nucleotide 14,308 in SC5A7) was cloned into the PstI site of pKC1132. The resulting plasmid (pIJ8715) contained the 3′ end of tal1 together with the complete zwf1 and opc1 genes and the 5′ end of pgi.
pIJ8715 was transferred by conjugation into M704 (Δzwf1). Exconjugants were selected with apramycin and nalidixic acid. After purification, colonies were grown nonselectively on SFM agar (three rounds of sporulation) and screened for loss of the apramycin resistance marker. Chromosomal DNA was isolated from candidate strains. Replacement of the in-frame deletion mutant allele by wild-type zwf1 was confirmed by Southern hybridization by using the 2.8-kb BamHI fragment contained in pIJ8720 as the probe.
Enzyme activity assays.
G6PDH (EC 1.1.1.49) assays were based on the production of NADPH, which was measured spectrophotometrically at 340 nm. The conditions used were those described by Lessie and Vander Wyk (19), except that 2-mercaptoethanol was omitted. 6PGDH (EC 1.1.1.43) activity was measured similarly by using either NAD+ or NADP+. 6-Phosphogluconolactonase was assayed by the method of Collard et al. (11), except that 50 mM Tris-HCl (pH 7.5) was used instead of HEPES (pH 7.1) Protein concentrations were measured by using the Bio-Rad protein assay reagent.
Diamide sensitivity assays.
Confluent lawns of strains were generated by pouring 3 ml of soft nutrient agar (containing approximately 106 Streptomyces spores) onto Difco nutrient agar plates. Paper discs containing 10 μl of either 0.1 or 0.5 M diamide (28) were added, and the plates were photographed after 30 h of incubation at 30°C.
Antibiotic and metabolite estimation.
ACT was measured by determining absorbance at 640 nm (molar extinction coefficient value at 640 nm [ɛ640], 25,320) as described by Bystrykh et al. (7). RED was measured in acidified methanol at 530 nm by using a molar extinction coefficient of 100,500 (17).
Fermentation experiments.
The cultivation conditions (in 3-liter Applicon fermentors with a working volume of 1 liter) used for production of ACT in a phosphate-limited minimal medium (containing 10 g of glucose per liter) were the conditions previously described (5) for S. lividans 1326/pIJ68. For analysis of RED production in 4-liter Chemap fermentors, 2.5 liters of phosphate-limited Evans minimal medium (13) containing 2 mM NaH2PO4 and 25 g of glucose per liter was sterilized for 20 min at 121°C and 500 rpm. The culture was grown at 28°C with stirring at 800 rpm with an aeration rate of 1 volume per volume of liquid per min. pH was controlled between 6.8 and 7.2 by adding 4 M HCl or 4 M NaOH. For estimation of dry cell weight (biomass), 2-ml samples of culture were vacuum filtered through preweighed Whatman GF/C filter paper, washed with 10 ml of distilled water, and dried at 90°C to constant weight. All measurements were done in triplicate. RED production was analyzed by high-performance liquid chromatography. Fermentation broth samples (2 ml) were collected and mixed with an equal amount of acidified methanol. After centrifugation (1,200 × g for 10 min) the supernatant was injected for high-performance liquid chromatography analysis with a 5 μm Beckman Ultrasphere octyldecyl silane C18 column (4.6 by 250 mm) by using a linear elution gradient ranging from 20% phase B (CH3OH plus 0.1% trifluoroacetic acid) in phase A (CH3OH-CH3CN-H2O [4:1:5] plus 0.1% trifluoroacetic acid) to 100% phase B in 12 min. The flow rate was 1 ml/min, and the injection volume was 10 μl. The column effluent was monitored at 533 nm. RED was purified and used as an external standard.
RESULTS
Deletion of zwf1.
A gene (zwf1) encoding a putative G6PDH was identified early in the S. coelicolor genome sequencing project in cosmid SC5A7. The putative zwf1 operon (Fig. 2a) is delineated at one end by a putative transcriptional regulator that is oriented away from the PPP gene cluster. Since zwf1 appears to lie within a polycistronic transcription unit, an in-frame deletion was made in the gene to eliminate Zwf1 activity without affecting the expression of the downstream genes (opc, pgi, gnd, and two hypothetical genes of unknown function). The resulting strain, M704 (Δzwf1), grew and sporulated normally.
