Abstract
We report the development of a nonantibiotic and nonpathogenic host-plasmid selection system based on lactococcal genes and threonine complementation. We constructed an auxotrophic Lactococcus lactis MG1363Δthr strain which carries deletions in two genes encoding threonine biosynthetic enzymes. To achieve plasmid-borne complementation, we then constructed the minimal cloning vector, pJAG5, based on the genes encoding homoserine dehydrogenase-homoserine kinase (the hom-thrB operon) as a selective marker. Using strain MG1363Δthr, selection and maintenance of cells carrying pJAG5 were obtained in threonine-free defined media. Compared to the commonly used selection system based on erythromycin resistance, the designed complementation system offers a competitive and stable plasmid selection system for the production of heterologous proteins in L. lactis. The potential of pJAG5 to deliver genes for expression in eukaryotes was evaluated by insertion of a mammalian expression unit encoding a modified green fluorescent protein. The successful delivery and expression of genes in human kidney fibroblasts indicated the potential of the designed nonantibiotic host-plasmid system for use in genetic immunization.
The continuous discovery of new vaccines and therapeutics poses a challenge to systems available for heterologous protein production. A suitable choice of host and production conditions is important for the manufacture of a pharmaceutical-grade product. We have recently developed a plasmid expression system for use in the lactic acid bacterium Lactococcus lactis (28). In this paper, we present a nonantibiotic alternative for selection of plasmid-harboring bacterial cells for use in heterologous gene expression.
Due to its food-grade status, L. lactis is attractive for both production and live delivery of vaccines and therapeutics. Recently, several examples of nondairy applications of this bacterium have been reported, and the development of plasmid-borne gene expression systems has made it possible to use L. lactis for the production of heterologous proteins (1, 5, 7, 11, 20, 21). Furthermore, L. lactis has been placed in a new class of live bacterial vaccine carriers derived from gram-positive, nonpathogenic, and noninvasive bacteria (reviewed in reference 33). Recently, live delivery was accomplished by feeding mice immunogen-synthesizing L. lactis organisms, thus obtaining mucosal delivery of the Helicobacter pylori urease subunit B antigen (22) and of the pneumococcal type 3 capsular polysaccharide antigen (12). The delivery of therapeutics using live recombinant L. lactis has been demonstrated by in situ secretion of interleukin-10 for treatment of colitis in mice (37).
Although L. lactis is generally regarded as safe, this status can be compromised by the introduction of foreign DNA necessary for the synthesis of recombinant proteins. Usually, high-copy-number plasmids are used for high-level expression of recombinant proteins (1, 5, 7, 11, 20, 21). A simple way to prevent plasmid loss is to use plasmid-encoded antibiotic resistance markers and grow the bacteria in the presence of antibiotics. The chief drawbacks of this approach are the potential loss of selective pressure as a result of antibiotic degradation (as in the case of β-lactamase) and contamination of the biomass or purified protein by antibiotics and resistance genes, which is unacceptable from a medical point of view.
Alternative genetic markers for L. lactis have been developed. Depending on the type of selection, they can be placed in two groups: resistance and complementation markers. Examples of resistance markers that confer immunity to an added agent, such as nisin (8) or the metal ions cadmium (Cd2+) (24) and copper (Cu2+) (23), have been designed for plasmid maintenance. Although some strains of L. lactis are naturally resistant to nisin and metal ions, the dominant nature of resistance markers makes them versatile, as they can be used in different lactococcal strains.
The use of auxotrophic markers is based on complementation of a mutation or deletion in the host chromosome and is therefore strain specific. In L. lactis, the first example was based on complementation of a lacF mutant strain deficient in lactose utilization (26). In two other systems, auxotrophic markers complement purine- and pyrimidine-auxotrophic strains using genes encoding nonsense tRNA suppressors (6, 36). In these systems, expression of the plasmid-borne suppressor tRNA gene allows read-through of a nonsense mutation(s) in the genes encoding purine or pyrimidine biosynthetic enzymes. Both systems permit selection in milk or other media that contain no, or small amounts of, purines or pyrimidines.
We previously constructed a threonine-auxotrophic derivative of the L. lactis MG1614 strain containing an internal deletion in the hom-thrB operon (27). The bicistronic hom-thrB operon encodes the homoserine dehydrogenase and homoserine kinase catalyzing two of the five steps converting aspartate to threonine. Thus, a hom-thrB strain cannot synthesize threonine and therefore needs an external source of threonine.
