Abstract
The biotransformation of the phytoanticipins 2-benzoxazolinone (BOA) and 2-hydroxy-1,4-benzoxazin-3-one (HBOA) by four endophytic fungi isolated from Aphelandra tetragona was studied. Using high-performance liquid chromatography-mass spectrometry, several new products of acylation, oxidation, reduction, hydrolysis, and nitration were identified. Fusarium sambucinum detoxified BOA and HBOA to N-(2-hydroxyphenyl)malonamic acid. Plectosporium tabacinum, Gliocladium cibotii, and Chaetosphaeria sp. transformed HBOA to 2-hydroxy-N-(2-hydroxyphenyl)acetamide, N-(2-hydroxyphenyl)acetamide, N-(2-hydroxy-5-nitrophenyl)acetamide, N-(2-hydroxy-3-nitrophenyl)acetamide, 2-amino-3H-phenoxazin-3-one, 2-acetylamino-3H-phenoxazin-3-one, and 2-(N-hydroxy)acetylamino-3H-phenoxazin-3-one. BOA was not degraded by these three fungal isolates. Using 2-hydroxy-N-(2-hydroxyphenyl)[13C2]acetamide, it was shown that the metabolic pathway for HBOA and BOA degradation leads to o-aminophenol as a key intermediate.
Plants have developed a number of mechanisms to overcome microbial diseases and herbivory, including the production of a variety of toxic metabolites. These may be constitutive secondary compounds already present in healthy plants (phytoanticipins) or synthesized de novo in response to pathogen attack (phytoalexins). The detoxification of these highly bioactive defense compounds is an important ability of many plant pathogens (13, 18).
Endophytes are bacterial or fungal microorganisms which colonize the healthy plant tissue inter- and/or intracellularly without causing any apparent symptoms of disease. They have been isolated from almost every host plant studied so far. The production of highly bioactive secondary metabolites was reported from several endophytes (16). Living inside the plant tissue they have to cope with the toxic defense compounds of plants. An adapted potential of biodegradation and a set of specific enzymes could allow them to subsist under these environmental conditions. Up to now only scarce research has been done in this field and only few experimental data are available. For example, aphelandrine, a macrocylic polyamine alkaloid found in the roots of different species of the genus Aphelandra (Acanthaceae) is metabolized by several endophytes, which were isolated from the roots of Aphelandra tetragona (21).
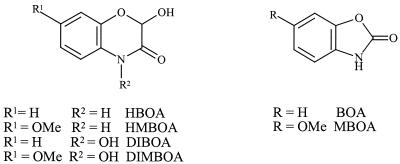
The benzoxazinones are a unique class of phytoanticipins occurring in the Gramineae, Acanthaceae, Ranunculaceae, and Scrophulariaceae families. Their role as defense compounds towards pests, like bacteria, fungi, and insects, is well documented in different cereals (Gramineae) (12). In Aphelandra sp. plants, the lactams 2-hydroxy-1,4-benzoxazin-3(2H)-one (HBOA) and 2-hydroxy-7-methoxy-1,4-benzoxazin-3(2H)-one (HMBOA), the hydroxamic acids 2,4-dihydroxy-1,4-benzoxazin-3(4H)-one (DIBOA) and 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3(4H)-one (DIMBOA) (Fig. 1), and the corresponding glucosides are accumulated (2). As soon as the integrity of the cells is ruptured, rapid deglucosylation leads to the aglucons. While the hydroxamic acids are chemically transformed into benzoxazolinone (2-benzoxazolinone [BOA] or 6-methoxy-benzoxazolinone [MBOA]) (Fig. 1), the lactams stay intact as HBOA and HMBOA (20). Thus, the invading pathogens, as well as the endophytes living inside the plant tissue, are confronted with the toxic benzoxazolinones BOA and MBOA and the less-toxic lactams HBOA and HMBOA. It was shown that phytopathogenic fungi can detoxify BOA and MBOA to N-(2-hydroxyphenyl)malonamic acid and N-(2-hydroxy-4-methoxyphenyl)malonamic acid, respectively (7, 9, 19). Additionally, 2-amino-3H-phenoxazin-3-one was identified as the transformation product of BOA by the fungal pathogen Gaeumannomyces graminis var. tritici (7) and by soil- and root-colonizing bacteria (6, 8). This compound and its acetyl derivative are known as the antibiotics questiomycin and N-acetylquestiomycin, respectively, which belong to the important group of actinomycin analogues. Recently, it was demonstrated that the metabolism of BOA and MBOA by Fusarium moniliforme, an endophytic fungus of corn (Zea mays), is identical to that of pathogenic fungi (22).
FIG. 1.
Structures of bezoxazinoids isolated from Aphelandra sp. DIMBOA, 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3(4H)-one.
In this work we report the biotransformation of the phytoanticipins BOA and HBOA by endophytes living inside the roots and shoots of A. tetragona. Several new products of oxidation, reduction, hydrolysis, acetylation, and nitration could be identified.
MATERIALS AND METHODS
Endophytic fungus isolation and cultivation.
Endophytic fungi were isolated from the roots and shoots of A. tetragona (Vahl) Nees (Acanthaceae) (21). Four endophytic fungi were used in this study: Plectosporium tabacinum (formerly Fusarium tabacinum), Gliocladium cibotii (CBS 372.93), Chaetosphaeria sp., and Fusarium sambucinum (tentatively identified). The stock cultures were maintained on malt extract agar (pH 5.7). Twenty milliliters of Czapek Dox broth medium (Sigma) (pH 5.7) supplemented with the allelochemicals (1 mM concentrations) was inoculated with three pieces (4-by-4-mm squares) of the mycelial plug from the margins of the agar plates. The cultures were incubated in 50-ml Erlenmeyer flasks in the dark at 25°C on a rotary shaker (100 rpm). The stock solutions (0.1 M) were prepared in ethanol. Previous experiments had indicated that the added amount of ethanol affected the fungal growth no more than 6%. Fungi in medium without any allelochemicals served as controls. The stability of the compounds in the medium was tested in uninoculated flasks (blanks). Each experiment was repeated three or four times with three replicates.
Sample preparation.
Two milliliters of the medium was taken at daily intervals and centrifuged to remove the fungal mycelium. The pH was adjusted to 3 with aqueous HCl (0.5 M), and the solution was applied to an Extrelut column (Merck) and eluted with ethyl acetate. The extract was concentrated in vacuo, and the residue was dissolved in 1 ml of methanol (MeOH) and analyzed by high-performance liquid chromatography (HPLC) (20 μl) and HPLC-mass spectrometry (MS).
Analytical procedures.
HPLC analyses were performed on an HP 1100 HPLC system (Hewlett-Packard Co., Palo Alto, Calif.). The HPLC conditions were as follows. A Nucleosil 100-5 C18 Macherey-Nagel column (250 by 3 mm) was used with a flow rate of 0.4 ml/min and Diode-Array-Detector settings of 280 and 400 nm. The mobile phase consisted of A (0.05% trifluoroacetic acid in H2O) and B (0.05% trifluoroacetic acid in MeOH). The gradient was as follows: 0 to 1 min, 3 to 20% B; 1 to 20 min, 20 to 100% B; and 20 to 25 min, 100% B.
