Abstract
Numerous Salmonella enterica and Escherichia coli O157:H7 outbreaks have been associated with contaminated sprouts. We examined how S. enterica serovars, E. coli serotypes, and nonpathogenic bacteria isolated from alfalfa sprouts grow on and adhere to alfalfa sprouts. Growth on and adherence to sprouts were not significantly different among different serovars of S. enterica, but all S. enterica serovars grew on and adhered to alfalfa sprouts significantly better than E. coli O157:H7. E. coli O157:H7 was essentially rinsed from alfalfa sprouts with repeated washing steps, while 1 to 2 log CFU of S. enterica remained attached per sprout. S. enterica Newport adhered to 3-day-old sprouts as well as Pantoea agglomerans and 10-fold more than Pseudomonas putida and Rahnella aquatilis, whereas the growth rates of all four strains throughout seed sprouting were similar. S. enterica Newport and plant-associated bacteria adhered 10- to 1,000-fold more than E. coli O157:H7; however, three of four other E. coli serotypes, isolated from cabbage roots exposed to sewage water following a spill, adhered to sprouts better than E. coli O157:H7 and as well as the Pseudomonas and Rahnella strains. Therefore, attachment to alfalfa sprouts among E. coli serotypes is variable, and nonpathogenic strains of E. coli to be used as surrogates for the study of pathogenic E. coli may be difficult to identify and should be selected carefully, with knowledge of the biology being examined.
Numerous food-borne diseases caused by Salmonella enterica and Escherichia coli serovar O157:H7 have been associated with contaminated alfalfa, clover, and bean sprouts (3, 13, 15, 17-19, 24, 27). For food production, seeds are grown into sprouts at ambient temperature in trays or rotating drums and are watered regularly during sprouting. The constant moisture, nutrients released by the sprouting seeds, and warm temperatures are conducive to the growth of human bacterial pathogens such as S. enterica and E. coli O157:H7 (1, 4, 5, 8, 11, 22).
Numerous studies have reported the growth of S. enterica and E. coli O157:H7 on sprouting seeds. We recently demonstrated that S. enterica strains grow to significantly higher levels on sprouting alfalfa seeds than E. coli O157:H7 when irrigation water is regularly refreshed (5). Our results suggested that S. enterica might reach higher numbers of bacteria on alfalfa sprouts in part because it adheres better to alfalfa sprouts and thus is not washed from the sprouts when the sprouts are irrigated. In this study, we have compared the adherence to alfalfa sprouts of the human pathogens S. enterica and E. coli and the plant-associated bacteria Rahnella aquatilis (10), Pseudomonas putida (7), and Pantoea agglomerans (10). We also have compared how these human pathogens and plant-associated bacteria colonize sprouting alfalfa seeds.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
Strains used in this study are listed in Table 1. S. enterica serovar Newport 96E01153C-TX and E. coli F4546 are clinical isolates from sprout-related outbreaks and were chosen as representative strains for the majority of experiments. The plant-associated bacteria R. aquatilis, P. putida, and P. agglomerans were isolated from commercially produced sprouts obtained directly from sprouting facilities prior to packaging. Bacteria were grown in, or plated on, Luria-Bertani (LB) or sorbitol-MacConkey medium. All media were obtained from Difco/BBL (Sparks, Md.). Antibiotics were obtained from Sigma (St. Louis, Mo.) and, when required, were incorporated into the medium at the following concentrations: kanamycin, 40 mg/liter; ampicillin, 100 mg/liter. Plasmid pKT-kan, in which a 131-bp nptII promoter fragment from Tn5 was fused to the green fluorescent protein gene (gfp) of plasmid pPROBE-KT, is a stable, broad-host-range vector that confers kanamycin resistance and green fluorescent protein expression (14). Plasmid pKT-kan was transformed into all strains listed in Table 1.
TABLE 1.
