Abstract
The expression of heterologous bacterial glycosyltransferases is of interest for potential application in the emerging field of carbohydrate engineering in gram-positive organisms. To assess the feasibility of using enzymes from gram-negative bacteria, the functional expression of the genes wbaP (formerly rfbP), wecA (formerly rfe), and wbbO (formerly rfbF) from enterobacterial lipopolysaccharide O-polysaccharide biosynthesis pathways was examined in Bacillus subtilis. WbaP and WecA are initiation enzymes for O-polysaccharide formation, catalyzing the transfer of galactosyl 1-phosphate from UDP-galactose and N-acetylglucosaminyl 1-phosphate from UDP-N-acetylglucosamine, respectively, to undecaprenylphosphate. The WecA product (undecaprenylpyrophosphoryl GlcNAc) is used as an acceptor to which the bifunctional wbbO gene product sequentially adds a galactopyranose and a galactofuranose residue from the corresponding UDP sugars to form a lipid-linked trisaccharide. Genes were cloned into the shuttle vectors pRB374 and pAW10. In B. subtilis hosts, the genes were effectively transcribed under the vegII promoter control of pRB374, but the plasmids were susceptible to rearrangements and deletion. In contrast, pAW10-based constructs, in which genes were cloned downstream of the tet resistance cassette, were stable but yielded lower levels of enzyme activity. In vitro glycosyltransferase assays were performed in Escherichia coli and B. subtilis, using membrane preparations as sources of enzymes and endogenous undecaprenylphosphate as an acceptor. Incorporation of radioactivity from UDP-α-d-14C-sugar into reaction products verified the functionality of WbaP, WecA, and WbbO in either host. Enzyme activities in B. subtilis varied between 20 and 75% of those measured in E. coli.
Glycoconjugates play a crucial role in many of the recognition, signaling, and adhesion events that take place at the surfaces of cells, with the oligosaccharide structures being the key to their functions (34). Manipulation of cell surface glycosylation patterns by carbohydrate-engineering techniques requires specific glycosyltransferases, glycosidases, nucleotide-sugar synthases, and transporters (12). Cell surface display of rationally designed glycosylation motifs is generally considered a versatile tool for applied research, leading to the expression of various antigenic determinants, tissue-targeting signals, or receptor mimics. For example, Paton et al. (30, 31) recently constructed a recombinant Escherichia coli that displayed a Shiga toxin receptor mimic on its surface. The high capacity of the engineered bacterium for adsorbing and neutralizing the toxin was attributed to the high density of receptor mimics displayed on the surface, which underlines the importance of multivalency for cell surface display.
Surface (S) layer-covered members of the family Bacillaceae are considered promising candidates for a combined cell surface display-carbohydrate-engineering approach. In general, S-layer glycoproteins are found as the outermost cell wall layer on several gram-positive bacteria (for reviews, see references 27 and 35). They assemble into two-dimensional crystalline arrays with the S-layer glycan chains protruding from the cell surface in a defined and regular manner. S-layers contain highly variable glycoconjugates and might be seen as gram-positive equivalents of lipopolysaccharides (LPS), which are essential and characteristic components of the outer membranes of gram-negative bacteria (47). The two classes of glycoconjugates show similarities with regard to the overall structure and constituent glycoses. The LPS of members of the family Enterobacteriaceae consists of three structural domains: the O-antigenic polysaccharide chain made of repeating units, the core oligosaccharide, and lipid A, serving as the anchor to the outer membrane. The S-layer glycans are also tripartite structures with the protein component replacing lipid A (35). Preliminary insights into S-layer glycan biosynthesis have provided evidence that there are also similarities on the molecular level. Genes encoding enzymes for the synthesis of nucleotide-sugar precursors are organized in operons within the corresponding cluster for the particular S-layer glycan (18, 19). While the molecular details have not yet been resolved, it is reasonable to speculate that assembly of the S-layer glycan will involve comparable glycosyltransferase activities. Besides regularity residing in the crystalline nature and multivalency of the S-layer lattices (35, 38), the Bacillaceae offer certain advantages of gram-positive expression systems. These include a high number of permissive sites for insertion of foreign protein sequences, conserved mechanisms for cell wall targeting, a simpler cell wall profile requiring only one translocation step, and a greater sturdiness due to the thicker cell wall (40). Thus, S-layer-carrying members of the Bacillaceae merit further investigation for their potential for regular, high-density cell surface display of tailored S-layer neoglycoconjugates.
So far, interspecies transfer of glycosyltransferases has been performed mainly between different gram-positive organisms. This includes the heterologous expression of an authentic immunological type 3 capsular polysaccharide (CPS) from Streptococcus pneumoniae in Lactococcus lactis (11). Only three of the four CPS biosynthesis genes, which have a cassette-like organization in S. pneumoniae, were found to be necessary for the formation of type 3 CPS in the host, implying a role of the type 3-specific synthase in the extracellular transport of the CPS or the existence of an alternative export system in L. lactis. Interestingly, upon expression of the bifunctional S. pneumoniae type 3 synthase in E. coli, novel glycolipids were formed (5). In contrast, the transfer of the 14.5-kb exopolysaccharide (EPS) gene cluster from Streptococcus thermophilus Sfi6 into the non-EPS-producing heterologous host L. lactis MG1363 yielded a modified EPS. The replacement of GlcpNAc by galactose was attributed to the lack of UDP-N-acetylglucosamine C4-epimerase activity in L. lactis, which would provide UDP-GalNAc for GalNAc incorporation into the EPS (10, 41). These findings imply that bacterial glycosyltransferases may have relaxed specificities for glycosyl donors and acceptors of related structures and that the functional heterologous expression of enzymes encoded even by whole gene clusters is possible, provided that the heterologous host possesses all necessary genetic information for precursor synthesis. Shuttling of glycosyltransferase genes has benefits not only for heterologous (poly)saccharide production or reengineering of glycan structures of the host, it also provides a useful tool for the functional characterization of glycosyltransferases in different host backgrounds. For instance, in vitro functional characterization of heptosyltransferase II (WaaF) of E. coli, which is involved in the synthesis of the inner core region of LPS, was accomplished after heterologous expression in the gram-positive host Corynebacterium glutamicum (13).
The utilization of S-layer glycoprotein arrays as multivalent surface display systems necessarily requires reengineering of S-layer glycans by heterologous glycosyltransferases. Thus, as an initial step in this long-term approach, the feasibility of the transfer of well-characterized glycosyltransferases from the Enterobacteriaceae into a gram-positive host was addressed. Due to the availability of molecular tools, non-S-layer-carrying Bacillus subtilis was chosen as an intermediate model host between the enterobacteria and the gram-positive S-layer-carrying bacilli for the heterologous expression of selected enzymes from LPS O-polysaccharide biosynthesis pathways. The advantages of employing B. subtilis as an expression host include its nonpathogenicity, the absence of significant codon bias, its extensively studied genetics, the presence of secretory mechanisms, and well-documented properties essential for gene expression (22). It was assumed that the undecaprenylphosphate (und-P) acceptor substrate required by the glycosyltransferases would be provided by the host because peptidoglycan synthesis follows the same und-P-dependent pathway in gram-positive and gram-negative bacteria (45). Both the reaction mechanisms and the reaction products of the selected enzymes are well characterized, which should allow the unambiguous assignment of their activities in the background of the enzyme repertoire and the general metabolism of B. subtilis.
