Abstract
Bcmfs1, a novel major facilitator superfamily gene from Botrytis cinerea, was cloned, and replacement and overexpression mutants were constructed to study its function. Replacement mutants showed increased sensitivity to the natural toxic compounds camptothecin and cercosporin, produced by the plant Camptotheca acuminata and the plant pathogenic fungus Cercospora kikuchii, respectively. Overexpression mutants displayed decreased sensitivity to these compounds and to structurally unrelated fungicides, such as sterol demethylation inhibitors (DMIs). A double-replacement mutant of Bcmfs1 and the ATP-binding cassette (ABC) transporter gene BcatrD was more sensitive to DMI fungicides than a single-replacement mutant of BcatrD, known to encode an important ABC transporter of DMIs. The sensitivity of the wild-type strain and mutants to DMI fungicides correlated with Bcmfs1 expression levels and with the initial accumulation of oxpoconazole by germlings of these isolates. The results indicate that Bcmfs1 is a major facilitator superfamily multidrug transporter involved in protection against natural toxins and fungicides and has a substrate specificity that overlaps with the ABC transporter BcatrD. Bcmfs1 may be involved in protection of B. cinerea against plant defense compounds during the pathogenic phase of growth on host plants and against fungitoxic antimicrobial metabolites during its saprophytic phase of growth.
Microorganisms in their natural environment need to protect themselves from adverse effects caused by natural toxic compounds. This also accounts for Botrytis cinerea Pres. ex Fr. [anamorph of Botryotinia fuckeliana (De Bary)], a plant-pathogenic fungus with a wide host range that can also grow as a saprophyte (8). Thus, the fungus has to cope with natural toxic compounds produced by host plants during pathogenesis and with antagonistic microorganisms during the saprophytic phase. ATP-binding cassette (ABC) and major facilitator superfamily (MFS) transporters can enable the fungus to survive exposure to toxic compounds. These membrane-bound proteins are known to provide protection against a wide range of natural toxic compounds and xenobiotics (12). ABC transporters use the energy of ATP hydrolysis to transport compounds over membranes. They may have a broad substrate range including unrelated chemicals such as sugars, inorganic ions, heavy metals, peptides, amino acids, oligopeptides, polysaccharides, proteins, and drugs (18). Transporters located in plasma membranes can transport toxic compounds from the inner leaflet of these membranes to the outer environment of cells, thereby reducing accumulation of the compounds in cells (14). ABC transporter activity in filamentous fungi involved in energy-dependent efflux of fungicides has been demonstrated for Aspergillus nidulans (3) and B. cinerea (33). Overexpression of ABC transporters can result in resistance to sterol demethylation inhibitors (DMIs) as reported for A. nidulans (3, 11), B. cinerea (17), Candida albicans (24), Penicillium digitatum (20), and Saccharomyces cerevisiae (18). MFS transporters may also prevent accumulation of toxic compounds in cells, but their activity is driven by the proton-motive force over membranes (21). MFS transporters from C. albicans (5) and S. cerevisiae (1) are involved in protection against exogenous toxic compounds, such as DMIs. In filamentous fungi, a number of MFS transporters are known to mediate the secretion of endogenously produced toxins (22), such as aflatoxin, cercosporin, Helminthosporium carbonum toxin (HC toxin), and trichothecenes by Aspergillus flavus (P. K. Chang, J. Yu, D. Bhatnagar, and T. E. Cleveland, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. O-31, p. 501, 1999), Cercospora kikuchii (6), Cochliobolus carbonum (23), and Fusarium sporotrichioides (2), respectively. This may result in self-protection of the producing organisms against these compounds. So far, a role of MFS transporters of filamentous fungi in protection against synthetic drugs, such as fungicides, has not been reported.
Recently we demonstrated that B. cinerea possesses multiple ABC and MFS transporter genes (16, 33) and showed that the ABC transporter BcatrB plays a role in protection against the plant defense compound resveratrol and phenylpyrrole fungicides (29). Similarly, the ABC transporter BcatrD provides protection against DMIs (17). Overexpression of these transporters in laboratory-generated mutants resulted in multidrug resistance to fungicides and unrelated chemicals (16). This mechanism may also apply to fungicide resistance development under field conditions (7, 19).
In this paper, we describe the isolation of the MFS gene Bcmfs1 from B. cinerea. We constructed Bcmfs1 replacement and overexpression mutants and phenotyped these mutants for sensitivity to compounds from different chemical classes. The differential sensitivity of the mutants to the DMI fungicide oxpoconazole correlated with expression levels of Bcmfs1 and with accumulation of the fungicide by germlings of the mutants. We propose that Bcmfs1 functions in protection against natural toxins, DMI fungicides, and other unrelated compounds. Hence, Bcmfs1 is the first MFS multidrug transporter of a filamentous fungus for which multiple substrates have been described.
MATERIALS AND METHODS
Fungal strains.
B. cinerea strain B05.10 (4), provided by P. Tudzynski (Institut für Botanik, Westfälische Wilhelms-Universität, Münster, Germany), is a haploid strain derived from SAS56 isolated by F. Faretra (Università of Bari, Bari, Italy). B05.10 was used as the parental isolate in all experiments. B05.10 and mutants constructed (Table 1) were maintained on malt extract agar plates Oxoid Ltd., Basingstoke, Hampshire, England) amended with 0.2% yeast extract (Oxoid) at 20°C. Formation of conidia was induced by irradiation with near-UV light for 24 h after 3 days of incubation and prolonged incubation for 3 to 7 days. Conidial suspensions were stored in 15% glycerol at −20°C.
TABLE 1.