The Zwf and 6PGDH activities present in crude extracts of mycelia of S. lividans 1326 and S. coelicolor M145 that had been grown for 40 h in YEME were determined. For both strains, specific activities of 9 to 10 nmol min−1 mg of protein−1 were observed for Zwf, while 6PGDH activity was two- to threefold higher. Enzyme assays with crude extracts of S. lividans M704 (Δzwf1) reproducibly produced a specific activity of 4 to 5 nmol min−1 mg of protein−1 (i.e., a reduction of about 50% compared to wild-type strain 1326). The residual activity was presumed to reflect the presence of another zwf gene (see below). The 6PGDH activity in M704 (Δzwf1) was the same as that in the wild-type strain.
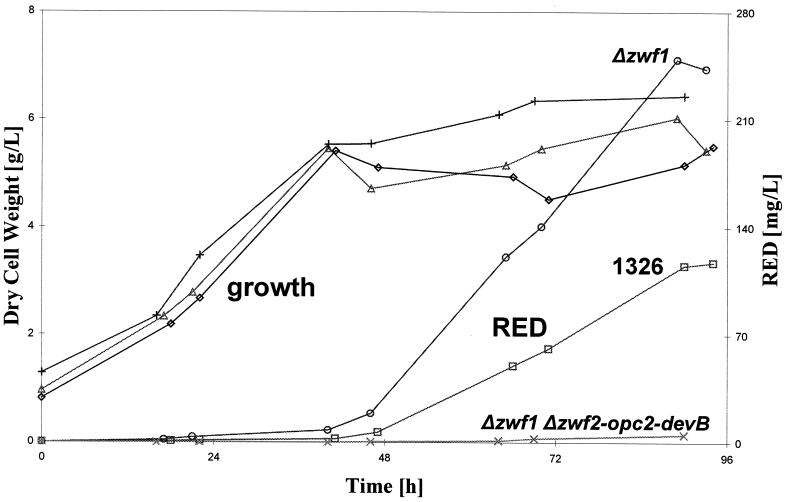
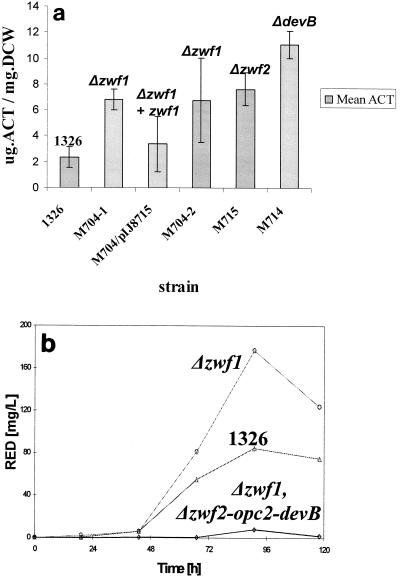
Protoplasts of M704 (Δzwf1) were transformed with multicopy plasmids carrying the pathway-specific activator genes for ACT (actII-ORF4 on pIJ68) or RED (redD on pIJ6014) biosynthesis. For RED production at the 4-liter bioreactor scale, M704/pJ6014 had a maximum volumetric productivity of ca. 250 mg · liter−1 after 90 h of fermentation, while control strain 1326/pIJ6014 had a maximum volumetric productivity of about 120 mg · liter−1 in the same period (Fig. 3). The growth and production kinetics were similar for the Δzwf1 mutant and the control strain, but the mutant produced twice as much RED. The specific productivities were 43 and 22 mg of RED per g (dry weight) of cells, respectively. Similar observations were made for production in shake flask fermentations, with the Δzwf1 mutant (M704) showing approximately two- to threefold greater productivity than control strain 1326 (Fig. 4a). The results were expressed in terms of the maximum specific ACT production (in milligrams of ACT per gram [dry weight] of cells). An independently isolated Δzwf1 mutant (M704-2) gave the same results. Similarly, shake flask fermentations with M704 Δzwf1 and 1326 containing pIJ6014 confirmed the RED fermentation results (Fig. 4b). The Δzwf mutant produced more than twice as much RED as the parental strain. A maximum volumetric productivity of about 180 mg · liter−1 was achieved after 90 h of fermentation of M704 (Δzwf1)/pIJ6014, compared with a maximum volumetric productivity of ca. 80 mg · liter−1 for 1326/pIJ6014. Lower antibiotic productivity at the shake flask scale than at the bioreactor scale was a constant feature of RED-producing strains during the RapidS Project (Jovetic and Marinelli, unpublished results).
FIG. 3.