In this paper, we report the construction of a plasmid selection system in L. lactis based on complementation of a threonine-auxotrophic strain using the hom-thrB operon. The plasmid is based only on a lactococcal replicon and the hom-thrB operon. We demonstrate its use for heterologous gene expression in L. lactis and for delivery of genes for in vitro expression in mammalian cells.
MATERIALS AND METHODS
Strains and growth conditions.
Strains and plasmids are listed in Table 1. Unless otherwise stated, Escherichia coli was grown at 37°C in Luria-Bertani medium (35), while L. lactis was grown at 30°C in M17 medium (Oxoid, Basingstoke, United Kingdom) supplemented with 0.5% glucose (GM17) or in synthetic LM3-30 medium (A. Vrang, unpublished data) containing 3% glucose and all amino acids except aspartic acid. When appropriate, either 100 μg of ampicillin/ml or 250 μg of erythromycin (Ery)/ml was added for E. coli and 1 μg of Ery/ml was added for L. lactis. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was used at a concentration of 160 μg/ml in agar plates for L. lactis. One-liter L. lactis cultures were cultivated for 30 h in LM3-30 in 2-liter fermentors at 30°C; 5 M KOH was automatically added to maintain the pH at 6, and the agitation rate was set at 300 rpm. Growth was monitored by measuring the optical density at 600 nm, and samples of supernatants were taken every hour. Complemented MG1363Δthr prototrophs were selected on 1.5% agarose plates containing LM3-30 without threonine using cells that had been washed in 0.9% NaCl to remove threonine.
TABLE 1.
Strains and plasmids
| Bacterium or plasmid | Characteristic(s) | Reference or source |
|---|---|---|
| L. lactis | ||
| MG1363 | 10 | |
| MG1614 | 10 | |
| MG1614Δthr | Δhom ΔthrB | 27 |
| MG1363Δthr | Δhom ΔthrB | This work |
| E. coli DH10B | Laboratory strain | Invitrogen |
| Plasmids | ||
| pGEM7Zf(+) | lacZ bla | Promega, Southampton, United Kingdom |
| pCR2.1 | lacZ bla kan | Invitrogen |
| pEGFP-N1 | CMV promoter-EGFP-SV40 poly(A); kan | Clontech, Palo Alto, Calif. |
| pG3E | pGEM-3Zf(−)::1.2 kb; ermL | 32 |
| pSMA231 | pGEM-7Zf(+)::1.8 kb; Δhom-thrB | 27 |
| pSMA507 | Δhom ΔthrB lacLM ermL | 27 |
| pSMBI109 | Citrate replicon-P170 promoter-SP310mut2; nucB ermL | A. Vrang, unpublished |
| pAK80 | Citrate replicon | 16 |
| pJAG1 | pCR2.1::0.6 kb; 5′ end of hom | This work |
| pJAG2 | pGEM-7Zf(+)::2.4 kb; hom-thrB | This work |
| pJAG3 | pG3E::1.8 kb; citrate replicon | This work |
| pJAG4 | pJAG3::2.4 kb; hom-thrB | This work |
| pJAG5 | Citrate replicon; hom-thrB | This work |
| pJAG6 | pJAG5::0.9 kb; P170 promoter-SP310mut2; nucB | This work |
| pJAG7 | pCR2.1::1.6 kb; CMV promoter-EGFP-SV40 pA | This work |
| pJAG8 | pJAG4::1.6 kb; CMV promoter-EGFP-SV40 pA | This work |
DNA isolation and manipulation.
Chromosomal (17) and plasmid (30) DNAs from L. lactis were prepared as described previously. E. coli plasmids were isolated using a plasmid extraction kit from Genomed (Bad Oeynhausen, Germany) as recommended by the manufacturer. L. lactis was made electrocompetent and transformed as described previously (14), while competent E. coli DH10B cells were purchased (Invitrogen, Groningen, The Netherlands) and transformed as recommended by the manufacturer. DNA restriction and modification enzymes (New England Biolabs, Beverly, Mass.) were used as recommended by the manufacturer.
Plasmid DNA was sequenced with a Thermo Sequenase fluorescently labeled primer cycle-sequencing kit (Amersham Pharmacia, Uppsala, Sweden), Cy5-labeled primers, and an ALFexpress DNA sequencer (Amersham Pharmacia).
Construction of the threonine-auxotrophic L. lactis MG1363Δthr strain.