The same HPLC system was used for HPLC-UV (DAD)-tandem MS (HPLC-MS/MS) as described above. The atmospheric pressure chemical ionization (APCI)-MS detector was interfaced directly to the output of the UV detector. APCI-MS was carried out with a Bruker ESQUIRE-LC quadrupole ion trap instrument (Bruker Daltonik, Bremen, Germany) connected to an APCI ion source (Hewlett Packard).
Electron impact (EI)-MS (at 70 eV) and chemical ionization (CI)-MS (with NH3 as the reactant gas) spectra were recorded on a Finnigan MAT 90. Electrospray ionization-MS spectra were recorded on a Finnigan TSQ 700 mass spectrometer.
Nuclear magnetic resonance (NMR) spectra were recorded at room temperature (RT) on a Bruker ARX-300 spectrometer (300 MHz for 1H and 75 MHz for 13C). Chemical shifts are reported in parts per million (δ scale), and tetramethylsilane was used as the internal standard.
Fungal growth assays.
The sensitivity of the fungi to BOA, HBOA, and their degradation products was determined by using the mycelial radial growth bioassay. An ethanol solution of the compounds tested was added to malt extract agar medium (final concentration of each compound, 0.1, 0.5, and 1 mM, respectively) and poured onto petri dishes. An agar plug (4 by 4 mm) of the fresh growing fungal mycelium was placed upside down on the center of each plate and incubated in the dark at 25°C. Fungal cultures growing on medium with the adequate amount of ethanol served as controls and were incubated simultaneously. The radius of the colony was measured at 24-h intervals until the mycelia grew to the edge of the ethanol-containing control plates or for a maximum of 8 days. The values given in Table 1 are percentages of inhibition calculated from the radius of the colony on the test medium relative to that on the ethanol-containing medium. Each experiment was repeated three times with three replicates.
TABLE 1.
Mycelial growth test of fungal cultures exposed to 1 mM BOA and HBOA and their main metabolitesa
| Compound | % of inhibition relative to control (SD) forb:
|
|||
|---|---|---|---|---|
| F. sambucinum | P. tabacinum | G. cibotii | Chaetosphaeria sp. | |
| BOA | 0 | 30.7 (1.9) | 32.5 (1.8) | 27.3 (3.0) |
| HBOA | 0 | 18.9 (2.0) | 9.2 (1.0) | 11.3 (1.5) |
| Metabolite 1 | —c | 10.5 (2.2) | 6.5 (1.5) | 6.6 (1.6) |
| Metabolite 2 | — | 10.8 (1.4) | 1.2 (1.8) | 4.4 (1.2) |
For details, see Materials and Methods.
The percentage of inhibition was calculated by using the following formula: % inhibition = 100 − [(growth on treated media/growth on control media) × 100]. The values were determined at the end of the assay period.
—, F. sambucinum does not produce these metabolites.
Chemicals.
Thin-layer chromatography plates (Silica Gel 60 F254) were from Merck. BOA, o-aminophenol, and N-(2-hydroxyphenyl)acetamide were purchased from Fluka, 99% [1,2-13C]glycine was purchased from Cambridge Isotope Laboratories, Inc., and acetoxyacetyl chloride (97%) was purchased from Aldrich. Synthetic HBOA, DIBOA, and N-(2-hydroxyphenyl)malonamic acid were kindly provided by D. Sicker (Institute of Organic Chemistry, University of Leipzig, Leipzig, Germany). For nitrite determination, the Quantofix nitrite test (Macherey-Nagel) was used.
Synthetic reference compounds. (i) 2-Hydroxy-N-(2-hydroxyphenyl)acetamide (compound 1). (a) 2-Benzoyloxyacetic acid.
2-Benzoyloxyacetic acid was prepared by a modified procedure described in reference 5. A mixture of benzoyl chloride (1.26 g), glycolic acid (1.2 g), and dioxane (3 ml) was refluxed for 30 min. The solvent was evaporated, H2O was added to the residue, and the mixture was cooled with ice. The crystalline compound was removed by filtration, washed with H2O and hexane, and recrystallized from the benzene-hexane mixture. Yield: 0.55 g (34%). Rf, 0.35 (CHCl3-MeOH-25% aqueous NH3, 2:1:0.1). 1H NMR (dimethyl sulfoxide [DMSO]-d6): 10.2 (br. s, COOH), 8.09 (d, J = 7.8 Hz, 2 arom. H), 7.50 to 7.65 (m, 1 arom. H), 7.45 (t, J = 7.8 Hz, 2 arom. H), 4.90 (s, COCH2O). 13C NMR: 173.42, 165.77 (C=O), 133.45 (arom. quat. C), 129.82, 128.84, 128.39 (arom. C), 60.45 (COCH2OH). Electrospray ionization-MS (negative mode): 179 ([M − H]−).
(b) 2-Benzoyloxyacetyl chloride.
2-Benzoyloxyacetyl chloride was prepared from 2-benzoyloxyacetic acid and SOCl2 by a previously published procedure (5).
(c) 2-Benzoyloxy-N-(2-hydroxyphenyl)acetamide.
To a mixture of o-aminophenol (160 mg), triethylamine (0.3 ml), and 4-dimethylaminopyridin (10 mg) in CH2Cl2 (3 ml), a solution of 2-benzoyloxyacetyl chloride (330 mg) in CH2Cl2 (2 ml) was introduced drop wise at 0°C with stirring. The mixture was stirred at RT for 2.5 h then evaporated to dryness. MeOH was added to the residue, and the crystallized compound was isolated by filtration and washed with MeOH. Yield: 120 mg (55%). Rf, 0.7 (CHCl3-MeOH, 9:1). 1H NMR (DMSO-d6): 9.84 (s, OH), 9.30 (s, NH), 7.35 to 8.05 (m, 6 arom. H), 6.55 to 6.95 (m, 3 arom. H), 4.89 (s, COCH2O). 13C NMR: 165.22, 165.07 (C=O), 147.42, 133.48, 129.09 (arom. quat. C), 129.23, 128.67, 125.51, 124.47, 121.50, 118.81, 115.2 (arom. C), 63.14 (COCH2OH). CI-MS: 289 (100, [M + NH4]+), 272 (70, [M + H]+).
(d) 2-Hydroxy-N-(2-hydroxyphenyl)acetamide (compound 1).
A mixture of 2-benzoyloxy-N-(2′-hydroxyphenyl)acetamide (90 mg), KOH (300 mg), and 50% aqueous MeOH (4 ml) was heated at 65°C for 1.5 h. The residue mixture was diluted with H2O, acidified with HCl, and extracted 6 times with CHCl3-isopropanol (8:2). The extract was evaporated, and the residue was dissolved in CHCl3-MeOH (95:5), introduced to a silica gel column, and eluted consecutively with CHCl3 and CHCl3-MeOH (95:5) to yield 40 mg (79%) of colorless crystals. Rf, 0.4 (CHCl3-MeOH, 9:1). HPLC-UV: retention time (Rt), 11.6 min; λmax, 204, 242, 283 nm; λmin, 226, 264 nm. 1H NMR (acetone-d6): 9.3 (br. s, NH), 9.1 (s, ArOH), 8.1 to 7 (m, 1 arom. H), 7.5 to 6.5 (m, 3 arom. H), 5.12 (t, J = 5.5 Hz, COCH2OH), 4.17 (d, J = 5.5 Hz, COCH2OH). 13C NMR: 171.50 (C=O), 147.75, 127.19 (2 quat. arom. C), 125.38, 121.37, 120.55, 116.85 (arom. C), 62.85 (COCH2OH). APCI-MS: 168 ([M + H]+), 150 ([M + H − H2O] +). CI-MS: 168 (100, [M + H]+), 185 (95, [M + NH4]+).
