Abstract
Arsenite [As(III)]-enriched anoxic bottom water from Mono Lake, California, produced arsenate [As(V)] during incubation with either nitrate or nitrite. No such oxidation occurred in killed controls or in live samples incubated without added nitrate or nitrite. A small amount of biological As(III) oxidation was observed in samples amended with Fe(III) chelated with nitrolotriacetic acid, although some chemical oxidation was also evident in killed controls. A pure culture, strain MLHE-1, that was capable of growth with As(III) as its electron donor and nitrate as its electron acceptor was isolated in a defined mineral salts medium. Cells were also able to grow in nitrate-mineral salts medium by using H2 or sulfide as their electron donor in lieu of As(III). Arsenite-grown cells demonstrated dark 14CO2 fixation, and PCR was used to indicate the presence of a gene encoding ribulose-1,5-biphosphate carboxylase/oxygenase. Strain MLHE-1 is a facultative chemoautotroph, able to grow with these inorganic electron donors and nitrate as its electron acceptor, but heterotrophic growth on acetate was also observed under both aerobic and anaerobic (nitrate) conditions. Phylogenetic analysis of its 16S ribosomal DNA sequence placed strain MLHE-1 within the haloalkaliphilic Ectothiorhodospira of the γ-Proteobacteria. Arsenite oxidation has never been reported for any members of this subgroup of the Proteobacteria.
Arsenic (As) is a known carcinogen in drinking water, occurring therein primarily as arsenate [As(V)] or as arsenite [As(III)], with the latter oxyanion having greater toxicity and hydrologic mobility than the former (10). Although various chemical agents can drive the redox reactions occurring between the As(V) and As(III) species (7, 31), it has been shown that specific microbiological detoxification mechanisms can achieve this as well (5, 30). Recent discoveries have revealed that the biochemical reduction or oxidation of these two inorganic As species can also be coupled with energy conservation in some prokaryotes. Thus, respiratory (dissimilatory) reduction of As(V) is found in several phylogenetically diverse anaerobic Bacteria and Crenarchaeota (28), while the aerobic oxidation of As(III) provides energy for the rapid growth of chemoautotrophic bacteria isolated from gold mines (34, 35).
Significant lithotrophic oxidative processes need not necessarily be coupled biochemically with oxygen. Such reactions can occur under anoxic conditions, provided that the oxidant has a higher electrochemical potential than the reductant. Examples of this phenomenon are the bacterial oxidation of Fe(II) with nitrate (44) and the oxidation of phosphite by sulfate reducers (36). Here we explore the potential of oxidants such as nitrate and Fe(III) to be coupled with the microbial oxidation of As(III) to As(V).
Arsenic-rich Mono Lake, California, was selected as the source of materials for investigation. The high content of dissolved inorganic arsenic (200 μM) in this stratified soda lake (pH 9.8; salinity, 70 to 90 g liter−1) is derived from hydrothermal sources feeding into the lake coupled with evaporative concentration that is characteristic of this arid region (24). As a result of the recent prolonged salinity-based stratification of the water column (20), bottom waters (the monimolimnion) have become anoxic and contain high concentrations of sulfide, ammonia, and methane (22, 26, 29). Calculations based on previous work indicated that dissimilatory reduction of As(V) in the lake's anoxic water column mineralizes 8 to 14% of annual phytoplankton primary production (29). Dissimilatory As(V) reduction, rather than chemical reactions, was also shown to be responsible for the observed change in arsenic species from As(V) in the oxic epilimnion to As(III) in the anoxic hypolimnion. However, a chemical disequilibrium occurs in the highly reducing (≥1.25 mM sulfide) bottom waters because about 5 μM As(V) is present therein, which suggested a chemical and/or microbial mechanism for its resupply, a phenomenon also noted in a diversity of other stratified aquatic systems, including the bottom waters of a meromictic crater lake (38), an As-contaminated freshwater lake (41), and a marine fjord (32). We first noted microbial oxidation of As(III) to As(V) in Mono Lake bottom waters amended with NO3− (18), prompting us to more closely scrutinize this phenomenon. We now report biological As(III) oxidation linked to NO3− or Fe(III) reduction in water samples from an As-rich lake, and we demonstrate chemoautotrophic growth of a novel bacterium, strain MLHE-1, with As(III) and NO3− serving as its electron donor and acceptor, respectively.
MATERIALS AND METHODS
Lake water incubations.
Samples from the lake's anoxic bottom waters were shipped chilled (∼5°C) on the day after sampling by overnight courier to Menlo Park, Calif., and stored and processed as described previously (18). Experiments were started within 1 month of sample collection. Water was placed in glass syringes, which were capped and injected with stock solutions of As(III) (added as NaAsO2), NaNO3, NaNO2, or Fe(III) chelated with nitrilotriacetic acid (NTA) (22) or, in the case of Mn(IV), as a suspension of MnO2 particles. The final concentrations achieved with these additions were 5 mmol liter−1. Samples were incubated in the dark at ∼20°C (18). Subsamples from time courses were passed through nylon centrifuge filters (pore size, 0.2 μm), frozen, and analyzed within 2 weeks (18). Abiotic controls consisted of lake water filtered through a sterile 0.2-μm-pore-size filter, followed by injection of a formalin solution (final concentration, 4%) into the sealed glass syringes.
Isolation and growth of strain MLHE-1.
An anaerobic enrichment culture was first established using full-strength Mono Lake water supplemented with 5 mM As(III) plus 5 mM NaNO3 by methods outlined previously (45). Growth, verified by increased turbidity and the loss of As(III) and accumulation of As(V), was noted in culture tubes, and the enrichment was transferred into sterilized (autoclaved) Mono Lake water supplemented with As(III) and NO3− as stated above. The enrichment was successfully carried out in this fashion for ∼4 months, with transfers occurring every 1 to 2 weeks. The enrichment was then transferred into a defined mineral salts medium (see below) and taken through several sequential transfers over the course of 2 months to dilute out the undefined components (i.e., ∼90 mg of dissolved organic carbon liter−1) from the lake water. The anaerobic basal salts medium of Switzer Blum et al. (45), which is both saline (109 g/liter) and alkaline (pH 9.8), was modified by eliminating yeast extract and other potential organic substrates (e.g., lactate), as well as reducing agents (e.g., cysteine-sulfide). Standard culture conditions for growth experiments used 5 mM NaH2AsO3 as the electron donor and 5 mM NaNO3 as the electron acceptor. The culture was purified by serial dilution. Other electron acceptors tested included Fe(III) chelated with NTA (23) and oxygen (air headspace) in lieu of NO3− (N2 headspace). Anaerobic growth was also examined with sulfide (5 mM) or H2 (10% in N2) as the electron donor in lieu of As(III). Cells were also grown under heterotrophic conditions using 5 mM acetate as the electron donor with either oxygen (air headspace) or 5 mM nitrate (N2 headspace) as the electron acceptor.