In the present study, we report on the shuttling of the enterobacterial O-polysaccharide biosynthesis genes wbaP (formerly rfbP), wecA (formerly rfe), and wbbO (formerly rfbF) from E. coli to the heterologous host B. subtilis. Using an in vitro transferase assay in combination with thin-layer chromatography (TLC), we demonstrate that both the activities and specificities of the encoded enzymes are maintained in the gram-positive host.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. The bacteria were grown at 37°C in Luria-Bertani (LB) broth or on LB agar supplemented, when appropriate, with ampicillin (Ap; 100 μg ml−1), kanamycin (Km; 10 μg ml−1), tetracycline (Tc; 15 μg ml−1 for E. coli and 7.5 μg ml−1 for B. subtilis), chloramphenicol (Cm; 30 μg ml−1), or gentamicin (Gm; 30 μg ml−1). For pRB374-based constructs, the recommended kanamycin concentration of 5 μg ml−1 was nonselective (4), so transformants were selected on media supplemented with 10 μg of kanamycin ml−1. In experiments involving E. coli CS1883 ΔgalE, the growth medium was supplemented with 0.1% (wt/vol) glucose and galactose (36). Spizizen's medium (39) for growth of B. subtilis was a solution containing 0.2% (NH4)2SO4, 1.4% K2HPO4, 0.6% KH2PO4, 0.1% sodium citrate-dihydrate, 0.02% MgSO4 · 7H2O, 0.5% glucose, and 1% Casamino Acids-yeast extract. Casamino Acids-yeast extract was made of 2% (wt/vol) Casamino Acids and 10% (wt/vol) yeast extract. TY medium was composed of 1.6% (wt/vol) tryptone, 1% (wt/vol) yeast extract, and 1% (wt/vol) NaCl.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype, phenotype, or description | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | K-12 φ80d lacZΔM15 endA1 recA1 hsdR17 (rK−mK−) supE44 thi-1 gyrA96 relA1 Δ(lacZYA-argF) U169 F− | Clonetech |
| 21548 | K-12 thr-1 leuB6 Δ(gpt-proA)66 hisG4 argE3 thi-1 rfbD1 lacY1 ara-14 galK2 xyl-5 mtl-1 mgl-51 rpsL31 kdgK51 supE44 rfe(wecA)::Tn10-48; Tcr | 26 |
| 2775 | Prototroph; serotype O8:K40 | B. Jann |
| CWG292 | 2775 derivative; wecA::Gm O−:K−; Gmr | 3 |
| CS1883 | K-12 thr-1 leuB6 Δ(gpt-proA)66 hisG4 argE3 thi-1 rfbD1 lacY1 ara-14 galK2 xyl-5 mtl-1 mgl-51 rpsL31 kdgK51 supE44 galE Δ(galOPE::Cm); Cmr | 36 |
| CWG288 | K-12 lacZ trp Δ(sbcB-rfb) upp rel rpsL galE:: Tn10 [λDE3]; Tcr | 120 |
| B. subtilisa | ||
| 1A748 | glgB::lacZ ΔM15 Km leu met hsdRI (R−M+); Kmr | BGSC |
| 1A751 | bglCΔ 102bglS ΔEV npr apr his | BGSC; 49 |
| Plasmids | ||
| pMAV11 | pACYC184 derivative carrying wecA from E. coli K-12; Cmr | 50 |
| pWQ19 | Cloning vector pRK404 derivative containing wbbM, glf, wbbN, and wbbO (formerly rfbCDEF) from K. pneumoniae O1 cloned on a 5.3-kb PstI fragment; Tcr | 7 |
| pWQ20 | pTrc99A containing wbbO | 7 |
| pJD132 | pBluescript SK derivative containing wbaP and flanking sequences from E. coli O9:K30 capsule biosynthesis cluster; Apr | J. Drummelsmith; Unpublished |
| pRB374 | 5.9-kb shuttle vector; colE1 ori for replication in E. coli; pUB110-derived ori for replication in gram-positive organisms; vegII promoter for E. coli and B. subtilis; selection; Apr in E. coli (100 μg ml−1) and Kmr in B. subtilis (10-50 μg ml−1) | E. Brown; 4 |
| pTS100 | pRB374 containing wbaP from pJD132 on a 1.7-kb XbaI/EcoRI fragment (TS-1/TS-2)b; Apr Kmr | This study |
| pTS103 | pRB374 containing wecA from pMAV11 on a 1.3-kb PstI fragment (TS-11/TS-6); Apr/Kmr | This study |
| pTS106 | pRB374 containing wbbO from pWQ19 on a 1.4-kb XbaI/KpnI fragment (TS-14/TS-15); Apr Kmr | This study |
| pTS107 | pTS103 containing wbbO from pWQ19 on a 1.4-kb XbaI/KpnI fragment (TS-14/TS-15); Apr/Kmr | This study |
| pAW10 | 3.9-kb shuttle vector; p15A ori for replication in E. coli; pAMα1 ori for replication in gram-positive organisms; Tetr (E. coli; 15 μg ml−1; B. subtilis, 5 μg ml−1) | D. Heinrichs; 42 |
| pTS108 | pAW10 containing wecA from pMAV11 on a 1.3-kb EcoRI/AccI fragment (TS-19/TS-16); Tcr | This study |
| pTS110 | pTS108 containing wbbO from pWQ19 on a 1.4-kb AccI/PstI fragment (TS-23/TS-22); Tcr | This study |
B. subtilis 168 strains as indexed by the Bacillus Genetic Stock Center (BGSC) accession number. B. subtilis 1A748 was chosen because of its protease deficiency; B. subtilis 1A751 was recommended for working with pRB374.
Primers for amplification of DNA fragments are given in parentheses. Primer sequences are listed in Table 2.
DNA manipulations.
Plasmid DNA was isolated from E. coli and B. subtilis using a QIAprep spin plasmid preparation kit (Qiagen) according to the manufacturer's instructions, except that B. subtilis cells were first recovered by centrifugation and incubated with 1 mg of lysozyme in 250 μl of 62 mM Tris-HCl buffer, pH 8.0, containing 1 mM EDTA and 25 μg of RNase for 30 min at 37°C to partially degrade the cell wall. All restriction enzymes were purchased from Invitrogen. Restriction endonuclease digestion was done according to the manufacturer's instructions, and digests were purified using a QIAquick PCR purification kit (Qiagen). Agarose gel electrophoresis was performed as described by Sambrook et al. (33). DNA fragments from agarose gels were purified using the Gene Clean III system from Bio 101 Inc., Vista, Calif.
Cloning of wbaP, wecA, and wbbO into shuttle vectors.
DNA fragments containing wbaP, wecA, and wbbO were amplified by PCR, using pJD132, pMAV11, and pWQ19, respectively, as templates. Oligonucleotides with the respective restriction sites were synthesized at the Guelph Molecular Supercentre; their sequences are listed in Table 2. PCRs were optimized for each primer pair using a GeneAmp PCR system 2400 (Perkin-Elmer, Norwalk, Conn.). The amplified fragments were ligated into the single-stranded-DNA shuttle vectors pRB374 (4) and pAW10 (46). The wbaP and wecA genes, together with upstream flanking DNA sequences, were cloned into pRB374 to produce pTS100 and pTS103, respectively. pTS107 was constructed by ligating wecA and wbbO into pRB374, whereas pTS106 contained only wbbO. pAW10-based constructs were made with wecA (pTS108) and a combination of wecA and in-frame wbbO (pTS110).
TABLE 2.
Oligonucleotides used for construction of plasmids
| Primera | Sequenceb | Start site on templatec |
|---|---|---|
| TS-1 (+) | GCGTATTctaGAGGAAATTATGACG (XbaI) | 6149 (pJD132) |
| TS-2 (−) | AAATCgAATTCTAAGAAAGAGCTTGGG (EcoRI) | 7877 (pJD132) |
| TS-11 (+) | CGCGCTGCAGATGTTAGGAAAATTC (PstI) | 1670 (pMAV11) |
| TS-6 (−) | CCGGTTCTGCAGGCATTGGTTGTG (PstI) | 2983 (pMAV11) |
| TS-14 (+) | TGCCGTAtcTaGATCCTGTCGAAGA (XbaI) | 3841 (pWQ19) |
| TS-15 (−) | CGCCTCCCAgGTacCACGTTACAGTAA (KpnI) | 5292 (pWQ19) |
| TS-19 (+) | TGGCGAatTcAGGAAAATTCCTGGAAT (EcoRI) | 1675 (pMAV11) |
| TS-16 (−) | CGGCCGGTaTaCCAGGCATTGGT (AccI) | 2986 (pMAV11) |
| TS-23 (+) | GCCGTAATTTGATCCTGTatAcGAAAATC (AccI) | 3842 (pWQ19) |
| TS-22 (−) | CGCCTCtgcAGTGACACGTTACAGTAA (PstI) | 5292 (pWQ19) |
Forward and reverse primers are indicated by plus and minus, respectively.