Bacterial strains used in this study
| Species, serovar, and/or serotype | Strain (USDA/ARS/PSM no.) | Descriptiona | Reference(s) or source |
|---|---|---|---|
| S. enterica serovar Baildon | 99A 23 (2247) | D2, clinical isolate, outbreak associated with tomatoes | S. Abbott, Microbial Diseases Laboratory, California Health Services |
| S. enterica serovar Cubana | 98A 9878 (1957) | G2, clinical isolate, outbreak associated with alfalfa sprouts | 15 |
| S. enterica serovar Havana | 98A 4399 (1958) | G2, clinical isolate, outbreak associated with alfalfa sprouts | 15 |
| S. enterica serovar Mbandaka | 99A1670 (1955) | C1, alfalfa seed isolate | California Health Services |
| S. enterica serovar Newport | 96E01152C-TX (1655) | C2, alfalfa sprouts isolate | 12, 27 |
| 2000-8384 (2362) | NVSL, Arizona, cattle isolate | Kathy Ferris | |
| 2000-6412 (2363) | NVSL, Colorado, cattle isolate | Kathy Ferris | |
| 2000-8892 (2364) | NVSL, Iowa, cattle isolate | Kathy Ferris | |
| 2000-6458 (2366) | NVSL, Kansas, cattle isolate | Kathy Ferris | |
| 2000-7535 (2365) | NVSL, Indiana, cattle isolate | Kathy Ferris | |
| S. enterica serovar Poona | 00A 3563 (2350) | G1, clinical isolate, outbreak associated with cantaloupe | California Health Services |
| S. enterica serovar Schwarzengrund | 96 E01152C-TX (1654) | B, alfalfa seed isolate, associated with an S. enterica serovar Newport outbreak, 1995-1996b | 12, 27 |
| E. coli O157:H7 | F4546 (2850) | Clinical isolate, outbreak associated with sprouts, 1997 | 6 |
| 96A 13466 (1239) | Clinical isolate, outbreak associated with apple cider | California Health Services | |
| C7927 | Clinical isolate, outbreak associated with apple cider | 26 | |
| H2439 | Clinical isolate, outbreak associated with apple cider | Timothy Barrett, Centers for Disease Control and Prevention | |
| 86-24 | Clinical isolate, outbreak associated with ground beef | 9 | |
| EDL933 (1272) | 4F, meat isolate | 16, 20 | |
| E. coli O13(w):H? | MW416 (2370) | Cabbage root isolate | 28 |
| E. coli O150:H? | MW418 (2372) | Cabbage root isolate | 28 |
| E. coli O137:H41 | MW421 (2375) | Cabbage root isolate | 28 |
| E. coli O?:H8,23,41 | MW424 (2378) | Cabbage root isolate | 28 |
| Pantoea agglomerans | SPS2F1 | Alfalfa sprout isolate | A. O. Charkowski et al., unpublished data |
| Pseudomonas putida | BM19 | Alfalfa sprout isolate | A. O. Charkowski et al., unpublished data |
| Rahnella aquatilis | SPS2F10 | Alfalfa sprout isolate | A. O. Charkowski et al., unpublished data |
NVSL, National Veterinary Services Laboratory.
Three S. enterica serovars were isolated from this seed lot, but only S. enterica serovar Newport was isolated from human patients.
Alfalfa seed sprouting.
Seeds for sprouting were obtained from International Specialty Supply (Cookeville, Tenn.), treated by continuous stirring in 3% (wt/vol) calcium hypochlorite (Fisher Scientific, Springfield, N.J.) at a ratio of 1 g of seeds to 5 ml of calcium hypochlorite for 15 min, and rinsed three times with sterile water. Approximately 50 seeds (approximately 0.1 g) were placed in a sterile 100- by 15-mm polystyrene petri plate (Fisher Scientific) with 20 ml of water and incubated at 25°C on a rotating shaker at 40 rpm. The water in which the seeds were sprouted was replaced daily with 20 ml of fresh sterile water.
Attachment assay.
Ten 3- to 5-day-old alfalfa sprouts, germinated as described above, were placed in 50-ml polystyrene tubes (Fisher Scientific). Bacteria from an 18-h culture grown on LB plates were diluted in sterile water, and 20 ml of various inocula were added to the sprouts. The inoculum levels were determined by plating 100 μl of each on LB plates containing kanamycin for strains with pKT-kan. The plates were incubated at 37°C overnight, and colonies were counted. Tubes of inoculated sprouts were shaken horizontally at 40 rpm in a Multitron orbital shaking incubator (ATR, Laurel, Md.) at 25°C for 4 h.
To determine the numbers of CFU per rinse and CFU per sprout, the inoculum was decanted to a sterile test tube and sprouts were rinsed by adding 10 ml of sterile water, gently shaking the polystyrene tube for 30 s, and decanting the liquid into a sterile test tube. Sprouts were rinsed three times, and individuals were placed in 500 μl of 1× phosphate-buffered saline (pH 7.4) and homogenized with a pestle connected to an electric drill (Black and Decker, Hampstead, Md.) or a MINIMITE cordless tool (Dremel, Racine, Wis.). The homogenate (500 μl) and rinse solutions (100 μl) were plated onto LB agar containing kanamycin and incubated at 37°C for 24 h, and colonies were enumerated. Five samples were examined per inoculum level for each experiment, and all experiments were repeated at least three times.