RED production in fermentors by wild-type and zwf mutant S. lividans strains carrying multiple copies of the pathway-specific activator gene for RED biosynthesis. Symbols: ▵, ◊, and +, dry weight of cells of Δzwf1 mutant M704, wild-type strain 1326, and Δzwf1 Δzwf2-opc2-devB mutant M706, respectively; ○, □, and ×, RED production by zwf1, wild-type, and Δzwf1 Δzwf2-opc2-devB strains, respectively.
FIG. 4.
Antibiotic production in shake flask fermentations with wild-type and mutant S. lividans strains. (a) Mean maximum specific ACT production (in micrograms of ACT per milligram [dry weight] of cells [DCW]) for each strain carrying pIJ68. The error bars indicate the standard errors of the means. The strains used and their genetic lesions are indicated. The strain in which Δzwf1 was complemented with wild-type DNA was designated M704/pIJ8715. (b) RED production by strains carrying pIJ6014. Symbols: ▵, wild-type strain; ⋄, Δzwf1 Δzwf2-opc2 devB strain; and ○, Δzwf1 strain.
M704 (Δzwf1)/pIJ68 took much longer to start rapid growth in 3-liter fermentors than 1326/pIJ68 took (data not shown). However, the specific ACT production (in milligrams of ACT per gram [dry weight] of cells) was substantially higher for the Δzwf1 mutant (500 ± 30 mg · g−1) than for the wild-type strain (390 ± 40 mg · g−1) in the stationary phase, in which production reached the highest volumetric rate. In this phase, the yield of ACT (in milligrams of ACT per gram of glucose consumed) was reproducibly 20% higher in M704 (Δzwf1)/pIJ68 cultures than in 1326/pIJ68 cultures (data not shown). Thus, even though ACT production started later in M704 (Δzwf1)/pIJ68, the Δzwf1 mutant exhibited better ACT production capability than the parental strain. The growth lag of M704 (Δzwf1)/pIJ68 (which was observed in four different experiments) was not observed with the RED-overproducing strain (Fig. 3) or with the zwf double mutant containing pIJ68. No significant differences in growth rates were observed between the strains in shake flask experiments. The reason for the lag in growth of the M704 (Δzwf1)/pIJ68 strain in the fermentors is not clear but may involve the particular inoculum and/or the specific growth conditions used in the ACT fermentor experiments.
Complementation of S. lividans M704 (Δzwf1).
To verify that the increased production did indeed result from the Δzwf1 mutation, the wild-type zwf1 gene was reintroduced into M704 (Δzwf1) by using a double-crossover strategy that was essentially the reverse of the procedure used to make the in-frame deletion. Protoplasts of the resulting strain were transformed with pIJ68 DNA. Shake flask fermentations (Fig. 4a) showed that the level of ACT production was lower than that in M704 (Δzwf1)/pIJ68 and similar to that in 1326/pIJ68, confirming that the Δzwf1 mutation was causally linked to the improvement in ACT production.
Deletion of zwf2.
The behavior of a Δzwf2 mutant was expected to be similar to that of the Δzwf1 strain, since the Zwf activity in the latter strain was approximately one-half that in 1326, suggesting that the two Zwf proteins might be equivalent isoforms. Enzyme assays with the Δzwf2 mutant, M715, confirmed that it had approximately one-half the Zwf activity of 1326 (data not shown). Shake flask fermentations of M715 (Δzwf2)/pIJ68 showed improved ACT biosynthesis (Fig. 4a), and specific ACT production was essentially equivalent to that observed in M704 (Δzwf1)/pIJ68. This result reinforced the observations made with the Δzwf1 mutants and indicated that the changes in antibiotic production reflected changes in the activity of the first step in the PPP.
Δzwf1 Δzwf2-opc2-devB mutants.
Mutants M706-1 and M706-2 (Fig. 2b) lacking zwf2 and devB together with the intervening gene (opc2) were constructed by using the Δzwf1 strain (M704). Since the gene having an unknown function that lies downstream of devB is oriented in the opposite direction, there should have been no polar effects on the expression of downstream genes. M706-1 and M706-2 (both Δzwf1 Δzwf2-opc2-devB) were cultured in YEME for 40 h, and the mycelia were examined for Zwf activity. There was no detectable Zwf activity, whereas 6PGDH activity was present at about the same level as it was in 1326 and M704 (Δzwf1).