Restriction sites are underlined. Lowercase letters indicate nucleotides introduced to construct the appropriate restriction sites (in parentheses).
Templates for PCRs are given in parentheses.
Genetic transformation methods.
Electroporation was carried out using a Bio-Rad Gene Pulser. Transformants were selected on LB plates, using antibiotic concentrations as indicated in Table 1. E. coli strains (70-μl aliquots of competent cells in 10% glycerol) were transformed with 0.1 to 0.5 μg of plasmid DNA in 2-mm-gap electroporation cuvettes (Bio-Rad) using standard electroporation conditions (400 Ω; 2.5 kV; 25 μF).
B. subtilis strains 1A748 and 1A751 were transformed by a modification of the method of Matsuno et al. (25) in which 50 ml of LB broth was inoculated with 1% of an overnight culture. The culture was incubated for approximately 3 h with shaking at 150 rpm at 37°C to an optical density at 600 nm (OD600) of 0.5. The cells were then harvested, washed twice with 1 mM HEPES, pH 7.0, and treated twice with electroporation buffer (1 mM HEPES, pH 7.0, 25% [vol/vol] polyethyleneglycol 8,000, 0.1 M mannitol). The pellet was resuspended in 250 μl of the same buffer to a final OD600 of 1.9, and 70-μl aliquots were used. Prior to electroporation, the freshly prepared cells were chilled on ice for 15 min, mixed with ∼1.5 μg of plasmid DNA in a precooled tube, and then transferred to a 2-mm-gap electroporation cuvette. After administration of a single pulse (200 Ω; 2.3 kV; 25 μF), the cells were immediately resuspended in 500 μl of LB broth and incubated for 10 min at 25°C. Subsequently, the cells were incubated for 2.5 h at 37°C with gentle shaking to allow phenotypic expression; 250-μl aliquots of the mixture were plated on LB plates supplemented with the appropriate antibiotic and incubated for 20 h at 37°C.
B. subtilis cells were also made competent by a modification of the protocol of Spizizen et al. (39). Twenty milliliters of Spizizen's medium was inoculated with 0.1% of an overnight culture. Incubation was continued into the stationary phase at 37°C with shaking at 190 rpm. Subsequently, 10 ml of Spizizen's medium supplemented with 1% (vol/vol) 250 mM CaCl2 and 1% (vol/vol) 250 mM MgCl2 was inoculated with 1 ml of the stationary-phase culture and incubated at 37°C and 110 rpm for 1.5 h (OD600, 0.3 to 0.4). Aliquots of 500 μl of culture were mixed with glycerol (final concentration, 20%) and frozen at −70°C. Prior to chemical transformation, 1 ml of cells was thawed on ice and treated with 10 μl of 100 mM EGTA, pH 7.0, for 5 min at 37°C. Subsequently, 1 μg of plasmid DNA was added, and this mixture was incubated for another 5 min. The cell suspension was transferred to a fresh tube containing 200 μl of preheated TY medium for incubation for 1 h at 37°C and 190 rpm, after which 300 μl was plated onto selective media.
Membrane preparation.
Membrane preparations provided the source of galactosyltransferase and N-acetylglucosaminyltransferase activities. The preparation protocol essentially followed the method described by Osborn et al. (29) with slight modifications. Briefly, a 100-ml overnight culture was diluted in 400 ml of LB broth and incubated with shaking at 37°C. Biomass from the mid- to late exponential phase (OD600, ∼1.5) was harvested by centrifugation and washed once with 500 ml of cold saline and then with 100 ml of cold buffer A (50 mM Tris acetate, pH 8.5, 1 mM EDTA, 1 mM dithiothreitol). Finally, the pellet was resuspended in 10 ml of cold buffer A. The cells were lysed by ultrasonication with intermittent cooling on ice. Sonication was performed with 5-s pulses and 10-s pauses, with a 2-min total duration of sonication for E. coli cells and 3 min for B. subtilis cells. Unlysed cells were removed by centrifugation at 4,000 × g for 10 min at 4°C, and the membrane fraction was collected from the cell-free lysate by centrifugation at 120,000 × g for 30 min at 4°C. The resulting membrane pellet was washed once with buffer A, centrifuged again, and finally resuspended in 800 μl of buffer A with gentle stirring for 30 min at 4°C. Aliquots of 50 μl, containing approximately 1.5 mg of membrane protein, were stored at −70°C until they were used. The protein content in membrane fractions was determined with the Folin-Ciocalteau reagent (23).
In vitro glycosyltransferase activity.
The in vitro glycosyltransferase assays were based on methods described previously (7). Enzyme activities were measured by the incorporation of radioactivity from UDP-α-d-[14C]galactopyranose (UDP-Galp; 278 mCi mmol−1; Perkin-Elmer) or UDP-α-d-[14C]N-acetylglucosamine (UDP-GlcpNAc; 10.2 mCi mmol−1; ICN) into chloroform-methanol (C-M)-extractable lipid-linked reaction products. The standard in vitro reaction mixture contained 50 μl of membranes and 70 pmol (∼45,000 cpm) of radiolabeled nucleosidediphosphate-sugar substrate in a total volume of 100 μl of buffer B (50 mM Tris acetate, pH 8.5, 1 mM EDTA, 1 mM MgCl2). To exclude substrate limitation in WbaP assays, unlabeled UDP-Galp was added to the mixtures, with the molar ratios of labeled to unlabeled substrate being 1:1, 1:3, and 1:8, respectively. In WbbO-assays, a fivefold molar surplus of unlabeled UDP-GlcNAc was supplied. Enzyme reactions were performed at 37°C and were terminated by the addition of 1.25 ml of C-M (3:2). The reaction times varied between 5 and 60 min in different assays. For C-M extraction, the reaction mixture was mixed vigorously for 3 min, and cell debris was removed by low-speed centrifugation in a benchtop centrifuge. The organic phase containing lipid-linked reaction products was transferred to a fresh tube, and the pellet was extracted a second time with 1.35 ml of C-M-distilled H2O (dH2O) (3:2:0.4). The organic phases from both extraction steps were treated with 150 μl of 40 mM MgCl2 with vigorous shaking for 5 min. The upper phase obtained after centrifugation was removed, and the lower organic phase was washed twice with 400 μl of pure solvent upper phase (PSUP) (C-M-dH2O-1 M MgCl2 [18:294:282:1]) (29). The organic phases from both extraction steps were combined, dried under nitrogen, resuspended in 50 μl of PSUP, and mixed with 2 ml of Ecolite scintillation fluid (ICN), and incorporated 14C was counted in a Tricarb 2000 liquid scintillation counter (Canberra Packard).
TLC of lipid-linked reaction products.
Lipid-linked reaction products were identified after separation of 10 μl of PSUP-resuspended extracts by TLC. Silica Gel 60 aluminum TLC plates (20 by 20 cm; thickness, 0.25 mm; Merck) were prerun in C-M (2:1), and extracts were separated using the solvent system C-M-dH2O (65:25:4); the plates were dried and then developed in the same solvent a second time (14). The plates were exposed for 2 days at −70°C to BioMax MR film (Kodak, Rochester, N.Y.), using a BioMax intensifying screen (Kodak). In addition, the separated lipids were visualized with iodine vapor, and carbohydrates and phosphorus were detected with the thymol reagent (1) and a molybdenum blue spray (Sigma), respectively.
Tricine SDS-PAGE and Western blot analysis.