Growth assay.
Alfalfa seeds were surface disinfested as described above, and the irrigation water was removed from the petri plates after 1 h and replaced with 20 ml of a solution of 106 CFU of bacteria per ml suspended in sterile water. The inoculum was removed from the petri plates after approximately 3 to 4 h and replaced with 20 ml of sterile water. Seeds were incubated at 25°C on a rotating shaker for 3 to 4 days, and the irrigation water was replaced daily with fresh sterile water. Sprout samples were taken daily after changing the water, and the number of CFU per sprout was determined as described above. The homogenates were plated onto LB agar containing kanamycin or onto sorbitol-MacConkey agar (for E. coli without pKT-kan). Three samples were examined per time point for each experiment, and all experiments were repeated at least three times.
Statistics.
Statistical analysis of the data was done with SAS PROC MIXED (version 8.2; SAS Institute Inc., Carey, N.C.) to allow estimation of different variances among strains or groups of strains when heterogeneity was significant (likelihood ratio test; P < 0.05). Linear models were fitted on log of average CFU versus log of inoculum, allowing both slopes and intercepts to vary among strains. When slopes did not differ (F test; P < 0.05), the model was reduced to one having a common slope. Strain comparisons were made, either among slopes or among response averages, with probability levels adjusted by the method of Tukey, Dunnett, or Bonferroni, depending on the type and number of comparisons being estimated.
RESULTS
S. enterica attaches as well as plant-associated bacteria and significantly better than E. coli to alfalfa sprouts.
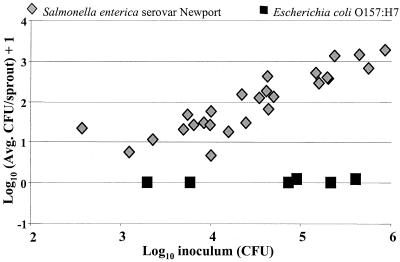
All of the strains used in this study were transformed with pKT-kan, a plasmid that confers kanamycin resistance and gfp expression. Alfalfa sprouts were inoculated with S. enterica serovar Newport(pKT-kan) 96E01153C-TX or E. coli F4546(pKT-kan) and incubated for 4 h at 25°C, and the number of bacteria attached to sprouts was determined. For all inoculum levels tested, higher populations of S. enterica serovar Newport(pKT-kan) than of E. coli F4546(pKT-kan) were recovered from rinsed alfalfa sprouts (Fig. 1) (P < 0.01). Moreover, we observed that S. enterica serovar Newport attached to sprouts in a linear manner over the four log units of inoculum tested (r2 = 0.82) (Fig. 1). Attachment assays were conducted with S. enterica serovar Newport 96E01153C-TX and E. coli F4546 to determine if the plasmid pKT-kan affected attachment on alfalfa sprouts. There was no significant difference in the total number of bacteria on sprouts for S. enterica serovar Newport and E. coli F4546 with or without the plasmid (data not shown).
FIG. 1.
Recovery (CFU per sprout) of S. enterica serovar Newport 96E01153C-TX and E. coli F4546 from 3-day-old alfalfa sprouts following 4-h adhesion assays. The experiment was repeated at least three times for each strain. Each experiment had multiple inoculation levels, and five sprout samples were taken for each level.