The threonine-auxotrophic MG1363Δthr strain was constructed as described previously(27) using the integration vector pSMA507 (Table 1). This plasmid contains an Ery resistance marker, a plasmid origin of replication from E. coli, the lacLM genes, and the L. lactis hom-thrB genes from MG1614 lacking the distal 310-bp end of hom and the proximal 57 bp of thrB (27). Plasmid pSMA507 was electroporated into L. lactis MG1363, and Ery-resistant transformants were isolated and cultured in GM17 without Ery for 75 generations. Cells were plated onto GM17 plates with X-Gal and screened for loss of the plasmid and concomitant loss of β-galactosidase activity. Chromosomal DNAs from white colonies and from an MG1363 wild-type colony were amplified to screen for deletions in the hom-thrB operon by PCR using the primers pThr-frw1 and pThr-rev1 (Table 2). PCR of strain MG1363 DNA gave rise to a 2,083-bp fragment, while DNA from the deletion mutant gave a 1,716-bp fragment.
TABLE 2.
Nucleotide sequences of PCR primers
| Primer | Sequencea |
|---|---|
| pThr-frw1 (XbaI) | 5′-TATCGTCTAGACTGATTAATCTGTCAGTAAAATAGAAG-3′ |
| pThr-rev1 | 5′-GCTACTTCTAAATTATTTGTC-3′ |
| pThr-rev2 (EcoRI) | 5′-ATTAAGAATTCCCAACAGATGTGTAATTTTATCAGATGAAAATGAATTAGCCAAAGTTCTTAAAATAGGAATACCCCC-3′ |
| pCMV-frw (SpeI) | 5′-TATCGACTAGTTAGTTATTAATAGTAATCAATTACGGGG-3′ |
| pBGH-rev (SpeI) | 5′-TATCGACTAGTTGATGAGTTTGGACAAACCACAACT-3′ |
| pNuc-frw (SacI) | 5′-TTCGCGAGCTCGAGGGGAAGTAATT-3′ |
| pNuc-rev (KpnI) | 5′-TATCGGGTACCCGATCTAAAAATTATAAAAGTGCCA-3′ |
| pM13-frw | 5′-GTAAAACGACGGCCAGT-3′ |
| pM13-rev | 5′-CAGGAAACAGCTATGAC-3′ |
Enzyme recognition sequences are underlined, and the altered EcoRI site in pThr-rev2 is italicized.
Cloning of the L. lactis hom-thrB operon.
Part of the hom-thrB operon of MG1614 was cloned in pSMA231, which contains 1,800 bp of the 3′ end of the hom gene and the complete thrB gene (27). The 5′ end of hom, including the promoter region, was PCR amplified with the primers pThr-frw1 and pThr-rev2 using MG1614 chromosomal DNA as a template. pThr-frw1 was designed to introduce an XbaI recognition sequence, and pThr-rev2 was designed to anneal to the region located 403 to 480 bp downstream of the start codon of hom. This region includes two EcoRI sites located 408 and 471 bp downstream of the hom start codon. To remove the former EcoRI site, pThr-rev2 was further designed to introduce an A-to-T substitution at this position (Table 2). The resulting PCR product was cloned into pCR2.1, giving rise to pJAG1, which was sequenced using the sequencing primers pM13-frw and pM13-rev. The 5′ end of hom was moved from pJAG1 into pSMA231 using XbaI and EcoRI, which resulted in pJAG2, harboring the complete hom-thrB operon.
Construction of the pJAG4 E. coli-L. lactis shuttle vector containing the hom-thrB marker.
Plasmid pG3E is derived from pGEM-3Zf(−) and confers Ery resistance in E. coli (32). A 1,757-bp EcoRI fragment from pAK80 containing the lactococcal minimal replicon from the citrate plasmid (31) was inserted into the EcoRI site of pG3E. The resulting pJAG3 conferred Ery resistance on transformed MG1363Δthr cells. pJAG2 was digested with XbaI and HindIII, and the complete hom-thrB operon was isolated on a 2,401-bp fragment and inserted into similarly digested pJAG3 to give pJAG4.
Construction of the pJAG5 L. lactis cloning vector.
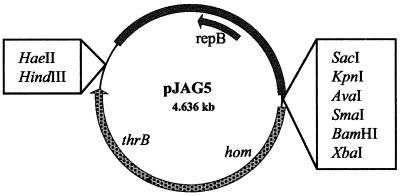
HaeII digestion of pJAG4 produced a 4,636-bp fragment containing the citrate replicon, the hom-thrB operon, and the polylinker. To favor self-religation, the isolated fragments were ligated in a large volume. The resulting vector, pJAG5 (Fig. 1), showed complementation of the MG1363Δthr strain on LM3-30 plates without threonine.