LPS preparations were made from sodium dodecyl sulfate (SDS)-proteinase K whole-cell lysates by a slight modification of the method of Hitchcock and Brown (15). Briefly, cells from 1 ml of an overnight culture, diluted to an OD600 of 1.0, were collected, washed with saline, and resuspended in 100 μl of lysis buffer (0.5 M Tris-HCl, pH 6.8, containing 2% [wt/vol] SDS and 10% [vol/vol] glycerol). The samples were boiled for 45 min prior to digestion with proteinase K at a final concentration of 0.5 μg ml−1 for 16 h at 55°C. Samples (2 to 10 μl) were loaded on precast 10 to 20% tricine SDS-polyacrylamide gels (Novex), and the polyacrylamide gel electrophoresis (PAGE) conditions were those recommended by the manufacturer. Silver staining (44) and Western immunoblotting (43) procedures have been described elsewhere. The production and specificity of anti-O8:K40 rabbit antibody was described previously (3). Alkaline phosphatase-conjugated antirabbit antibody was used as the second antibody, and detection was performed with nitroblue tetrazolium chloride-5-bromo-4-chloro-3-indolylphosphate.
RESULTS AND DISCUSSION
Cloning strategy considerations.
For expression of the enterobacterial O-polysaccharide biosynthesis enzymes WbaP, WecA, and WbbO in the gram-positive host B. subtilis, a shuttle vector approach was chosen. The initial cloning steps were performed in E. coli DH5α, and the activities of the constructs were verified in vivo in E. coli mutants with characterized defects in the respective genes prior to transferring the genes to the gram-positive host, where no such mutants would be available. Due to generally low transformation frequencies when B. subtilis is used as the primary host, plasmids pRB374 and pAW10 were compared with regard to transformation efficiency and maintenance of structural stability. pRB374 is a 5.9-kb E. coli-B. subtilis shuttle vector which contains the colE1 ori for E. coli and the plus ori for gram-positive bacteria derived from pUB110 (4). The genes were cloned into the pUC18-derived multicloning site of pRB374. The multicloning site is flanked by the transcriptional terminators T1 of E. coli rrnB and t0 of phage λ, which assure termination of 90% of RNA synthesis in B. subtilis. In the pBR374-based constructs, the genes were under the transcriptional control of the B. subtilis vegII promoter, allowing expression of genes with functional ribosome binding sites in both E. coli and B. subtilis. pAW10 is a 3.9-kb E. coli-Staphylococcus aureus shuttle vector which contains the p15A ori for E. coli and the gram-positive ori of pAMα1 from Enterococcus faecalis (46). To provide a vector-encoded promoter in pAW10, the genes were cloned downstream and in the same orientation as the tetracycline resistance gene cassette, affording the possibility of transcriptional read-through.
B. subtilis strains 1A748 and 1A751 were the selected hosts for heterologous expression of the glycosyltransferases. Due to its chromosomal kanamycin resistance, the protease-deficient strain 1A748 was transformed only with plasmids from the pAW10 series; strain 1A751 was transformed with all shuttle vector-based constructs. Transformation of B. subtilis with recombinant plasmids required specially adapted protocols for chemical transformation as well as for electrotransformation. The observed transformation efficiencies varied between 1.5 × 103 and 1 × 104 transformants per μg of plasmid DNA from the pAW10 series when the competent cells treated with Spizizen's salts were used. In contrast, competent cells stabilized with polyethyleneglycol 8,000 yielded reproducible but lower transformation rates of 5 × 102 CFU μg−1. Transformations with the larger pRB374-based constructs led to transformation rates nearly 1 order of magnitude below those obtained with the pAW10-based constructs. The choice of B. subtilis strain did not influence this result.
Vector stability is an absolute requirement for cloning and heterologous gene expression. The pAW10-based constructs pTS108 and pTS110 displayed no instability over at least 50 generations in transformed B. subtilis strains. Even under nonselective conditions, at least 30% of transformed B. subtilis 1A748 cells and approximately 20% of transformed B. subtilis 1A751 cells still retained the plasmid after 50 generations. Plasmid DNA isolated from cultures of either transformed B. subtilis strain yielded the expected restriction digest pattern (not shown). Thus, these plasmids did not undergo rearrangements in B. subtilis and were relatively stable in transformants. For the pRB374 shuttle vector, structural integrity was reported to be maintained over a period of 30 generations (4); however, after 50 generations, deletions of the pRB374-based constructs pTS100, pTS103, pTS106, and pTS107 became evident in B. subtilis 1A751. As inferred from agarose gels, cells with deletion derivatives outgrew those with intact plasmids in overnight cultures. The amount of intact plasmid after 50 generations was estimated to be only 15% of the total extracted from the culture under selective conditions. The mechanisms underlying the instability are unclear, but in an E. coli-Thermus thermophilus shuttle vector, the E. coli sequences contributed to the instability in distinct hosts (9).
The design of improved shuttle vectors and the utilization of chromosomal integration should circumvent this problem of vector instability in future. For instance, integration into the Thermoanaerobacterium sp. chromosome via homologous recombination has been accomplished only recently using pUC-based suicide vectors (24). The demonstration that this approach is also applicable to a thermophilic, anaerobic, S-layer-carrying organism (Thermoanaerobacterium thermosaccharolyticum) (24) might open up a useful alternative for the transformation of other meso- and thermophilic S-layer-carrying members of the Bacillaceae.
Expression of WbaP and WecA.
WbaP and WecA participate in the biosynthesis of enterobacterial O-polysaccharides. They are both predicted to be integral membrane proteins; they are structural homologues, as well as functional homologues, in initiating O-polysaccharide synthesis (37). Both enzymes utilize the und-P lipid acceptor. Reactions involved in the biosynthesis of O-polsyaccharides were first decribed in Salmonella enterica serogroups B and E. The galactosyltransferase WbaP (formerly RfbP) catalyzes the reversible transfer of Galp 1-phospate from the precursor UDP-Galp to und-P as the initial synthetic reaction for each O-heteropolysaccharide repeat unit (47). Interestingly, there are a number of structural homologues of WbaP in systems involved in capsular and exopolysaccharide synthesis in various bacteria (47). These include the E. coli K30 antigen whose biosynthetic system provided the WbaP homologue studied here (8). The GlcpNAc 1-phosphate transferase WecA (formerly Rfe), the only other known initiating enzyme, was first characterized in the biosynthesis of enterobacterial common antigen (ECA) (26). WecA also transfers GlcpNAc residues found in the heteropolysaccharide O units of several E. coli serotypes (2), Shigella dysenteriae type 1 (17), and Shigella flexneri (2). These O units are assembled sequentially and elongated by blockwise polymerization of the undecaprenylpyrophosphate (und-PP)-linked repeat units at the reducing terminus of the growing polymer in a Wzy-dependent process. Interestingly, WecA also initiates formation of polymannan in E. coli O8 and O9 (16, 32) and polygalactan (d-galactan I) in Klebsiella pneumoniae O1 (6), even though the O units of these homopolysaccharides do not contain GlcpNAc. In these cases, und-PP-GlcNAc provides a primer for assembly of the repeat unit domain of the glycan by a processive glycosyltransfer mechanism (polymerization by growth at the nonreducing terminus) (reviewed in reference 47). Thus, WecA and WbaP provide important prototypes for obtaining insights into the initiation step of O-polysaccharide synthesis.