B. cinerea strains used in this study
| Strain | Character | Reference |
|---|---|---|
| B05.10 | Wild-type strain | 4 |
| ΔBcatrB4 | BcatrB replacement mutant derived from B05.10 carrying the hygromycin resistance cassette | 29 |
| ΔBcatrD-8 | BcatrD replacement mutant derived from B05.10 carrying the hygromycin resistance cassette | 17 |
| ΔBcmfs1-16 and ΔBcmfs1-18 | Bcmfs1 replacement mutants derived from B05.10 carrying the hygromycin resistance cassette | This study |
| OV1-23, OV1-48, and OV1-13 | Bcmfs1 overexpression mutants derived from B05.10 carrying the hygromycin resistance cassette with a low, medium, and high level of resistance to oxpoconazole, respectively | This study |
| HR-9 | Reference strain derived from B05.10 carrying an ectopic integration of the hygromycin resistance cassette | 17 |
| ΔBΔ1-22 | BcatrB and Bcmfs1 double-replacement mutant derived from ΔBcatrB4 carrying both the hygromycin and nourseothricin resistance cassettes | This study |
| ΔDΔ1-45 | BcatrD and Bcmfs1 double replacement mutant derived from ΔBcatrD-8 carrying both the hygromycin and nourseothricin resistance cassettes | This study |
| HNR-4 | Reference strain derived from B05.10 carrying an ectopic integration of both the hygromycin and nourseothricin resistance cassettes | This study |
Plasmids carrying a hygromycin resistance cassette (pLOB1) and a nourseothricin resistance cassette (pNR2) were gifts from Jan van Kan, Sander Schouten, and Ilona Kars (Laboratory of Phytopathology, Wageningen University, Wageningen, The Netherlands). pNR2 is derived from pNR1 (kindly provided by Paul and Bettina Tudzynski, Westfälische Wilhelms-Universität, Münster, Germany). pLOB1 carries the Escherichia coli hygromycin phosphotransferase-encoding gene hph, and pNR2 carries the Streptomyces noursei nourseothricin acetyltransferase-encoding gene nat-1 (Werner-Bioagents, Jena, Germany) under control of the A. nidulans oliC promoter and a B. cinerea β-tubulin transcription terminator fragment.
Compounds.
Oxpoconazole, iprodione, and prochloraz (technical grade) were obtained from Ube Industries, Ltd. (Ube, Yamaguchi, Japan), captan and tebuconazole were obtained from Bayer AG (Leverkusen, Germany), cyprodinil and pyrifenox were obtained from Syngenta (Stein, Switzerland), fenarimol was obtained from Eli Lilly and Company (Indianapolis, Ind.), and fluazinam was obtained from ISK Bioscience Co. (Mentor, Ohio). Barbaloin, camptothecin, cercosporin, colchicine, cycloheximide, 8-methoxypsoralen, reserpine, rhodamine 6G, rose bengal, toluidine blue, and vincamine were purchased from Sigma (St. Louis, Mo.), globulol and patchoulol were purchased from Fluka Chemie AG (Buchs, Switzerland), and hypericin and pseudohypericin were purchased from Planta Naturstoffe (Vienna, Austria).
Cloning of Bcmfs1.
Cloning of Bcmfs1 was performed following an approach similar to that described for BcatrD (17). An Expressed Sequence Tags (EST) fragment of Bcmfs1 (Fig. 1A) obtained by PCR amplification was used for screening of a genomic library of strain SAS56 in λEMBL3, provided by A. ten Have (Laboratory of Phytopathology, Wageningen University). Positive and purified phages were subcloned in pBluescript II SK and sequenced. DNA manipulations were performed according to standard methods (25). Escherichia coli strain DH5α was used for propagation of the constructs.
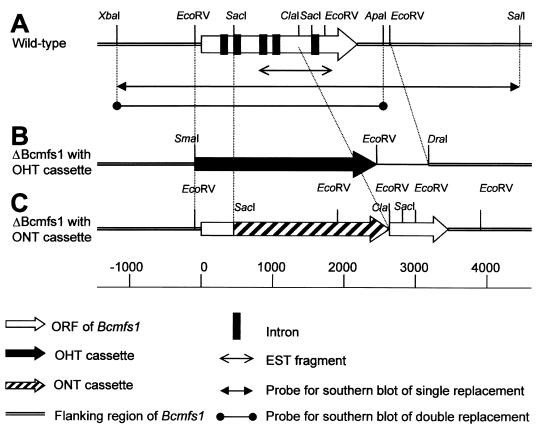
FIG. 1.
Physical map of Bcmfs1 in genomic DNA from B. cinerea wild-type strain B05.10 (A) and replacement mutant of Bcmfs1 with the hygromycin-resistance (OHT) cassette (B) or the nourseothricin-resistance (ONT) cassette (C). Southern blots of DNA from single-replacement mutants and double-replacement mutants of Bcmfs1 were hybridized with the 5.5-kb XbaI-SalI fragment and the 3.6-kb XbaI-ApaI fragment, respectively. Northern blots were hybridized with the EST fragment of Bcmfs1.
cDNA was amplified by reverse transcription-PCR using the SUPERSCRIPT One-Step reverse transcription-PCR with PLATINUM Taq system (Life Technologies Inc., Breda, The Netherlands). Homology of the putative protein sequence derived from Bcmfs1 cDNA with other MFS proteins was calculated using the Clustal method by the program Megalign (DNAstar, Madison, Wis.).
Construction of Bcmfs1 replacement mutants.
In an 8.5-kb XbaI-SalI subclone containing the full-length Bcmfs1 gene and its flanking regions, the 2.4-kb EcoRV fragment containing Bcmfs1 was replaced by the hygromycin-resistance cassette (OHT cassette) from pLOB1 to construct the replacement vector pΔ1-H (Fig. 1B). Before transformation, the plasmid was linearized with XhoI. Transformation was performed as described for replacement of the BcatrD gene (17).
Construction of double-replacement mutants.
In an 8.5-kb XbaI-SalI subclone, the 1.1-kb SacI-ClaI fragment was replaced by the nourseothricin resistance cassette (ONT cassette) from pNR to construct the replacement vector pΔ1-N (Fig. 1C). For the construction of double-replacement mutants, protoplasts from ΔBcatrB4 (29) or ΔBcatrD-8 (17) were transformed with 1 μg of pΔ1-N. Before transformation, the plasmid was linearized with XhoI. The selection and purification of putative transformants were performed by three successive transfers on malt extract agar amended with 100 mg of nourseothricin (Werner-Bioagents) liter−1 followed by single-spore isolation.
Construction of Bcmfs1 overexpression mutants.
A subclone containing the 5.5-kb XbaI-SalI fragment (Fig. 1A) in pBluescript II SK was used to generate overexpression mutants by cotransformation with pLOB1 to protoplasts of B05.10 as described previously (17).
Southern and Northern blot analysis.