(ii) N-(2-Hydroxy-5-nitrophenyl)acetamide (compound 3) and N-(2-hydroxy-3-nitrophenyl)acetamide (compound 4).
Compounds 3 and 4 were prepared with some modifications according to the method described by King (10, 11). To a suspension of N-(2-hydroxyphenyl)acetamide (150 mg), a 65% aqueous solution of HNO3 (0.4 ml) was added under cooling conditions with ice. At 0°C, the mixture was stirred for 15 min, diluted with H2O, stirred for another 5 min, and filtered. The crystals were washed with H2O and dried to yield 57 mg (31%) of N-(2-hydroxy-5-nitrophenyl)acetamide. The mother liquor was evaporated to dryness, and the residue dissolved in the benzene-acetone mixture was introduced to the silica gel column and eluted consecutively with CHCl3-acetone (4:1) to yield 34 mg (19%) of N-(2-hydroxy-3-nitrophenyl)acetamide as yellow crystals.
(a) N-(2-Hydroxy-5-nitrophenyl)acetamide (compound 3).
Rf, 0.3 (CHCl3-MeOH, 9:1). HPLC-UV: Rt, 14.5 min; λmax, 228, 257, 319 nm; λmin, 220, 243, 278 nm. 1H NMR (DMSO-d6): 11.54 (br. s, OH), 9.43 (br. s, NH), 8.93 [s, C(6)H], 2.15 (s, CH3), 7.88, 7.8 [2d, J = 8.9 Hz, C(4)H], 7.02 [d, J = 8.9 Hz, C(3)H]. 13C NMR: 169.2 (C=O), 153.55, 139.05, 126.74 (arom. quat. C), 120.24, 116.38, 114.54 (arom. C), 23.7 (CH3). CI-MS: 214 (100, [M + NH4]+), 197 (10, [M + H]+).
(b) N-(2-Hydroxy-3-nitrophenyl)acetamide (compound 4).
Rf, 0.6 (CHCl3-MeOH, 9:1). HPLC-UV: Rt, 15.2 min; λmax, 238, 295, 355 nm; λmin, 218, 270, 332 nm. 1H NMR (DMSO-d6): 9.71 (br. s, NH), 7.98, 7.99 [2d, J = 8 Hz, C(4)H], 7.7, 7.69 [2d, J = 8 Hz, C(6)H], 7.0 [t, J = 8 Hz, C(5)H], 2.14 (s, CH3). 13C NMR: 169.6 (C=O), 144.0, 137.4, 129.4 (arom. quat. C), 128.3, 120.0, 119.0 (arom. C), 23.4 (CH3). EI-MS: 196 (30, [M]·+), 154 (100, [M − CH2=C=O]·+).
(iii) 2-Amino-3H-phenoxazin-3-one (compound 7).
2-Amino-3H-phenoxazin-3-one was prepared from o-aminophenol and p-benzoquinone according to the method described in reference 14. Rf, 0.5 (CHCl3-acetone, 5:1). HPLC-UV: Rt, 19.7 min; λmax, 224, 437 nm; λmin, 301, 353 nm; λsh, 270 nm. APCI-MS: 213 ([M + H]+).
(iv) 2-Acetylamino-3H-phenoxazin-3-one (compound 8).
2-Acetylamino-3H-phenoxazin-3-one was prepared from 2-amino-3H-phenoxazin-3-one according to the method described in reference 6. Rf, 0.6 (CHCl3-acetone, 5:1). HPLC-UV: Rt, 21.3 min; λmax, 240, 404 nm; λmin, 211 nm. EI-MS: 254 (30, [M]·1), 212 (100, [M − CH2=C=O]·+).
(v) Data for compound 9 [2-(N-hydroxy)acetylamino-3H-phenoxazin-3-one].
HPLC-UV: Rt, 18.8 min; λmax, 250, 408 nm; λmin, 302 nm. APCI-MS: 271 ([M + H]+); MS/MS 229 (100, [M − CH2=C=O + H]+), 253 (10, [M − H2O + H]+).
(vi) 2-(2-Hydroxyacetyl)amino-3H-phenoxazin-3-one (compound 10). (a) 2-Acetoxyacetylamino-3H-phenoxazin-3-one.
To a suspension of 2-amino-3H-phenoxazin-3-one (100 mg) in pyridine (5 ml), a solution of 2-acetoxyacetyl chloride (0.1 ml) in CH2Cl2 (1 ml) was added at 0°C and stirred under cooling conditions for 1 h. The crystalline product was removed by filtration and washed with ethanol and diethyl ether. Yield: 119 mg (81%) of orange crystals. Rf, 0.7 (CHCl3-acetone, 5:2). 1H NMR (CDCl3): 9.23 (br. s, NH), 8.44 [s, C(4′)H], 7.9 to 7.5 (m, 4 arom. H), 6.47 [s, C(1′)H], 4.76 (s, COCH2O), 2.28 (s, CH3). CI-MS: 313 ([M + H]+).
(b) 2-Hydroxyacetylamino-3H-phenoxazin-3-one (compound 10).
A suspension of 21 mg of 2-acetoxy-N-(3-oxo-3H-phenoxazin-2-yl)acetamide in a mixture of dimethylformamide (1 ml), and a 50% aqueous solution of K2CO3 (0.1 ml) was heated at 50°C for 30 min and then diluted with H2O. The orange crystalline product was removed by filtration, washed with H2O, and dried. The product was dissolved in CH2Cl2 and purified by column chromatography on silica gel (CH2Cl2-acetone, 10:1) to yield 6 mg (33%). Rf, 0.4 (CHCl3-acetone, 5:1). HPLC-UV: Rt, 19.9 min; λmax, 235, 403 nm; λmin, 210, 293 nm. 1H NMR (CF3COOD): 8.86 [s, C(1′)H], 8.1 to 7.65 (m, 4 arom. H), 7.04 [s, C(4′)H], 4.75 (s, COCH2). CI-MS: 271 ([M + H]+).
(vii) 2-Hydroxy-N-(2-hydroxyphenyl)[1,2-13C]acetamide (1,2-13C-labeled compound 1). (a) Benzyl 2-amino[1,2-13C]acetate hydrochloride ([1,2-13C]glycine benzylester hydrochloride).