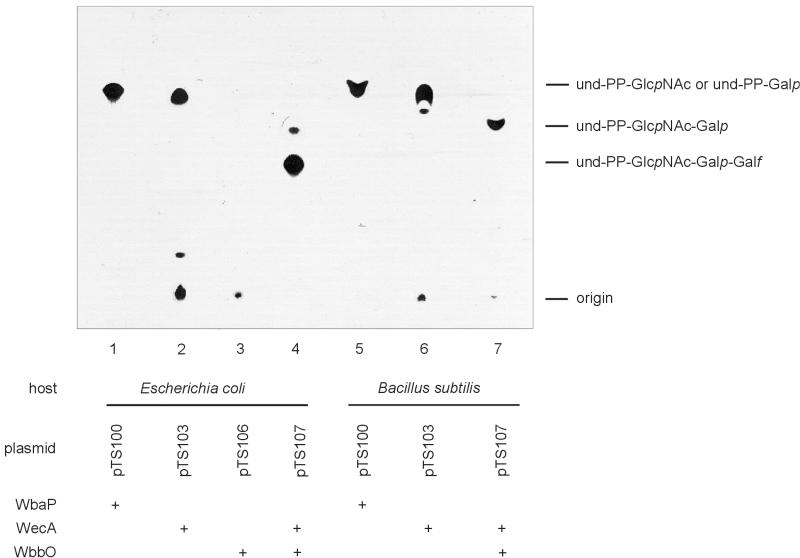
The galactosyltransferase WbaP was cloned on a 1.7-kb XbaI/EcoRI fragment into the shuttle vector pRB374 to give the 7.6-kb plasmid pTS100. pTS100 was transformed into E. coli CWG288 and B. subtilis 1A751, and transferase assays were carried out with the respective membranes as a source of WbaP to form the predicted C-M-extractable lipid-linked saccharide from the 14C-labeled sugar nucleotide precursor. The yield of the reaction product (und-PP-α-d-Galp) was measured and given as an indication of enzyme activity. In both the E. coli CWG288 and the B. subtilis 1A751 backgrounds, transfer of radioactivity from UDP-α-d-[14C]Galp occurred in the absence of other exogenous precursors, as expected in a process during which WbaP is the initiating transferase. Incorporation of radioactivity was measured after a reaction time of 20 min using E. coli(pTS100) and B. subtilis(pTS100) membranes, giving 15.1 and 3.0 pmol, respectively, of incorporated 14C-sugar per mg of membrane protein (Table 3). To exclude putative limitation of the nucleotide sugar precursor, in another experiment, the reaction mixtures were supplemented with 70 pmol of unlabeled UDP-Galp precursor in addition to the same amount of UDP-α-d-[14C]Galp. The amounts of incorporated radioactivity from UDP-α-d-[14C]Galp generated with E. coli CWG288 and B. subtilis membranes were 62 and 60%, respectively, of the values obtained in the first assay using UDP-α-d-[14C]Galp as the sole precursor. Although the amount of incorporated radioactivity decreased in these experiments as expected, when the dilution of the radiolabeled substrate is accounted for, the absolute levels of Gal incorporation into lipid intermediates showed a slight increase (Table 3). From these data, it appears that in reactions containing a 1:1 molar ratio of labeled and unlabeled substrates, the amounts of 14C incorporation in the initial reactions were slightly affected by substrate limitation. However, the presence of a sufficient amount of substrate in the assay mixtures was supported by the incorporation data derived from assays containing a threefold or even an eightfold molar surplus of unlabeled nucleotide sugar precursor (data not shown). In either experiment, the overall activity of WbaP in B. subtilis 1A751 was approximately 19% of the enzyme activity in E. coli CWG288 (Table 3). Measurement of WbaP activity in E. coli utilized the GalE− host, CWG288, to ensure direction of the UDP-α-d-[14C]Galp substrate specifically to und-PP-α-d-Galp formation and prevent the possible redirection of radiolabel from the nucleotide sugar precursor via UDP-α-d-[14C]Glcp into other glycoconjugates. B. subtilis possesses a galE homologue which encodes a protein that is 57% identical to the product of the E. coli gene (42). GalE is assumed to be essential for the growth of B. subtilis in medium containing either glucose or galactose, and there are indications that accumulation of UDP-Gal might be toxic for B. subtilis in vivo (21). However, in vitro transferase assays using membrane preparations of B. subtilis 1A751(pTS100) as a source of enzyme clearly demonstrated that the shuttled enzyme WbaP can utilize UDP-Galp as a substrate to form und-PP-Galp in the B. subtilis background (Table 3). The migration behavior of the C-M-extractable reaction products from both organisms on TLC plates (Fig. 1, lanes 1 and 5) further indicated that identical product formation had occurred in both bacterial backgrounds. Thus, WbaP was functionally expressed in B. subtilis 1A751, implying that WbaP catalyzed formation of und-PP-α-d-Galp. Furthermore, this product was not modified by the endogenous enzyme repertoire of the gram-positive host.
TABLE 3.
Incorporation of galactose and N-acetylglucosamine, respectively, from UDP-linked sugars into lipid-linked intermediates
| Plasmid | Enzyme | Predicted product | Reaction time (min) | Incorporation of sugara
|
B. subtilis/E. coli (%) | |||
|---|---|---|---|---|---|---|---|---|
|
E. coli
|
B. subtilis
|
|||||||
| Gal | GlcNAc | Gal | GlcNAc | |||||
| pTS100 | WbaPb | und-PP-Galp | 20 | 15.1 | 3.0 | 19.9 | ||
| 20c | 18.8 | 3.6 | 19.3 | |||||
| pTS103 | WecAd | und-PP-GlcpNAc | 20 | 24.0 | 9.5 | 39.5 | ||
| pTS108 | WecAd | und-PP-GlcpNAc | 20 | 23.7 | 5.0 | 21.1 | ||
Sugar incorporation was calculated from incorporated radioactivity from UDP-α-d-[14C]Gal and UDP-α-d-[14C]GlcNAc, respectively. Incorporation rates are given in picomoles per milligram of membrane protein. The amount of incorporation of radioactivity into controls was subtracted; controls accounted for <0.5% of the radioactivity determined with the respective plasmid. The means from three assays are listed; values varied ± 1%.
Membranes from E. coli CWG288 and B. subtilis 1A751 were used as sources of enzymes in transferase assays.
The molar ratio of UDP-α-d-[14C]Gal to UDP-Gal was 1:1.
Membranes from E. coli CWG292 and B. subtilis 1A751 were used as sources of enzymes in transferase assays.
FIG. 1.
TLC autoradiogram showing und-P-linked intermediates synthesized in an in vitro assay and extracted with C-M (3:2). Membrane preparations of E. coli strains and B. subtilis 1A751 provided the sources of the various glycosyltransferases. The relevant enzymes in each reaction mixture are identified (+) below the appropriate lane, and predicted reaction products are indicated to the right of the frame. In WbaP and WbbO assays (lanes 1, 3, 4, 5, and 7), radioactivity was incorporated from UDP-α-d-[14C]Galp; in WecA assays (lanes 2 and 6), radioactivity was incorporated from UDP-α-d-[14C]GlcpNAc. Enzyme activities were assayed in membranes from E. coli strains CWG288 (lane 1), CWG292 (lane 2), 21548 (lane 3), and CS1883 (lane 4) and from B. subtilis 1A751 (lanes 5 to 7). No signal was detected in control cells without a plasmid (not shown).
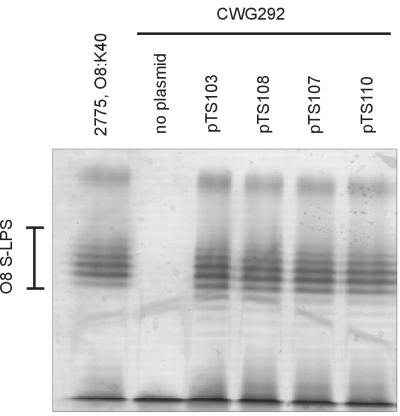
For documentation of WecA activity, pTS103, pTS107, pTS108, and pTS110 were electroporated in the E. coli wecA mutant strain CWG292. The O8:K40 antigens produced by the parent of E. coli CWG292 (E. coli 2775) are both dependent on WecA activity, and in vivo assays are available in a wecA mutant host to confirm activity of plasmid-encoded WecA. Proteinase K-digested whole-cell lysates were prepared and examined on silver-stained tricine SDS-PAGE gels. Plasmid-encoded WecA was capable of fully restoring O8 smooth LPS (S-LPS) production in E. coli, giving an SDS-PAGE profile indistinguishable from that of the wild-type strain E. coli 2775 (Fig. 2). These results were confirmed by Western immunoblotting (not shown). Thus, the wecA gene was actively transcribed from both the pBR374- and the pAW10-based shuttle constructs.