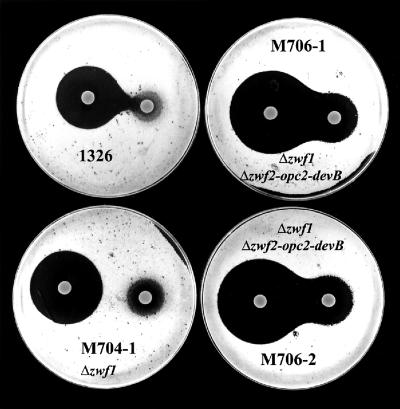
The abilities of these strains to generate NADPH were assessed by testing their sensitivities to diamide (which causes the formation of toxic cytoplasmic disulfides in low-molecular-weight thiols and in proteins). In S. coelicolor, such oxidative stress is neutralized by thioredoxin reductase, which uses NADPH to reduce the deleterious disulfide bonds (28). When challenged with diamide, M704 (Δzwf1) showed a small but reproducible increase in sensitivity compared to 1326 (Fig. 5). The double zwf mutant strains M706-1 and M706-2 (Δzwf1 Δzwf2-opc2-devB) showed much larger zones of inhibition, indicating a further increase in sensitivity to diamide.
FIG. 5.
Increased diamide sensitivity of S. lividans mutant strains. The sensitivities of the strains to inhibition of growth by diamide are indicated by zones of clearing (around the diamide-containing discs) on lawns of growth. The larger zones were observed around the discs containing the higher concentrations of diamide (0.5 M compared to 0.1 M).
M706-1 (Δzwf1 Δzwf2-opc2-devB) protoplasts were transformed with the transcriptional activator plasmids (pIJ68 and pIJ6014) and with the corresponding vector (pIJ486) as a control. When grown on phosphate-limited Evans medium containing 1.5% agar, the resulting strains appeared to be slightly blue and dark red for the pIJ68 and pIJ6014 derivatives, respectively, indicating that they could still make antibiotic in spite of the double zwf deletions. Growth of M706 (Δzwf1 Δzwf2-opc2-devB)/pIJ68 in a fermentor resulted in less antibiotic production than growth of either the single Δzwf1 mutant (M704) or the wild-type control strain containing the same plasmid. A specific level of ACT production of only 80 ± 9 mg of ACT per g (dry weight) of cells was observed for the M706 (Δzwf1 Δzwf2-opc2-devB) mutant, compared to values of 390 and 500 mg of ACT per g (dry weight) of cells for the wild-type and M704 (Δzwf1) strains, respectively (data not shown). M706 (Δzwf1 Δzwf2-opc2-devB)/pIJ68 grew at a rate similar to that of 1326/pIJ68, but ACT production reached a plateau at 140 h and did not increase further, as it did in M704 (Δzwf1)/pIJ68.
Similarly, RED productivity in M706 (Δzwf1 Δzwf2-opc2-devB)/pIJ6014 was drastically reduced compared to that in 1326/pIJ6014 and that in M704 (Δzwf1)/pIJ6014 (Fig. 3). The growth patterns of the mutant and control strains were similar for the first 48 h of cultivation. The control strain then started to accumulate RED, whereas M706 (Δzwf1 Δzwf2-opc2-devB)/pIJ6014 showed a further increase in biomass but produced only traces of the red pigment. The reduction in antibiotic production was confirmed by shake flask experiments for both ACT (data not shown) and RED (Fig. 4b).
Deletion of devB.
Since the zwf double mutant [M706 (Δzwf1Δzwf2-opc2-devB)] did not produce as much antibiotic as the single-mutant Δzwf1 strain (M704), a mutant was made in which only devB was inactivated; this mutant was designated M714. A mutation in devB might improve antibiotic production capability, since such a strain might show a significantly reduced flux through the PPP without suffering such a large reduction in the ability to produce NADPH.
DevB activity was not detected in the devB mutants M714 (ΔdevB) and M706 (Δzwf1 Δzwf2-opc2-devB). In these mutants, the rate of hydrolysis of 6-phosphogluconolactone was indistinguishable from the observed rate of spontaneous hydrolysis (data not shown). In contrast, the Zwf activity of M714 (ΔdevB) was not significantly different from that of wild-type strain 1326, whereas there was no detectable Zwf activity in M706 (Δzwf1 Δzwf2-opc2-devB). The results of these enzyme assays are in complete agreement with the results expected for the mutations. Shake flask fermentation cultures of M714 (ΔdevB)/pIJ68 had a specific ACT productivity significantly higher than that of any other strain tested in this study (Fig. 4a).