Dark H14CO3− fixation.
Cell suspensions of strain MLHE-1 were prepared by growing anaerobic batch cultures (2 liters) on As(III) plus NO3− and washing them with basal salts medium lacking these components. Cells were resuspended in 360 ml, giving a concentration factor of 5.5-fold (final cell density, 3.8 × 108 cells ml−1). The basal salts medium was used to resuspend the cells but was modified to have a lower content of NaHCO3 (0.5 mM) plus Na2CO3 (1.0 mM) to allow for better incorporation of 14C into the cells. The suspensions were dispensed into serum bottles (20 ml into 57-ml bottles) and sealed. All manipulations were made in an anaerobic glove box. Suspensions were incubated with or without As(III) and NO3− (0.5 mM each), and NaH14CO3 (5 μCi/bottle) was added as a radiotracer. After 12 h of incubation in the dark (20°C) with gentle rotary shaking (100 rpm), cells were passed through a 0.45-μm-pore-size filter, and the filter was washed with ∼5 ml of the basal salts. Filters were acid fumed overnight in a desiccator, and radioactivity was counted with a liquid scintillation spectrometer.
Analytical methods.
The arsenic species in the lake water and culture experiments were determined by ion chromatography and high-performance liquid chromatography (13, 18, 23, 45). Nitrate, nitrite, and sulfate were also analyzed by ion chromatogaphy using a Dionex model DX 500 with an AS9HC column and a 9 mM sodium carbonate eluent. Sulfide was determined by spectrophotometry (9). Acetate was determined with an enzymatic bioanalysis-food analysis acetate kit (Roche). Cell counts were achieved with acridine orange (17). Scanning electron micrographs of strain MLHE-1 were prepared as outlined previously (40) with a LEO model 983 field emission scanning electron microscope. Hydrogen was measured by injecting syringe-sampled headspace gases into an HNU model 310 gas chromatograph equipped with a thermal conductivity detector and a 0.32- by 548-cm silica gel column (oven temperature, 50°C) with an argon carrier (flow rate, 20 ml min−1).
DGGE.
MLHE-1 cells were separated from culture medium by centrifugation. The cell pellet was resuspended in lysis buffer, and DNA was extracted and purified as described by Ferrari and Hollibaugh (16). We used PCR-denaturing gradient gel electrophoresis (DGGE) analysis to check the purity of the culture prior to sequencing. A portion of the 16S rRNA gene encompassing a hypervariable region was amplified with primers 356f (forward, CCTACGGGAGGCAGCAG, eubacterial) and 517r (reverse, ATTACCGCGGCTGCTGG, universal). A 40-bp GC clamp (CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCC) was added to the 5′ end of primer 356f, and fluorescein was attached to the 5′ end of primer 517r. PCR conditions were similar to those used by Ferrari and Hollibaugh (16). DGGE was performed with a CBS Scientific (Del Mar, Calif.) DGGE system as described by Bano and Hollibaugh (3). Gels were scanned with an FMBIO II (Hitachi) gel scanner set to measure fluorescein fluorescence.
Sequencing and phylogenetic analysis.
A large fragment of the 16S rRNA gene was amplified with primers 9f (forward, GAGTTTGATCCTGGCTCAG) and 1492r (reverse, GGTTACCTTGTTACGACTT). The nucleotide sequence of this fragment was determined at the University of Georgia Molecular Genetics Instrumentation Facility by using primers 9f and 1492r, with 356f as an internal primer that overlaps part of the sequence of each of the other two primers as outlined previously (3). The PCR product was also cloned and sequenced as described previously (3), and the sequence obtained from clones was identical to that obtained directly from the PCR product. Sequences were aligned by using the Genetics Computer Group Inc. package (Wisconsin package version 10.0, 1999) and compared to known sequences by using BLAST (1). Phylogenetic trees were inferred, and bootstrap analysis (100 replicates) was performed with the PHYLIP package (15) using evolutionary distances (Jukes-Cantor distances) and the neighbor-joining method.
A partial sequence of the gene encoding the large subunit of ribulose-1,5′-biphosphate carboxylase/oxygenase (RuBisCo) was obtained by PCR amplification of a portion of the gene with the RuBisCO form 1 cbbL gene primers described by Elsaied and Naganuma (14). The forward 20-mer primer (5′GACTTCACCAAAGACGACGA-3′) corresponds to nucleotide positions 595 to 615 of the Anabaena strain 7120 cbbL gene (accession number L02520), while the reverse 20-mer primer (5′-TCGAACTTGATTTCTTTCCA-3′) corresponds to nucleotide positions 1387 to 1405 of the same Anabaena strain 7120 cbbL gene (11). This primer set amplifies an approximately 800-bp fragment of the cbbL gene. PCRs were performed as described above (16S rRNA gene sequence) using the following conditions: initial denaturation of the template DNA at 94°C for 2 min, followed by 30 cycles of denaturation (1 min at 94°C), annealing (1 min at 49°C), and extension (3 min at 72°C). The PCR product was purified using Wizard PCR preparations (Promega) and sequenced in both directions.
Nucleotide sequence accession number.
The nucleotide sequence that we obtained has been deposited in GenBank under accession number AF406554.
RESULTS
Experiments with Mono Lake water.