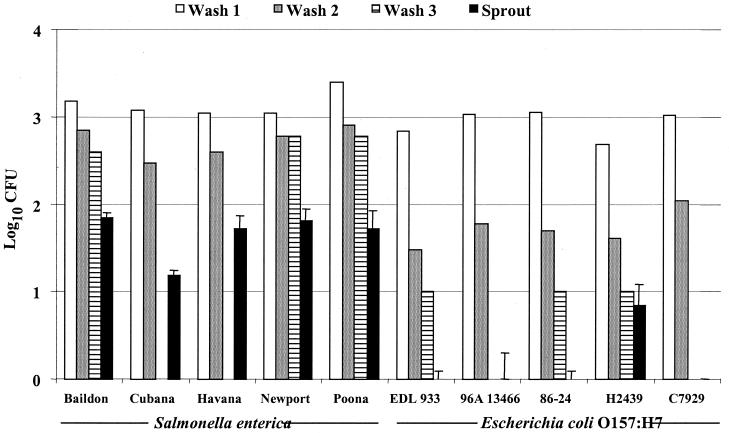
To examine whether the inability of E. coli F4546(pKT-kan) to attach to sprouts was unique to this particular strain, alfalfa sprouts were inoculated with five additional strains of E. coli O157:H7 (see Table 1 for strain details). For all inoculum levels tested, there was no significant difference in the number of CFU of bacteria attached to sprouts among the five different E. coli O157:H7 strains tested (F4546, 96A 13466, C7927, H2439, 86-24, and EDL 933) (Fig. 2) (P = 0.89). Moreover, five different strains of S. enterica Newport were tested (2000-8384, 2000-6412, 2000-8892, 2000-7535, and 2000-6458), and there was no significant difference in the number of bacteria attached to alfalfa sprouts (P = 0.86) (data not shown). In addition, alfalfa sprouts were inoculated with six other S. enterica serovars, including Baildon, Cubana, Havana, Mbandaka, Poona, and Schwarzengrund, and there was no significant difference in the number of CFU attached to sprouts among the six different serovars of S. enterica (Fig. 2) (P = 0.28). However, comparison among experiments with similar inocula revealed differences in the populations of bacteria which are removed in the rinse solutions between S. enterica and E. coli O157:H7 but not among the serovars of S. enterica or strains of E. coli O157:H7. Furthermore, most E. coli O157:H7 cells were removed from the sprouts by the second 10-ml rinse (Fig. 2).
FIG. 2.
Recovery (CFU per sprout and CFU per rinse) of S. enterica serovars Baildon, Cubana, Havana, Newport 96E01153C-TX, and Poona and E. coli EDL933, 1239, 86-24, H2439, and C7927 from 3-day-old alfalfa sprouts following 4-h adhesion assays. Data represent those from a typical experiment with an inoculation level of 103 CFU, with three sprout samples taken for each strain. Error bars indicate standard deviations. The experiment was repeated at least three times for each strain.
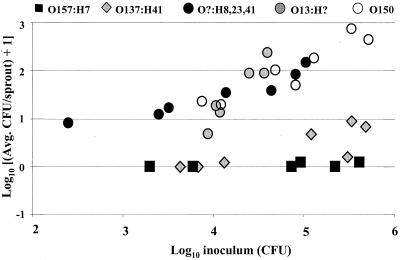
To determine whether the inability to attach to alfalfa sprouts was unique to E. coli O157:H7, alfalfa sprouts were inoculated with four additional serotypes of E. coli isolated from plant tissue (see Table 1 for strain details) and the number of bacteria attached to sprouts was determined. For all inoculum levels tested, the number of CFU recovered from the sprouts of E. coli serotypes O?:H8,23,41, O13:H?, and O150 was significantly higher (P < 0.01) than for E. coli O157:H7 (strain F4546) (Fig. 3). For low inoculum levels (103 to 104 CFU), E. coli O137:H41 and O157:H7 attached in a similar manner; however, at higher inoculum levels (105 to 106 CFU), E. coli O137:H41 attached at significantly higher levels (P < 0.01) than O157:H7.
FIG. 3.
Recovery (CFU per sprout) of E. coli F4546, MW421, MW424, MW416, and MW418 from 3-day-old alfalfa sprouts following 4-h adhesion assays. The experiment was repeated at least three times for each strain. Each experiment had multiple inoculation levels, and five sprout samples were taken for each level.
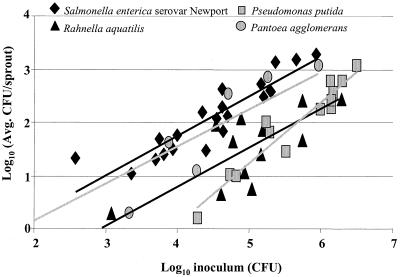
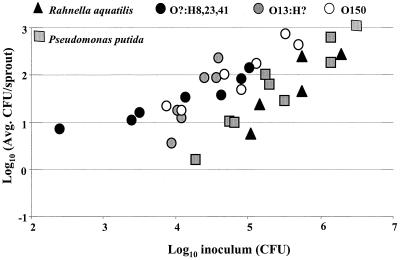
In order to compare S. enterica attachment to bacteria that are commonly associated with plants, alfalfa sprouts were also inoculated with three bacterial strains that had been isolated from alfalfa sprouts and transformed with pKT-kan: P. putida(pKT-kan), P. agglomerans(pKT-kan), or R. aquatilis(pKT-kan). The number of P. agglomerans(pKT-kan) bacteria attached to sprouts was higher than that of either P. putida(pKT-kan) or R. aquatilis(pKT-kan) (P = 0.005), both of which attached similarly (P = 0.69) (Fig. 4). At every inoculum level tested, significantly higher numbers of S. enterica serovar Newport(pKT-kan) 96E01153C-TX than of P. putida(pKT-kan) and R. aquatilis(pKT-kan) attached to alfalfa sprouts (Fig. 4) (P < 0.05). However, there was no significant difference between the number of CFU of S. enterica serovar Newport(pKT-kan) 96E01153C-TX and P. agglomerans(pKT-kan) (P = 0.62). Moreover, the numbers of attached E. coli serotype O?:H8,23,41, O13:H?, and O150 bacteria were similar to those of P. putida(pKT-kan) and R. aquatilis(pKT-kan) at all inocula tested (Fig. 5) (P < 0.01).