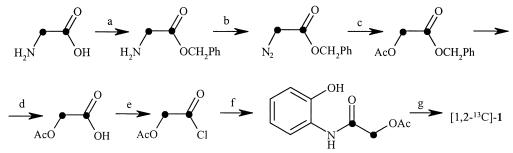
The schema of the synthesis of 2-hydroxy-N-(2-hydroxyphenyl)[1,2-13C]acetamide (1,2-13C-labeled compound 1) is shown in Fig. 2. SOCl2 (5 ml) was introduced drop wise to a cooled (5°C) suspension of commercially available [1,2-13C]glycine (480 mg) in benzylic alcohol (15 ml). The suspension was stirred under cooling conditions for an additional 15 min and then heated at 120°C for 2 h. After cooling with ice, diethyl ether was added to the mixture until it became cloudy. The crystallized benzyl 2-amino[1,2-13C]acetate hydrochloride (1.04 g, 83%) was removed by filtration, washed several times with diethyl ether, and dried. 1H NMR (DMSO-d6): 8.75 (br. s, NH3+), 7.55 to 7.25 (m, 5 arom. H), 5.23 (d, J = 3 Hz, PhCH2O), 3.83 (dd, J = 138 Hz, 6.4 Hz, N13CH213C=O). 13C NMR: 167 (d, J = 61 Hz, 13C=O), 39.45 (d, J = 62 Hz, N13CH2). CI-MS: 168 ([M + H]+).
FIG. 2.
Schema of the synthesis of 2-hydroxy-N-(2-hydroxyphenyl)[1,2-13C]acetamide (1,2-13C-labeled compound 1). Ph, phenyl; •, 13(-atom; Ac, acetyl.
(b) Benzyl diazo[1,2-13C]acetate.
CH2Cl2 (15 ml) was added to a −10°C cooled solution of benzyl 2-amino[1,2-13C]acetate hydrochloride (990 mg, 0.0049 mol) in H2O (10 ml). To the vigorously stirred mixture, first a solution of NaNO2 (0.5 g, 0.0073 mol) in H2O (5 ml) was added drop wise and then a 5% aqueous solution of H2SO4 (2.4 ml) was added drop wise. The mixture was stirred at 0°C for 20 min and then transferred to a previously cooled (at −20°C) separator funnel. The lower yellow-colored organic layer was transferred to a saturated aqueous NaHCO3 solution. The water phase was extracted once more with CH2Cl2. The combined organic extracts were washed with saturated aqueous NaHCO3 solution, dried (Na2SO4), and evaporated at RT to yield 562 mg (65%) of yellow oil. 1H NMR (CDCl3): 7.45 to 7.25 (m, 5 arom. H), 5.19 (d, J = 3.5 Hz, PhCH2O), 4.77 (d, J = 203 Hz, 13CHN2). 13C NMR: 166 (d, J = 97 Hz, 13C=O), 46.12 (d, J = 96 Hz, 13CHN2). CI-MS: 196 (5, [M + NH4]+), 108 (51), 91 (100).
(c) Benzyl 2-acetoxy[1,2-13C]acetate.
A solution of benzyl diazo[1,2-13C]acetate (500 mg) in CH2Cl2 (3 ml) was added drop wise at 0°C to a stirred mixture of acetic acid (3 ml) containing 3 drops of BF3-diethyl etherate. The yellow color immediately disappeared, and N2 was released. The mixture was stirred for an additional 2 h, diluted with CHCl3, transferred to a separator funnel, washed with saturated aqueous NaHCO3 solution, dried (Na2SO4), and evaporated to yield 579 mg (98%) of pale yellow oil. 1H NMR (CDCl3): 7.45 to 7.25 (m, 5 arom. H), 5.19 (d, J = 3 Hz, PhCH2O), 4.63 (dd, J = 144 Hz, 5 Hz, O13CH213C=O), 2.15 (s, CH3). 13C NMR: 167.7 (d, J = 64 Hz, 13C=O), 60.73 (d, J = 65 Hz, 13CHN2). CI-MS: 228 ([M + NH4]+).
(d) 2-Acetoxy[1,2-13C]acetate.
PdIC (10%, 100 mg) was added to a solution of benzyl 2-acetoxy[1,2-13C]acetate (529 mg) in MeOH (25 ml). The suspension was stirred in an H2 atmosphere at RT for 1 h, filtered, and evaporated to yield 292 mg (97%) of colorless oil. 1H NMR (CDCl3): 8.4 (br. s, 13COOH), 4.64 (dd, J = 145 Hz, 4.4 Hz, O13CH213C=O), 2.16 (s, CH3). 13C NMR: 173 (d, J = 62 Hz, 13C=O), 60.4 (d, J = 62 Hz, O13CH213C=O). CI-MS: 138 ([M + NH4]+).
(e) 2-Acetoxy[1,2-13C]acetyl chloride.
A mixture of 2-acetoxy[1,2-13C]acetic acid (259 mg), SOCl2 (2 ml), and benzene (3 ml) was refluxed for 1 h and evaporated under reduced pressure at RT. The residual oil (303 mg, 100%) was dissolved in 2 ml of CH2Cl2 without purification, and this solution was introduced to the next synthetic step.
(f) 2-Acetoxy-N-(2-hydroxyphenyl)[1,2-13C]acetamide.
To a suspension of o-aminophenol (480 mg) in a mixture of CH2Cl2 (3 ml), triethylamine (1 ml), and 4-dimethylaminopyridine (20 mg), a solution of 2-acetoxy[1,2-13C]acetyl chloride (303 mg) in CH2Cl2 (2 ml) was introduced drop wise at 0°C. The mixture was stirred under cooling conditions for 1 h, introduced directly into a silica gel column, and eluted consecutively with CHCl3 (1% ethanol) and CHCl3-MeOH (95:5) to yield 260 mg (56%) of colorless solid. Rf, 0.75 (CHCl3-MeOH, 9:1). 1H NMR (DMSO-d6): 9.82 (br. s, NH), 9.23 (br. s, OH), 7.81 (d, J = 8 Hz, 1 arom. H), 7.5 to 6.65 (m, 3 arom. H), 4.7 (dd, J = 145 Hz, 4 Hz, O13CH213C=O), 2.12 (s, CH3). 13C NMR: 165 (d, J = 55 Hz, 13C=O), 62.5 (d, J = 55 Hz, O13CH213C=O). CI-MS: 212 (100, [M + H]+), 229 (9, [M + NH4]+).
(g) 2-Hydroxy-N-(2-hydroxyphenyl)[1,2-13C]acetamide (1,2-13C-labeled compound 1).
A mixture of 2-acetoxy-N-(2′-hydroxyphenyl)[1,2-13C]acetamide (200 mg), KOH (200 mg), and MeOH (3 ml) was heated at 60°C for 20 min, acidified with an MeOH solution of HCl, and evaporated. The residue was dissolved in a mixture of CHCl3-MeOH (9:1), introduced directly into a silica gel column, and eluted with CHCl3-MeOH (95:5) to yield 150 mg of a solid residue. The crystalline compound was dissolved in MeOH, and the solution was decolorized with activated charcoal, filtered, and evaporated to yield 146 mg (91%) of colorless crystals. Rf, 0.4 (CHCl3-MeOH, 9:1). HPLC-UV: Rt, 11.6 min; λmax, 204, 242, 283 nm; λmin, 226, 264 nm. 1H NMR (acetone-d6): 9.3 (br. s, NH), 9.14 (br. s, ArOH), 8.02 (d, J = 8 Hz, 1 arom. H), 7.5 to 6.7 (m, 3 arom. H), 5.15 (br. s, 13COH), 4.16 (dd, J = 140 Hz, 4 Hz, O13CH213C=O). 13C NMR: 171 (d, J = 60 Hz, 13C=O), 62.8 (d, J = 54 Hz, O13CH213C=O). CI-MS: 170 (100, [M + H]+), 187 (75, [M + NH4]+).