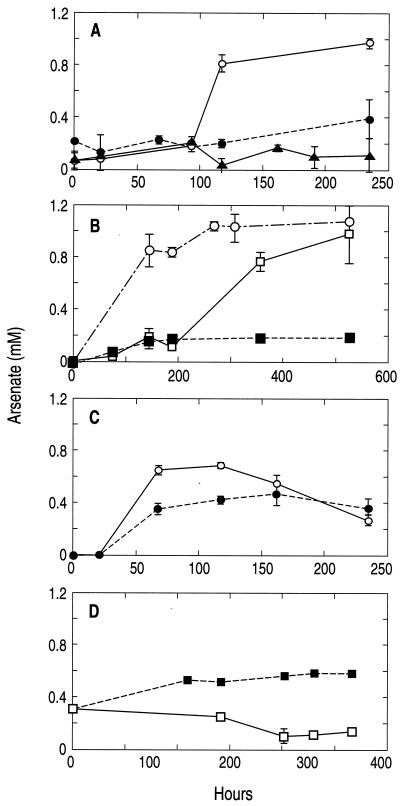
Nitrate-supplemented waters quantitatively converted As(III) to As(V), while killed controls formed only small amounts of As(V) (Fig. 1A). In the live samples, nitrate was completely reduced to nitrite (data not shown). By the end of the experiment, the accumulated nitrite in the three water samples (4.6 ± 1.1 mM) was close to the amount of nitrate initially added (5.4 ± 0.3 mM). We also observed oxidation of 1 mM As(III) with 5 mM nitrite, although there was a considerable lag period (∼200 h) before it was evident (Fig. 1B). We did not determine the end product of nitrite reduction.
FIG. 1.
Formation of As(V) in anoxic lake water samples amended with 1 mM As(III). Note that the time scales (x axis) vary between the four panels. (A) Samples supplemented with 5 mM NaNO3 added as the terminal electron acceptor to both live and killed controls, plus a live control without NO3−. Symbols: ○, live with nitrate; •, killed with nitrate; ▴, live without nitrate. (B) Samples amended with nitrate (○) or nitrite (□), and killed controls with nitrite (▪). (C) Live (○) and killed (•) controls amended with 5 mM Fe(III)-NTA. (D) Live (□) and killed (▪) controls amended with MnO2. Symbols represent the means for three separately incubated water samples, and error bars indicate ± 1 standard deviation.
Live samples amended with Fe(III)-NTA initially formed an orange precipitate, indicating that a portion of the chelated iron did not remain in solution but precipitated as FeOOH. After 7 days the live samples progressively turned a dark brown, suggesting biological reduction to Fe(II) and precipitation as FeCO3. The abiotic controls, however, remained orange for the duration of the incubation. Live samples initially produced As(V) with time, but after ∼120 h these levels declined (Fig. 1C). Arsenate production in the live samples initially exceeded the As(III) oxidation that occurred in abiotic controls.
Addition of MnO2 particles to samples resulted in its obvious presence as a black precipitate. Over time (∼12 days) the abundance of this precipitate in the live samples decreased markedly, while it remained at its initial high density in the controls. Significant levels of As(V) (∼300 μM) were detected in both the live samples and a filter-sterilized control at the beginning of the experiment (Fig. 1D), which reflected an elapsed time of an hour or two between the times of initial injection and the first sampling. After 300 h of incubation, however, the levels of As(V) in the live samples declined to ∼100 μM, while that in the control increased steadily to ∼600 μM.
Isolation of and growth experiments with strain MLHE-1.
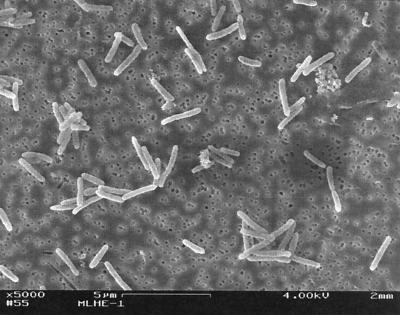
Successful transfer of the lake water-based enrichment into the basal salts medium was achieved, and after growth was evident, a serial dilution was performed, with growth and As(III) oxidation evident at the 10−8 dilution. Uniform cell morphology, as determined by microscopic examination, was observed at this high dilution. DGGE analysis of the DNA extracted from the culture gave a single band, and the 16S ribosomal DNA sequence obtained from this DNA was unambiguous, indicating that the culture was pure. Growth on As(III) plus NO3− generated weak turbidity (A680 = 0.07), but cell density could be increased (A680 > 0.10) by more additions of electron donor plus acceptor. Cells consisted of short, motile rods (Fig. 2) that stained gram negative.
FIG. 2.
Scanning electron micrograph of strain MLHE-1. Bar, 5 μm.
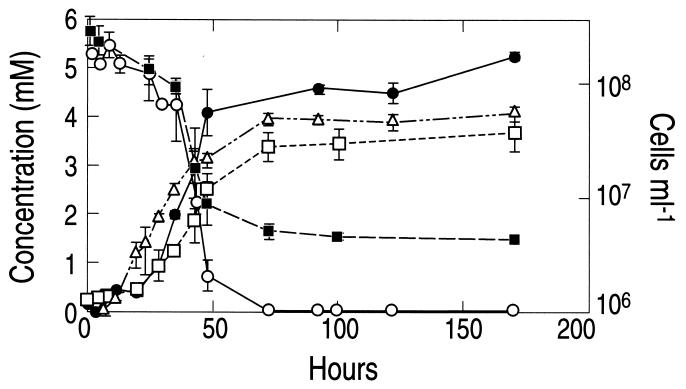
The isolate, strain MLHE-1, was able to achieve growth by oxidation of As(III) to As(V) with the reduction of equivalent quantities of nitrate to nitrite (Fig. 3). This indicated that a two-electron transfer occurred between As(III) and nitrate according to the following equation:
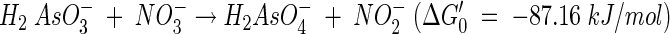 |
(1) |
FIG. 3.
Anaerobic growth of strain MLHE-1 with As(III) as the electron donor and nitrate as the electron acceptor. Symbols: •, As(V); ○, As(III); ▪, nitrate; □, nitrite; ▵, cells. Symbols represent the means for three separate cultures, and error bars indicate ± 1 standard deviation.
The doubling time and specific growth rate under these conditions were, respectively, 8.1 h and 0.09 h−1. No further reduction of NO2− was observed and no growth occurred in controls that lacked either nitrate or As(III), and no abiotic oxidation of As(III) occurred in sterile controls (not shown). In addition, we did not observe anaerobic growth of strain MLHE-1 with Fe(III)-NTA as the electron acceptor with As(III) as the electron donor (data not shown). Neither did we observe significant growth or As(III) oxidation under an air atmosphere (oxygen as the electron acceptor). After 93 h of incubation under an air atmosphere, only a small amount of As(V) accumulated (0.1 ± 0.05 mM; n = 3), and cell densities at the beginning of the incubation (5.8 × 106 ± 1.6 × 106 cells ml−1) were about the same as those after 93 h (5.2 × 106 ± 3.0 × 106 cells ml−1).