FIG. 2.
WecA-dependent synthesis of serotype O8 LPS in E. coli CWG292. Proteinase K-digested whole-cell lysates were prepared for examination of LPS on silver-stained SDS-PAGE gels. The plasmids are indicated above the lanes; E. coli strain 2775 was the positive control. Serotype O8 S-LPS was restored in the WecA-deficient strain E. coli CWG292 by the plasmids pTS103 and pTS108. Expression of WecA and O8 S-LPS formation were also documented when the gene was encoded by plasmids containing both the wecA and the wbbO genes (pTS107 and pTS110).
UDP-GlcpNAc::und-P GlcpNAc 1-phosphatetransferase assays were carried out with E. coli CWG292 and B. subtilis strains 1A748 and 1A751 harboring the respective wecA-bearing plasmids. WecA-catalyzed incorporation of radioactivity from UDP-α-d-[14C]GlcpNAc into the predicted product provided evidence for both the pTS103- and pTS108-encoded enzyme in E. coli CWG292 and in B. subtilis (Table 3). No difference in the utilization of the radioactive substrate was observed with the two B. subtilis strains (only the data for 1A751 are shown). As with WbaP, WecA activity was lower in B. subtilis than in E. coli. Even though pTS103 and pTS108 catalyzed almost identical amounts of product formation in E. coli CWG292, [14C]GlcpNAc incorporation in the B. subtilis 1A751 reaction product was significantly lower when wecA was encoded by pTS108 (39 and 21% of the E. coli value for pTS103 and pTS108, respectively). One explanation for this observation might be the strength of the vegII promoter in pRB374-based plasmids. Reaction products were extracted from E. coli and B. subtilis membranes. The majority of the resulting und-PP-α-d-[14C]GlcpNAc intermediate migrated as a single component in TLC (Fig. 1, lanes 2 and 6). In E. coli CWG292 membranes, trace amounts of larger material were detected near the origin (Fig. 1, lane 2), which likely reflected extended ECA intermediates, as would be predicted in an enzyme preparation that would contain residual amounts of the other ECA precursors (14).
Expression of sequential activity from WecA-WbbO.
While wecA, the structural gene for a tunicamycin-sensitive UDP-GlcpNAc::und-P GlcpNAc 1-phosphatetransferase, is located in the ECA biosynthesis locus, wbbO (formerly rfbF) is the last of six genes contained in the 6.6-kb his-linked O-polysaccharide biosynthesis locus in K. pneumoniae and is required for the expression of d-galactan I (6, 7). WbbO directs the WecA intermediate und-PP-α-d-GlcpNAc into d-galactan I biosynthesis. It is a novel bifunctional galactosyltransferase capable of sequentially transferring an α-d-Galp and a β-d-galactofuranose (Galf) residue from the corresponding UDP sugars to und-PP-α-d-GlcpNAc, giving rise to the trisaccharide β-d-Galf-(1→3)-α-d-Galp-(1→3)-β-d-GlcpNAc linked to und-PP (14). WbbO shows no activity in a wecA mutant host due to absence of the und-PP-α-d-GlcpNAc acceptor. The galactofuranosyltransferase activity of WbbO is dependent on the availability of the UDP-Galf precursor and thus on the activity of a UDP-galactopyranose mutase (20). This enzyme, which is encoded by the glf gene of the O-polysaccharide cluster, catalyzes the reversible interconversion of UDP-Galp to UDP-Galf, with the furanosidic form being thermodynamically disfavored (28).
The functional expression of WecA encoded by pTS107 and pTS110 was assessed in vivo using E. coli CWG292 (Fig. 2), and WbbO activity was tested in the E. coli K-12 strain CS1883 (Fig. 3). Expression of WbbO in this strain results in a core modification due to the transfer of GlcpNAc-Galp-Galf to the host lipid A core (7). It was clearly evident by tricine SDS-PAGE of LPS from E. coli CS1883(pTS107) and E. coli CS1883(pTS110) that the plasmid-encoded products result in an additional band migrating slightly more slowly than that of the lipid A core of E. coli CS1883 (Fig. 3). Plasmid pWQ20 containing wbbO cloned behind the trc promoter (7) served as a control. The staining intensity of the additional core band was lower when the host was transformed with pTS110 in comparison to pTS107 and pWQ20. This could reflect either decreased promoter strength or decreased copy number in the pAW10-based construct relative to pTS107 and pWQ20. To document the dependence of WbbO activity upon the lipid-linked saccharide acceptor und-PP-α-d-GlcpNAc formed by WecA, E. coli 21548 (wecA::Tn10) was transformed with the plasmids pTS106 (wecA mutant) and pTS107. As was expected, pTS107 resulted in modified lipid A core, whereas pTS106 did not (not shown). Due to chromosomal tetracycline resistance of E. coli 21548, this experiment was not carried out with pAW10-based plasmids.
FIG. 3.

WbbO-mediated modification of the LPS of E. coli CS1883. Proteinase K-digested whole-cell lysates were prepared for examination of LPS on silver-stained SDS-PAGE gels. The plasmids are indicated above the lanes. E. coli strain CS1883(pWQ20) was used as the positive control for expression of WbbO. Plasmids containing wbbO (pWQ20, pTS107, and pTS110) result in an LPS core modification reflected in an additional band migrating slightly more slowly than that of the lipid A core of E. coli CS1883 (indicated by the arrow).
Membranes from E. coli CS1883 ΔgalE harboring the appropriate plasmids (pTS107 and pTS110) were used as a source of WbbO galactosyltransferase enzyme. Typically, a fivefold molar surplus of unlabeled UDP-GlcpNAc was provided in WbbO assays to assure the formation of the lipid-saccharide acceptor required by the enzyme. Prior to determination of WbbO activity, a standard WecA assay (20-min reaction time) was carried out in E. coli CWG292. By comparing the amounts of incorporated radioactivity, it became evident that WecA activities were slightly lower when originating from pTS107 and pTS110 than when originating from their parent plasmids. As has already been observed with pTS103 and pTS108, similar incorporation of radioactivity was obtained for pTS107 and pTS110 in E. coli. In B. subtilis, however, WecA activity was lower when the enzyme was encoded by pTS110 (Table 4). WbbO activities determined in a 20-min assay are given in Table 4. WbbO activities in B. subtilis 1A751 reached in average 20% of activity compared to E. coli CS1883 when originating from pTS107 and an average 12% of activity when originating from pTS110. To allow direct comparison of WecA and WbbO activities in the same E. coli host, the wecA mutant strain 21548 was used. From membranes of E. coli 21548(pTS107), the incorporation of α-d-[14C]GlcpNAc and α-d-[14C]Gal into C-M-extractable product occurred in an approximate molar ratio of 1:1.8, indicative of the presence of the predicted trisaccharide β-d-Galf-(1→3)-α-d-Galp-(1→3)-β-d-GlcpNAc linked to und-PP. This finding was supported by the α-d-[14C]GlcpNAc-to-α-d-[14C]Gal ratios obtained by combining data from WecA and WbbO assays in E. coli CWG292 and CS1883, respectively (Table 4). The migration of the major product extracted from E. coli CS1883(pTS107) on TLC is consistent with this conclusion. A small amount of und-PP-α-d-GlcpNAc-α-d-Galp was also detected. In B. subtilis, the α-d-[14C]GlcpNAc-to-α-d-[14C]Gal ratio was approximately 1 for both pTS107 and pTS110. The faster migration of the extraction product from B. subtilis 1A751(pTS107) relative to that from E. coli CS1883(pTS107) was consistent with the product und-PP-α-d-GlcpNAc-α-d-Galp (Fig. 1) and comigrated with the minor product in E. coli. The synthesis of the smaller product in B. subtilis reflects the absence of glf in its genome and the inability to form the UDP-Galf necessary for synthesis of an und-PP-linked trisaccharide.