FIG. 1.
Minimal cloning vector pJAG5. The arrows indicate the direction of transcription. repB encodes the replication protein of the citrate replicon, and hom and thrB encode homoserine dehydrogenase and homoserine kinase, respectively. Only unique restriction sites are indicated.
Construction of the pJAG6 expression vector containing the Staphylococcus aureus nuclease gene.
We have constructed plasmid pSMBI109, which contains a gene cassette harboring the inducible P170 promoter (A. Vrang et al., unpublished), the optimized signal peptide SP3mut2 (34; P. Ravn, J. Arnau, S. M. Madsen, A. Vrang, H. Israelsen, unpublished data), and the S. aureus nucB nuclease (Nuc) gene (4). This gene cassette was PCR amplified using the pNuc-frw and pNuc-rev primers, which introduced terminal 5′ SacI and 3′ KpnI recognition sequences. The resulting 914-bp fragment was digested with SacI and KpnI and ligated to the similarly digested pJAG5, resulting in pJAG6.
Construction of pJAG8 for eukaryotic expression of the green fluorescent protein.
The gene encoding the red-shifted variant of the green fluorescent protein (EGFP) was isolated from pEGFP-N1 using PCR and the primers pCMV-frw and pBGH-rev. Both primers contained terminal SpeI sequences. The resulting ∼1,650-bp fragment included the cytomegalovirus (CMV) promoter and enhancer regions and the simian virus 40 (SV40) polyadenylation signal sequence (SVpA). This fragment was cloned into pCR2.1, producing pJAG7. The EGFP expression cassette was isolated as an SpeI fragment from pJAG7 and ligated into XbaI-digested pJAG5 to produce pJAG8.
Plasmid stability.
To test the segregational stability of pJAG5 during exponential growth, transformed MG1363Δthr cells were grown for 100 generations under nonselective conditions. Every 10 generations, diluted samples were cultured on plates with and without threonine. During fermentation, segregational stability was tested at the transition to stationary phase and again 15 h after the onset of stationary phase. Strains with pJAG6 were tested on LM3-30 medium with and without threonine, and strains with pSMBI109 were tested on GM17 medium with and without Ery.
Nuclease activity determinations.
Nuc activities were determined by incubation of culture supernatants with sonicated salmon DNA as a substrate followed by precipitation in ice-cold perchloric acid and measurement of absorbance at 260 nm (Vrang et al., unpublished). One unit of Nuc is defined as the amount of nuclease that will produce 1 μmol of acid-soluble polynucleotide per min from native DNA.
In vitro expression of EGFP from pJAG8 in human kidney cells.
Adherent human 293 kidney fibroblast cells were incubated in RPMI medium (Gibco, New York, N.Y.) supplemented with 10% fetal calf serum for 24 h at 37°C in the presence of 5% CO2. The cells were transfected with pJAG8 using the Effectene transfection kit (Qiagen, Hilden, Germany) as described by the manufacturer. Expression of EGFP was visualized by fluorescence microscopy at 488 nm 24 h after transfection.
RESULTS
Construction of a threonine-auxotrophic L. lactis MG1363Δthr strain.
To construct MG1363Δthr, we used the integration vector pSMA507, which harbors a truncated hom-thrB operon and lacLM and is unable to replicate in L. lactis. First, transformed cells viable on GM17 plates containing X-Gal and Ery should have pSMA507 integrated into their chromosomes due to homologous recombination into the hom-thrB genes and should form blue colonies. Second, without antibiotic pressure, cells whose chromosomes would undergo a second homologous recombination between neighboring sets of hom-thrB genes should form white Ery-sensitive colonies. Following this strategy, genomic DNAs from 10 white colonies were analyzed by PCR, which for all isolates indicated the presence of an internal hom-thrB deletion. Phenotypic tests on plates without threonine confirmed the threonine auxotrophy of these isolates, one of which was named strain MG1363Δthr.
Construction and testing of a hom-thrB-complementing shuttle vector, pJAG4.