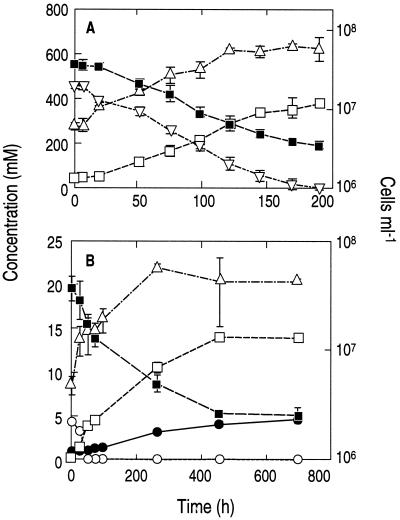
Strain MLHE-1 also exhibited a capacity for anaerobic, chemoautotrophic growth with either H2 (Fig. 4A) or sulfide (Fig. 4) serving as the electron donor and with NO3− as the electron acceptor. Growth on H2 revealed a reasonably close 1:1 stoichiometry between H2 oxidized (0.45 mM) and NO3− reduced (0.36 mM) to NO2− (0.34 mM). The doubling time was 37 h, and the specific growth rate was 0.019 h−1. No growth occurred in controls without H2 or NO3− (not shown). Growth on sulfide resulted in the production of sulfate, while NO3− was reduced to NO2−. There was a 1:1 stoichiometry between the sulfide-sulfate and NO3−-NO2− couples, with a factor of 4 evident between sulfide oxidized and NO3− reduced, indicating the occurrence of an eight-electron transfer reaction. No growth occurred in controls without sulfide (not shown). Because the kinetics of sulfide loss were much more rapid than those of sulfate production, the occurrence of intermediary oxidation states of sulfur (e.g., sulfite or thiosulfate) as transient intermediates in this oxidation is implied, although we did not investigate this possibility. The doubling time for growth on sulfide was 74 h, and the specific growth rate was 0.009 h−1. Clearly, growth of strain MLHE-1 on As(III) was more rapid than that with either H2 or sulfide.
FIG. 4.
Anaerobic growth of strain MLHE-1 with nitrate as the electron acceptor and the inorganic electron donors hydrogen (A) and sulfide (B). Symbols: ▪, nitrate; □, nitrite; ▿, hydrogen; ▵, cells; ○, sulfide; •, sulfate. Symbols represent the means for three cultures, and error bars indicate ± 1 standard deviation.
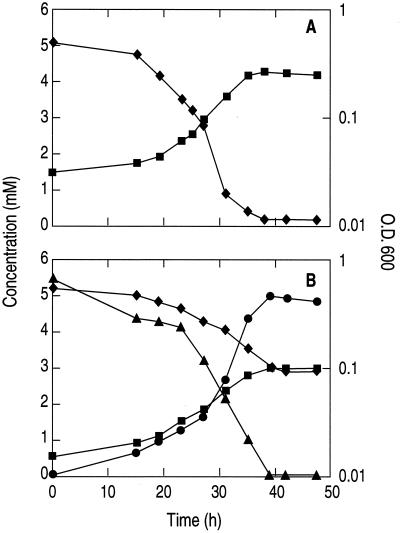
Heterotrophic growth of MLHE-1 with acetate as the electron donor and oxygen (air atmosphere) or NO3− as the electron acceptor is shown in Fig. 5A and B, respectively. When NO3− was provided, it was reduced to NO2− and no further reduction was evident. The generation time during exponential aerobic growth was 5.5 ± 0.2 h (average from four experiments), while during anaerobic growth on NO3− it was 9.5 ± 1 h (average from three experiments). Growth did not occur when air, nitrate, or acetate was omitted from the medium (data not shown).
FIG. 5.
Heterotrophic growth of strain MLHE-1 with acetate as the electron donor under aerobic conditions (A) and anaerobic conditions with nitrate as the electron acceptor (B). Symbols: ♦, acetate; ▴, nitrate; •, nitrite; ▪, optical density at 600 nm (O.D. 600).
Phylogenetic alignment.
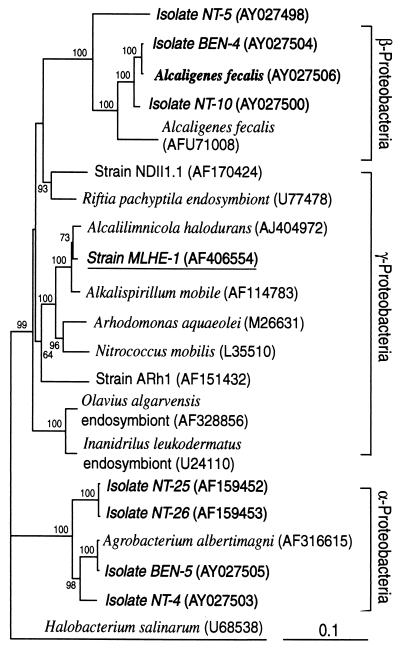
Phylogenetic analysis of the partial 16S ribosomal DNA sequence that we obtained from strain MLHE-1 revealed that it was affiliated with the Ectothiorhodospiraceae of the γ-Proteobacteria (Fig. 6). Sequence similarities indicate that it is most closely related to the haloalkaliphiles Alcalilimnicola halodurans (98.6%) and Alkalispirillum mobile (98.5%).
FIG. 6.
Phylogenetic relationships of strain MLHE-1 based on phylogenetic analysis of its 16S rRNA gene sequence. Aerobic As(III) oxidizers, as summarized by Santini et al. (34), are in boldface, and the tree includes the chemoautotrophic strains of the α-Proteobacteria related to the genus Agrobacterium and the heterotrophic strains of the β-Proteobacteria related to the genus Alcaligenes (30). The tree is unrooted, with Halobacterium salinarum as the outgroup. Numbers adjacent to nodes indicate bootstrap support for the branch (100 iterations; values of <50 are not shown), and the bar at the bottom indicates the number of nucleotide substitutions per position.
Dark H14CO3− fixation and RuBisCo sequences.