Southern and Northern blot analyses were performed as described previously (17). In Southern blot experiments, 5 μg of genomic DNA was digested with EcoRV and hybridized with the 5.5-kb XbaI-SalI probe to characterize Bcmfs1 single-replacement mutants or the 3.6-kb XbaI-ApaI probe to characterize double-replacement mutants as presented in Fig. 1A. In Northern expression analysis experiments, 10 μg of total RNA was loaded on agarose gel (1.6%), and the blots were hybridized with the EST fragment of Bcmfs1 (Fig. 1A).
Phenotype assay.
The phenotype of replacement and overexpression mutants was studied by investigating their sensitivity to compounds in radial growth experiments as described previously (7). Drops of spore suspension (3 μl) of B. cinerea (106 conidia ml−1) were inoculated on plates with synthetic medium amended with chemicals from 100× concentrated stock solutions in methanol. The plates were incubated at 20°C in the dark for 3 days. The sensitivity to camptothecin, cercosporin, and other photosensitizers was investigated in the light as well as in the dark. Effective concentrations inhibiting radial growth by 50% (EC50s) of chemicals were calculated from dose-response curves using Excel 97. Experiments were repeated three times, and statistical analysis of the EC50s was performed by the least-significant-difference test (t test).
Accumulation of oxpoconazole.
Accumulation experiments were performed as described previously (13). Germling suspensions (4 mg wet weight per ml) in 0.05 M potassium phosphate buffer (pH 6.0) containing d-glucose (10 g liter−1) were preincubated on a reciprocal shaker at 20°C and 180 rpm for 20 min. [14C]oxpoconazole (initial external concentration, 30 μM; 750 Bq nmol−1) was added from a 100× concentrated stock solution in methanol. Samples (5 ml) taken from the suspensions at various time intervals were collected and washed three times with the same buffer on a GF6 microfiber glass filter (Schleicher & Schuell, Dassel, Germany). Radioactivity in mycelium was extracted with scintillation liquid (LUMASAFE PLUS, LUMAC∗LSC B.V., Groningen, The Netherlands) for 1 day and counted in a liquid scintillation spectrometer BECKMAN LS6000TA (Beckman Coulter Inc., Fullerton, Calif.).
Virulence assay.
Detached leaves of tomato (cv. Moneymaker Cf4) were placed in florist foam on wet paper in plastic chambers. Drops of spore suspensions (1 μl) of B. cinerea (2 × 106 conidia ml−1) in B5 medium [1% sucrose, 10 mM (NH4)H2PO4, 0.31% Gamborg B5 medium elements (Duchefa, Haarlem, The Netherlands)] were spotted onto the surface of the tomato leaves. The wild-type isolate B05.10 and the mutants were inoculated on two halves of the same leaf. Inoculated leaves were incubated in closed boxes at 20°C in the dark. Diameters of lesions were measured 3 days after inoculation. Experiments were performed twice.
Nucleotide sequence accession number.
The full-length Bcmfs1 gene of B. cinerea has been submitted to GenBank under accession number AF238225.
RESULTS
Cloning of Bcmfs1.
Screening of a phage library of B. cinerea with an EST fragment of Bcmfs1 (Fig. 1A) resulted in the selection of a phage containing the full-length Bcmfs1 gene. Comparison of the sequence of genomic DNA and cDNA revealed that Bcmfs1 contains a 1,794-bp open reading frame (ORF) interrupted by five introns. The introns vary in size from 53 to 92 bp and are distributed over the whole ORF of Bcmfs1 (Fig. 1A). The 5′ and 3′ sequences of these introns match known intron sequences from filamentous fungi (31). The putative ORF of Bcmfs1 has two ATG codons at the 5′ end. Hence, the first codon may not belong to the ORF of Bcmfs1.
The BLAST database (http://www.ncbi.nlm.nih.gov/BLAST/) provided by the National Center for Biotechnology Information demonstrated that Bcmfs1 is homologous to other MFS transporters, such as aflT from Aspergillus parasiticus (54.1% identity), CFP1 from C. kikuchii (36.6% identity), TOXA from C. carbonum (35.4% identity), and ORF10 from Aspergillus terreus (35.6% identity). Hydropathy analysis (http://www.ch.embnet.org/software/TMPRED_form.html) provided by the Swiss Institute of Bioinformatics predicts that Bcmfs1 has 14 transmembrane domains (data not shown).
Bcmfs1 single-replacement mutants.
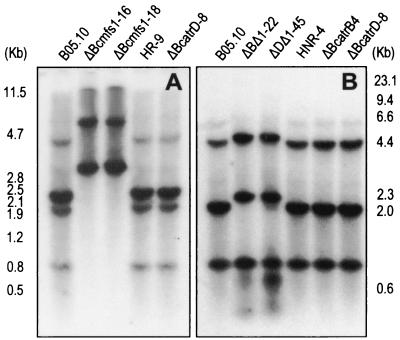
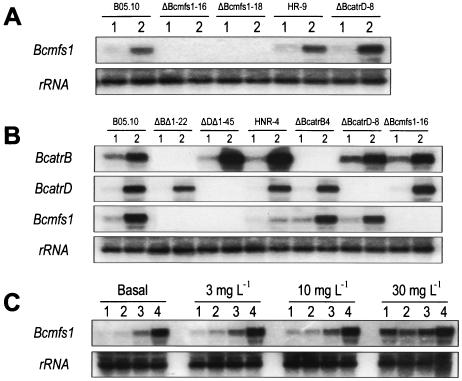
Protoplasts of B. cinerea strain B05.10 were transformed with linearized pΔ1-H (Fig. 1B). Thirty putative transformants were selected and purified by single-spore isolation. Southern blot analysis of genomic DNA from these transformants revealed that several strains were homokaryotic transformants with a site-specific integration, including ΔBcmfs1-16 and ΔBcmfs1-18 (Fig. 2A). The reference strain HR-9 carrying the OHT cassette and BcatrD replacement mutant ΔBcatrD-8 (17) showed the same bands as the parental strain, B05.10 (Fig. 2A). The expression of the Bcmfs1 replacement mutants ΔBcmfs1-16 and ΔBcmfs1-18 was investigated by Northern analysis with the EST fragment of Bcmfs1 (Fig. 1A) as a probe. The basal level of expression of Bcmfs1 in the parental strain B05.10, the reference strain HR-9, and ΔBcatrD-8 was low but strongly induced after treatment of germlings with oxpoconazole (30 mg liter−1) for 60 min. In ΔBcmfs1-16 and ΔBcmfs1-18, neither basal nor oxpoconazole-induced expression was observed (Fig. 3A).