RESULTS
Preliminary studies of growth inhibition by the fungitoxic plant anticipins BOA and HBOA with 20 endophytic fungi from A. tetragona led to the selection of four different isolates for further studies. P. tabacinum and G. cibotii showed a pronounced growth reduction, Chaetosphaeria sp. showed a slight growth reduction, and F. sambucinum showed no mycelial growth reduction when cultivated on malt agar plates containing 1 mM BOA or HBOA.
The degradation products of HBOA and BOA were analyzed by HPLC, HPLC-MS, and MS/MS. The structures of the metabolites were deduced from the data obtained and proven by comparison with the synthetic reference compounds.
Metabolism of BOA.
Three of the four endophytic fungi tested, P. tabacinum, G. cibotii, and Chaetosphaeria sp., were unable to metabolize BOA. After an incubation time of 24 days, the content of BOA remained unchanged and no metabolites were detected.
When F. sambucinum was cultivated in medium supplemented with 1 mM BOA (Rt = 14.9 min) a more-polar compound (Rt = 11.5 min) was detected by HPLC after 10 days. By HPLC-MS and MS/MS analysis and comparison with the synthetic reference, N-(2-hydroxyphenyl)malonamic acid (compound 6) was identified (Fig. 3). After 14 days, BOA had disappeared from the medium while the concentration of compound 6 had continually increased (final concentration detected, 0.3 mM). No further transformation occurred during an additional 10 days of cultivation, indicating that metabolite compound 6 is a stable terminal product.
FIG. 3.
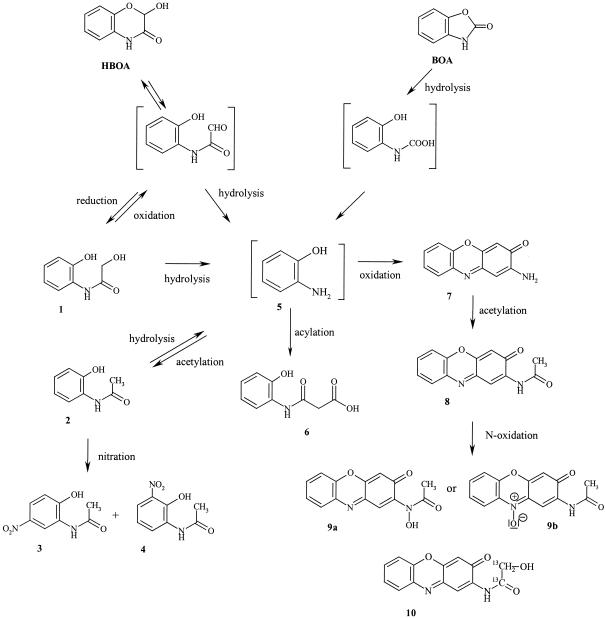
Proposed schema of biotransformation of HBOA and BOA by F. sambucinum, P. tabacinum, G. cibotii, and Chaetosphaeria sp. Metabolite compounds 1 to 10 were identified as degradation products in the medium.
Metabolism of HBOA.
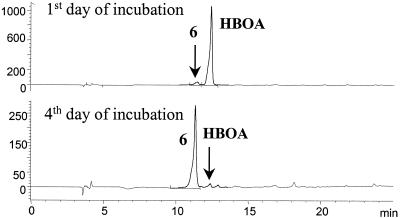
N-(2-Hydroxyphenyl)malonamic acid (compound 6) was also the sole metabolite produced by F. sambucinum from HBOA. The complete transformation to compound 6 was accomplished after 4 days of cultivation (Fig. 4).
FIG. 4.
HPLC chromatograms of samples taken after 1 and 4 days of incubation of F. sambucinum with HBOA (initial concentration, 1 mM) monitored at 280 nm. HBOA is transformed to N-(2-hydroxyphenyl)malonamic acid (compound 6) (final concentration, 0.31 ± 0.03 mM, determined with an external standard). Values on the y axis are in milli-absorption units.
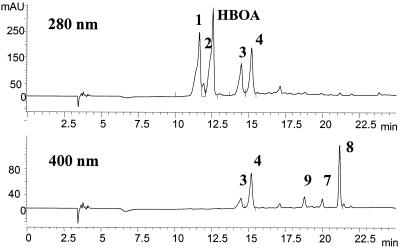
When P. tabacinum, G. cibotii, and Chaetosphaeria sp. were cultivated in the medium containing HBOA, the accumulation of several metabolites was observed. Figure 5 shows the HPLC-UV chromatograms of the medium monitored at 280 and 400 nm after HBOA transformation by G. cibotii, the isolate producing the broadest spectrum of metabolites.
FIG. 5.
HPLC chromatogram of the medium after 10 days of incubation of G. cibotii with HBOA monitored at 280 and 400 nm. The 1 mM concentration of HBOA corresponds to 1,200 mAU. The numbering of compounds corresponds to that of Fig. 3. Metabolite compound 2 is almost transformed to compounds 3 and 4. At 400 nm, metabolite compounds 7, 8, and 9 are detected. Metabolite compound 7 is almost changed to compound 8, and compound 8 starts to be further transformed to compound 9. Values on the y axis are in milli-absorption units.
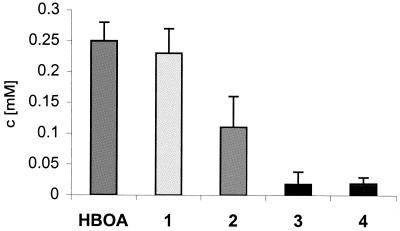
After 3 days of cultivation of G. cibotii with HBOA (Rt = 12.6 min), a significant amount of a more-polar compound (Rt = 11.6 min) was detected in the medium (compound 1) (Fig. 3) and its concentration increased continually while that of HBOA was decreasing. A second degradation product (compound 2) (Rt = 12 min) was observed after 6 days. During the further cultivation, compound 2 was transformed to its p- and o-nitro derivatives (compounds 3 and 4) (Rt = 14.5 and 15.2 min, respectively). Twenty-five percent of the HBOA stayed in the medium unchanged (even after 24 days), and compounds 1, 2, 3, and 4 were detected as the main metabolites (Fig. 6). The cultures of P. tabacinum and Chaetosphaeria sp. degraded HBOA completely within 14 days and compounds 1 and 2 were the two main metabolites. In the course of all experiments, the consumption of compound 1 and the accumulation of compound 2 were observed.
FIG. 6.