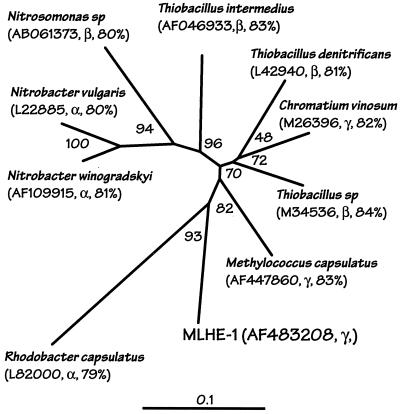
Suspensions of As(III)-grown cells demonstrated dark 14CO2 fixation. Thus, 14C counts on filters containing cells incubated with NO3− plus As(III) gave 2,041 ± 238 dpm (mean from three cell suspensions ± 1 standard deviation), while ∼10-fold-lower counts were measured in the following controls: cells incubated without any additions (190 ± 38 dpm), cells with only As(III) added (212 ± 46 dpm), or mixtures without cells but with both nitrate and As(III) added (135 ± 46 dpm). Phylogenetic analysis of the MLHE-1 cbbL gene sequence (Fig. 7) placed it in the form 1 cluster of RuBisCO (39). BLAST analysis (1) indicated that it was most closely related to a Thiobacillus-like organism (accession number M34536) (86% similarity; 554 of 638 bp) whose 16S rRNA sequence identified it as a member of the β-Proteobacteria (43). It was also 85% similar (552 of 642 bp) to Methylococcus capsulatus (accession number AF447860).
FIG. 7.
Phylogenetic relationship between a partial sequence of the cbbL gene from strain MLHE-1 and sequences from representative isolates. The neighbor-joining tree is unrooted and is based on analysis of 744 bp of nucleotide sequence. The accession numbers of sequences used to construct the tree, the phylogenetic affiliations of the isolates (16S rRNA genes), and the BLAST (1) similarity of the MLHE-1 gene to the isolate gene are given in parentheses. The scale bar indicates an evolutionary distance of 0.1, and numbers at branch points indicate bootstrap support as in Fig. 6.
DISCUSSION
The stimulation of As(III) oxidation with NO3−
(Fig. 1A) demonstrates a clear biological enhancement of this phenomenon. Similar results were obtained with nitrite (Fig. 1B), although there was a long lag time before As(III) oxidation was evident. Presumably this lag represented a period of adaptation of the resident flora to the high concentrations (5 mM) of added nitrite, a strong oxidant with well-recognized antimicrobial properties. We did not pursue the metabolic fate of nitrite, though it was most likely further reduced to either dinitrogen gas or ammonia. Our interest in As(III) oxidation with nitrate in Mono Lake grew out of earlier observations of what, at first glance, appeared to be an inhibitory effect of nitrate upon dissimilatory As(V) reduction (18). The observation that nitrate apparently also strongly inhibited As(V) reduction in anoxic estuarine sediments (13) suggests that the potential for As(III) oxidation by anaerobes may be distributed more widely in nature than just within the confines of this unusual soda lake (see below).
The ecological importance of nitrate as an in situ oxidant for As(III) in Mono Lake is not clear because the epilimnion of Mono Lake is severely nitrogen limited (21). This notwithstanding, very high rates of ammonia oxidation occur in the water column (22), and nitrifying bacteria are also present therein (46). This paradox can be explained by concurrent high rates of consumption by NO3−-respiring bacteria occurring in a tightly coupled nitrification-denitrifcation cycle. The results of our present study indicate that NO3− has the potential to serve as an electron acceptor for in situ As(III) oxidation in this system. Future experiments should examine quantitative relationships between NO3− production, NO3− consumption, and As(III) oxidation in Mono Lake, as was recently reported for another lake (37).
We also examined whether Fe(III) or Mn(IV) could serve as an electron acceptor for As(III) oxidation. Low concentrations of dissolved Fe(III) and Mn(IV) are generally observed in Mono Lake, reaching only as high as 10 and 0.4 μM, respectively (24, 25). It is well known that Mn(IV) is a very potent chemical oxidant of As(III), while Fe(III) is a contrastingly poor one (7). We detected As(V) production in both live and killed controls in the experiments with Fe(III) (Fig. 1C). The more rapid initial accumulation of As(V) in the live samples suggests that oxidation was augmented by biological coupling. The As(V) concentrations declined in the live samples after 120 h, and a slight decline was noted in controls after 160 h. The fact that As(V) concentrations never exceeded 0.6 mM in this experiment but reached 1 mM in the experiments with NO3− and NO2− (Fig. 1A and B) suggests significant adsorption of As(V) to the precipitated Fe(III). In addition, any As(V) formed in the live samples would also be subject to dissimilatory reduction back to As(III) at the expense of other electron donors, such as dissolved organic carbon (18), which explains its rapid removal in contrast to the sterile controls. This view is reinforced by our observations of the color changes of the precipitate that implied the formation of FeCO3 in the live samples (see Results).
The persistence of As(V) in solution in the NO3− amendments (Fig. 1A) but not in the Fe(III) amendments (Fig. 1C) is curious. The reason for this disparity between the treatments was not immediately obvious, because sufficient equivalents of electron acceptor were provided with both the Fe(III) [5 mM e− equivalents for reduction to Fe(II)] and NO3− (40 mM e− equivalents for reduction to NH3) to consume all of the reducing equivalents generated from the oxidation of 1 mM As(III) to As(V) (2 mM e− equivalents). However, these waters contain other potential electron donors besides the added As(III), including ∼90 mg of dissolved organic carbon liter−1 (12), a portion of which may be available to serve as electron donors for microorganisms capable of dissimilatory As(V) reduction (18). In addition, the bottom waters contain abundant reduced compounds (e.g., sulfide, ammonia, and methane) that could also be used as chemoautotrophic or one-carbon electron donors. Therefore, the supply of electron donors appears to have exceeded that of added electron acceptor in experiments with Fe(III)-NTA, and the As(V) formed by As(III) oxidation was subjected to dissimilatory reduction back to As(III). In contrast, the supply of electron acceptor in the NO3− amendments exceeded the amount of reducing equivalents, and the arsenic remained present as As(V).
The effects of chemical oxidation of As(III) by solid-phase Mn(IV) were immediately apparent as a result of the 0.3 mM As(V) generated at the start of the experiment (Fig. 1D) and the continued increase of As(V) over time in the killed control. The rate of abiotic As(III) oxidation was constrained by adsorption of As(V) to the MnO2 particles. In contrast, the decline of As(V) over the course of the incubation in the live samples indicates its removal by dissimilatory reduction. Dissimilatory reduction with Mn(IV) as the electron acceptor was also occurring, as evidenced by the visual thinning of the amount of MnO2 precipitate in the live samples but not in the controls. As was the case with nitrate, it is not possible from these preliminary experiments to gauge the in situ importance of either Fe(III) or Mn(IV) as an oxidant of As(III) in Mono Lake. However, each element clearly does have the potential to oxidize As(III) in the matrix that comprises Mono Lake water.