FIG. 2.
Southern blot analysis of DNA from B. cinerea. Genomic DNA (5 μg) was digested with EcoRV and hybridized with a 5.5-kb XbaI-SalI probe (A) and with a 3.6-kb XbaI-ApaI probe (B). Results obtained with parental strain B05.10, Bcmfs1 replacement mutants ΔBcmfs1-16 and ΔBcmfs1-18, reference mutant HR-9, BcatrD replacement mutant ΔBcatrD-8, BcatrB and Bcmfs1 double-replacement mutant ΔBΔ1-22, BcatrD and Bcmfs1 double-replacement mutant ΔDΔ1-45, reference mutant HNR-4, and BcatrB replacement mutant ΔBcatrB4 are shown.
FIG. 3.
Northern blot analysis of total RNA (10 μg) from germlings of B. cinerea. (A) Parental strain B05.10, Bcmfs1 replacement mutants ΔBcmfs1-16 and ΔBcmfs1-18, reference mutant HR-9, and BcatrD replacement mutant ΔBcatrD-8. Basal levels of expression (lanes 1) and expression levels after treatment with 30 mg of oxpoconazole liter−1 (lanes 2) are shown. (B) Parental strain B05.10, BcatrB and Bcmfs1 double-replacement mutant ΔBΔ1-22, BcatrD and Bcmfs1 double-replacement mutant ΔDΔ1-45, reference mutant HNR-4, BcatrB replacement mutant ΔBcatrB4, BcatrD replacement mutant ΔBcatrD-8, and Bcmfs1 replacement mutant ΔBcmfs1-16. Basal levels of expression (lanes 1) and expression levels after treatment with 30 mg of oxpoconazole liter−1 (lanes 2) are shown. (C) Parental strain B05.10 (lanes 1) and the Bcmfs1 overexpression mutants OV1-23 (lanes 2), OV1-48 (lanes 3), and OV1-13 (lanes 4). Basal and induced expression levels after treatment with 3, 10, and 30 mg of oxpoconazole liter−1 are shown. RNA was hybridized with the EST probe specific for Bcmfs1 (Fig. 1A). Equal loading of lanes with RNA was checked by subsequently probing the same blot with 28S rRNA.
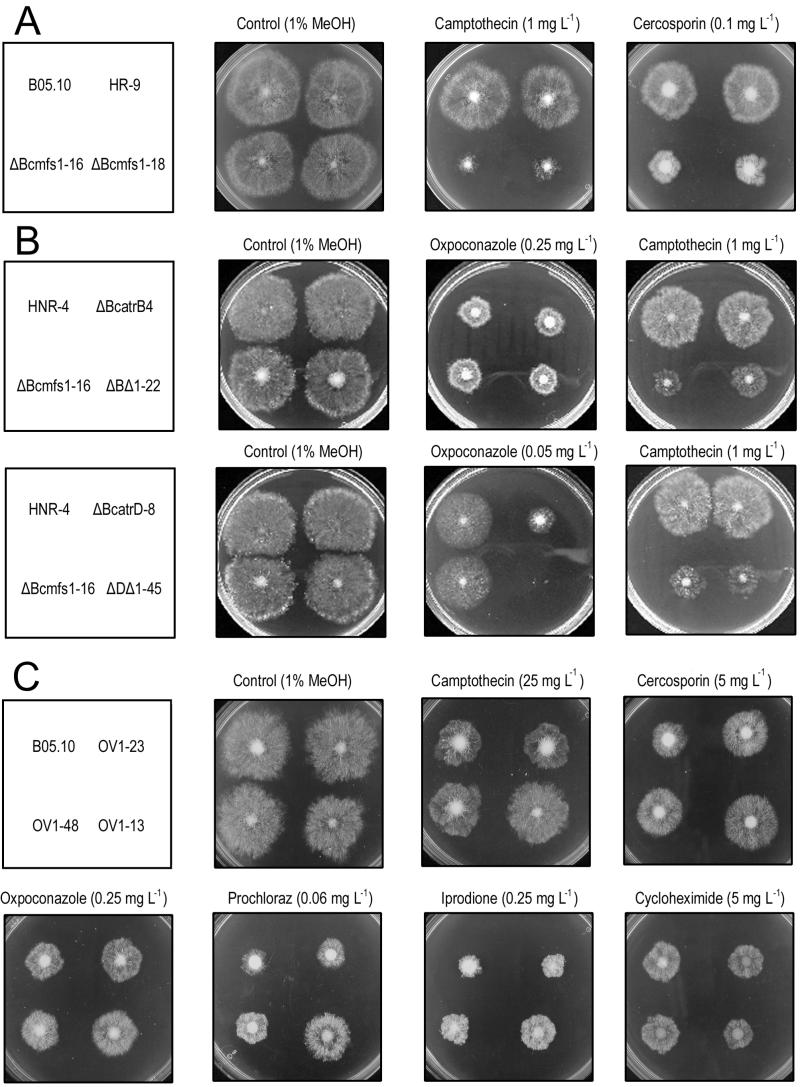
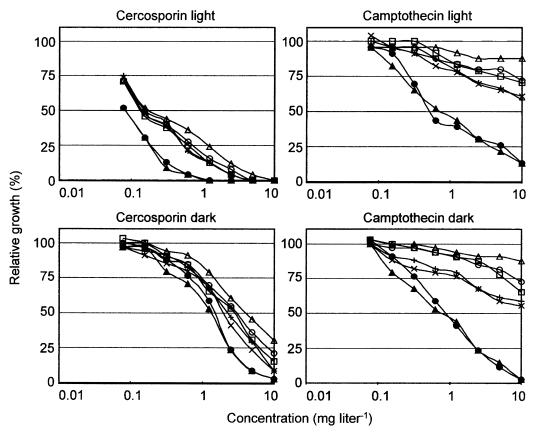
Radial growth tests demonstrated that ΔBcmfs1-16 and ΔBcmfs1-18 had an increased sensitivity to the alkaloid camptothecin (a fungitoxic compound from Camptotheca acuminata) and the perylenequinone cercosporin (a host-specific toxin produced by C. kikuchii), while the reference strain HR-9 displayed almost the same sensitivity to these compounds as the parental strain, B05.10 (Fig. 4A; Table 2). As expected, the fungitoxic activity of the photosensitizer cercosporin was higher in the light than in the dark (Fig. 5). The fungitoxic activity of other photosensitizers, such as cercosporin analogues (hypericin and pseudohypericin), rose bengal, toluidine blue, 8-methoxypsoralen, and alkaloids (barbaloin, colchicine, reserpine, and vincamine), was similar for all strains tested and was not influenced by light (data not shown). The activity of fungicides (captan, cyprodinil, fluazinam, iprodione, oxpoconazole, prochloraz, and tebuconazole) and other fungitoxic compounds (cycloheximide and rhodamine 6G) was similar for the wild-type strain and all Bcmfs1 single-replacement mutants tested (results shown only for ΔBcmfs1-16 in Table 2). Similar results were obtained with the botrydial analogues patchoulol or globulol (32) (data not shown).