FIG. 4.
Recovery (CFU per sprout) of S. enterica serovar Newport 96E01153C-TX, P. putida, P. agglomerans, and R. aquatilis from 3-day-old alfalfa sprouts following 4-h adhesion assays. The experiment was repeated at least three times for each strain. Each experiment had multiple inoculation levels, and five sprout samples were taken for each level.
FIG. 5.
Recovery (CFU per sprout) of P. putida, P. agglomerans, R. aquatilis, and E. coli MW421, MW424, MW416, and MW418 from 3-day-old alfalfa sprouts following 4-h adhesion assays. The experiment was repeated at least three times for each strain. Each experiment had multiple inoculation levels, and five sprout samples were taken for each level.
Growth of plant-associated bacteria, S. enterica, and serotypes of E. coli on alfalfa sprouts.
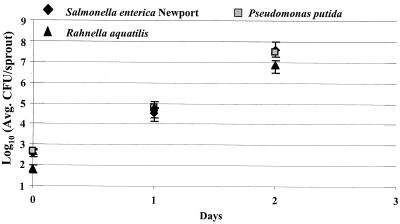
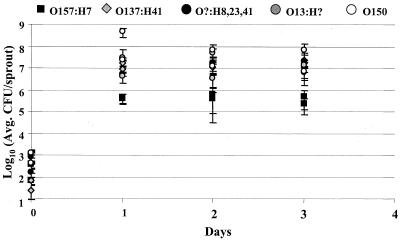
Alfalfa seeds were inoculated with each strain of plant-associated bacteria or E. coli and sprouted for 3 days, and samples were tested daily to determine if growth on alfalfa sprouts was correlated with the observed attachment differences. There was no significant difference in growth among plant-associated bacteria, P. putida (BM19), P. agglomerans (SPS2F1), and R. aquatilis (SPS2F10). Growth of the plant-associated bacteria was similar to that of S. enterica serovar Newport 96E01153C-TX (Fig. 6). E. coli O157:H7 was reduced in growth compared to the other E. coli serotypes tested (Fig. 7), but its growth was similar to that in earlier experiments (5).
FIG. 6.
Recovery (CFU per sample) of S. enterica serovar Newport 96E01153C-TX, P. putida, and R. aquatilis from sprouting alfalfa following a 4-h inoculation period and growth over 2 days. Data represent those from a typical experiment The experiment was repeated at least three times, and three sprout samples were taken for each strain. Error bars indicate standard deviations.
FIG. 7.
Recovery (CFU per sample) of E. coli F4546, MW421, MW424, MW416, and MW418 from sprouting alfalfa following a 4-h inoculation period and growth over 3 days. Data represent those from a typical experiment. The experiment was repeated at least three times, and three sprout samples were taken for each strain. Error bars indicate standard deviations.
DISCUSSION
In a naturally contaminated alfalfa seed lot epidemiologically linked to a food-borne disease outbreak, it was estimated that approximately 1 most probable number of S. enterica in 100 g of seed (approximately 1 in 40,000 seeds) actually harbored the pathogen (12). However, as the seeds are germinated, the S. enterica spread through the irrigation water to contaminate the entire batch of sprouts. This hypothesis is supported by multiple reports of human pathogen contamination of sprouts without isolation of the pathogens from seed but with epidemiological data to implicate the seed (3, 13, 15, 19, 27). These reports are substantiated by laboratory observation of S. enterica recovered from previously sterile irrigation water used to irrigate contaminated seed. In fact, testing of irrigation water is the recommended method for testing sprouts for human pathogens. Our attachment assays were designed to explore the ability of human pathogens, which spread among sprouting seeds via water contaminated by a small number of contaminated seeds, to adhere to previously uncontaminated sprouts and not be removed by rinsing steps. We have demonstrated that S. enterica, some E. coli serotypes, and plant-associated bacteria colonize and adhere to alfalfa sprouts and that there are differences in their ability to attach. The number of bacteria attached to the sprouts increased with the inoculum for all of the bacterial strains tested except the E. coli O157:H7 strains. The 4-h attachment assay does not differentiate between bacterial attachment to plant tissue or other bacterial cells; however, from a food safety perspective, the result on human health is inconsequential.