Compounds detected in the medium of G. cibotii incubated for 16 days with HBOA (initial concentration, 1 mM). The numbering of compounds corresponds to that of Fig. 3. Concentrations (c) were determined by HPLC analysis with the external standards. Values represent means and standard deviations (error bars) of triplicate experiments with three replicates.
In all three fungal cultures, the HBOA degradation was accompanied by the formation of less-polar, colored metabolites. The color of the medium had changed after 3 days, and the signal of metabolite compound 7 appeared on the chromatogram (Rt = 19.7 min) (Fig. 5) (400 nm). With further cultivation, compound 7 was readily transformed to its N-acetylated derivative (compound 8) (Rt = 21.3 min), and this metabolite was found in the samples of P. tabacinum and G. cibotii. Metabolite compound 8 is further transformed to its N-hydroxylated derivative (compound 9) (Rt = 18.8 min). The concentration of the colored metabolites in the medium reached a maximum of 10 μM.
The results of BOA and HBOA transformation for each endophytic fungal isolate are summarized in Table 2.
TABLE 2.
Overview of the biotransformation of BOA and HBOA by fungal isolates
| Compound | Metabolite(s) produced by:
|
||
|---|---|---|---|
| F. sambucinum | P. tabacinum and G. cibotii | Chaetosphaeria sp. | |
| BOA | 6 | None | None |
| HBOA | 6 | 1, 2, 3,a 4,a 7, 8, 9, 10b | 1, 2, 7 |
Metabolites 3 and 4 appear only in the medium of G. cibotii.
Metabolite 10 appears in detectable amounts only with 13C2-labeled compound 1 in the culture of P. tabacinum.
Identification of metabolite compounds 1 to 4 and 7 to 9 (Fig. 3) produced by P. tabacinum, G. cibotii, and Chaetosphaeria sp.
Metabolite compound 1 showed, after HPLC-MS analysis, a quasimolecular ion ([M + H]+) with an m/z of 168, which is 2 amu (atomic mass unit) higher than the quasimolecular ion of HBOA. Since HBOA is a cyclic hemiacetal, it seemed likely that this compound is the product of its reduction to the corresponding hydroxyacetyl derivative, 2-hydroxy-N-(2-hydroxyphenyl)acetamide (compound 1). Comparison with the synthetically prepared compound 1 confirmed this deduction.
The quasimolecular ion ([M + H]+) with an m/z of 152 of metabolite compound 2 is 16 amu less than that of metabolite compound 1, which suggested the acetyl derivative compound 2 [N-(2-hydroxyphenyl)acetamide]. This was proven by using commercially available compound 2 as a standard.
Metabolite compounds 3 and 4 were identified as N-(2-hydroxy-5-nitrophenyl)acetamide and N-(2-hydroxy-3-nitrophenyl)acetamide, respectively. Both signals showed the same quasimolecular ion ([M + H]+) with an m/z of 197. The even number of the molecular mass was evidence for the even number of nitrogen atoms in these compounds. Additionally, the MS/MS analyses gave in both cases the same fragment ion (m/z, 155), which corresponds to ([M − CH2=C=O + H]+). The suggested structures of compounds 3 and 4 were proven by using synthetic standards. The orientation of the nitro group in the p and o positions toward the phenolic O atom, but not toward the N atom, indicates that the substrate has been N-acetylated before the aromatic nitration.
An additional degradation product appeared first in metabolite compound 7 with the quasimolecular ion ([M + H]+) with an m/z of 213, which pointed at 2-amino-3H-phenoxazin-3-one. As proof of this assumption, compound 7 was synthesized and its analytical data were compared with that of the detected metabolite. Metabolite compound 8, with a quasimolecular ion ([M + H]+) with an m/z of 255 and an MS/MS fragment with an m/z of 213 ([M − CH2=C=O + H]+), corresponds to the N-acetylated derivative of compound 7, namely 2-acetylamino-3H-phenoxazin-3-one (compound 8), and it was similarly identified by comparison with the synthetically prepared standard.
Metabolite compound 9 gave a quasimolecular ion ([M + H]+) with an m/z of 271, which is 16 amu more than that of metabolite compound 8 and obviously corresponds to its hydroxylated derivative. The MS/MS fragmentation of the parent ion yields a main fragment with an m/z of 229 (100, [M − CH2=C=O + H]+), which is 16 amu more than the quasimolecular ion of metabolite compound 7 ([M + H]+, m/z of 213). Since the UV absorption spectrum of compound 9 is similar to that of compound 8, the additional O atom cannot be connected with the chromophore of the molecule and suggests a possible N oxidation product. There are two possible positions for N oxidation in molecules of compound 8, on the amide NH and at N-10 (Fig. 3, structures 9a and b). As the MS/MS fragmentation of the parent ion (with an m/z of 271) yields a daughter ion with an m/z of 253 (10, [M + H − H2O]+), structure 9a is favored. A dehydration fragment of the quasimolecular ion corresponding to the alternative structure 9b is very unlikely (3). Unfortunately, all attempts for synthetic preparation of 9a and 9b were unsuccessful.
Metabolism of compounds 1 and 2.
In order to establish the further transformation pathway of the two main metabolite compounds 1 and 2, G. cibotii, P. tabacinum, and Chaetosphaeria sp. were cultivated on media supplemented with these compounds.
Interestingly, when metabolite compound 1 was added to the medium, its partial reverse oxidation to HBOA (approximately 20% of compound 1) was observed after 3 days. After 10 days of cultivation, the same compounds as for HBOA degradation were detected (HBOA and compounds 1 to 4 and 7 to 9).
In the case of metabolite compound 2, approximately 70% was degraded by G. cibotii within 6 days and compounds 3, 4, 8, and 9 were detected. No degradation of metabolite compound 2 was observed with P. tabacinum and Chaetosphaeria sp.
Pathway of biotransformation.
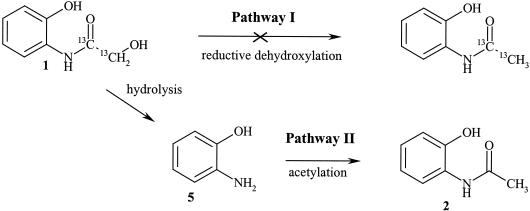
The mechanism of the reaction leading from metabolite compound 1 to compound 2 was established. The labeled metabolite compound 1 {2-hydroxy-N-(2-hydroxyphenyl)[1,2-13C2]acetamide} was fed to the fungal cultures in order to determine whether compound 1 is transformed to compound 2 via an enzymatic activation of the hydroxyl group at position 2 (possible phosphorylation) followed by a reductive dehydroxylation (pathway I) or via an initial hydrolysis yielding o-aminophenol (compound 5), which is enzymatically acetylated further (pathway II) (Fig. 7). For this experiment, the cultures of P. tabacinum, G. cibotii, and Chaetosphaeria sp. were used.
FIG. 7.
Two possible pathways for the transformation of 2-hydroxy-N-(2-hydroxyphenyl)acetamide (compound 1) to N-(2-hydroxyphenyl)acetamide (compound 2). Compound 5 corresponds to o-aminophenol.