FIG. 4.
Activity of compounds in radial growth experiments. (A) Parental strain B05.10, reference mutant HR-9, and Bcmfs1 replacement mutants ΔBcmfs1-16 and ΔBcmfs1-18. (B) Reference mutant HNR-4, single gene replacement mutants (ΔBcatrB4, ΔBcatrD-8, and ΔBcmfs1-16), and double gene replacement mutants (ΔBΔ1-22 and ΔDΔ1-45). (C) Wild-type strain B05.10 and Bcmfs1 overexpression mutants (OV1-23, OV1-48, and OV1-13).
TABLE 2.
Activity of compounds on radial growth of B. cinerea
| Chemical class | Compound | EC50 (mg liter−1)a of compound for:
|
|||
|---|---|---|---|---|---|
| B05.10 | ΔBcmfs1-16 | OV1-13 | HR-9 | ||
| DMIs | Oxpoconazole | 0.151 a | 0.189 a | 0.330 b | 0.161 a |
| Prochloraz | 0.031 ab | 0.025 a | 0.088 c | 0.032 ab | |
| Tebuconazole | 0.161 a | 0.151 a | 0.337 b | 0.136 a | |
| Anilinopyri- midine | Cyprodinil | 0.0029 a | 0.0022 a | 0.0060 b | 0.0025 a |
| Dicarboximide | Iprodione | 0.057 a | 0.054 a | 0.148 b | 0.056 a |
| Phthalimide | Captan | 6.29 a | 6.30 a | 12.6 b | 5.58 a |
| Phenylpyridyl- amine | Fluazinam | 0.0021 a | 0.0021 a | 0.0037 b | 0.0019 a |
| Antibiotic | Cycloheximide | 3.42 b | 3.55 b | 1.94 a | 3.10 ab |
| Xenobiotic | Rhodamine 6G | 1.53 ab | 1.88 b | 1.16 a | 1.47 ab |
Means followed by the same letters in the same rows indicate that figures do not differ significantly (P = 0.05).
FIG. 5.
Activity of cercosporin and camptothecin on relative growth of B. cinerea wild-type strain B05.10 (+), reference transformant HR-9 (×), two Bcmfs1 replacement mutants, ΔBcmfs1-16 (•) and ΔBcmfs1-18 (▴), and Bcmfs1 overexpression mutants OV1-23(□), OV1-48 (○), and OV1-13 (▵).
Double gene replacement mutants.
Protoplasts of the BcatrB replacement mutant ΔBcatrB4 (29) and the BcatrD replacement mutant ΔBcatrD-8 (17) were transformed with linearized pΔ1-N (Fig. 1C). Southern analysis of genomic DNA from the putative transformants digested with EcoRV revealed that ΔBΔ1-22 and ΔDΔ1-45 were homokaryotic transformants (Fig. 2B). Northern analysis of the transformants demonstrated that basal and oxpoconazole-induced expression of Bcmfs1 was not detectable in mutants ΔBΔ1-22 and ΔDΔ1-45 (Fig. 3B).
The sensitivity of the double-replacement mutant ΔDΔ1-45 to the DMI fungicide oxpoconazole was higher than that of the single-replacement mutant ΔBcatrD-8 (Fig. 4B; Table 3). This was not observed for the double-replacement mutant ΔBΔ1-22 (Fig. 4B). Differential activities against ΔDΔ1-45 and ΔBcatrD-8 were also observed with other DMI fungicides, such as fenarimol, prochloraz, pyrifenox, and tebuconazole (Table 3). In contrast, camptothecin did have activity similar to that of ΔBcmfs1-16, ΔBΔ1-22, and ΔDΔ1-45 (Fig. 4B).
TABLE 3.
Activity of compounds on radial growth of B. cinerea
| Chemical class | Compound | EC50 (mg liter−1)a of compound for:
|
|||
|---|---|---|---|---|---|
| HNR-4b | ΔBcatrD-8 | ΔBcmfs1-16 | ΔDΔ1-45 | ||
| DMIs | Oxpoconazole | 0.114 c | 0.035 b | 0.107 c | 0.017 a |
| Prochloraz | 0.026 c | 0.014 b | 0.016 b | 0.005 a | |
| Tebuconazole | 0.110 c | 0.087 b | 0.105 c | 0.035 a | |
| Pyrifenox | 0.046 b | 0.036 b | 0.044 b | 0.021 a | |
| Fenarimol | 0.869 c | 0.374 b | 0.780 c | 0.242 a | |
| Alkaloid | Camptothecin | >10 b | >10 b | 0.68 a | 0.70 a |
Means followed by the same letters in the same rows indicate that figures do not differ significantly (P = 0.05).
EC50 values of compounds for wild-type strain B05.10 and HNR-4 do not differ significantly (data not shown).
Bcmfs1 overexpression mutants.
Protoplasts of B. cinerea strain B05.10 were transformed with the plasmid carrying the full-length Bcmfs1 gene (Fig. 1A). The expression of Bcmfs1 in putative transformants was investigated by Northern blot analysis. Transformants with three different levels of expression were selected and arbitrarily classified as low (OV1-23), medium (OV1-48), and high (OV1-13) (Fig. 3C). Induced expression levels of Bcmfs1 in these transformants after treatment with oxpoconazole (3, 10, and 30 mg liter−1) for 60 min correlated with the basal levels of expressions (Fig. 3C).