Fewer than 10 CFU of E. coli O157:H7 per sprout were associated with individual alfalfa sprouts regardless of the inoculum level, suggesting that E. coli O157:H7 strains are not able to attach to alfalfa sprouts as well as S. enterica serovars and plant-associated bacteria. Moreover, our data demonstrate the removal of most E. coli O157:H7 cells from association with sprouts following gentle rinsing. Previously, we demonstrated that S. enterica serovars grew on alfalfa sprouts significantly better than E. coli O157:H7 with frequent irrigation (5). These results suggest that S. enterica has an advantage over E. coli O157:H7 for attachment to sprouting seeds and 3-day-old sprouts, and this result could partially explain why the majority of sprout-associated outbreaks have been caused by S. enterica (2). One distinct difference between S. enterica and E. coli O157:H7 is the ability to produce aggregative fimbriae (curli). Both genera have curli genes; however, single-base-pair csgD promoter mutations leave ≥95% of E. coli O157:H7 without curli (25). Curli may play a role in the attachment of S. enterica to sprouts, as curli are induced in an environment similar to a plant surface, low temperature, and low osmolarity (21).
In the 4-h attachment assay, S. enterica attached to 3-day-old sprouts as well as a P. agglomerans strain did and 10-fold more than P. putida and R. aquatilis strains did, whereas the growth rates of all four strains throughout seed sprouting were similar. These results demonstrate that bacterial attachment to 3-day- old sprouts is not predictive of the ability to colonize sprouts and suggest that additional attachment mechanisms are used when bacteria grow in the presence of sprouting seeds over several days. Earlier reports of S. enterica serovar Typhimurium attachment to lettuce leaves at numbers similar to those for Pseudomonas fluorescens, a common plant epiphyte (23), may have been hindered in their ability to distinguish the effects of bacterial growth from initial attachment. Our 4-h attachment assay aimed to model the ability of S. enterica released from contaminated seed into irrigation water to attach to 3-day-old sprouts. Our data revealed no significant difference in the abilities of S. enterica Newport strains or S. enterica serovars isolated from different hosts, animal or plant, to attach to alfalfa sprouts. Furthermore, the linear relationship between inoculum levels and populations attached to sprouts suggests that S. enterica utilizes both attachment sites on the sprout and attachment to bound bacterial cells, therefore establishing an infinite number of colonization sites for itself.
Although E. coli O157:H7 was severely limited in its ability to adhere to sprouts, three of four other E. coli serotypes isolated recently from cabbage roots attached to sprouts as well as the P. putida and R. aquatilis strains. These results confirm that there are biological differences among E. coli serotypes and are consistent with results of attachment studies with lettuce seedlings (28). Therefore, these studies demonstrate that nonpathogenic strains of E. coli to be used as surrogates for the study of pathogenic E. coli may be difficult to identify and should be selected carefully, with knowledge of the biology being examined.
Although these results show that E. coli O157:H7 can be easily rinsed from 3-day-old alfalfa sprouts, they do not suggest a diminished risk of human infection by E. coli O157:H7 in association with alfalfa sprouts. Earlier work (5) clearly shows that small populations of E. coli O157:H7 (i.e., 100 to 101 CFU) can grow to high populations over several days, depending on environmental conditions that are likely to occur between production and consumption. With regard to the sprout producer, our data may suggest that generous washing could rinse most E. coli O157:H7 cells from contaminated sprouts; however, if the seed contamination was high, again small populations could persist or multiply in transport or storage and therefore continue to be a risk for human infection. Therefore, this work highlights differences among the biologies of S. enterica, E. coli, and plant-associated bacteria in association with alfalfa sprouts; nevertheless, the risk of human infection remains.