DISCUSSION
We demonstrated that modulation of Zwf activity has a considerable impact on the antibiotic production capability of S. lividans strains which were engineered to overproduce ACT or RED. Blocking PPP activity by deleting both zwf genes resulted in a marked reduction in antibiotic production. However, when Zwf activity was halved (as in the individual Δzwf1 and Δzwf2 mutants), antibiotic production increased. The latter observation was surprising, given the positive correlation between methylenomycin production and PPP activity in S. coelicolor (26). The results suggest that the production of ACT and RED under the conditions used in these experiments is not limited by the supply of NADPH. Presumably, the NADPH required for growth of the double zwf mutant is adequately supplied by tricarboxylic acid cycle activity (e.g., by isocitrate dehdrogenase) and/or transhydrogenase activity (which can reversibly interchange the levels of NADH and NADPH under appropriate conditions). Homologues of genes encoding the two putative transhydrogenase subunits are present in the S. coelicolor genome sequence (pntA and pntB; accession number AL450289), although there has been no biochemical confirmation of their function.
The apparently normal growth characteristics of M706 (Δzwf1 Δzwf2-opc2-devB) and its ACT- and RED-overproducing derivatives confirm that the cellular requirements for nucleotide biosynthesis can be adequately met by the reversible transketolase reactions using glycolytic intermediates. While pentose synthesis was unaffected in mammalian cells carrying targeted disruptions of G6PDH (29), the susceptibility of these cells to oxidative stress was markedly enhanced. The increased sensitivity of the S. lividans zwf mutants to diamide is indicative of a reduced capacity for NADPH synthesis. However, diamide would also be expected to inactivate the putative PntAB transhydrogenase heterodimer (PntB contains a single cysteine residue). Hence, treatment with this agent may not be representative of the metabolic events occurring during fermentations, in which transhydrogenase activity may augment the supply of NADPH. Less antibiotic was produced by the multiply mutant strain M706 (Δzwf1 Δzwf2-opc2-devB) than by the single zwf mutants M704 (Δzwf1) and M715 (Δzwf2). This suggests that there is a requirement for some PPP activity (presumably to produce a minimal level of additional NADPH, which cannot be sufficiently supplied by other possible routes) for the production of both ACT and RED. It seems likely that the improvement in antibiotic productivity in the single zwf mutants reflects a difference in the rate of carbon flux between the PPP and glycolysis rather than a difference in NADPH production. This interpretation is consistent with the results of chemostat experiments performed with S. lividans 1326 containing either pIJ68 or pIJ6014, in which an inverse correlation was noted between the PPP flux and the levels of production of ACT or RED (2). Jonsbu et al. (17) observed a similar relationship between reduced PPP flux and the production of nystatin by Streptomyces noursei and concluded that the available supply of NADPH was greater than the amount required (41 mmol of NADPH g of nystatin−1). For ACT synthesis, the amount of NADPH required was estimated to be 9.5 mmol g of ACT−1 (4). A similar analysis for the RED pathway (Butler, unpublished data) suggested that 30 mmol of NADPH g of RED−1 is required (reflecting the highly reduced nature of the acyl side chain). The strains examined in this study produced larger amounts of antibiotic (ACT, ca. 5 to 7 g · liter−1; RED, ca. 240 mg · liter−1) than the nystatin producer (6 to 7 mg · liter−1). However, even at these higher levels the demand for NADPH (66.5, 7.5, and 0.29 mmol · liter−1 for ACT, RED, and nystatin, respectively) appears to become limiting only for ACT and RED production in M706 (Δzwf1 Δzwf2-opc2-devB). It would be interesting to assess whether the biosynthesis of more reduced macrolides (directed by cloned biosynthetic genes in S. lividans) would show a greater dependence on Zwf levels, since these compounds often have a much greater biosynthetic requirement for NADPH. Reduced flux (compared to that in the wild-type strain) through the PPP would lower the loss of carbon (as CO2), which might lead to higher levels of the glycolytic intermediates required for the production of malonyl coenzyme A, ATP, and potentially other intermediates required for antibiotic production. Hence, providing that the appropriate NADPH availability is achieved, this effect of improved antibiotic production may be generally applicable.
The S. coelicolor genome also contains a gene whose product is homologous to a flavin-dependent G6PDH. This activity has been observed in mycobacteria and Nocardia spp. (32) and has been shown to use a flavin cofactor (F420) rather than NADPH. Purwantini et al. detected no activity in S. lividans, so it is unlikely that this flavin-dependent enzyme contributes to carbon flux through the PPP. The activity was assayed by using the F420 purified from Mycobacterium thermoautotrophicum, and it is formally possible that a structurally different cofactor might be required for the Streptomyces enzyme.