Overexpression mutants possessed a decreased sensitivity to camptothecin, cercosporin, DMI fungicides, cyprodinil, iprodione, captan, and fluazinam compared to wild-type strain B05.10. Surprisingly, the same mutant showed an increased sensitivity to the antibiotic cycloheximide (Fig. 4C; Table 2).
Accumulation of oxpoconazole.
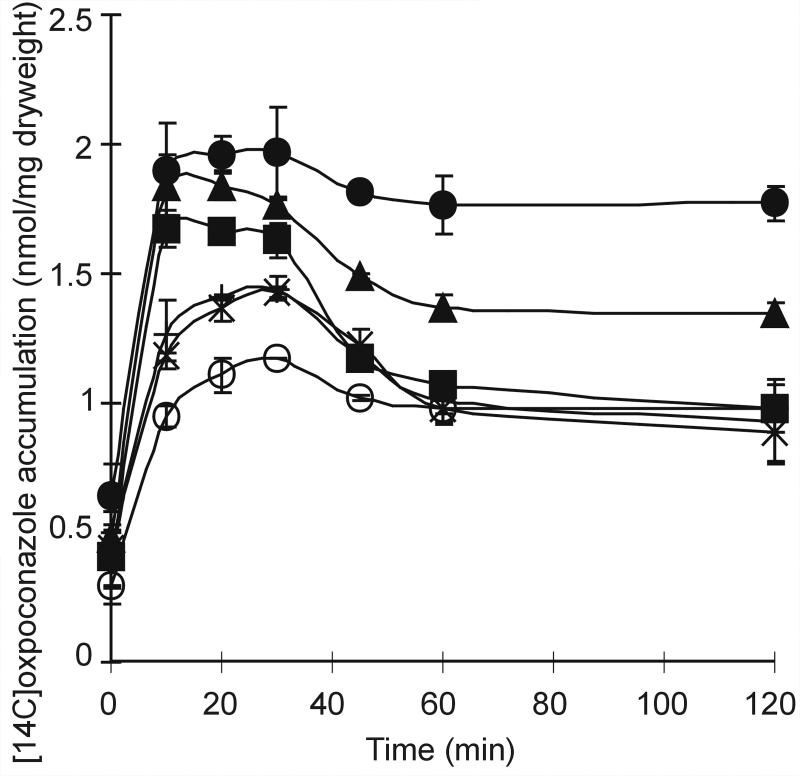
The accumulation of oxpoconazole by germlings of B. cinerea wild-type strain B05.10 was transient in time. The mutants tested also showed this phenomenon, though to a varying extent. The initial accumulation (up to 40 min of incubation) of oxpoconazole by all strains tested correlated with sensitivity to oxpoconazole in radial growth experiments (Fig. 6; Tables 2 and 3). The correlation coefficient (r2) between accumulation of oxpoconazole after 20 min of incubation and the EC50 of oxpoconazole for the different strains was calculated to be 0.813. Strikingly, the steady-state level of accumulation of oxpoconazole (after 60 min of incubation) by ΔBcatrD-8 and ΔDΔ1-45 always remained higher than that by all other strains tested (Fig. 6). Furthermore, the level of accumulation by ΔDΔ1-45 was significantly higher than that by ΔBcatrD-8.
FIG. 6.
Accumulation of oxpoconazole (30 μM) by germlings of B. cinerea wild-type strain B05.10 (+), reference transformant HNR-4 (×), BcatrD single-replacement mutants ΔBcatrD-8 (▴), Bcmfs1 single-replacement mutants ΔBcmfs1-16 (▪), BcatrD and Bcmfs1 double-replacement mutant ΔDΔ1-45 (•), and Bcmfs1 overexpression mutants OV1-13 (○).
Virulence assay.
Virulence of all mutants tested (ΔBcmfs1-16, ΔBcmfs1-18, ΔBΔ1-22, ΔDΔ1-45, OV1-23, OV1-48, OV1-13, HR-9, and HNR-4) on detached tomato leaves was similar to that of the parental strain, B05.10 (data not shown).
DISCUSSION
Bcmfs1 is a new member of the MFS genes family encoding transporters with 14 transmembrane domains. Phenotypic characterization of replacement and overexpression mutants indicated that the transporter provides protection against the alkaloid camptothecin, the photosensitizer cercosporin (a perylenequinone toxin), and DMI fungicides. Accumulation of the DMI fungicide oxpoconazole by germlings of these mutants and the parental strain, B05.10, and their sensitivity to oxpoconazole correlated with the expression of Bcmfs1. These results demonstrate that Bcmfs1 is a multidrug transporter involved in protection against a wide range of chemicals.
Bcmfs1 has a high homology with aflT from A. parasiticus, CFP1 from C. kikuchii, and TOXA from C. carbonum. The homology with CFP1 may reflect why both Bcmfs1 and CFP1 are involved in protection against cercosporin. Like Bcmfs1, MFS proteins from yeasts, such as BenR (15) and FLU1 (5) from C. albicans and FLR1 (1) from S. cerevisiae, transport DMI fungicides. However, these three yeast MFS proteins have a low level of homology to Bcmfs1, suggesting that there is no obvious relation between homology and substrate specificity.
The role of bcmfs1 in transport of DMI fungicides became obvious only after functional inactivation in a ΔBcatrD mutant (Fig. 4B; Table 3). A similar phenomenon has been reported with C. albicans for the ABC transporter CDR2, which showed a phenotype only in mutants with a ΔCDR1 background (28). This can be ascribed to redundancy of transporters with an overlap in substrate specificity. Previously, we demonstrated that BcatrD is the major transporter of DMI fungicides in B. cinerea (17). Hence, we assume that the lack of phenotype of ΔBcmfs1 mutants with respect to sensitivity to DMIs is due to compensating activity of BcatrD. However, such compensating activity does not seem to be accompanied by increased transcription of BcatrD, since basal and induced transcript levels of the gene were similar in strains B05.10 and ΔBcmfs1-16 (Fig. 3B). The conclusion that Bcmfs1 mediates transport of DMIs is supported by the observation that the Bcmfs1-overexpressing mutants showed a significant reduction in DMI sensitivity (Fig. 4C; Table 2). BcatrB is not a DMI transporter (29, 33). Still, expression of BcatrB is induced by treatment with DMI fungicides (16), indicating that inducers of expression of BcatrB are not necessarily a substrate of the encoded proteins. Similar phenomena have been described for other ABC genes (17, 29).