Details of plasmid construction are described in Materials and Methods and Table 1. For replication in L. lactis, the minimal replicon from the citrate plasmid of Lactococcus lactis subsp. lactis biovar diacetylactis was cloned into pG3E to give pJAG3, which conferred Ery resistance on transformed MG1363Δthr cells. The assembled hom-thrB operon from pJAG2 was then added to give pJAG4. MG1363Δthr transformed with pJAG4 carrying hom-thrB was able to grow on medium without threonine, demonstrating complementation. To compare the transformation efficiencies of the hom-thrB and Ery resistance markers on pJAG4, transformed cells were spread on LM3-30 plates without threonine and LM3-30 plates with Ery, respectively. The experiment was done twice in triplicate and showed that the numbers of CFU were similar (hom-thrB complementation, 106 ± 9 CFU; Ery selection, 112 ± 11 CFU) on the two types of plates, indicating that hom-thrB selection was as efficient as Ery selection.
Construction and testing of the minimal lactococcal complementation vector, pJAG5.
We aimed at constructing a minimal complementation vector based solely on lactococcal DNA which contained only complementing genes, an origin of replication, and a versatile polylinker and no antibiotic resistance gene or nonlactococcal DNA, such as an E. coli origin of replication. Therefore, the citrate replicon, the polylinker, and hom-thrB were isolated from pJAG4 on a single fragment, which was religated and electroporated into strain MG1363Δthr. Prototrophic colonies were isolated on LM3-30 plates without threonine, and a subsequent plasmid analysis confirmed the structure of the resulting cloning vector, pJAG5 (Fig. 1). This plasmid was stably maintained under nonselective conditions, since no plasmid loss was observed during cultivation for >100 generations in LM3-30 medium.
Secretion of S. aureus nuclease in fermentations.
The capacity of the designed complementation system for use in plasmid-borne gene expression was compared to that of the currently used selection system based on antibiotic resistance. As a reporter gene, we used nucB fused to the optimized SP3mut2 signal peptide sequence. The reporter gene was transcribed from the pH- and growth-phase-regulated P170 promoter (28). We analyzed the level of nuclease production using this reporter gene in two vectors, both containing the theta-type citrate replicon but harboring either the hom-thrB-complementing marker (pJAG6) or the Ery resistance marker (pSMBI109). These plasmids were tested in MG1363 and MG1363Δthr under different growth conditions, and the levels of Nuc activity were compared after 30 h of fermentation and at an optical density at 600 nm of approximately 6 (Table 3). First, the influence of a chromosomal hom-thrB deletion was investigated by comparing the Nuc expression of pSMBI109-transformed MG1363 and MG1363Δthr strains in the presence of threonine. As the fermentation showed identical levels of Nuc activity in the two strains, the use of a hom-thrB deletion mutant did not affect the level of expression under nonselective conditions. Second, we compared pJAG6(hom+-thrB+) and pSMBI109(ermL+) in MG1363Δthr under the same conditions. The results showed that the Nuc activities using these plasmids in the auxotrophic strain were also identical. Third, we compared the two plasmid-host systems under selective conditions and found that the Nuc activity of the MG1363Δthr/pJAG6 system was slightly higher than that of the MG1363/pSMBI109 system (13.2 ± 0.8 versus 10.5 ± 0.5 U/ml). This result indicated that endogenous synthesis of threonine did not affect heterologous gene expression. However, the growth rate of MG1363Δthr/pJAG6 was slightly (<10%) impaired under these conditions. Relief of the selective pressure by the presence of threonine, however, did not lead to pJAG6-free MG1363Δthr cells, as the numbers of CFU on LM3-30 plates with and without threonine were similar (data not shown).
TABLE 3.
Nuclease determinations during fermentation
| Strain | Plasmid | Conditionsa | Nuc (u/ml ± SD)b |
|---|---|---|---|
| MG1363Δthr | pJAG6 | −Thr | 13.2 ± 0.8 |
| pJAG6 | +Thr | 11.8 ± 0.8 | |
| pSMBI109 | +Thr | 10.9 ± 0.8 | |
| MG1363 | pSMBI109 | +Thr | 11.1 ± 0.4 |
| pSMBI109 | +Thr, Ery | 10.5 ± 0.5 | |
| pSMBI109 | −Thr | 11.8 ± 0.3 |
−Thr, without threonine; +Thr, with threonine; Ery, with Ery.
Nuc activities are presented as the means of three Nuc activity determinations.
The results show that the complementation system offers an effective and stable plasmid selection system for production of heterologous Nuc in L. lactis.
Expression of green fluorescent protein in human kidney fibroblasts.