The improvement in ACT productivity observed in the devB mutant (M714) also suggests that it is changes in flux through the PPP that are largely responsible for the effects on antibiotic production observed here. Loss of the activity of DevB is likely to have much more impact on flux through the PPP than mutations in any one of the zwf genes have, because DevB is the only protein known to be catalytically involved in hydrolysis of 6-phosphogluconolactone (23). The results also suggest that there are no significant adverse effects produced by potential accumulation of the toxic compound 6-phosphogluconolactone (33) in the organism under the conditions examined. Although the lactone can undergo spontaneous hydrolysis, the low rate of this reaction would still be likely to cause a significant reduction in flux through the PPP. NADPH production by the Zwf activities may be affected (either directly or indirectly) by the accumulation of 6-phosphogluconolactone. Therefore, it is not yet possible to attribute the improvement in antibiotic production solely to a reduction in carbon flux through the PPP rather than to a reduction in the available NADPH concentration. It would be interesting to probe these interpretations experimentally by performing a metabolic flux analysis of strains in which PPP activity is inducibly expressed (39) and to examine the effects of induction on antibiotic production at a variety of times during fermentation. Overexpression of the zwf genes appears to require coexpression of the adjacently located (presumably cotranscribed) opc gene. In contrast to the successful production of human G6PDH (1) for crystallography, attempts to overproduce both the Zwf proteins of S. coelicolor in E. coli produced soluble but inactive protein (Butler, unpublished). This implies that the streptomycete Zwf proteins are assembled into heteromeric active proteins with their corresponding Opc proteins. This biochemical organization occurs in C. glutamicum (25), and the OpcA protein in Nostoc punctiforme ATCC 29133 (15) appears to play a role as an allosteric activator of the Zwf protein. In contrast, the 6-phosphogluconolactonase was expressed as a soluble, active protein in E. coli (Butler, unpublished), suggesting that no cofactors specific for the Streptomyces enzyme are necessary for this enzymatic activity.
While the results of Obanye et al. (26) suggest that methylenomycin production should be reduced in the mutants described here, the effect on the synthesis of more highly reduced compounds remains to be determined. Other metabolic approaches have also been used to enhance secondary metabolite production in actinomycetes (9, 20, 24, 31). The mutations and strains described here add to the available repertoire of tools that can be used to increase the yields of a potentially wide range of secondary metabolites.
Acknowledgments
This work was supported by EU Cell Factory grant B104-CT96-0332 to Mervyn Bibb (coordinated by R. Luiten) and BBSRC grant 208/P14580.
We are grateful to K. F. Chater, D. A. Hopwood, M. Elliot, and G. Sawers for comments on the manuscript. M. J. Buttner, T. Palmer, S. Rawsthorne, and A. Smith are thanked for helpful discussions. We thank P. Jakimowicz, T. Kieser, and E. Takano for help with preparation of figures. We also thank J. K. M. Brown for help with statistical analysis (using the Genstat software) and S. Riches for help with preparation of the manuscript.
Footnotes
This paper is dedicated to the memory of Pieter Postma for his immense contribution to the field of bacterial physiology. He was a true friend and colleague who will be missed enormously.
REFERENCES