Bcmfs1-overexpressing mutants display reduced sensitivity to various unrelated fungicides (Fig. 4C; Table 2). This suggests that the multidrug transporter Bcmfs1 has a low substrate specificity for these products and that loss of the Bcmfs1 function in deletion mutants can be compensated for by other transporters. Mutant OV1-13 has a slightly increased sensitivity to cycloheximide (Fig. 4C; Table 2). A similar phenomenon was observed in multidrug-resistant mutants of A. nidulans with resistance to DMIs and increased sensitivity to dithiocarbamate fungicides and the antibiotic phleomycin (3). We hypothesize that the increased sensitivity displayed by the overexpression mutant could be due to the fact that MFS transporters function as not only efflux but also influx transporters (14).
The accumulation of oxpoconazole by germlings of B. cinerea was transient in time. The initial accumulation (up to 40 min) by OV1-13 was lower than that by B05.10 (Fig. 6), and the accumulation by all strains correlated with their sensitivity to the fungicide (Fig. 6; Tables 2 and 3). The steady-state level of oxpoconazole accumulation (after 60 min) by ΔDΔ1-45 was significantly higher than that by ΔBcatrD-8. These observations indicate that mutations in Bcmfs1 indeed functionally affect efflux of oxpoconazole. Besides BcatrD and Bcmfs1, additional transporters in B. cinerea may exist that play a role in efflux of DMI fungicides. This assumption is based on the observation that the double-replacement mutant ΔDΔ1-45 still displays a transient accumulation profile (Fig. 6), which suggests that efflux activity still proceeds to a weak extent. The transporter gene involved might be BcatrG and/or BcatrK, since expression of these genes was induced by DMI fungicides (16). Such a situation would indicate that multiple transporter proteins mediate the transport of a particular compound. A similar phenomenon has been described for C. albicans, which possesses at least four transporter genes involved in efflux of DMIs. These include the ABC transporter genes CDR1 (24) and CDR2 (27) and the MFS genes CaBenR (26) and FLU1 (5).
It is probable that B. cinerea developed transporter systems during evolution to cope with natural toxic compounds. However, in this context it is difficult to understand why camptothecin and cercosporin are substrates of Bcmfs1. Camptothecin is an alkaloid compound with antitumor activity isolated from Chinese tree C. acuminata. This plant is not known as a host of B. cinerea. It might be that plant species within the wide host range of B. cinerea contain the same or related alkaloids. Cercosporin is a natural photoactivated toxin produced by Cercospora species (9), and similar compounds are not known for B. cinerea. It is not likely that they are produced during pathogenesis, since necrotic symptoms incited by B. cinerea are light independent. Bcmfs1 is also not a general transporter of photosensitizers, as shown for Snq2 (34). Hence, the potency of Bcmfs1 for transport of cercosporin is hard to explain. A number of MFS transporters from filamentous fungi homologous to Bcmfs1 can function as virulence factors. This is reported for the cercosporin transporter from C. kikuchii (6), the HC toxin transporter from C. carbonum (23), and the transporter of trichothecenes from F. sporotrichioides (2). MFS genes involved in secretion of HC toxin and trichothecenes are located in a gene cluster carrying genes that encode enzymes involved in biosynthesis of these toxins. A role for Bcmfs1 in secretion of endogenous toxins is not obvious, since DNA sequences flanking Bcmfs1 did not reveal the presence of genes involved in toxin biosynthesis. Botrydial, produced by B. cinerea, is toxic to sweet pepper (10). This toxin might be a substrate of Bcmfs1, although disruption of Bcmfs1 did not increase the sensitivity to botrydial analogues, such as patchoulol and globulol (32). The virulence of all Bcmfs1 mutants tested on detached tomato leaves was similar to that of the parental strain, B05.10. For these reasons, the intrinsic function of Bcmfs1 is still obscure. Such a function might become obvious upon testing the virulence of replacement mutants on a wide range of host plants. These studies are being performed in current research, but so far no phenotype with respect to host virulence has been found. It is also possible that Bcmfs1 functions in protection against antibiotics produced by antagonistic microorganisms during its saprophytic phase of growth. Such a function has recently been reported for BcatrB of B. cinerea in protection against phenazine antibiotics produced by Pseudomonas species (30). This hypothesis is currently being tested for Bcmfs1 and other ABC and MFS transporters of B. cinerea.
Acknowledgments
We thank Tycho Vermeulen for amplifying the Bcmfs1 EST fragment, Alan Andrade and Lute-Harm Zwiers for fruitful discussions, Jan van Kan, Sander Schouten, and Arjen ten Have for advice on manipulation of B. cinerea, and Pierre de Wit for critical reading of the manuscript.
REFERENCES
- 1.Alarco, A. M., I. Balan, D. Talibi, N. Mainville, and M. Raymond. 1997. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J. Biol. Chem. 272:19304-19313. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, N. J., S. P. McCormick, and T. M. Hohn. 1999. TRI12, a trichothecene efflux pump from Fusarium sporotrichioides: Gene isolation and expression in yeast. Mol. Gen. Genet. 261:977-984. [DOI] [PubMed] [Google Scholar]
- 3.Andrade, A. C., G. Del Sorbo, J. G. M. van Nistelrooy, and M. A. De Waard. 2000. The ABC transporter AtrB from Aspergillus nidulans mediates resistance to all major classes of fungicides and some natural toxic compounds. Microbiology 146:1987-1997. [DOI] [PubMed] [Google Scholar]
- 4.Buttner, P., F. Koch, K. Voigt, T. Quidde, S. Risch, R. Blaich, B. Bruckner, and P. Tudzynski. 1994. Variations in ploidy among isolates of Botrytis cinerea: implications for genetic and molecular analyses. Curr. Genet. 25:445-450. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese, D., J. Bille, and D. Sanglard. 2000. A novel multidrug efflux transporter gene of the major facilitator super family from Candida albicans (FLU1) conferring resistance to fluconazole. Microbiology 146:2743-2754. [DOI] [PubMed] [Google Scholar]
- 6.Callahan, T. M., M. S. Rose, M. J. Meade, M. Ehrenshaft, and R. G. Upchurch. 1999. CFP, the putative cercosporin transporter of Cercospora kikuchii, is required for wild type cercosporin production, resistance, and virulence on soybean. Mol. Plant-Microbe Interact. 12:901-910. [DOI] [PubMed] [Google Scholar]
- 7.Chapeland, F., R. Fritz, C. Lanen, M. Gredt, and P. Leroux. 1999. Inheritance and mechanisms of resistance to anilinopyrimidine fungicides in Botrytis cinerea (Botryotinia fuckeliana). Pestic. Biochem. Physiol. 64:85-100. [Google Scholar]
- 8.Coley-Smith, J. R., K. Verhoeff, and W. R. Jarvis. 1980. The biology of Botrytis. Academic Press, Inc., London, United Kingdom.