We established an anaerobic enrichment culture and carried it through several transfers. We chose NO3− as the terminal electron acceptor in lieu of Fe(III) for practical reasons, as it is both soluble and a stronger oxidant. Repeated transfers in artificial medium eliminated the dissolved organics carried over from the lake water by dilution. The enrichment was purified by serial dilution, and its purity was confirmed by the presence of a single band of DNA on a DGGE gel.
The 1:1 balance achieved during growth of MLHE-1 between (i) NO3− and As(III) removed and (ii) NO2− and As(V) produced (Fig. 2) verifies the two-electron transfer outlined in equation 1. Therefore, because no growth occurred in live controls without NO3− or As(III), strain MLHE-1 must be a chemoautotroph. This ability was confirmed by the incorporation of H14CO3− in the dark into cell material compared with controls. Further evidence was its ability to grow using H2 or sulfide as an electron donor in lieu of As(III) (Fig. 3). The ability to obtain a PCR product with cbbL-specific primers and phylogenetic analysis of the fragment obtained confirm it to be a RuBisCO sequence and provide the final unequivocal evidence for the autotrophic capabilities of strain MLHE-1 (Fig. 7). Strain MLHE-1 was unable to grow as an autotroph with As(III) and Fe(III)-NTA. Thus, any As(III) oxidation linked to Fe(III) reduction that we observed in Mono Lake water (Fig. 1C) was probably carried out by other components of the lake's microbial flora. The chemoautotrophic oxidation of As(III) with NO3− by strain MLHE-1 is therefore analogous to the oxidation of Fe(II) with NO3− by anaerobic bacteria (44).
Strain MLHE-1 aligns with the γ-Proteobacteria, as a member of the Ectothiorhodospira, most closely related to Alkalispirillum mobile (98.5%) and Alcalilimnicola halodurans (98.6%) but distant from known aerobic strains of As(III)-oxidizing bacteria (Fig. 6). Members of the Ectothiorhodospira are usually halophilic, anaerobic phototrophs or, in the case of Alkalispirillum mobile, an aerobic chemoheterotroph (33). Chemoautotrophy or the ability to metabolize arsenic oxyanions has not been described previously for these organisms.
The ability of strain MLHE-1 to grow using acetate as its electron donor with oxygen or NO3− as its electron acceptor means that it is both a facultative chemoautotroph and a facultative anaerobe. Failure to observe aerobic or microaerophilic growth with As(III) would suggest that growth on this electron donor can be achieved only under anaerobic conditions. Aerobic, chemoautotrophic growth on As(III) has been reported for strain NT-26, a member of the α-Proteobacteria that has a doubling time (7.6 h) comparable to that of strain MLHE-1 (7.9 h) (35). Santini et al. (34) isolated several new species of aerobic As(III)-oxidizing bacteria from gold mines in Australia. These isolates, which comprise both autotrophs and heterotrophs that oxidize As(III), as well as several previously described species (30), are shown in Fig. 6, where they cluster in either the α- or β-Proteobacteria. It is clear that strain MLHE-1 is not taxonomically related to the microorganisms in either of these groups. It remains to be seen if anaerobic As(III) oxidation like that carried out by strain MLHE-1 is a broadly polyphyletic phenomenon, like that of dissimilatory As(V) reduction (28), or more narrowly distributed. It is not yet known if the facultative chemoautotroph strain NT-26 can grow under anaerobic conditions with As(III) and NO3− (J. M. Santini, unpublished data).
The microbial oxidation of As(III) with NO3− as the terminal electron acceptor is probably a phenomenon that is widespread in nature. For example, the mass balances of As(III), NO3−, and Fe(II) in the As-contaminated, freshwater Mystic Lake indicated that As(III) in the sediments was biologically oxidized to As(V) when the lake was stratified (37). Injection of As(III) into the suboxic groundwater zone at a U.S. Geological Survey experimental site on Cape Cod, Massachusetts, resulted in its down-gradient oxidation to As(V) (42). Although those authors speculated that this oxidation was caused by interaction with Mn(IV) or Fe(III) minerals, the groundwater also contained considerable NO3− (200 μM) derived from treated sewage effluent, which would have been sufficient to allow for the oxidation of the injected 100 μM As(III).
The microbial oxidation of As(III) with NO3− as a terminal electron acceptor has implications for the mobility of arsenic in aquifers that have a low dissolved organic matter content. Mildly reducing subsurface systems that receive NO3− either from fertilizer application or from sewage effluent will be poised to retard the transport of As(V) because of its strong adsorption to minerals. In addition, the oxidative dissolution of arsenopyrites could occur under mildly reducing conditions with NO3− in lieu of oxygen. Hence, this phenomenon may prove to be of importance in understanding the mechanisms of arsenic speciation and mobilization in the subsurface aquifers of As-contaminated locales like those of Bangladesh, which can contain both NO3− and arsenic (27). Organisms like strain MLHE-1 seem well adapted to survive in subsurface environments because of their “flexible” metabolism. The ability to grow with diverse inorganic electron donors allows for its survival in the absence of organic matter and enables it to colonize barren regions, while its heterotrophic mode would sustain it when there is an abundance of organic substrates.
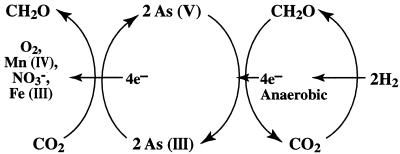
The oxidation of As(III) with NO3− means that there can be a complete anaerobic cycle of arsenic between its +5 and +3 oxidation states that supports the growth of both arsenate-respiring and arsenite-oxidizing prokaryotes (Fig. 8). It is curious that both the oxidative and reductive sides of this cycle have representative autotrophs, as typified by the As(III)-oxidizing strain MLHE-1 and by the As(V)-reducing Pyrobaculum arsenaticum, which uses H2 (19). Such an arsenic-based metabolism allows for the fixation of CO2 into living organic matter in the absence of photosynthesis. This is analogous to the recent discovery of a subsurface H2-based methanogenic community (6). Like that of H2, the metabolism of arsenic by microorganisms may have exobiological implications. Hydrothermal fluids often contain relatively high concentrations (∼10 to 50 μM) of arsenic (47) as well as H2 and/or sulfide. The E0′ of the As(V)-As(III) couple is +60 mV, which allows for biological As(III) oxidation to be linked to more-positive pairs such as NO3−-NO2− (+430 mV) or Fe(III)-Fe(II) (+100 mV) (4). Molecular oxygen is absent from all bodies in the solar system except Earth, but other substances on these anoxic worlds could initiate the oxidation of As(III) by generating biochemical oxidants such as NO3− and Fe(III). Strong oxidants are present in Martian soils, and Chyba and Hand (8) have speculated on mechanisms for the formation of oxidants on Europa. Arsenic is present in meteorites from Mars (2). Therefore, planetary bodies (e.g., Mars and Europa) that have (or had) both liquid water and volcanic activity present in their geologic history could conceivably have evolved a microbial ecosystem based on the metabolism of arsenic.