- 1.Au, S. W. N., C. E. Naylor, S. Gover, L. Vandeputte-Rutten, D. A. Scopes, P. J. Mason, L. Luzzatto, V. M. S. Lam, and M. J. Adams. 1999. Solution of the structure of tetrameric human glucose-6-phosphate dehydrogenase by molecular replacement. Biol. Crystallogr. D 55:826-834. [DOI] [PubMed] [Google Scholar]
- 2.Avignone-Rossa, C., J. White, A. Kuiper, P. W. Postma, M. J. Bibb, and M. J. Texeira de Mattos. 2002. Carbon flux distribution in chemostat cultures of Streptomyces lividans. Metab. Eng. 4:138-150. [DOI] [PubMed] [Google Scholar]
- 3.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. Nagaraj Rao, and B. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 4.Bruheim, P., M. J. Butler, and T. E. Ellingsen. 2002. A theoretical analysis of the biosynthesis of actinorhodin in a hyper-producing Streptomyces lividans strain cultivated on various carbon sources. Appl. Microbiol. Biotechnol. 58:735-742. [DOI] [PubMed] [Google Scholar]
- 5.Bruheim, P., H. Sletta, M. J. Bibb, J. White, and D. W. Levine. 2002. High yield actinorhodin production in fed-batch culture by a Streptomyces lividans strain over-expressing the pathway-specific activator gene actII-ORF4. J. Ind. Microbiol. Biotechnol. 28:103-111. [DOI] [PubMed] [Google Scholar]
- 6.Butler, M. J., J. Deutscher, P. W. Postma, T. J. G. Wilson, A. Galinier, and M. J. Bibb. 1999. Analysis of a ptsH homologue from Streptomyces coelicolor A3(2). FEMS Microbiol. Lett. 177:279-288. [DOI] [PubMed] [Google Scholar]
- 7.Bystrykh, L. V., M. A. Fernandez-Moreno, J. K. Herrema, F. M. Malpartida, D. A. Hopwood, and L. Dijkhuizen. 1996. Production of actinorhodin-related “blue pigments” by Streptomyces coelicolor A3(2). J. Bacteriol. 178:2238-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chater, K. F. 1990. The improving prospects for yield increase by genetic engineering in antibiotic-producing streptomycetes. Bio/Technology 8:115-121. [DOI] [PubMed] [Google Scholar]
- 9.Chen, G., G.-Y. Wang, X. Li, B. Waters, and J. E. Davies. 2000. Enhanced production of microbial metabolites in the presence of dimethyl sulfoxide. J. Antibiot. 53:1145-1153. [DOI] [PubMed] [Google Scholar]
- 10.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-543. [DOI] [PubMed] [Google Scholar]
- 11.Collard, F., J.-F. Collet, I. Gerin, M. Veiga-da-Cunha, and E. Van Schaftingen. 1999. Identification of the cDNA encoding human 6-phosphogluconolactonase, the enzyme catalyzing the second step of the pentose phosphate pathway. FEBS Lett. 459:223-226. [DOI] [PubMed] [Google Scholar]
- 12.Dekleva, M. L., and W. R. Strohl. 1988. Biosynthesis of ɛ-rhodomycinone from glucose by Streptomyces C5 and comparison with intermediary metabolism of other polyketide-producing streptomycetes. Can. J. Microbiol. 34:1235-1240. [DOI] [PubMed] [Google Scholar]
- 13.Evans, C. G. T., D. Herbet, and D. W. Tempest. 1970. The continuous culture of microorganisms. 2. Construction of a chemostat. Methods Microbiol. 2:277-327. [Google Scholar]
- 14.Hagen, K. D., and J. C. Meeks. 2001. The unique cyanobacterial protein OpcA is an allosteric effector of glucose-6-phosphate dehydrogenase in Nostoc punctiforme ATCC29133. J. Biol. Chem. 276:11477-11486. [DOI] [PubMed] [Google Scholar]
- 15.Hager, P. W., H. W. Calfee, and P. V. Phibbs. 2000. The Pseudomonas aeruginosa devB/SOL homolog, pgl, is a member of the hex regulon and encodes 6-phosphogluconolactonase. J. Bacteriol. 182:3934-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopwood, D. A. 1997. Genetic contributions to understanding polyketide synthases. Chem. Rev. 97:2465-2498. [DOI] [PubMed] [Google Scholar]
- 17.Jonsbu, E., B. Christiensen, and J. Nielsen. 2001. Changes of in vivo fluxes through central metabolic pathways during the production of nystatin by Streptomyces noursei in batch culture. Appl. Microbiol. Biotechnol. 56:93-100. [DOI] [PubMed] [Google Scholar]
- 18.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, United Kingdom.