- 9.Daub, M. E., and M. Ehrenshaft. 2000. The photoactivated Cercospora toxin cercosporin: contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 38:461-490. [DOI] [PubMed] [Google Scholar]
- 10.Deighton, N., I. Muckenschnabel, A. J. Colmenares, I. G. Collado, and B. Williamson. 2001. Botrydial is produced in plant tissues infected by Botrytis cinerea. Phytochemistry 57:689-692. [DOI] [PubMed] [Google Scholar]
- 11.Del Sorbo, G., A. C. Andrade, J. G. M. van Nistelrooy, J. A. L. van Kan, E. Balzi, and M. A. De Waard. 1997. Multidrug resistance in Aspergillus nidulans involves novel ATP-binding cassette transporters. Mol. Gen. Genet. 254:417-426. [DOI] [PubMed] [Google Scholar]
- 12.Del Sorbo, G., H. Schoonbeek, and M. A. De Waard. 2000. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fung. Genet. Biol. 30:1-15. [DOI] [PubMed] [Google Scholar]
- 13.De Waard, M. A., and J. G. M. van Nistelrooy. 1988. Accumulation of SBI fungicides in wild-type and fenarimol-resistant isolates of Penicillium italicum. Pestic. Sci. 22:371-382. [Google Scholar]
- 14.Driessen, A. J. M., B. P. Rosen, and W. N. Konings. 2000. Diversity of transport mechanisms: common structural principles. Trends Biochem. Sci. 25:397-401. [DOI] [PubMed] [Google Scholar]
- 15.Fling, M. E., J. Kopf, A. Tamarkin, J. A. Gorman, H. A. Smith, and Y. Koltin. 1991. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol. Gen. Genet. 227:318-329. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi, K., H. Schoonbeek, H. Sugiura, and M. A. De Waard. 2001. Multidrug resistance in Botrytis cinerea associated with decreased accumulation of the azole fungicide oxpoconazole and increased transcription of the ABC transporter gene BcatrD. Pestic. Biochem. Physiol. 70:168-179. [Google Scholar]
- 17.Hayashi, K., H. Schoonbeek, and M. A. De Waard. 2002. Expression of the ABC transporter BcatrD from Botrytis cinerea reduces sensitivity to sterol demethylation inhibitor fungicides. Pestic. Biochem. Physiol. 73:110-121. [Google Scholar]
- 18.Kolaczkowski, M., A. Kolaczowska, J. Luczynski, S. Witek, and A. Goffeau. 1998. In vivo characterization of the drug resistance profile of the major ABC transporters and other components of the yeast pleiotropic drug resistance network. Microb. Drug Resist. 4:143-158. [DOI] [PubMed] [Google Scholar]
- 19.Leroux, P., F. Chapeland, D. Desbrosses, and M. Gredt. 1999. Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop Prot. 18:687-697. [Google Scholar]
- 20.Nakaune, R., K. Adachi, O. Nawata, M. Tomiyama, K. Akutsu, and T. Hibi. 1998. A novel ATP-binding cassette transporter involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Appl. Environ. Microbiol. 64:3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitkin, J. W., D. G. Panaccione, and J. D. Walton. 1996. A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology 142:1557-1565. [DOI] [PubMed] [Google Scholar]
- 24.Prasad, R., P. De Wergifosse, A. Goffeau, and E. Balzi. 1995. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27:320-329. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1996. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 40:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 29.Schoonbeek, H., G. Del Sorbo, and M. A. De Waard. 2001. The ABC transporter BcatrB affects the sensitivity of Botrytis cinerea to the phytoalexin resveratrol and the fungicide fenpiclonil. Mol. Plant-Microbe Interact. 14:562-571. [DOI] [PubMed] [Google Scholar]
- 30.Schoonbeek, H., J. M. Raaijmakers, and M. A. De Waard. Fungal ABC transporters and microbial interactions in natural environments. Mol. Plant-Microbe Interact., in press. [DOI] [PubMed]
- 31.Unkles, S. E. 1992. Gene organization in industrial filamentous fungi, p. 28-53. In J. R. Kinghorn and G. Turner (ed.), Applied molecular genetics of filamentous fungi. Blackie, Glasgow, United Kingdom.
- 32.Vallejo, I., L. Rebordinos, I. G. Collado, and J. M. Cantoral. 2001. Differential behavior of mycelial growth of several Botrytis cinerea strains on either patchoulol- or globulol-amended media. J. Phytopathol. 149:113-118. [Google Scholar]
- 33.Vermeulen, T., H. Schoonbeek, and M. A. De Waard. 2001. The ABC transporter BcatrB from Botrytis cinerea is a determinant of the activity of the phenylpyrrole fungicide fludioxonil. Pest Manag. Sci. 57:393-402. [DOI] [PubMed] [Google Scholar]
- 34.Ververidis, P., F. Davrazou, G. Diallinas, D. Georgakopoulos, A. K. Kanellis, and N. Panopoulos. 2001. A novel putative reductase (Cpd1p) and the multidrug exporter Snq2p are involved in resistance to cercosporin and other singlet oxygen-generating photosensitizers in Saccharomyces cerevisiae. Curr. Genet. 39:127-136. [DOI] [PubMed] [Google Scholar]