Acknowledgments
We thank Sharon Abbott, California Department of Health Services, Berkeley; Marian Wachtel, USDA-ARS Beltsville Area Research Center, Beltsville, Md.; and Kathy Ferris, USDA-ARS Animal Health Systems Research, Clay Center, Nebr., for providing strains. We thank William Miller for providing plasmid pKT-kan and Robert Mandrell, USDA-ARS WRRC, Albany, Calif., for critical review of the manuscript.
REFERENCES
- 1.Andrews, W. H., P. B. Mislivec, C. R. Wilson, V. R. Bruce, P. L. Poelma, R. Gibson, M. W. Trucksess, and K. Young. 1982. Microbial hazards associated with bean sprouting. J. Assoc. Off. Anal. Chem. 65:241-248. [PubMed] [Google Scholar]
- 2.Anonymous. 1999. Guidance for industry: reducing microbial food safety hazards for sprouted seeds and guidance for industry: sampling and microbial testing of spent irrigation water during sprout production. Fed. Reg. 64:57893-57902. [Google Scholar]
- 3.Breuer, T., D. H. Benkel, R. L. Shapiro, W. N. Hall, M. M. Winnett, M. J. Linn, J. Neimann, T. J. Barrett, S. Dietrich, F. P. Downes, D. M. Toney, J. L. Pearson, H. Rolka, L. Slutsker, and P. M. Griffin. 2001. A multistate outbreak of Escherichia coli O157:H7 infections linked to alfalfa sprouts grown from contaminated seeds. Emerg. Infect. Dis. 7:977-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro-Rosas, J., and E. F. Escartin. 2000. Survival and growth of Vibrio cholerae O1, Salmonella typhi, and Escherichia coli O157:H7 in alfalfa sprouts. J. Food Sci. 65:162-165. [Google Scholar]
- 5.Charkowski, A. O., J. D. Barak, C. Z. Sarreal, and R. E. Mandrell. 2002. Growth and colonization patterns of Salmonella enterica and Escherichia coli O157:H7 on alfalfa sprouts and the effects of sprouting temperature, inoculum dose, and frequency of irrigation on bacterial levels. Appl. Environ. Microbiol. 68:3114-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Como-Sabetti, K., S. Reagan, S. Allaire, K. Parrott, C. M. Simonds, S. Hrabowy, B. Ritter, W. Hall, J. Altamirano, R. Martin, F. Downes, G. Jennings, R. Barrie, M. F. Dorman, N. Keon, M. Kucab, A. Al Shab, B. Robinson-Dunn, S. Dietrich, L. Moshur, L. Reese, J. Smith, K. Wilcox, J. Tilden, G. Wojtala, J. D. Park, M. Winnett, L. Petrilack, L. Vasquez, S. Jenkins, E. Barrett, M. Linn, D. Woolard, R. Hackler, H. Martin, D. McWilliams, B. Rouse, S. Willis, J. Rullan, J. Miller, G. S. Henderson, J. Pearson, J. Beers, R. Davis, and D. Saunders. 1997. Outbreaks of Escherichia coli O157:H7 infection associated with eating alfalfa sprouts—Michigan and Virginia, June-July 1997. Morb. Mortal. Wkly. Rep. 46:741-744. [Google Scholar]
- 7.de Groot, A., I. Heijnen, H. de Cock, A. Filloux, and J. Tommassen. 1994. Characterization of type IV pilus genes in plant growth-promoting Pseudomonas putida WCS358. J. Bacteriol. 176:642-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi, M., S. Golding, S. Yaron, and K. R. Matthews. 2001. Use of green fluorescent protein expressing Salmonella Stanley to investigate survival, spatial location, and control on alfalfa sprouts. J. Food Prot. 64:1891-1898. [DOI] [PubMed] [Google Scholar]
- 9.Griffin, P. M., S. M. Ostroff, R. V. Vauxe, K. D. Greene, J. G. Wells, J. H. Lewis, and P. A. Blake. 1988. Illness associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann. Intern. Med. 109:705-712. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton-Miller, J. M., and S. Shah. 2001. Identity and antibiotic susceptibility of enterobacterial flora of salad vegetables. Int. J. Antimicrob. Agents 18:81-83. [DOI] [PubMed] [Google Scholar]
- 11.Hara-Kudo, Y., H. Konuma, M. Iwaki, F. Kasuga, Y. Sugita-Konishi, Y. Ito, and S. Kumagai. 1997. Potential hazard of radish sprouts as a vehicle of Escherichia coli O157:H7. J. Food Prot. 60:1125-1127. [DOI] [PubMed] [Google Scholar]
- 12.Inami, G. B., and S. E. Moler. 1999. Detection and isolation of Salmonella from naturally contaminated alfalfa seeds following an outbreak investigation. J. Food Prot. 62:662-664. [DOI] [PubMed] [Google Scholar]