- 19.Lessie, T. G., and J. C. Vander Wyk. 1972. Multiple forms of Pseudomomas multivorans glucose-6-phosphate and 6-phosphogluconate dehydrogenases: differences in size, pyridine nucleotide specificity and susceptibility to inhibition by adenosine 5′-triphosphate. J. Bacteriol. 110:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lombo, F., B. Pfeifer, T. Leaf, S. Ou, Y. S. Kim, D. E. Cane, P. Licari, and C. H. Khosla. 2001. Enhancing the atom economy of polyketide biosynthetic processes through metabolic engineering. Biotechnol. Prog. 17:612-617. [DOI] [PubMed] [Google Scholar]
- 21.MacNeil, D. J., K. M. Gewain, C. L. Ruby, G. Dezeny, P. Gibbons, and T. MacNeil. 1992. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilising a novel integration vector. Gene 111:61-68. [DOI] [PubMed] [Google Scholar]
- 22.Mahr, K., G. P. van Wezel, C. Svensson, U. Krengel, M. J. Bibb, and F. Titgemeyer. 2000. Glucose kinase of Streptomyces coelicolor A3(2): large-scale purification and biochemical analysis. Antonie Leeuwenhoek 78:253-261. [DOI] [PubMed] [Google Scholar]
- 23.Miclet, E., V. Stoven, P. A. Michels, F. R. Opperdoes, J.-M. Lallemand, and F. Duffieux. 2001. NMR spectroscopic analysis of the first two steps of the pentose-phosphate pathway elucidates the role of 6-phosphogluconolactonase. J. Biol. Chem. 276:34840-34846. [DOI] [PubMed] [Google Scholar]
- 24.Minas, W., P. Brunker, P. T. Kallio, and J. E. Bailey. 1998. Improved erythromycin production in a genetically engineered industrial strain of Saccharopolyspora erythraea. Biotechnol. Prog. 14:561-566. [DOI] [PubMed] [Google Scholar]
- 25.Moritz, B., K. Striegel, A. A. De Graaf, and H. Sahm. 2000. Kinetic properties of the glucose-6-phosphate and 6-phosphogluconate dehydrogenases from Corynebacterium glutamicum and their applications for predicting pentose phosphate pathway flux. Eur. J. Biochem. 267:3442-3452. [DOI] [PubMed] [Google Scholar]
- 26.Obanye, A. I. C., G. Hobbs, D. C. J. Gardner, and S. G. Oliver. 1996. Correlation between carbon flux through the pentose phosphate pathway and production of the antibiotic methylenomycin in Streptomyces coelicolor A3(2). Microbiology 142:133-137. [DOI] [PubMed] [Google Scholar]
- 27.Paget, M. S. B., L. Chamberlin, A. Atrih, S. J. Foster, and M. J. Buttner. 1999. Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 181:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paget, M. S. B., J.-G. Kang, J.-H. Roe, and M. J. Buttner. 1998. σR, an RNA polymerase sigma factor that modulates expression of the thioredoxin system in response to oxidative stress in Streptomyces coelicolor A3(2). EMBO J. 17:5776-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandolfi, P. P., F. Sonati, R. Rivi, P. Mason, F. Grosveld, and L. Luzzatto. 1995. Targeted disruption of the housekeeping gene encoding glucose-6-phosphate dehydrogenase (G6PD): G6PD is dispensible for pentose synthesis but essential for defense against oxidative stress. EMBO J. 14:5209-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passantino, R., A.-M. Puglia, and K. F. Chater. 1991. Additional copies of the actII regulatory gene induce actinorhodin production in pleiotropic bld mutants of Streptomyces coelicolor A3(2). J. Gen. Microbiol. 137:2059-2064. [Google Scholar]
- 31.Pfeifer, B. A., S. J. Admiraal, H. Gramajo, D. E. Cane, and C. H. Khosla. 2001. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291:1790-1792. [DOI] [PubMed] [Google Scholar]
- 32.Purwantini, E., T. Gillis, and L. Daniels. 1997. Presence of F420-dependent glucose-6-phosphate dehydrogenase in Mycobacterium and Nocardia species, but absence in Streptomyces and Corynebacterium species and methanogenic Archaea. FEMS Microbiol. Lett. 146:129-134. [DOI] [PubMed] [Google Scholar]
- 33.Rakitzis, E. T., and P. Papandreou. 1998. Reactivity of 6-phosphogluconolactone with hydroxylamine: the possible involvement of glucose-6-phosphate dehydrogenase in endogenous glycation reactions. Chem.-Biol. Interact. 113:205-216. [DOI] [PubMed] [Google Scholar]
- 34.Salas, J. A., L. M. Quiros, and C. Hardisson. 1984. Pathways of glucose catabolism during germination of Streptomyces spores. FEMS Microbiol. Lett. 22:229-233. [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 36.Stryer, L. 1995. Biochemistry, 4th ed. W. H. Freeman and Company, New York, N.Y.
- 37.Sundaram, S., H. Karakaya, D. J. Scanlan, and N. H. Mann. 1998. Multiple oligomeric forms of glucose-6-phosphate dehydrogenase in cyanobacteria and the role of OpcA in the assembly process. Microbiology 144:1549-1556. [DOI] [PubMed] [Google Scholar]
- 38.Takano, E., H. C. Gramajo, E. Strauch, N. Andres, J. White, and M. J. Bibb. 1992. Transcriptional regulation of the redD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2). Mol. Microbiol. 6:2797-2804. [DOI] [PubMed] [Google Scholar]
- 39.Takano, E., J. White, C. J. Thompson, and M. J. Bibb. 1995. Construction of thiostrepton-inducible, high-copy-number expression vectors for use in Streptomyces spp. Gene 166:133-137. [DOI] [PubMed] [Google Scholar]