By HPLC-MS analysis it could be shown that only HBOA originated directly from labeled compound 1. The metabolite compounds 2, 3, 4, 7, and 8 contained no 13C2-labeled fragment. This fact strongly suggests that the biotransformation pathway of metabolite compound 1 leads via o-aminophenol as the general intermediate (pathway II) (Fig. 7). The results from the incubation of the fungal isolates with o-aminophenol-yielding metabolite compounds 2, 6, 7, or 8 also supported this affirmation (Fig. 3).
A new metabolite was detected (Rt = 19.9 min) when P. tabacinum was cultivated with labeled compound 1. This metabolite has a UV spectrum similar to that of compound 8 (Fig. 3). Its quasimolecular ion ([M + H]+) with an m/z of 273 is 16 amu (2 amu more for 13C2) higher than that of metabolite compound 8 and indicates an additional hydroxylation (the same as for compound 9). But in this case, the hydroxy functionality is part of the lost fragment, as shown by the MS/MS fragmentation yielding a fragment ion with an m/z of 213 ([M − OH—13CH=13C=O + H]+), which corresponds to protonated aminophenoxazinone (compound 7). These data led to the deduction of metabolite compound 10. A synthetically prepared unlabeled compound 10 as a standard proved this hypothesis. Obviously, the 13C2-labeled compound 1 is more stable for enzymatic hydrolysis, and only some amount of it undergoes hydrolysis to o-aminophenol (compound 5), which is further oxidatively coupled with the unhydrolyzed molecule to yield compound 10. It seems that in the case of P. tabacinum, the labeled compound 1 manifested remarkable isotopic effects, because not only the presence of the new compound 10 but also the time and rate of its transformation differs from that of the unlabeled one.
Biological activity of BOA, HBOA, and metabolite compounds 1 and 2.
The biological activity of BOA, HBOA, and metabolite compounds 1 and 2 was determined by utilizing the mycelial growth inhibition assay. Three concentrations were tested (1, 0.5, and 0.1 mM), and the results with the 1 mM concentration are shown in Table 1.
All four fungi were more sensitive to BOA than to HBOA. Both compounds demonstrated the strongest impact on the mycelial growth at the initial phase of cultivation (e.g., 77% inhibition by BOA on G. cibotii). After that, the fungi were able to adapt or at least to reduce their effect. In comparison to HBOA, its degradation of metabolite compounds 1 and 2 already showed less impact on the mycelial growth at the beginning of the assay period.
DISCUSSION
The defense compounds HBOA and BOA have been isolated in significant amounts from the roots of A. tetragona (2, 17). It is known that virulent phytopathogenic fungi of different cereals are able to transform BOA to less-toxic metabolites (7). In this work, we report that endophytic fungi isolated from the roots and shoots of A. tetragona metabolize BOA and HBOA to a series of different compounds (Table 2).
Since the spontaneous degradation product of DIBOA, namely BOA, is considered the terminal defense compound in the plant-parasite interaction, we cultivated all four endophytic isolates on medium supplemented with BOA. Surprisingly, even after a long incubation time (24 days) P. tabacinum, G. cibotii, and Chaetosphaeria sp. did not show any degradation. However, F. sambucinum transformed BOA completely within 14 days into N-(2-hydroxyphenyl)malonamic acid (compound 6), a compound already known to be a degradation product of BOA by fungal pathogens of maize and wheat (7, 9, 19, 22). F. sambucinum was also the only one which showed no growth inhibition with 1 mM BOA, whereas P. tabacinum, G. cibotii, and Chaetosphaeria sp. were inhibited (Table 1). The incapacity of the fungi to detoxify BOA correlates with the reduced ability to grow in the presence of this allelochemical. Recently, 29 species of Fusarium were assessed for their tolerance to a 10-times-higher concentration of BOA (1 mg/ml in contrast to 0.13 mg/ml in the present paper). Two strains of F. sambucinum isolated from a potato were tested, but none of them was able to grow on media containing 1.0 mg of BOA per ml (9). The F. sambucinum strain living as an endophyte in A. tetragona is apparently adapted to the toxic metabolites of the plant. Unfortunately, the question of how the endophytic isolates P. tabacinum, G. cibotii, and Chaetosphaeria sp. can adapt to BOA without degrading cannot be answered at present.
In the roots of A. tetragona, HBOA was detected in a remarkable concentration (0.06% fresh weight) (17). In the course of the present study, all four tested fungi were incubated together with 1 mM HBOA. Surprisingly F. sambucinum also transforms HBOA completely into N-(2-hydroxyphenyl)malonamic acid (compound 6) within 4 days. This is the first time that this compound is described as the degradation product of HBOA.
The incubation of P. tabacinum, G. cibotii, and Chaetosphaeria sp. on medium containing HBOA led to a large spectrum of metabolites. Two main metabolites (compounds 1 and 2) are clearly degradation products of HBOA since the fungal isolates were not sensitive to them. Metabolite compound 1 appeared in the medium as a result of an enzymatic reduction of HBOA. It is remarkable that if compound 1 was added to the medium, it was partially reconverted to HBOA.
In the course of the incubation with HBOA, metabolite compound 1 is transformed into compound 2. Using 13C2-labeled compound 1, it was clearly shown that its transformation involves a hydrolytic step, yielding o-aminophenol (compound 5), which is further transformed (by acylation, nitration, and oxidation) to the established metabolites (compounds 2 to 10) (Fig. 3). Thus, o-aminophenol appears to be the key intermediate in all of these biotransformations. This affirmation was supported also by results from direct incubation of the fungal isolates with o-aminophenol, yielding metabolite compounds 2, 6, 7, or 8.
o-Aminophenol (compound 5) can be spontaneously oxidized to 2-amino-3H-phenoxazin-3-one (compound 7) (1). Compound 7 and its N-acetyl derivative, compound 8, have been mentioned earlier in connection with BOA degradation by root-infecting fungi of wheat (7), soil bacteria, and root-colonizing bacteria of wheat (6, 8). In our case, compounds 7 and 8 were found only during the experiments with HBOA, whereas with BOA no aminophenoxazinone-related compounds were detected. Moreover, a new derivative of compound 8 was found while G. cibotii or P. tabacinum additionally hydroxylated it at the amidic N atom (compound 9a).
The incubation of G. cibotii with HBOA and metabolite compound 1 or 2 resulted in two nitro derivatives of compound 2 (compounds 3 and 4). The source of the nitro functionality seems to be the inorganic nitrate present in the cultivation medium, as it has been previously described for Streptomyces fumanus, producing dioxapyrrolomycin (4). Recent studies of the biotransformation of tocopherols by Streptomyces catenulae revealed that the nitro derivatives, which were isolated from the culture medium, were formed by a combination of enzymatic nitrate reduction to nitrite followed by a nonenzymatic nitration (15). Since nitrite was detected in the cultivation medium of G. cibotii, but not in the three other cultures (data not shown), it can be assumed that the nitration of compound 2 follows the same mechanism.