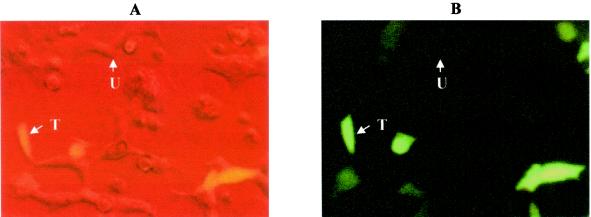
To test whether pJAG5 was capable of transient transfection of eukaryotic cells and gene delivery for in situ expression, we cloned the reporter cassette harboring the CMV promoter, the EGFP gene, and the SVpA polyadenylation signal sequence into pJAG5, resulting in pJAG8. Introduction of pJAG8 into the nuclei of the cells was facilitated by using lipid-formulated plasmid. It should be noted that pJAG8 contains no eukaryotic selection marker or replication system for maintenance in fibroblast cells. The fibroblasts were visualized by phase-contrast microscopy, but only transfected cells expressing the cytoplasmic reporter EGFP were visible on epifluorescence micrographs (Fig. 2). The successful transfection of eukaryotic cells and the detectable expression of EGFP (Fig. 2) demonstrated the utility of the pJAG5 cloning vector for gene delivery and in situ expression in eukaryotic cells.
FIG. 2.
Adherent human 293 kidney fibroblasts transfected with pJAG8. (A) Phase-contrast micrograph illuminated at 488 nm. (B) Epifluorescence micrograph of the same region. U indicates a nonexpressor cell, while T indicates a transfected cell expressing EGFP. Magnification, ×400.
DISCUSSION
While the presence of antibiotic resistance genes on recombinant plasmids allows for efficient selection and maintenance in transformed cells, their use is undesirable in medical biotechnology, as even trace amounts of β-lactams, such as penicillin, or macrolides, such as Ery, can cause anaphylactic shock in sensitized persons (9, 18). Furthermore, the continued spread of antibiotics and their resistance genes, leading to multiresistant pathogens, is a topic of public concern. Here, we describe the development of a nonantibiotic plasmid selection system based on complementation for use in L. lactis. The system includes (i) a minimal cloning vector (pJAG5) encoding threonine biosynthetic enzymes and harboring a lactococcal theta-type replicon and a polylinker and (ii) a threonine-auxotrophic L. lactis MG1363 host strain, permitting selection in threonine-free growth media such as the synthetic LM3-30. Notably, pJAG5 is highly stable and can be maintained without selective pressure for >100 generations. This is most likely due to the theta-type mode of replication, which gives structural and segregational stability (19). Furthermore, L. lactis vectors of the theta family have narrow host ranges (15, 25, 39), preventing or at least reducing horizontal plasmid transfer to other microorganisms.
Potential drawbacks of a selection system dependent on complementation of an auxotrophic strain are growth rate reduction and decrease in heterologous protein production caused by the necessity of endogenous threonine synthesis under selective conditions. However, both the growth profiles and the Nuc expression of the hom-thrB-complemented threonine-auxotrophic strain were similar in media with and without threonine, indicating minimal pleiotropic effects. Furthermore, our designed antibiotic-free selection system is as effective as the Ery-dependent system and can be employed when antibiotic-free conditions are desired, for instance, during production of proteins or plasmid DNA for medical use or production of recombinant L. lactis for use in live delivery of vaccines or therapeutics.
The application of pJAG5 (Fig. 1) in eukaryotic gene expression was analyzed using the green fluorescent protein (2). An optimized (3) and codon-modified (13) EGFP gene controlled by the constitutive CMV promoter (29) was used for high-level expression and brighter fluorescence. The small gene size (714 bp) and the ease with which EGFP expression can be visualized (no substrate needs to be added) make it ideal for studying plasmid-mediated gene transfer. As shown in Fig. 2, we demonstrated EGFP expression in human kidney fibroblasts using pJAG8 transfection. This shows that the minimal pJAG5 vector can be used for delivery and in situ expression of eukaryotic genes, which to our knowledge is the first example of an L. lactis-derived plasmid being successfully used for gene expression in eukaryotic cells. Since genetic immunization (reviewed in references 38 and 40) involves the direct delivery of plasmid DNA into patients to elicit an immune response toward the encoded antigen, the use of a vector without an antibiotic resistance marker is obviously desirable. We are investigating the potential use of the pJAG5 vector in gene delivery for genetic immunization.