TABLE 4.
Incorporation of galactose and N-acetylglucosamine from UDP-linked sugars into lipid-linked intermediates, demonstrating the sequential actions of WecA and WbbO
| Plasmid | Enzymes | Predicted producta | Reaction time (min) | Incorporation of sugard
|
B. subtilis/E. coli (%) | |||
|---|---|---|---|---|---|---|---|---|
|
E. coli
|
B. subtilis
|
|||||||
| GlcNAc | Gal | GlcNAc | Gal | |||||
| pTS107 | WecAb + WbbOc | und-PP-GlcpNAc-Galp-(Galf) | 20 | 16.4 | 29.8 | 6.3 | 5.7 | 19.1 |
| pTS110 | WccAb + WbbOc | und-PP-GlcpNAc-Galp-(Galf) | 20 | 14.1 | 25.6 | 4.0 | 3.2 | 12.5 |
Galf residue is added if UDP-galactopyranose mutase is provided by the host.
Membranes from E. coli CWG292 were used as a source of enzyme in transferase assays.
Membranes from E. coli CS1883 and B. subtilis 1A751 were used as sources of enzymes in transferase assays.
Sugar incorporation was calculated from incorporated radioactivity from UDP-α-d-[14C]Gal and UDP-α-d-[14C]GlcNAc. Incorporation rates are given in picomoles per milligram of membrane protein. The amount of incorporation of radioactivity into controls was subtracted; controls accounted for <0.5% of the radioactivity determined with the respective plasmid. The means from three assays are listed; values varied ± 1%.
Conclusions.
Heterologous expression of glycosyltransferases in gram-positive bacteria is a highly desirable aim for functional characterization of the transferases. Furthermore, heterologous glycosyltransferases might have benefits in reengineering of S-layer glycoproteins for their future utilization as carbohydrate surface display systems.
To address the feasibility of the transfer of well-characterized glycosyltransferases from enterobacteria into a gram-positive host, two initiating enzymes of O-polysaccharide biosynthesis (WbaP and WecA) and one chain-extending enzyme (WbbO) were chosen. B. subtilis was used as a model gram-positive host in a shuttle vector-based approach. All three enzymes were functional in B. subtilis, as demonstrated by in vitro glycosyltransferase assays and the TLC evidence of isolated reaction products. Enzyme activities in B. subtilis varied between 20 and 75% of those observed in E. coli, perhaps reflecting differences in copy numbers of plasmids and promoter strengths in E. coli and B. subtilis. Activity was obtained from two sequentially acting enzymes (WecA and WbbO). However, the bifunctionality of WbbO seen in E. coli was not achieved in B. subtilis due to the lack of UDP-Galf substrate in that organism.
These experiments represent a necessary first step towards transformation and chromosomal integration of glycosyltransferase genes in gram-positive S-layer-covered bacteria for remodeling of their cell surface carbohydrates. Applications of such tailored S-layer neoglycoconjugates might include the fields of vaccine design (38) and receptor mimetics (30, 31).
Acknowledgments
We thank David Heinrichs and Eric Brown for providing shuttle vectors. B. subtilis strains 1A751 and 1A748 were kindly provided by Daniel Zeigler from the Bacillus Genetic Stock Center, Columbus, Ohio. The technical assistance of Corin Forrester and Bärbel Schröfelbauer is gratefully acknowledged.
This work was supported by the Austrian Science Fund, projects P12966-MOB and P14209-MOB (to P.M.), the Zentrum für Internationale Beziehungen, Universität für Bodenkultur Wien, “Mittel zur Pflege von Universitätspartnerschaften,” Projects 1/99 and 8/99 (to C.S.), the Hochschuljubiläumsstiftung der Stadt Wien, Project H-148/2001 (to C.S.), and funding from the Natural Sciences and Engineering Research Council of Canada (to C.W.). C.W. is the recipient of a Canada Research Chair.
REFERENCES
- 1.Adachi, S. 1965. Thin-layer chromatography of carbohydrates in the presence of bisulfite. J. Chromatogr. 17:295-299. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, D. C., and M. A Valvano. 1994. Role of the rfe gene in the biosynthesis of the Escherichia coli O7-specific lipopolysaccharide and other O-specific polysaccharides containing N-acetylglucosamine. J. Bacteriol. 176:7079-7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amor, P. A., and C. Whitfield. 1997. Molecular and functional analysis of genes required for expression of group IB K antigens in Escherichia coli: characterization of the his-region containing gene cluster for multiple cell-surface polysaccharides. Mol. Microbiol. 26:145-161. [DOI] [PubMed] [Google Scholar]
- 4.Brückner, R. 1992. A series of shuttle vectors for Bacillus subtilis and Escherichia coli. Gene 122:187-192. [DOI] [PubMed] [Google Scholar]
- 5.Cartee, R. T., W. T. Forsee, J. W. Jensen, and J. Yother. 2001. Expression of the Streptococcus pneumoniae type 3 synthase in Escherichia coli. J. Biol. Chem. 276:48831-48839. [DOI] [PubMed] [Google Scholar]
- 6.Clarke, B. R., and C. Whitfield. 1992. Molecular cloning of the rfb region of Klebsiella pneumoniae serotype O1:K20: the rfb gene cluster is responsible for synthesis of d-galactan I O polysaccharide. J. Bacteriol. 174:4614-4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke, B. R., D. Bronner, W. J. Keenleyside, W. B. Severn, J. C. Richards, and C. Whitfield. 1995. Role of Rfe and RfbF in the initiation of biosynthesis of d-galactan I, the lipopolysaccharide O antigen from Klebsiella pneumoniae serotype O1. J. Bacteriol. 177:5411-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummelsmith, J., and C. Whitfield. 1999. Gene products required for surface expression of the capsular form of the group 1 K antigen in Escherichia coli (O9a:K30). Mol. Microbiol. 5:1321-1332. [DOI] [PubMed] [Google Scholar]
- 9.Fridjonsson, O., and R. Mattes. 2001. Production of recombinant α-galactosidases in Thermus thermophilus. Appl. Environ. Microbiol. 67:4192-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germond, J.-E., M. Delley, N. D'Amico, and S. J. F. Vincent. 2001. Heterologous expression and characterization of the exopolysaccharide from Streptococcus thermophilus Sfi6. Eur. J. Biochem. 268:5149-5156. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert, C., K. Robinson, R. W. F. Le Page, and J. M. Wells. 2000. Heterologous expression of an immunogenic pneumococcal type 3 capsular polysaccharide in Lactococcus lactis. Infect. Immun. 68:3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grabenhorst, E., P. Schlenke, S. Pohl, M. Nimtz, and H. S. Conradt. 1999. Genetic engineering of recombinant glycoproteins and the glycosylation pathway in mammalian host cells. Glycoconj. J. 16:81-97. [DOI] [PubMed] [Google Scholar]
- 13.Gronow, S., W. Brabetz, and H. Brade. 2000. Comparative functional characterization in vitro of heptosyltransferase I (WaaC) and II (WaaF) from Escherichia coli. Eur. J. Biochem. 267:6602-6611. [DOI] [PubMed] [Google Scholar]
- 14.Guan, S., A. J. Clarke, and C. Whitfield. 2001. Functional analysis of the galactosyltransferases required for biosynthesis of d-galactan I, a component of the lipopolysaccharide O1 antigen of Klebsiella pneumoniae. J. Bacteriol. 183:3318-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jann, K., G. Goldemann, C. Weisgerber, C. Wolf-Ullisch, and S. Kanegasaki. 1982. Biosynthesis of the O9 antigen of Escherichia coli. Initial reaction and overall mechanism. Eur. J. Biochem. 127:157-164. [DOI] [PubMed] [Google Scholar]