In conclusion, the endophytic fungi extensively metabolize HBOA. Three of the fungal isolates have similar biochemical potencies for transforming HBOA into several metabolites and no capacity to open the oxazolone ring of BOA. One of them, F. sambucinum, detoxified BOA and HBOA to the same product. Nine metabolites were identified, and six of them have been unknown until now in connection with benzoxazinoids. The fact that three of nine metabolites detected during the transformation of HBOA have been previously described as degradation products of BOA led to the suggestion that the same metabolic pathway is used for the degradation of these two benzoxazinoids. In the present study, we manifest that there is a unified metabolic pathway starting with the hydrolysis of the hemiacetal ring of HBOA or the oxazolone ring of BOA and leading in both cases to o-aminophenol, which followed by acylation yields N-(2-hydroxyphenyl)acetamide or N-(2-hydroxyphenyl)malonamic acid. The enzymes catalyzing the biotransformation of BOA and HBOA are presumed to be N-acetyl- and N-malonyl-transferases as well as oxidases and reductases, e.g., cytochrome P-450 monooxygenases. While the first are well-known detoxification enzymes for xenobiotics in living systems, the latter are often highly specific biocatalysts. Their potential for biotransformations could be of interest in biotechnology, and further studies are worthwhile.
Acknowledgments
We thank Dieter Sicker (Institute of Organic Chemistry, University of Leipzig) for providing the reference samples of HBOA, DIBOA, and N-(2-hydroxyphenyl)malonamic acid and Ulf Thrane (BioCentrum-DTU, Technical University of Denmark, Lyngby), Orlando Petrini (Pharmaton SA, Lugano, Switzerland), and Walter Gams (Centraalbureau voor Schimmelcultures, The Netherlands) for the identification of fungal isolates.
This research was supported by the Swiss National Science Foundation, Helmut Legerlotz-Stiftung, and UBS AG, Zürich, Switzerland (Das Forschungsprojekt wurde unterstützt durch UBS AG im Auftrag eines Kunden).
REFERENCES
- 1.Barry, C. E., III, P. G. Nayar, and T. P. Begley. 1989. Phenoxazinone synthase: mechanism for the formation of the phenoxazinone chromophore of actinomycin. Biochemistry 28:6323-6333. [DOI] [PubMed] [Google Scholar]
- 2.Baumeler, A., M. Hesse, and C. Werner. 2000. Benzoxazinoids-cyclic hydroxamic acids, lactams and their corresponding glucosides in the genus Aphelandra (Acanthaceae). Phytochemistry 53:213-222. [DOI] [PubMed] [Google Scholar]
- 3.Bild, N., and M. Hesse. 1967. Notiz über die Massenspektren von N-oxiden. Helv. Chim. Acta 50:1885-1892. [DOI] [PubMed] [Google Scholar]
- 4.Carter, G. T., J. A. Nietsche, J. J. Goofman, M. J. Torrey, T. S. Dunne, M. M. Siegel, and D. B. Borders. 1989. Direct biochemical nitration in the biosynthesis of dioxapyrrolomycin. A unique mechanism for the introduction of nitro groups in microbial products. J. Chem. Soc. D 1989:1271-1273. [Google Scholar]
- 5.Concilio, C., A. Tezza, and E. G. Trivellato. 1957. Esters of chloramphenicol. I. Water-insoluble acylglycolates. Farmaco (Pavia) Ed. Sci. 12:655-662. [PubMed] [Google Scholar]
- 6.Friebe, A., I. Wieland, and M. Schulz. 1996. Tolerance of Avena sativa to the allelochemical benzoxazolinone. Degradation of BOA by root-colonizing bacteria. Angew. Bot. 70:150-154. [Google Scholar]
- 7.Friebe, A., V. Vilich, L. Hennig, M. Kluge, and D. Sicker. 1998. Detoxification of benzoxazolinone allelochemicals from wheat by Gaeumannomyces graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and Fusarium culmorum. Appl. Environ. Microbiol. 64:2386-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagliardo, R. W., and W. S. Chilton. 1992. Soil transformation of 2(3H)-benzoxazolone of rye into phytotoxic 2-amino-3H-phenoxazin-3-one. J. Chem. Ecol. 18:1683-1691. [DOI] [PubMed] [Google Scholar]
- 9.Glenn, A. E., D. M. Hinton, I. E. Yates, and C. W. Bacon. 2001. Detoxification of corn antimicrobial compounds as the basis for isolating Fusarium verticillioides and some other Fusarium species from corn. Appl. Environ. Microbiol. 67:2973-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King, H. 1927. Trypanocidal action and chemical constitution. Part VI. Amphoteric s-carbamidoarylarsinic acids. J. Chem. Soc. 1927:1049-1060. [Google Scholar]
- 11.Maleski, R. 1993. Improved procedures for the preparation of 2-nitro-5-methoxyphenol and 6-methoxy-2(3H)-benzoxazolone from 3-methoxyphenol. Synth. Commun. 23:343-348. [Google Scholar]
- 12.Niemeyer, H. M. 1988. Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defence chemicals in the gramineae. Phytochemistry 27:3349-3358. [Google Scholar]
- 13.Osbourn, A. E. 1999. Antimicrobial phytoprotectants and fungal pathogens: a commentary. Fungal Genet. Biol. 26:163-168. [DOI] [PubMed] [Google Scholar]
- 14.Osman, A.-M., and I. Bassiouni. 1960. Synthesis of oxazolo-phenoxazines. J. Am. Chem. Soc. 82:1607-1609. [Google Scholar]
- 15.Rousseau, B., L. Dostal, and J. P. N. Rosazza. 1997. Biotransformations of tocopherols by Streptomyces catenulae. Lipids 32:79-84. [DOI] [PubMed] [Google Scholar]
- 16.Tan, R. X., and W. X. Zou. 2001. Endophytes: a rich source of functional metabolites. Nat. Prod. Rep. 18:448-459. [DOI] [PubMed] [Google Scholar]
- 17.Todorova, M., C. Werner, and M. Hesse. 1994. Enzymatic phenol oxidation and polymerization of the spermine alkaloid aphelandrine. Phytochemistry 37:1251-1256. [Google Scholar]
- 18.VanEtten, H. D., R. W. Sandrock, C. C. Wasmann, S. D. Soby, K. McCluskey, and P. Wang. 1995. Detoxification of phytoanticipins and phytoalexins by phytopathogenic fungi. Can. J. Bot. 73:S518-S525. [Google Scholar]
- 19.Vilich, V., B. Löhndorf, R. A. Sikora, and A. Friebe. 1999. Metabolism of benzoxazolinone allelochemicals of Zea mays by Fusarium subglutinans. Mycol. Res. 103:1529-1532. [Google Scholar]
- 20.Virtanen, A. I., and P. K. Hietala. 1960. Precursors of benzoxazolinone in rye plants. Acta Chem. Scand. 14:499-504. [Google Scholar]
- 21.Werner, C., O. Petrini, and M. Hesse. 1997. Degradation of the polyamine alkaloid aphelandrine by endophytic fungi isolated from Aphelandra tetragona. FEMS Microbiol. Lett. 155:147-153. [DOI] [PubMed] [Google Scholar]
- 22.Yue, Q., C. W. Bacon, and M. D. Richardson. 1998. Biotransformation of 2-benzoxazolinone and 6-methoxy-benzoxazolinone by Fusarium moniliforme. Phytochemistry 48:451-454. [Google Scholar]