FIG. 8.
Proposed biogeochemical cycle of arsenic between its +5 and +3 oxidation states as mediated by microorganisms. Note that chemoautotrophic microorganisms occupy anaerobic niches on both the reductive and oxidative sides, using H2 and As(III) as electron donors, respectively, to gain energy for the fixation of CO2 into cell carbon.
Acknowledgments
This research was supported by the USGS National Research Program and a NASA Exobiology grant (to R.S.O.) and by a U.S. National Science Foundation grant (MCB 99-77886) to J.T.H.).
We are grateful to J. F. Stolz and H. L. Ehrlich for their constructive comments on an earlier draft of the manuscript, and we thank D. Senn, H. Hemond, D. Nordstrom, and J. Kargel for helpful discussions and A. Jacobs for technical assistance.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Banin, A., B. C. Clark, and H. Wanke. 1992. Surface chemistry and mineralogy, p. 594-625. In H. H. Kieffer, B. M. Jakowsky, C. W. Snyder, and M. S. Matthews (ed.), Mars. University of Arizona Press, Tucson.
- 3.Bano, N., and J. T. Hollibaugh. 2000. Diversity and distribution of DNA with affinity to ammonia-oxidizing bacteria of the β-subclass of the class Proteobacteria in the Arctic Ocean. Appl. Environ. Microbiol. 66:1960-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bard, A. J., R. Parsons, and J. Jordon. 1985. Standard potentials in aqueous solutions. Marcel Dekker, New York, N.Y.
- 5.Cervantes, C., G. Ji, J. L. Ramírez, and S. Silver. 1994. Resistance to arsenic compounds in microorganisms. FEMS Microbiol. Rev. 15:355-367. [DOI] [PubMed] [Google Scholar]
- 6.Chapelle, F. H., K. O'Neill, P. M. Bradley, B. A. Methé, S. A. Ciufo, L. L. Knobel, and D. R. Lovley. 2002. A hydrogen-based subsurface microbial community dominated by methanogens. Nature 415:312-315. [DOI] [PubMed] [Google Scholar]
- 7.Cherry, J. A., A. U. Shaikh, D. E. Tallman, and R. V. Nicholson. 1986. Arsenic species as an indicator of redox conditions in groundwater. J. Hydrol. 261:15030-15038. [Google Scholar]
- 8.Chyba, C., and K. P. Hand. 2001. Life without photosynthesis. Science 292:2026-2027. [DOI] [PubMed] [Google Scholar]
- 9.Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-459. [Google Scholar]
- 10.Cullen, W. R., and K. J. Reimer. 1989. Arsenic speciation in the environment. Chem. Rev. 89:713-764. [Google Scholar]
- 11.Curtis, S. E., and R. Haselkorn. 1983. Isolation and sequence of the gene for the larger subunit of ribulose-1,5-biphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc. Natl. Acad. Sci. USA 80:1835-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domagalski, J. L., H. P. Eugster, and B. F. Jones. 1990. Trace metal geochemistry of Walker, Mono, and Great Salt Lakes, p. 315-353. In R. J. Spencer and I-Ming Chou (ed.), Fluid-mineral interactions: a tribute to H. P. Eugster. Special publication no. 2. The Geochemical Society, St. Louis, Mo.
- 13.Dowdle, P. R., A. M. Laverman, and R. S. Oremland. 1996. Bacterial dissimilatory reduction of arsenic(V) to arsenic(III) in anoxic sediments. Appl. Environ. Microbiol. 62:1664-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsaied, H., and T. Naganuma. 2001. Phylogenetic diversity of ribulose-1,5-bisphosphate carboxylase/oxygenase large-subunit genes from deep-sea microorganisms. Appl. Environ. Microbiol. 67:1751-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package), 3.5c ed. University of Washington, Seattle.
- 16.Ferrari, V. C., and J. T. Hollibaugh. 1999. Distribution of microbial assemblages in the central Arctic Ocean basin studied by PCR/DGGE: analysis of a large data set. Hydrobiologia 40:55-68. [Google Scholar]
- 17.Hobbie, J. E., R. L. Daley, and S. Jaspar. 1977. Use of Nuclepore filters for counting bacteria for fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoeft, S. E., F. Lucas, J. T. Hollibaugh, and R. S. Oremland. 2002. Characterization of microbial arsenate reduction in the anoxic bottom waters of Mono Lake, California. Geomicrobiol. J. 19:1-18. [Google Scholar]
- 19.Huber, R., M. Sacher, A. Vollmann, H. Huber, and D. Rose. 2000. Respiration of arsenate and selenate by hyperthermophilic Archaea. Syst. Appl. Microbiol. 23:305-314. [DOI] [PubMed] [Google Scholar]
- 20.Jellison, R., J. Romero, and J. M. Melack. 1998. The onset of meromixis during restoration of Mono Lake, California: unintended consequences of reducing water diversions. Limnol. Oceanogr. 43:706-711. [Google Scholar]
- 21.Jellison, R., and J. M. Melack. 1988. Photosynthetic activity of phytoplankton and its response to meromixis in Mono Lake, California. Hydrobiologia 158:69-88. [Google Scholar]
- 22.Joye, S. M., T. L. Connell, L. G. Miller, R. S. Oremland, and R. A. Jellison. 1999. Oxidation of ammonia and methane in an alkaline, saline lake. Limnol. Oceanogr. 44:178-188. [Google Scholar]
- 23.Laverman, A. M., J. Switzer Blum, J. K. Schaefer, E. J. Philips, D. R. Lovley, and R. S. Oremland. 1995. Growth of strain SES-3 on arsenate and other diverse electron acceptors. Appl. Environ. Microbiol. 61:3556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maest, A. S., S. P. Pasilis, L. G. Miller, and D. K. Nordstrom. 1992. Redox geochemistry of arsenic and iron in Mono Lake, California. p. 507-511 In Y. K. Kharaka and A. S. Maest (ed.), Water-rock interaction. WRI-7. A. A. Balkema Publishers, Rotterdam, The Netherlands.