- 17.Klena, J. D., and C. A. Schnaitman. 1993. Function of the rfb gene cluster and the rfe gene in the synthesis of O antigen by Shigella dysenteriae 1. Mol. Microbiol. 9:393-402. [DOI] [PubMed] [Google Scholar]
- 18.Kneidinger, B., M. Graninger, A. Puchberger, P. Kosma, and P. Messner. 2001. Biosynthesis of nucleotide-activated d-glycero-d-manno-heptose. J. Biol. Chem. 276:20935-20944. [DOI] [PubMed] [Google Scholar]
- 19.Kneidinger, B., M. Graninger, G. Adam, A. Puchberger, P. Kosma, S. Zayni, and P. Messner. 2001. Identification of two GDP-6-deoxy-d-lyxo-4-hexulose reductases synthesizing GDP-d-rhamnose in Aneurinibacillus thermoaerophilus L420-91T. J. Biol. Chem. 276:5577-5583. [DOI] [PubMed] [Google Scholar]
- 20.Köplin, R., J.-R. Brisson, and C. Whitfield. 1997. UDP-galactofuranose precursor required for formation of the lipopolysaccharide O antigen of Klebsiella pneumoniae serotype O1 is synthesized by the product of the rfbDKPO1 gene. J. Biol. Chem. 272:4121-4128. [DOI] [PubMed] [Google Scholar]
- 21.Krispin, O., and R. Allmansberger. 1998. The Bacillus subtilis galE gene is essential in the presence of glucose and galactose. J. Bacteriol. 180:2265-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam, K. H. E., K. C. Chow, and W. K. R. Wong. 1998. Construction of an efficient Bacillus subtilis system for the extracellular production of heterologous protein. J. Biotechnol. 63:167-177. [DOI] [PubMed] [Google Scholar]
- 23.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 24.Mai, V., and J. Wiegel. 2000. Advances in development of a genetic system for Thermoanaerobacterium spp.: expression of genes encoding hydrolytic enzymes, development of a second shuttle vector, and integration of genes into the chromosome. Appl. Environ. Microbiol. 66:4817-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuno, Y., T. Ano, and M. Shoda. 1992. High-efficiency transformation of Bacillus subtilis NB2, an antifungal antibiotic iturin producer, by electroporation. J. Ferment. Bioeng. 73:261-264. [Google Scholar]
- 26.Meier-Dieter, U., R. Starman, K. Barr, H. Mayer, and P. D. Rick. 1990. Biosynthesis of enterobacterial common antigen in Escherichia coli. Biochemical characterization of Tn10 insertion mutants defective in enterobacterial antigen synthesis. J. Biol. Chem. 265:13490-13497. [PubMed] [Google Scholar]
- 27.Messner, P., and C. Schäffer. p. 51-124. Prokaryotic glycoproteins. In W. Herz, H. Falk, G. W. Kirby, R. E. Moore, and C. Tamm (ed.), Progress in the chemistry of organic natural products, vol. 85. Springer-Verlag, New York, N.Y. [DOI] [PubMed]
- 28.Nassau, P. M., S. L. Martin, R. E. Brown, A. Weston, D. Monsey, M. R. McNeil, and K. Duncan. 1996. Galactofuranose biosynthesis in Escherichia coli K-12: identification and cloning of UDP-galactopyranose mutase. J. Bacteriol. 178:1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborn, J. N., M. A. Cynkin, J. M. Gilbert, L. Muller, and M. Singh. 1972. Synthesis of bacterial O-antigens. Methods Enzymol. 28:583-601. [Google Scholar]
- 30.Paton, A. W., R. Morona, and J. C. Paton. 2000. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat. Med. 6:265-270. [DOI] [PubMed] [Google Scholar]
- 31.Paton, A. W., R. Morona, and J. C. Paton. 2001. Neutralization of Shiga toxins Stx1, Stx2c, and Stx2e by recombinant bacteria expressing mimics of globotriose and globotetraose. Infect. Immun. 69:1967-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rick, P. D., G. L. Hubbard, and K. Barr. 1994. Role of the rfe gene in the synthesis of the O8 antigen in Escherichia coli K-12. J. Bacteriol. 176:2877-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.Saxon, E., and C. Bertozzi. 2001. Chemical and biological strategies for engineering cell surface glycosylation. Annu. Rev. Cell Dev. Biol. 17:1-23. [DOI] [PubMed] [Google Scholar]
- 35.Schäffer, C., and P. Messner. 2001. Glycobiology of surface-layer proteins. Biochimie 83:591-599. [DOI] [PubMed] [Google Scholar]
- 36.Schnaitmann, C. A., and E. A. Austin. 1990. Efficient incorporation of galactose into lipopolysaccharides by Escherichia coli K-12 strains with polar galE mutations. J. Bacteriol. 172:5511-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnaitman, C. A., and J. D. Klena. 1993. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol. Rev. 57:655-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleytr, U. B., P. Messner, D. Pum, and M. Sára. 1999. Crystalline bacterial cell surface layers (S-layers): from supramolecular cell structure to biomimetics and nanotechnology. Angew. Chem. Int. Ed. 38:1034-1054. [DOI] [PubMed] [Google Scholar]
- 39.Spizizen, J., B. Reilly, and A. Evans. 1966. Microbial transformation and transfection. Annu. Rev. Microbiol. 20:371-400. [DOI] [PubMed] [Google Scholar]
- 40.Ståhl, S., and M. Uhlen. 1997. Bacterial surface display: trends and progress. Trends Biotechnol. 15:185-192. [DOI] [PubMed] [Google Scholar]
- 41.Stingele, F., S. J. F. Vincent, E. J. Faber, J. W. Newell, J. P. Kamerling, and J.-R. Neeser. 1999. Introduction of the exopolysaccharide gene cluster from Streptococcus thermophilus Sfi6 into Lactococcus lactis MG1363: production and characterization of an altered polysaccharide. Mol. Microbiol. 32:1287-1295. [DOI] [PubMed] [Google Scholar]
- 42.Thoden, J. B., P. A. Frey, and H. M. Holden. 1996. Crystal structures of the oxidized and reduced forms of UDP-galactose 4-epimerase isolated from E. coli. Biochemistry 35:2557-2566. [DOI] [PubMed] [Google Scholar]
- 43.Towbin, M. T., T. Staehelin, and G. Gordon. 1979. Electrophoretic transfer of proteins from acrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai, G. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 45.van Heijenoort, J. 2001. Formation of glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology 11:25R-36R. [DOI] [PubMed] [Google Scholar]
- 46.Wada, A., and H. Watanabe. 1998. Penicillin-binding protein 1 of Staphylococcus aureus is essential for growth. J. Bacteriol. 180:2759-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, L., D. Liu, and P. R. Reeves. 1996. C-terminal half of Salmonella enterica WbaP (RfbP) is the galactosyl-1-phosphate transferase domain catalyzing the first step of O antigen synthesis. J. Bacteriol. 178:2598-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitfield, C. 1995. Biosynthesis of lipopolysaccharide O antigens. 1995. Trends Microbiol. 3:178-185. [DOI] [PubMed] [Google Scholar]
- 49.Wolf, M., A. Geczi, O. Simon, and R. Borris. 1995. Genes encoding xylan and beta-glucan hydrolysing enzymes in Bacillus subtilis: characterization, mapping and construction of strains deficient in lichenase, cellulase, and xylanase. Microbiology 141:281-290. [DOI] [PubMed] [Google Scholar]
- 50.Yao, Z., and M. A. Valvano. 1994. Genetic analysis of the O-specific lipopolysaccharide biosynthesis region (rfb) of Escherichia coli K-12 W3110: identification of genes that confer group 6 specificity to Shigella flexneri serotypes Y and 4a. J. Bacteriol. 176:4133-4143. [DOI] [PMC free article] [PubMed] [Google Scholar]