- 13.Mahon, B. E., A. Ponka, W. N. Hall, K. Komatsu, S. E. Dietrich, A. Siitonen, G. Cage, P. S. Hayes, M. A. Lambert-Fair, N. H. Bean, P. M. Griffin, and L. Slutsker. 1997. An international outbreak of Salmonella infections caused by alfalfa sprouts grown from contaminated seeds. J. Infect. Dis. 175:876-882. [DOI] [PubMed] [Google Scholar]
- 14.Miller, W. G., J. H. Leveau, and S. E. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 15.Mohle-Boetani, J. C., J. A. Farrar, S. B. Werner, D. Minassian, R. Bryant, S. Abbott, L. Slutsker, and D. J. Vugia. 2001. Escherichia coli O157 and Salmonella infections associated with sprouts in California, 1996-1998. Ann. Intern. Med. 135:239-247. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien, A. O., T. A. Lively, M. E. Chen, S. W. Rothman, and S. B. Formal. 1983. Escherichia coli 0157:H7 strains associated with hemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet 1:702. [DOI] [PubMed] [Google Scholar]
- 17.O'Mahony, M., J. Cowden, B. Smyth, D. Lynch, M. Hall, B. Rowe, E. L. Teare, R. E. Tettmar, A. M. Rampling, M. Coles, R. J. Gilbert, E. Kingcott, and C. L. R. Bartlett. 1990. An outbreak of Salmonella Saint-Paul infection associated with beansprouts. Epidemiol. Infect. 104:229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proctor, M. E., M. Hamacher, M. L. Tortorello, J. R. Archer, and J. P. Davis. 2001. Multistate outbreak of Salmonella serovar Muenchen infections associated with alfalfa sprouts grown from seeds pretreated with calcium hypochlorite. J. Clin. Microbiol. 39:3461-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puohiniemi, R., T. Heiskanen, and A. Siitonen. 1997. Molecular epidemiology of two international sprout-borne Salmonella outbreaks. J. Clin. Microbiol. 35:2487-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 21.Romling, U., W. D. Sierralta, K. Eriksson, and S. Normark. 1998. Multicellular and aggregative behavior of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249-264. [DOI] [PubMed] [Google Scholar]
- 22.Stanley, T. L., C. D. Ellermeier, and J. M. Slauch. 2000. Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar Typhimurium survival in Peyer's patches. J. Bacteriol. 182:4406-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeuchi, K., C. M. Matute, A. N. Hassan, and J. F. Frank. 2000. Comparison of the attachment of Escherichia coli O157:H7, Listeria monocytogenes, Salmonella typhimurium, and Pseudomonas fluorescens to lettuce leaves. J. Food Prot. 63:1433-1437. [DOI] [PubMed] [Google Scholar]
- 24.Taormina, P. J., L. R. Beuchat, and L. Slutsker,. 1999. Infections associated with eating seed sprouts: an international concern. Emerg. Infect. Dis. 5:626-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhlich, G. A., J. E. Keen, and R. O. Elder. 2001. Mutations in the csgD promoter associated with variations in curli expression in certain strains of Escherichia coli O157:H7. Appl. Environ. Microbiol. 67:2367-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uljas, H. E., and S. C. Ingham. 1999. Combinations of intervention treatments resulting in 5-log10-unit reductions in numbers of Escherichia coli O157:H7 and Salmonella typhimurium DT104 organisms in apple cider. Appl. Environ. Microbiol. 65:1924-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Beneden, C. A., W. E. Keene, R. A. Strang, D. H. Werker, A. S. King, B. Mahon, K. Hedberg, A. Bell, M. T. Kelly, V. K. Balan, W. R. Mac Kenzie, and D. Fleming. 1999. Multinational outbreak of Salmonella enterica serotype Newport infections due to contaminated alfalfa sprouts. JAMA 281:158-162. [DOI] [PubMed] [Google Scholar]
- 28.Wachtel, M. R., L. C. Whitehand, and R. E. Mandrell. 2002. Prevalence of Escherichia coli associated with a cabbage crop inadvertently irrigated with partially treated sewage wastewater. J. Food Prot. 65:471-475. [DOI] [PubMed] [Google Scholar]