Acknowledgments
We thank Lasse Vinner and Sylvie Corbet for helpful discussions. We acknowledge Anne Cathrine Steenbjerg, Annemette Jørgensen, and Birgit Johansen for excellent technical assistance and thank Bjarne Albrechtsen for critical reading of the manuscript.
REFERENCES
- 1.Bermudez-Humaran, L. G., P. Langella, A. Miyoshi, A. Gruss, R. T. Guerra, D. O.-L. Montes, and Y. Le Loir. 2002. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl. Environ. Microbiol. 68:917-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalfie, M., Y. Tu, G. Euskirchen, W. W. Ward, and D. C. Prasher. 1994. Green fluorescent protein as a marker for gene expression. Science 263:802-805. [DOI] [PubMed] [Google Scholar]
- 3.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 4.Davis, A., I. B. Moore, D. S. Parker, and H. Taniuchi. 1977. Nuclease B. A possible precursor of nuclease A, an extracellular nuclease of Staphylococcus aureus. J. Biol. Chem. 252:6544-6553. [PubMed] [Google Scholar]
- 5.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickely, F., D. Nilsson, E. B. Hansen, and E. Johansen. 1995. Isolation of Lactococcus lactis nonsense suppressors and construction of a food-grade cloning vector. Mol. Microbiol. 15:839-847. [DOI] [PubMed] [Google Scholar]
- 7.Djordjevic, G. M., and T. R. Klaenhammer. 1998. Inducible gene expression systems in Lactococcus lactis. Mol. Biotechnol. 9:127-139. [DOI] [PubMed] [Google Scholar]
- 8.Froseth, B. R., and L. L. McKay. 1991. Molecular characterization of the nisin resistance region of Lactococcus lactis subsp. lactis biovar diacetylactis DRC3. Appl. Environ. Microbiol. 57:804-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher, D. M., and D. P. Sinn. 1983. Penicillin-induced anaphylaxis in a patient under hypotensive anaesthesia. Oral Surg. Oral Med. Oral Pathol. 56:361-364. [DOI] [PubMed] [Google Scholar]
- 10.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NDO712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gil, M. T., I. Perez-Arellano, J. Buesa, and G. Perez-Martinez. 2001. Secretion of the rotavirus VP8∗ protein in Lactococcus lactis. FEMS Microbiol. Lett. 203:269-274. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert, C., K. Robinson, R. W. Le Page, and J. M. Wells. 2000. Heterologous expression of an immunogenic pneumococcal type 3 capsular polysaccharide in Lactococcus lactis. Infect. Immun. 68:3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas, J., E. C. Park, and B. Seed. 1996. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr. Biol. 6:315-324. [DOI] [PubMed] [Google Scholar]
- 14.Holo, H., and I. F. Nes. 1995. Transformation of Lactococcus by electroporation. Methods Mol. Biol. 47:195-199. [DOI] [PubMed] [Google Scholar]
- 15.Horng, J. S., K. M. Polzin, and L. L. McKay. 1991. Replication and temperature-sensitive maintenance functions of lactose plasmid pSK11L from Lactococcus lactis subsp. cremoris. J. Bacteriol. 173:7573-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Israelsen, H., S. M. Madsen, A. Vrang, E. B. Hansen, and E. Johansen. 1995. Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector, pAK80. Appl. Environ. Microbiol. 61:2540-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen, E., and A. Kibenich. 1992. Isolation and characterization of IS1165, an insertion sequence of Leuconostoc mesenteroides subsp. cremoris and other lactic acid bacteria. Plasmid 27:200-206. [DOI] [PubMed] [Google Scholar]
- 18.Jorro, G., C. Morales, J. V. Braso, and A. Pelaez. 1996. Anaphylaxis to erythromycin. Ann. Allergy Asthma Immunol. 77:456-458. [DOI] [PubMed] [Google Scholar]
- 19.Kiewiet, R., S. Bron, K. de Jonge, G. Venema, and J. F. Seegers. 1993. Theta replication of the lactococcal plasmid pWVO2. Mol. Microbiol. 10:319-327. [PubMed] [Google Scholar]
- 20.Kok, J. 1996. Inducible gene expression and environmentally regulated genes in lactic acid bacteria. Antonie Leeuwenhoek 70:129-145. [DOI] [PubMed] [Google Scholar]