- 25.Mason, D. T. 1967. Limnology of Mono Lake, California. Univ. Calif. Publ. Zool. 83:1-110. [Google Scholar]
- 26.Miller, L. G., R. Jellison, R., R. S. Oremland, and C. W. Culbertson. 1993. Meromixis in Mono Lake. 3. Breakdown of stratification and biogeochemical response to overturn. Limnol. Oceanogr. 38:1040-1051. [Google Scholar]
- 27.Nickson, R. T., J. M. MacArthur, P. Ravenscroft, W. G. Burgess, and K. M. Ahmed. 2000. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl. Geochem. 15:403-413. [Google Scholar]
- 28.Oremland, R. S., D. K. Newman, B. W. Kail, and J. F. Stolz. 2001. Bacterial respiration of arsenate and its significance in the environment, p. 273-296. In W. T. Frankenberger, Jr. (ed.), Environmental chemistry of arsenic. Marcel Dekker, New York, N.Y.
- 29.Oremland, R. S., P. R. Dowdle, S. Hoeft, J. O. Sharp, J. K. Schaefer, L. G. Miller, J. Switzer Blum, R. L. Smith, N. S. Bloom, and D. Wallschlaeger. 2000. Bacterial dissimilatory reduction of arsenate and sulfate in meromictic Mono Lake, California. Geochim. Cosmochim. Acta 64:3073-3084. [Google Scholar]
- 30.Osborne, F. H., and H. L. Ehrlich. 1976. Oxidation of arsenite by a soil isolate of Alcaligenes. J. Appl. Bacteriol. 41:295-305. [DOI] [PubMed] [Google Scholar]
- 31.Oscarson, D. W., P. M. Huang, C. Defosse, and A. Herbillon. 1981. Oxidative power of Mn (IV) and Fe (III) oxides with respect to As (III) in terrestrial and aquatic environments. Nature 291:50-51. [Google Scholar]
- 32.Peterson, M. L., and R. Carpenter. 1983. Biogeochemical processes affecting total arsenic and arsenic species distributions in an intermittently anoxic fjord. Mar. Chem. 12:295-321. [Google Scholar]
- 33.Rijkenberg, M. J. A., R. Kort, and K. J. Hellingwerf. 2001. Alkalispirillum mobile gen. nov., spec. nov., an alkaliphilic non-phototrophic member of the Ectothiorhodospiraceae. Arch. Microbiol. 175:369-375. [DOI] [PubMed] [Google Scholar]
- 34.Santini, J. M., L. I. Sly, A. Wen, D. Comrie, P. De Wulf-Durand, and J. M. Macy. 2002. New chemolithoautotrophic arsenite oxidizing bacteria isolated from Australian gold mining environments—phylogenetic relationships. Geomicrobiol. J. 19:67-76. [Google Scholar]
- 35.Santini, J. M., L. I. Sly, R. D. Schnagl, and J. M. Macy. 2001. A new chemoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl. Environ. Microbiol. 66:92-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schink, B., and M. Friedrich. 2000. Phosphite oxidation by sulfate reduction. Nature 406:37. [DOI] [PubMed] [Google Scholar]
- 37.Senn, D. B., and H. F. Hemond. 2002. Nitrate controls on iron and arsenic in an urban lake. Science 296:2373-2376. [DOI] [PubMed] [Google Scholar]
- 38.Seyler, P., and J.-M. Martin. 1989. Biogeochemical processes affecting arsenic species distribution in a permanently stratified lake. Environ. Sci. Technol. 23:1258-1263. [Google Scholar]
- 39.Shively, J. M., G. van Keulen, and W. G. Meijer. 1998. Something from almost nothing. Carbon dioxide fixation in chemoautotrophs. Annu. Rev. Microbiol. 52:191-230. [DOI] [PubMed] [Google Scholar]
- 40.Smith, R. L., F. S. Strohmaier, and R. S. Oremland. 1985. Isolation of anaerobic oxalate degrading bacteria from freshwater lake sediments. Arch. Microbiol. 14:8-13. [Google Scholar]
- 41.Spliethoff, H. M., R. P. Mason, and H. F. Hemond. 1995. Interannual variability in the speciation and mobility of arsenic in a dimictic lake. Environ. Sci. Technol. 29:2157-2161. [DOI] [PubMed] [Google Scholar]
- 42.Stadler, S., S. Jann, R. Höhn, M. Isenbeck-Schröter, V. W. Niedan, C. Scholz, A. Tretner, J. A. Davis, and D. B. Kent. 2001. Tracer tests with As(III) in the oxic and suboxic groundwater zones at the USGS Cape Cod site, Mass., USA, p. 1013-1016. In R. Cidu (ed.), Water-rock interaction. WRI-10. A. A. Balkema Publishers, Rotterdam, The Netherlands.
- 43.Stein, J. L., M. Haygood, and H. Felbeck. 1990. Nucleotide sequence and expression of a deep sea ribulose 1,5 bisphosphate carboxylase gene cloned from a chemoautotrophic bacterial endosymbiont. Proc. Natl. Acad. Sci. USA 87:8850-8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Straub, K. L., M. Benz, B. Schink, B., and F. Widdel. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 62:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Switzer Blum, J., A. Burns Bindi, J. Buzzelli, J. F. Stolz, and R. S. Oremland. 1998. Bacillus arsenicoselenatis sp. nov. and Bacillus selenitireducens sp. nov.: two haloalkaliphiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Arch. Microbiol. 171:19-30. [DOI] [PubMed] [Google Scholar]
- 46.Ward, B. B., D. Martino, C. Diaz, and S. B. Joye. 2000. Analysis of ammonia-oxidizing bacteria from hypersaline Mono Lake, California, on the basis of 16S rRNA sequences. Appl. Environ. Microbiol. 66:2873-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkie, J. A., and J. G. Hering. 2000. Rapid oxidation of geothermal arsenic (III) in streamwaters of the eastern Sierra Nevada. Environ. Sci. Technol. 34:4747-4753. [Google Scholar]