- 21.Kuipers, O. P., P. G. de Ruyter, M. Kleerebezem, and W. M. de Vos. 1997. Controlled overproduction of proteins by lactic acid bacteria. Trends Biotechnol. 15:135-140. [DOI] [PubMed] [Google Scholar]
- 22.Lee, M. H., Y. Roussel, M. Wilks, and S. Tabaqchali. 2001. Expression of Helicobacter pylori urease subunit B gene in Lactococcus lactis MG1363 and its use as a vaccine delivery system against H. pylori infection in mice. Vaccine 19:3927-3935. [DOI] [PubMed] [Google Scholar]
- 23.Leelawatcharanas, V., L. G. Chia, P. Charoenchai, N. Kunajakr, C.-Q. Liu, and N. W. Dunn. 1997. Plasmid-encoded copper resistance in Lactococcus lactis. Biotechnol. Lett. 19:639-643. [Google Scholar]
- 24.Liu, C. Q., N. Khunajakr, L. G. Chia, Y. M. Deng, P. Charoenchai, and N. W. Dunn. 1997. Genetic analysis of regions involved in replication and cadmium resistance of the plasmid pND302 from Lactococcus lactis. Plasmid 38:79-90. [DOI] [PubMed] [Google Scholar]
- 25.Lucey, M., C. Daly, and G. Fitzgerald. 1993. Identification and sequence analysis of the replication region of the phage resistance plasmid pCI528 from Lactococcus lactis subsp. cremoris UC503. FEMS Microbiol. Lett. 110:249-256. [DOI] [PubMed] [Google Scholar]
- 26.MacCormick, C. A., H. G. Griffin, and M. J. Gasson. 1995. Construction of a food-grade host/vector system for Lactococcus lactis based on the lactose operon. FEMS Microbiol. Lett. 127:105-109. [DOI] [PubMed] [Google Scholar]
- 27.Madsen, S. M., B. Albrechtsen, E. B. Hansen, and H. Israelsen. 1996. Cloning and transcriptional analysis of two threonine biosynthetic genes from Lactococcus lactis MG1614. J. Bacteriol. 178:3689-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen, S. M., J. Arnau, A. Vrang, M. Givskov, and H. Israelsen. 1999. Molecular characterization of the pH-inducible and growth phase-dependent promoter P170 of Lactococcus lactis. Mol. Microbiol. 32:75-87. [DOI] [PubMed] [Google Scholar]
- 29.Manthorpe, M., F. Cornefert-Jensen, J. Hartikka, J. Felgner, A. Rundell, M. Margalith, and V. Dwarki. 1993. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum. Gene Ther. 4:419-431. [DOI] [PubMed] [Google Scholar]
- 30.O'Sullivan, D. J., and T. R. Klaenhammer. 1993. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus lactis and Lactobacillus spp. Appl. Environ. Microbiol. 59:2730-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen, M. L., K. R. Arnved, and E. Johansen. 1994. Genetic analysis of the minimal replicon of the Lactococcus lactis subsp. lactis biovar diacetylactis citrate plasmid. Mol. Gen. Genet. 244:374-382. [DOI] [PubMed] [Google Scholar]
- 32.Petersen, A., J. Josephsen, and M. G. Johnsen. 1999. TPW22, a lactococcal temperate phage with a site-specific integrase closely related to Streptococcus thermophilus phage integrases. J. Bacteriol. 181:7034-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozzi, G., and J. M. Wells. 1997. Gram-positive bacteria, vaccine vehicles for mucosal immunization. Springer-Verlag, Berlin, Germany.
- 34.Ravn, P., J. Arnau, S. M. Madsen, A. Vrang, and H. Israelsen. 2000. The development of TnNuc and its use for the isolation of novel secretion signals in Lactococcus lactis. Gene 242:347-356. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Sørensen, K. I., R. Larsen, A. Kibenich, M. P. Junge, and E. Johansen. 2000. A food-grade cloning system for industrial strains of Lactococcus lactis. Appl. Environ. Microbiol. 66:1253-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steidler, L., W. Hans, L. Schotte, S. Neirynck, F. Obermeier, W. Falk, W. Fiers, and E. Remaut. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 38.Tighe, H., M. Corr, M. Roman, and E. Raz. 1998. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol. Today 19:89-97. [DOI] [PubMed] [Google Scholar]
- 39.von Wright, A., S. Wessels, S. Tynkkynen, and M. Saarela. 1990. Isolation of a replication region of a large lactococcal plasmid and use in cloning of a nisin resistance determinant. Appl. Environ. Microbiol. 56:2029-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468. [DOI] [PubMed] [Google Scholar]